Effect of Oily Sludge Treatment with Molten Blast Furnace Slag on the Mineral Phase Reconstruction of Water-Quenched Slag Properties
Abstract
:1. Introduction
2. Materials and Methods
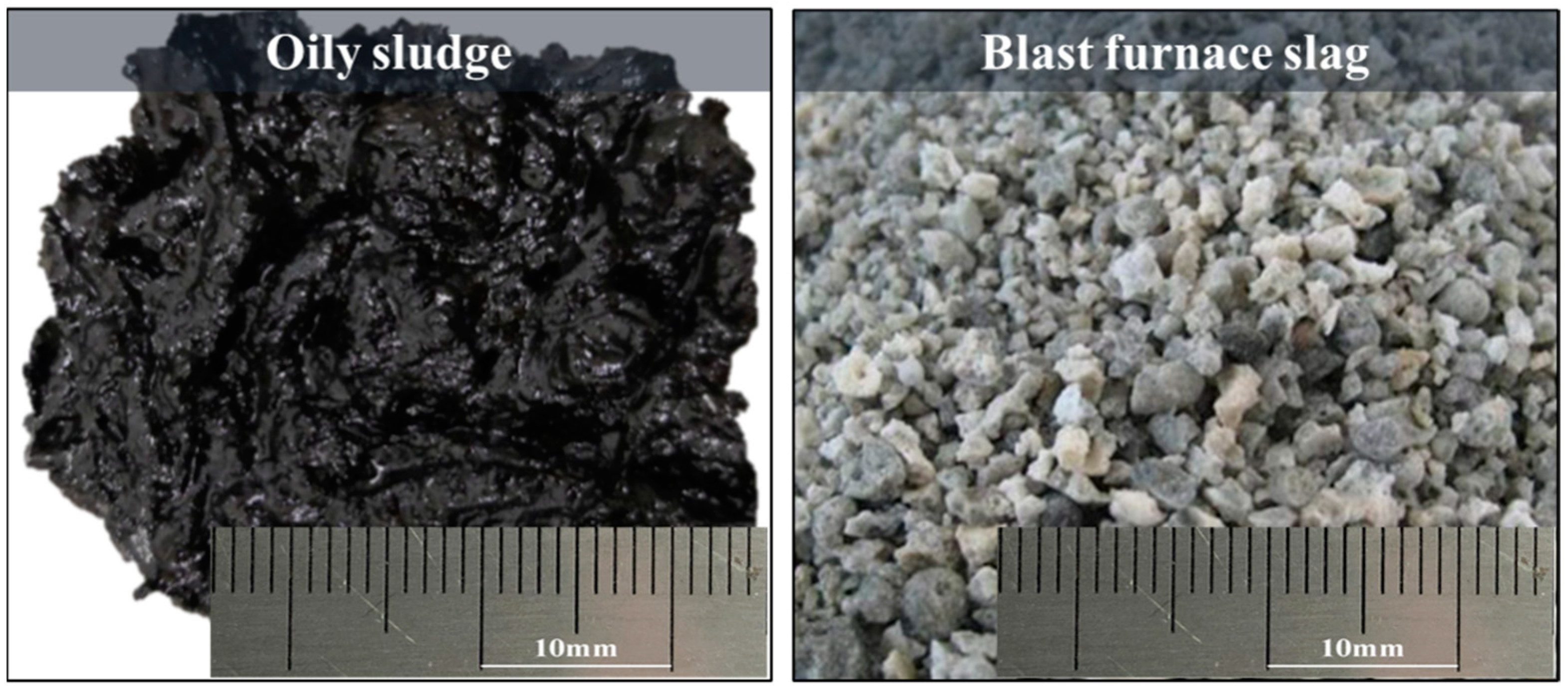
2.1. Raw Materials
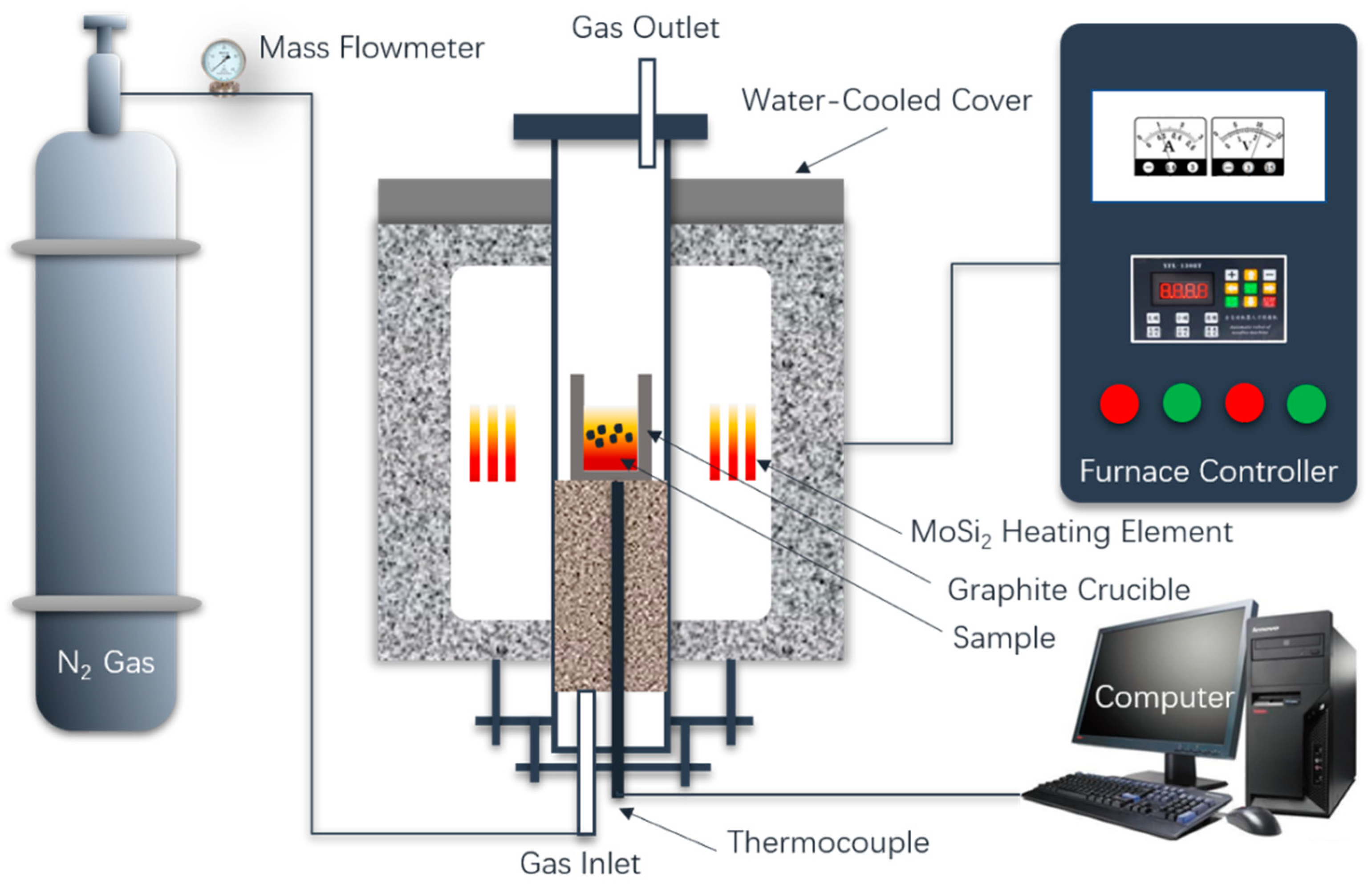
2.2. Experimental Methods
3. Results
