Assessment of Corrosion Performance of Steel Rebar in Snail Shell Ash Blended Cements under Marine Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Preparation and Characterization of SSA
2.2.1. Preparation Methodology of SSA from Raw Snail Shell
2.2.2. Characterization of SSA
2.3. Preparation of the Cement Extracts Using SSA
2.4. Preparation of Steel Rebar
2.5. Evaluation of Corrosion Performance of Steel Rebars in Various Cement Extracts
2.5.1. Electrochemical Methods
2.5.2. Gravimetric Weight Loss Method
2.6. Characterization of Corrosion Products
2.7. Casting of Concrete Specimens
Evaluation of Mechanical Properties
3. Results and Discussion
3.1. Characterization of SSP and SSA
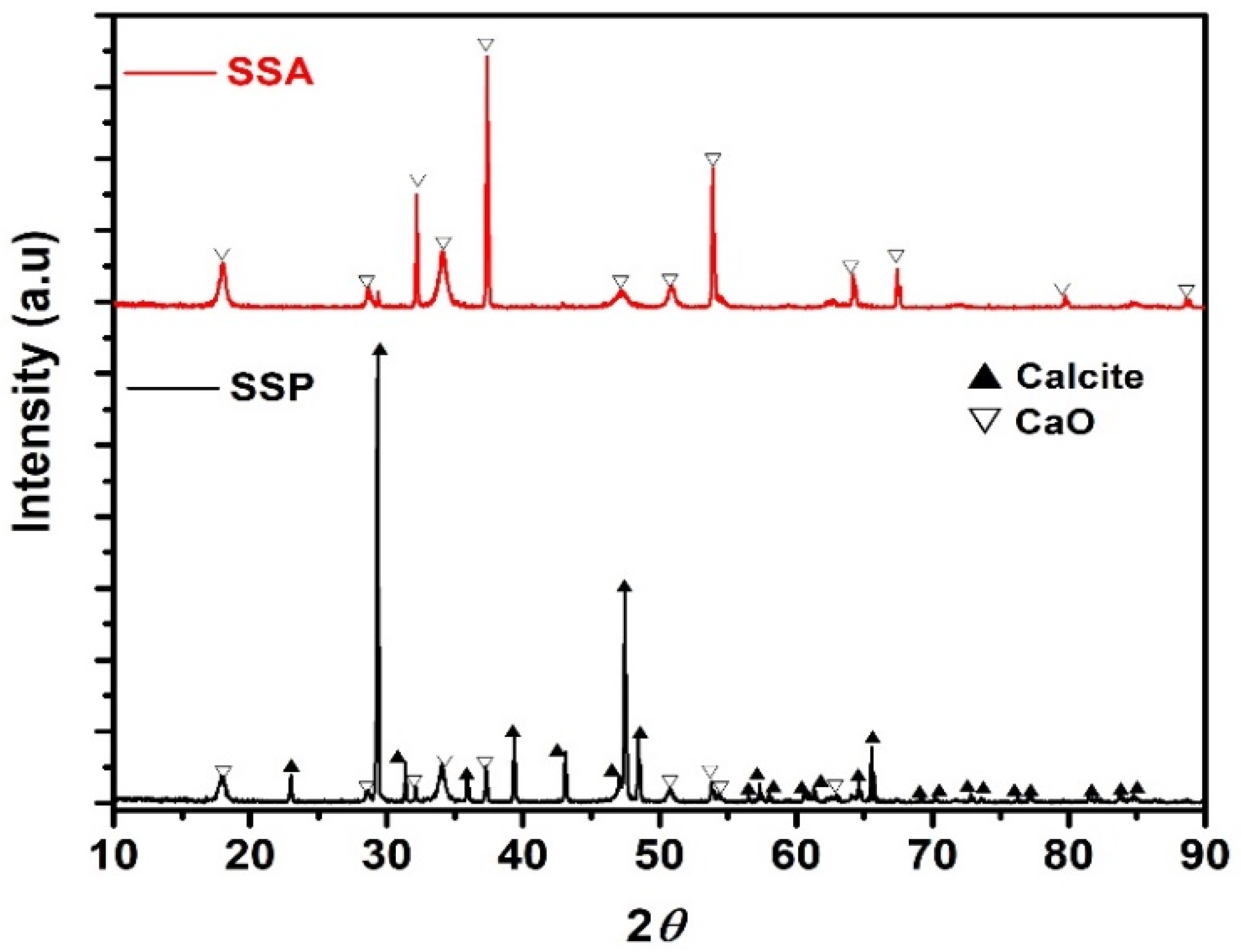
3.1.1. X-ray Diffraction
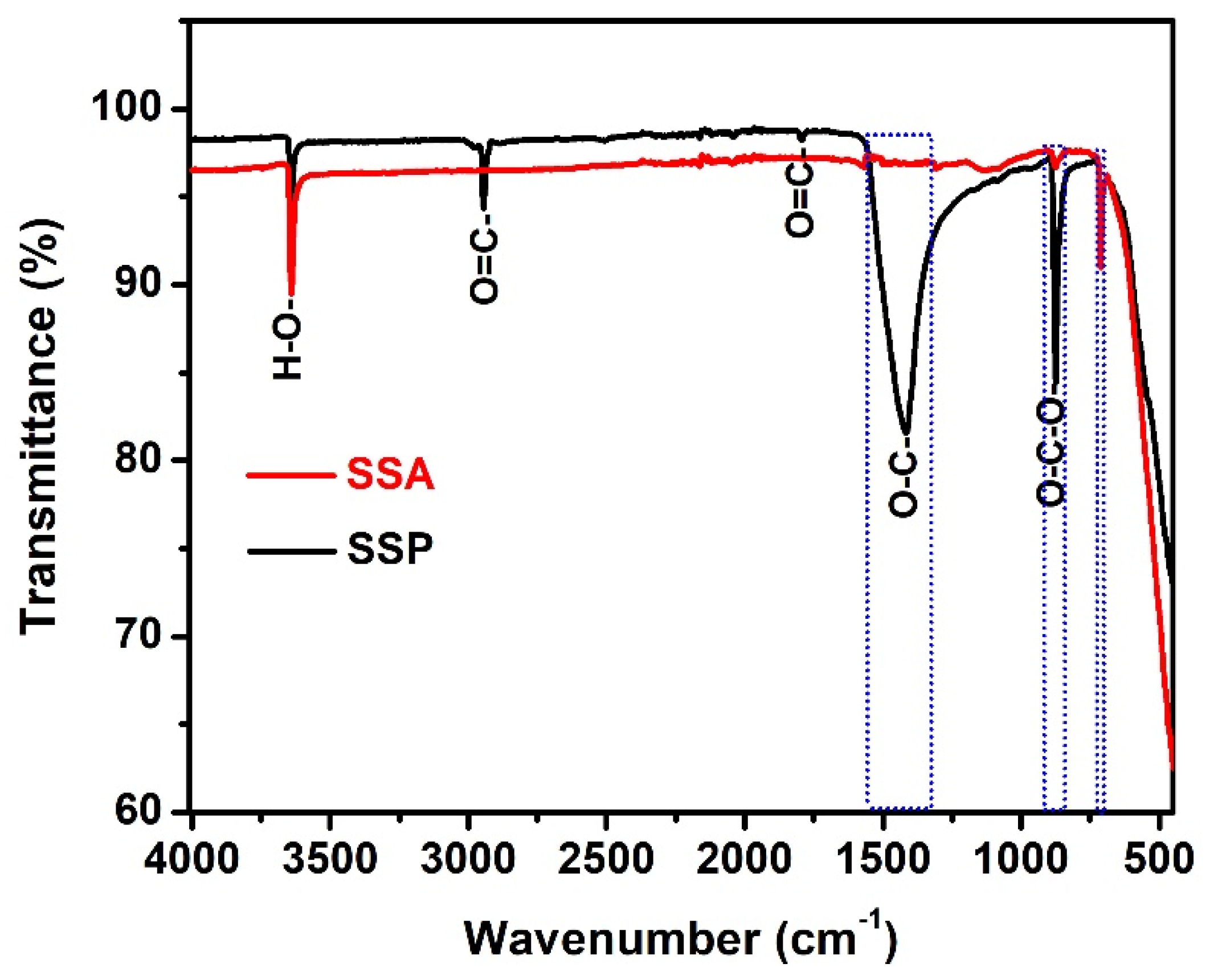
3.1.2. Fourier Transform Infrared Spectroscopy