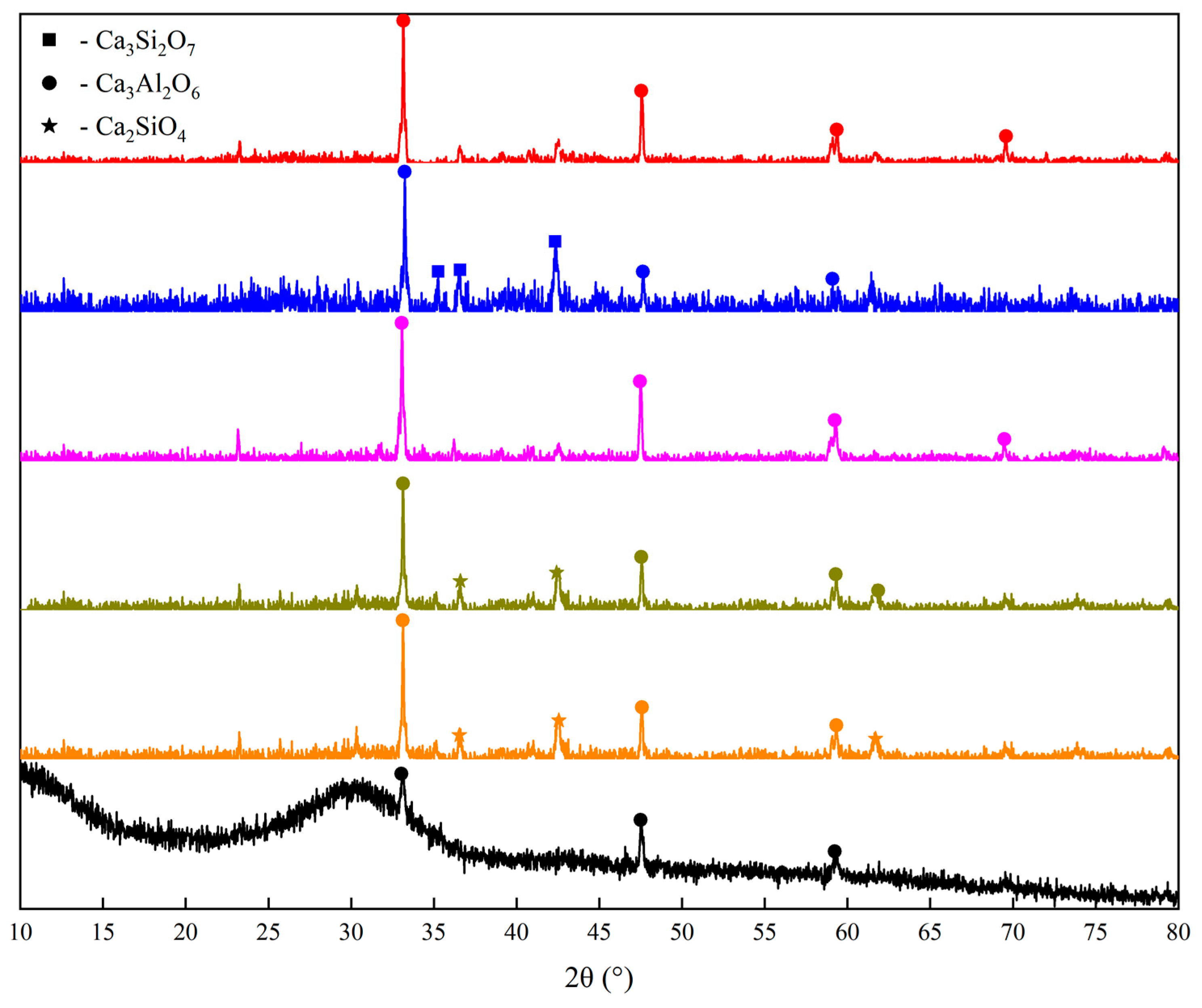
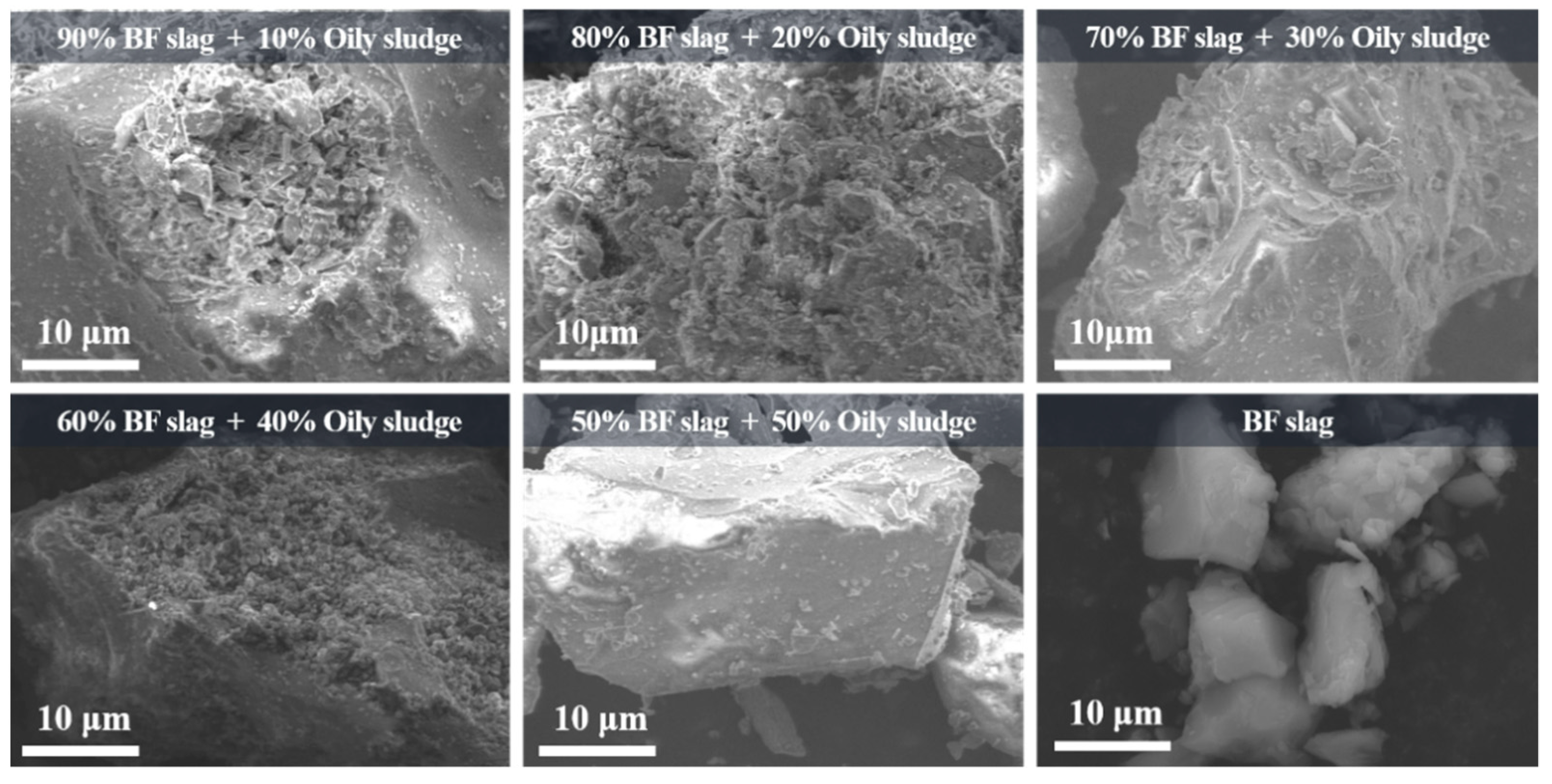
3.1. Analysis of Phase and Morphology of the Slag after Pyrolysis (BFS-P)