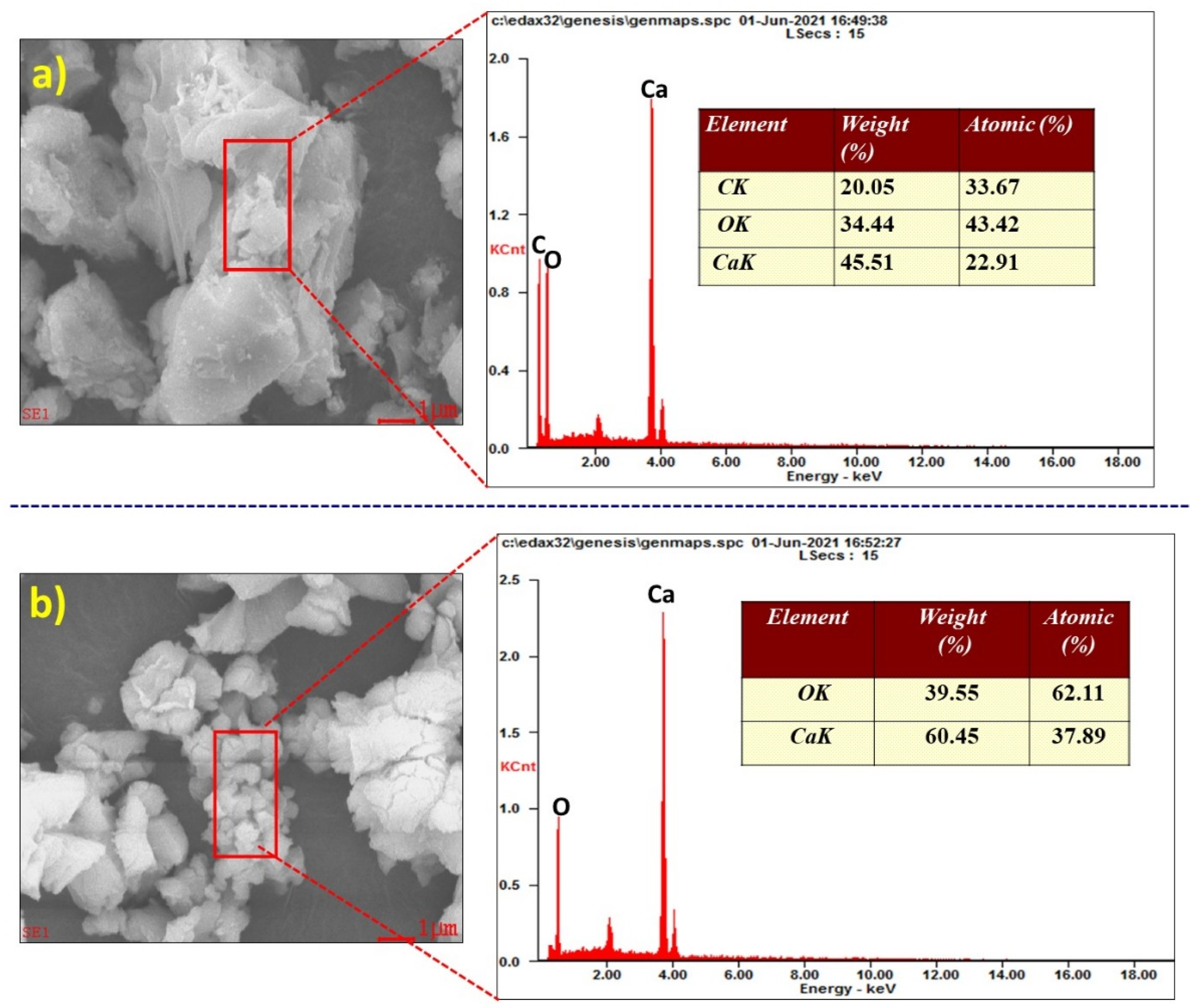
3.1.3. Scanning Electron Microscopy/Energy Dispersive X-ray Spectroscopy
3.2. Evaluation of Corrosion Performance of Steel Rebars