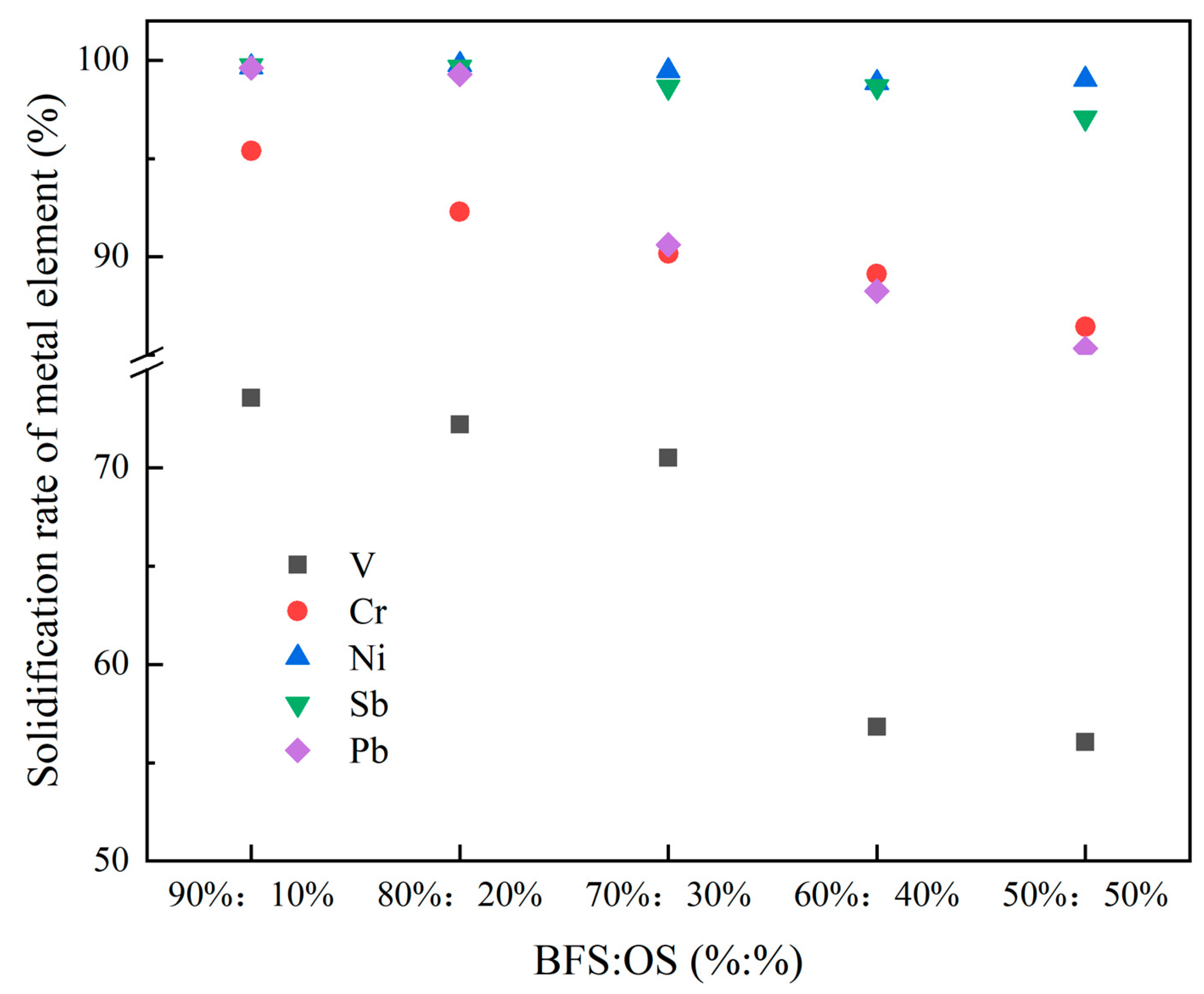
3.2. Analysis of the Solidification Rate and Stability of Heavy Metals in the Reconstruction Slag
- c1: concentration of heavy metals in the BFS-P, mg/kg;
- c2: concentration of heavy metals in BFS, mg/kg;
- c0: concentration of heavy metals in OS, mg/kg;
- m0: mass of OS, kg;
- m2: mass of BFS added, kg.