3.2.1. Electrochemical Techniques
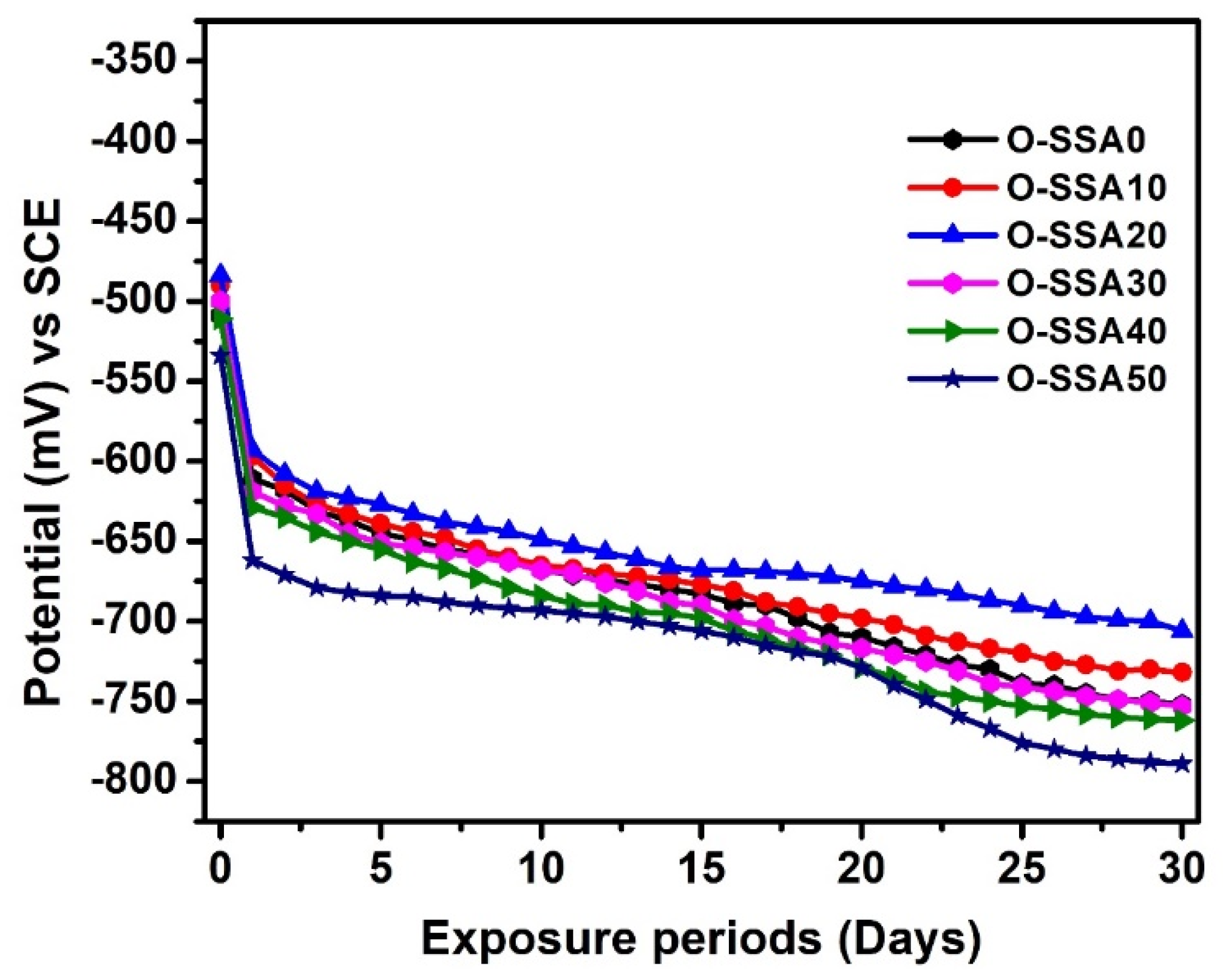
Chrono-Potential Studies
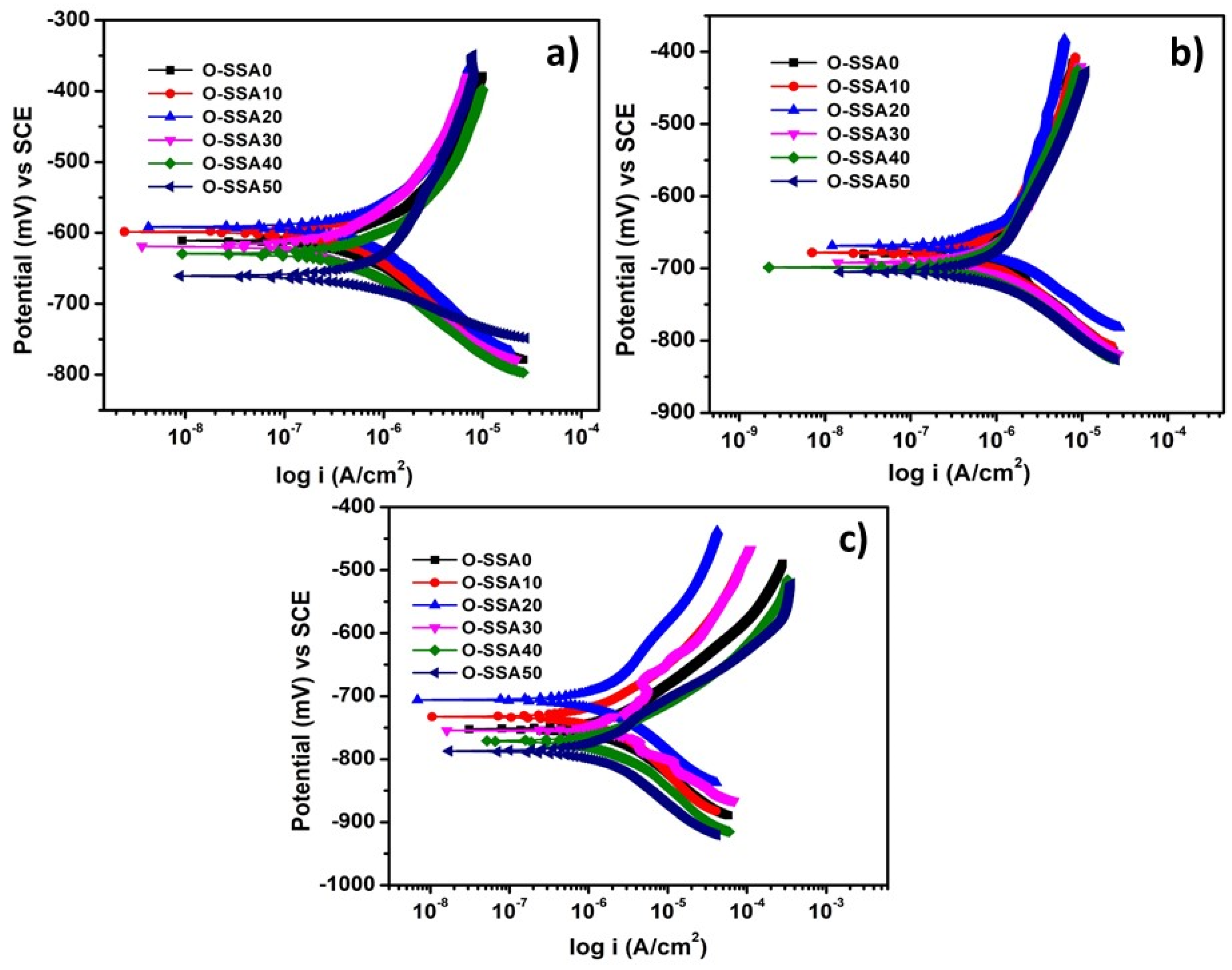
Potentiodynamic Polarization Studies
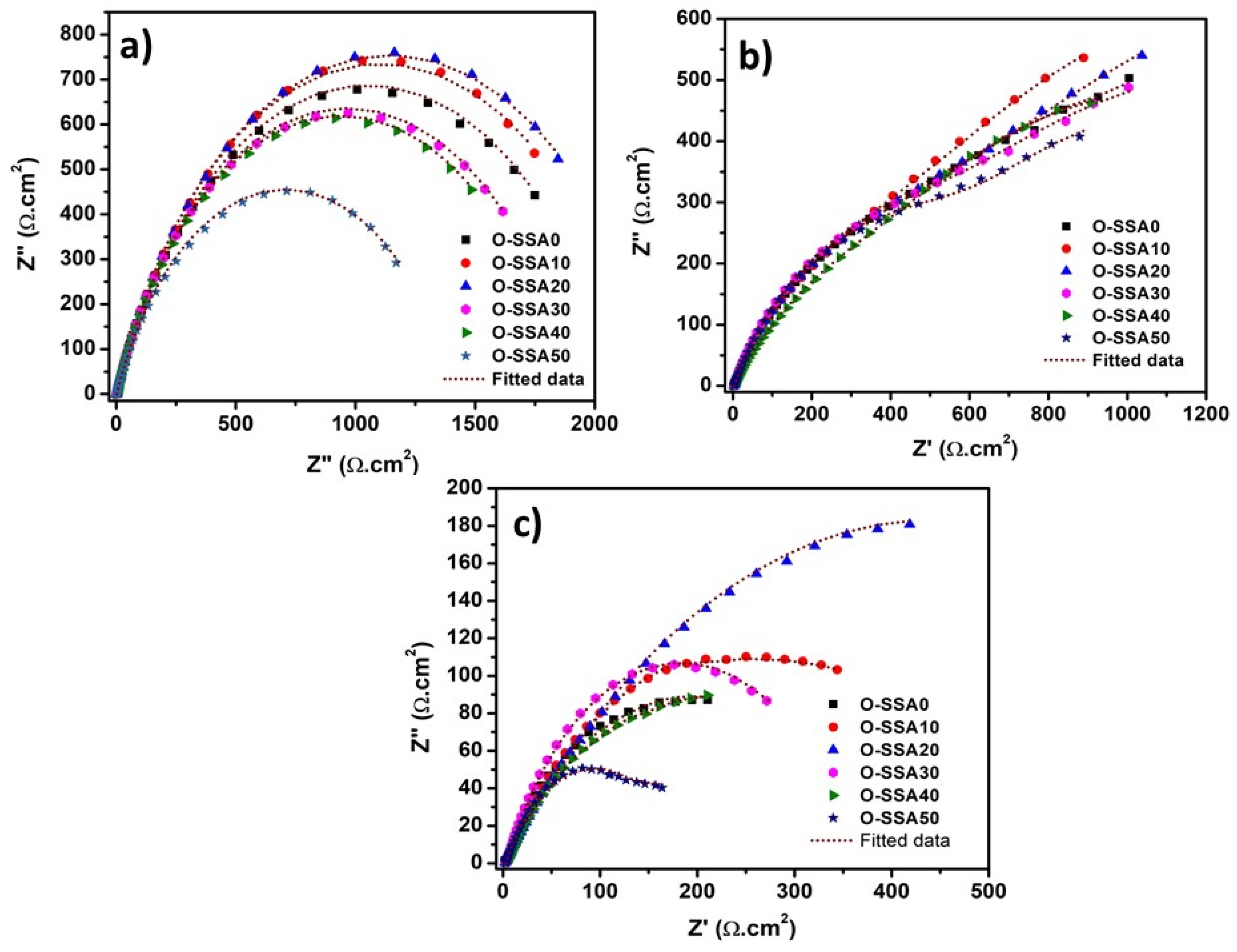