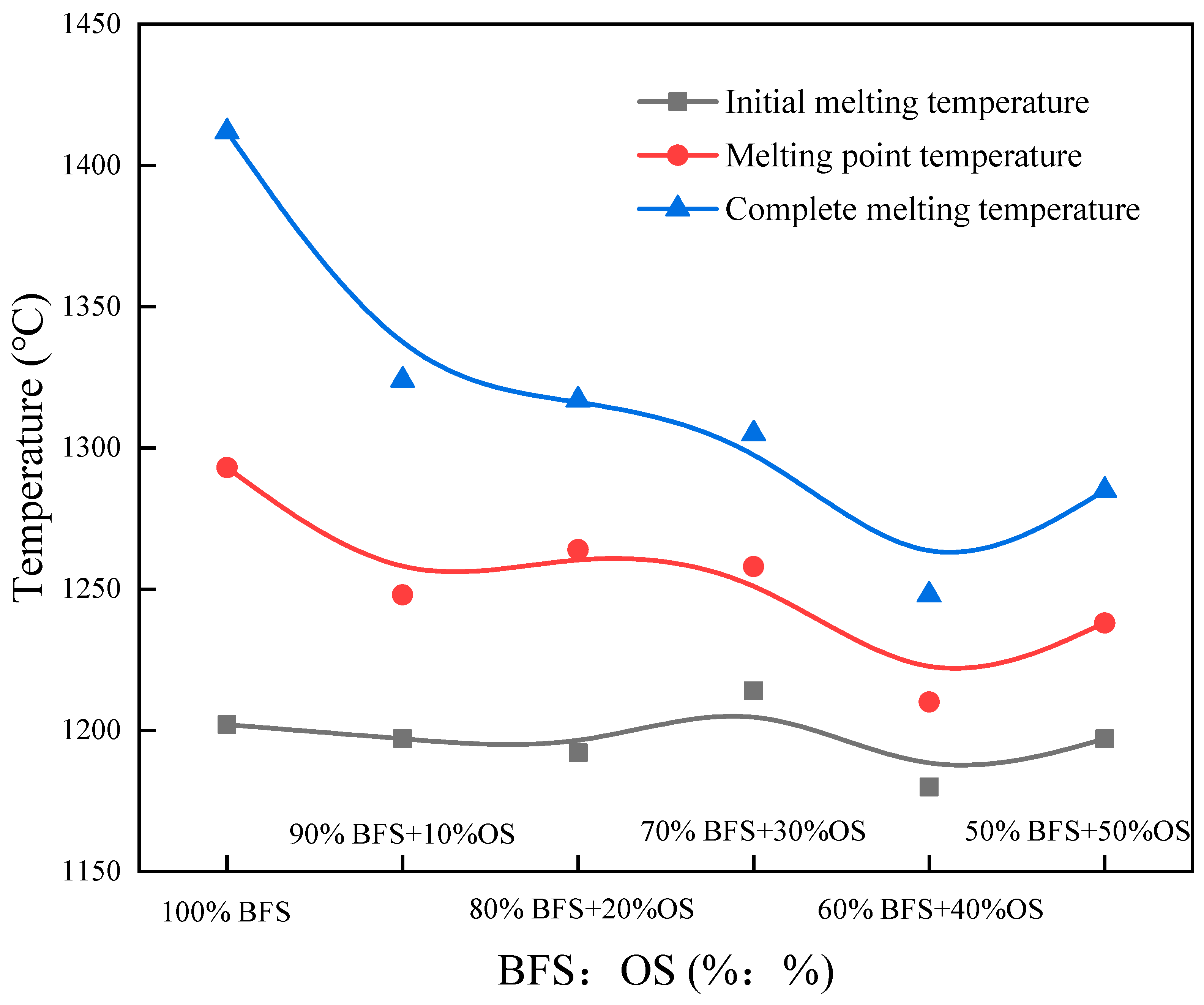
3.3. Reconstruction Slag Melting Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.X.; Qi, Y.H.; Yan, D.L.; Hu, C.Q.; Zhang, X.X. Energy-Saving and Environmental Protection of Ironmaking System in China. Iron Steel 2006, 41, 1–5. [Google Scholar]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Nicolas, R.S.; Hamdan, S.; Jannie, S.J.; Deventer, V. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Ball, A.S.; Stewart, R.J.; Schliephake, K. A review of the current options for the treatment and safe disposal of drill cuttings. Waste Manag. Res. 2012, 30, 457–473. [Google Scholar] [CrossRef]
- Wang, L.; Quan, H.; Li, Q. Effect of Solid Waste-Petroleum Coke Residue on the Hydration Reaction and Property of Concrete. Materials 2019, 12, 1216. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhang, S.; Steenari, B.-M.; Ekberg, C. Controlling the Composition and Magnetic Properties of Nano-SrFe12O19 Powder Synthesized from Oily Cold Mill Sludge by the Citrate Precursor Method. Materials 2019, 12, 1250. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.J.; Li, P.; Lei, W. Thermodynamic Analysis of Blast Furnace Slag Waste Heat-Recovery System Integrated with Coal Gasification. JOM 2015, 67, 1079–1085. [Google Scholar] [CrossRef]
- Long, X.; Zhang, G.; Han, L. Dewatering of floated oily sludge by treatment with rhamnolipid. Water Res. 2013, 47, 4303–4311. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yu, Q.; Qin, Q. The effects of slag compositions on the coal gasification reaction in molten blast furnace slag. Energy Sources 2014, 36, 73–79. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhu, X. A review of waste heat recovery technologies towards molten slag in steel industry. Appl. Energy 2013, 112, 956–966. [Google Scholar] [CrossRef]
- Kasai, E.; Kitajima, T.; Akiyama, T.; Yagi, J.; Saito, F. Rate of methane-steam reforming reaction on the surface of molten BF slag-for heat recovery from molten slag by using a chemical reaction. ISIJ Int. 1997, 37, 1031–1036. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gong, Z.; Wang, Z.; Li, X.; Liu, J.; Tang, C.; Chu, Z. Pyrolysis performance and kinetic analysis of oily sludge. J. Therm. Anal. Calorim. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Li, Q. The situation and challenges for deepwater oil and gas exploration and exploitation in China. China Offshore Oil Gas 2006, 18, 130–133. [Google Scholar]
- Yong, J. Characteristics of Oily Sludge and Several Treatment Methods. Environ. Prot. Oil Gas Fields 2005, 15, 38–41. [Google Scholar]
- Gordalla, B.C.; Ewers, U.; Frimmel, F.H. Hydraulic fracturing: A toxicological threat for groundwater and drinking-water. Environ. Earth Sci. 2013, 70, 3875–3893. [Google Scholar] [CrossRef]
- Hui, K.L.; Tang, J.; Lu, H.J.; Xi, B.D.; Qu, C.T.; Li, J. Status and prospect of oil recovery from oily sludge: A review. Arab. J. Chem. 2020, 13, 6523–6543. [Google Scholar] [CrossRef]
- Yang, J.P.; Wang, W.J.; Yang, J. Development and prospect of oily sludge treatment. Oxid. Commun. 2015, 38, 2216–2224. [Google Scholar]
- Huang, Z.; Xu, Z.; Quan, Y. A review of treatment methods for oil-based drill cuttings[C]//IOP Conference Series: Earth and Environmental Science. IOP Publ. 2018, 170, 022074. [Google Scholar]
- Zhao, D.F.; Zhao, C.C.; Shuai, L.U. The Study on Oily Sludge Coking Process. Res. Environ. 2000, 2, 55–57. [Google Scholar]
- Kuang, S.; Zhong, X.U. Pollution characteristics of polycyclic aromatic hydrocarbons (PAHs) in oily sludge from the Zhongyuan Oilfield and its peripheral soils. Chin. J. Geochem. 2009, 28, 176–183. [Google Scholar] [CrossRef]
- Liu, X.; Niu, H.; Yan, H. Research and application of high-efficiency eco-engineering rural sewage treatment system. Trans. Chin. Soc. Agric. Eng. 2013, 29, 184–191. [Google Scholar]
- Sil, A.; Wakadikar, K.; Kumar, S. Toxicity characteristics of drilling mud and its effect on aquatic fish populations. J. Hazard. Toxic Radioact. Waste 2012, 16, 51–57. [Google Scholar] [CrossRef]
- Naik, B.S.; Srinivasarao, N.B.; Mishra, I.M.; Bhattacharya, S.D. Biodegradation of total petroleum hydrocarbons from oily sludge. Bioremediat. J. 2011, 15, 140–147. [Google Scholar] [CrossRef]
- Wei, P.F.; Zou, B.; Chen, J. Study on consolidation of waste drilling fluids of Jianghan Oilfield. Chem. Bioeng. 2003, 6, 34–35. [Google Scholar]
- Hou, B.; Xie, S.X.; Chen, M. The treatment of refinery heavy oil sludge. Pet. Sci. Technol. 2013, 31, 458–464. [Google Scholar] [CrossRef]
- Taiwo, E.A.; Otolorin, J.A. Oil recovery from petroleum sludge by solvent extraction. Pet. Sci. Technol. 2009, 27, 836–844. [Google Scholar] [CrossRef]


| CaO | SiO2 | Al2O3 | MgO | R(CaO/SiO2) |
|---|---|---|---|---|
| 43.64 | 36.36 | 11.80 | 8.05 | 1.20 |
| Proximate Analysis (Weight Content %) | Heat Value (MJ/Kg) | |||
|---|---|---|---|---|
| Moisture | Volatiles | Ash | Fixed Carbon | |
| 30.16 | 40.88 | 24.47 | 3.48 | 28.44 |
| Metal Element | V | Cr | Ni | Cu | Zn | Cd | Sb | Pb |
|---|---|---|---|---|---|---|---|---|
| Oily sludge (mg/kg) | 132.5 | 165.4 | 225.3 | 75.7 | 218.4 | 12.2 | 101.4 | 482.7 |
| GB 15618-2018 (mg/kg) | — | 350.0 | 190.0 | 200.0 | 300.0 | 0.8 | 40.0 | 170.0 |
| No. | Mixture Ratio (BFS:OS) | Temperature (°C) | Total Mass of Sample (g) |
|---|---|---|---|
| 1 | 90%:10% | 1450 | 100 |
| 2 | 80%:20% | 1450 | 100 |
| 3 | 70%:30% | 1450 | 100 |
| 4 | 60%:40% | 1450 | 100 |
| 5 | 50%:50% | 1450 | 100 |
| Sample No. | Leaching Content (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|
| V | Cr | Cu | Zn | Ni | Sb | Pb | |
| 1 | 0.04 ± 0.003 | 0.34 ± 0.005 | — | — | 3.45 ± 0.007 | 1.63 ± 0.004 | — |
| 2 | — | 0.02 ± 0.001 | — | — | 0.80 ± 0.004 | 6.26 ± 0.002 | 0.69 ± 0.002 |
| 3 | — | — | — | — | 0.65 ± 0.003 | 0.95 ± 0.003 | 1.92 ± 0.007 |
| 4 | — | — | — | — | 0.07 ± 0.005 | 2.85 ± 0.002 | — |
| 5 | — | 0.37 ± 0.006 | — | — | 0.04 ± 0.003 | 2.46 ± 0.003 | — |
| (GB5085.3-2007) (3) (mg/kg) | — | 10.00 | — | — | 10.00 | 10.00 | 3.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Zhang, K.; Wu, X.; Ling, Q.; Hu, J.; Li, X.; Liu, H. Effect of Oily Sludge Treatment with Molten Blast Furnace Slag on the Mineral Phase Reconstruction of Water-Quenched Slag Properties. Materials 2021, 14, 7285. https://doi.org/10.3390/ma14237285
Qin Y, Zhang K, Wu X, Ling Q, Hu J, Li X, Liu H. Effect of Oily Sludge Treatment with Molten Blast Furnace Slag on the Mineral Phase Reconstruction of Water-Quenched Slag Properties. Materials. 2021; 14(23):7285. https://doi.org/10.3390/ma14237285
Chicago/Turabian StyleQin, Yuelin, Ke Zhang, Xinlong Wu, Qingfeng Ling, Jinglan Hu, Xin Li, and Hao Liu. 2021. "Effect of Oily Sludge Treatment with Molten Blast Furnace Slag on the Mineral Phase Reconstruction of Water-Quenched Slag Properties" Materials 14, no. 23: 7285. https://doi.org/10.3390/ma14237285
APA StyleQin, Y., Zhang, K., Wu, X., Ling, Q., Hu, J., Li, X., & Liu, H. (2021). Effect of Oily Sludge Treatment with Molten Blast Furnace Slag on the Mineral Phase Reconstruction of Water-Quenched Slag Properties. Materials, 14(23), 7285. https://doi.org/10.3390/ma14237285