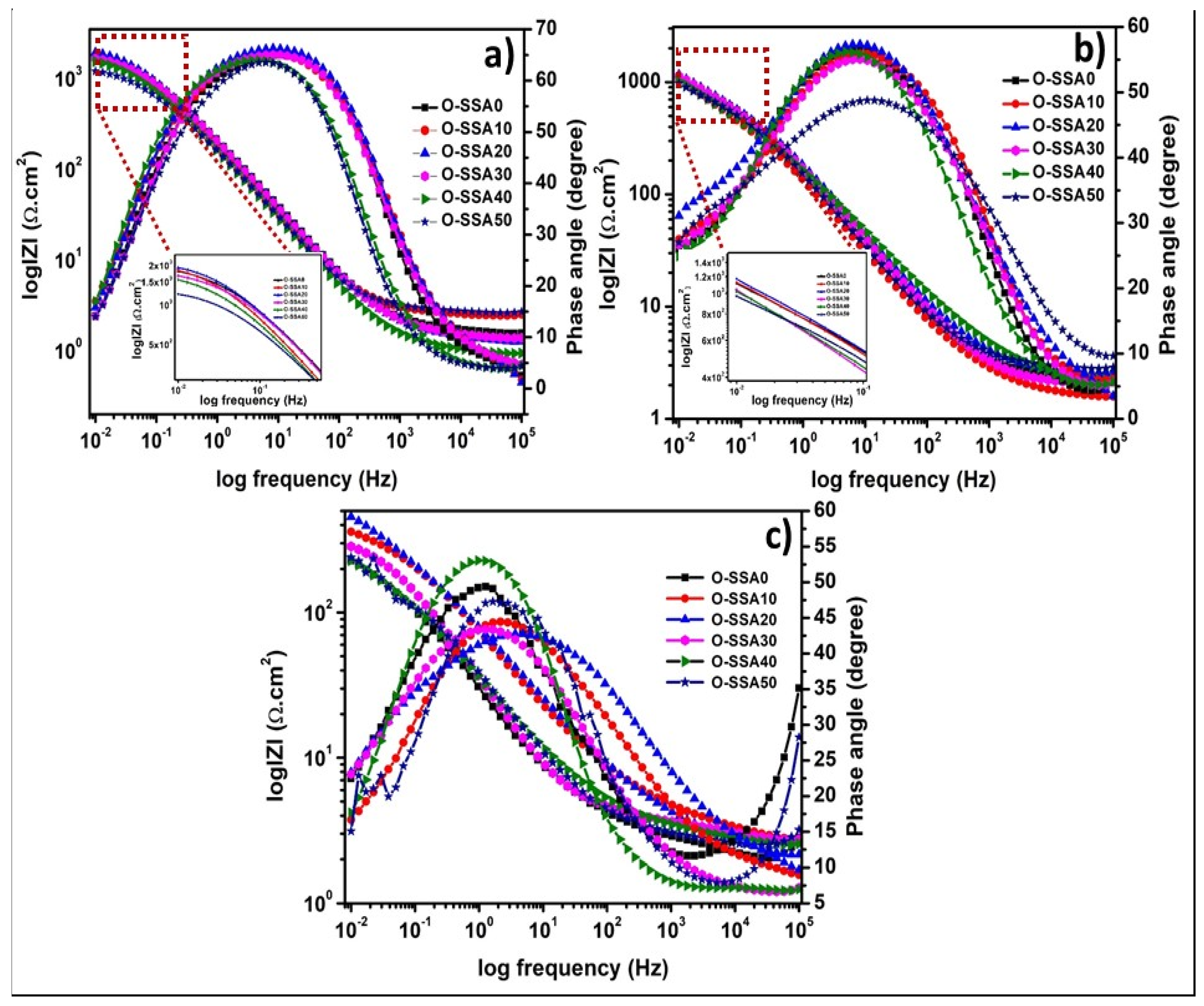
Electrochemical Impedance Spectroscopy
3.2.2. Gravimetric Weight Loss Method
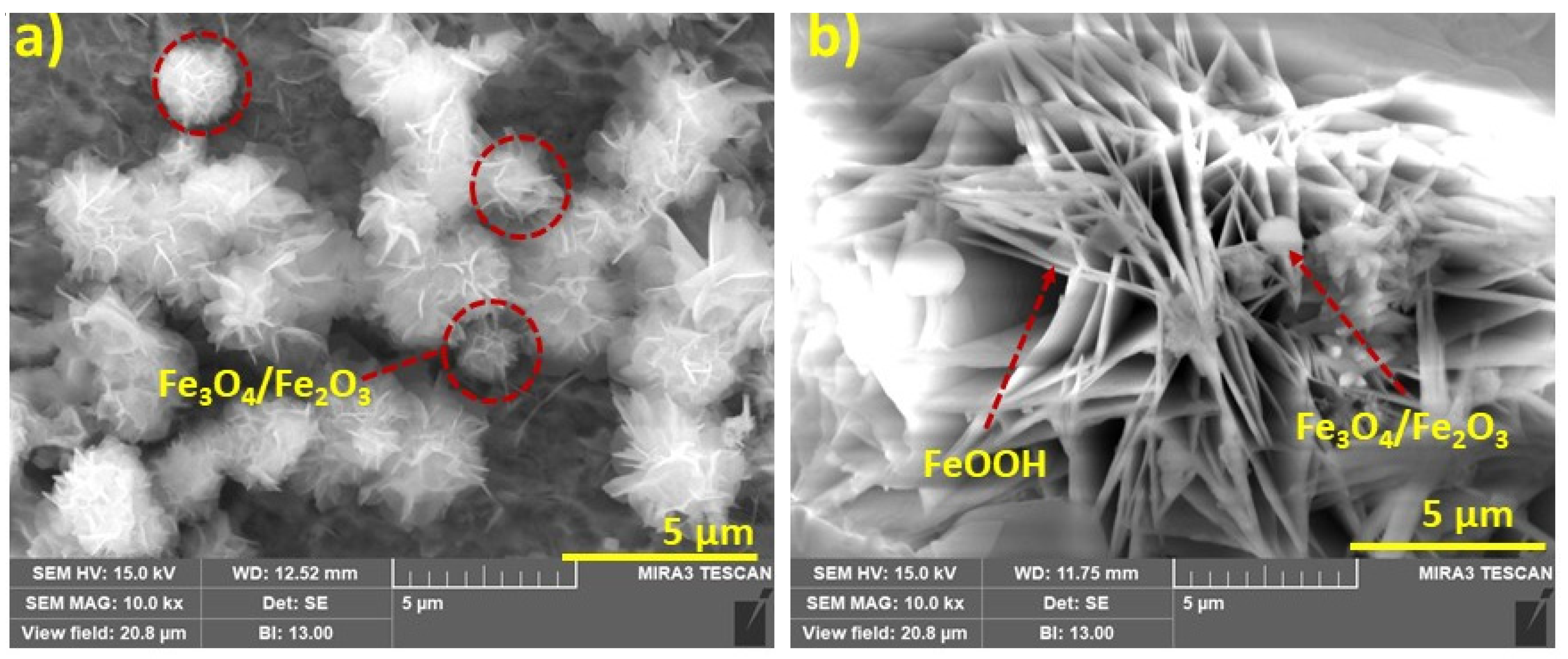
3.3. Surface Characterization of Steel Rebars
3.4. Mechanical Properties of Concrete
3.4.1. Compressive Strength Test
3.4.2. Split Tensile Strength Test
3.5. Extrapolation Modeling
3.5.1. Extrapolation Modeling for Compressive Strength with Later Age
3.5.2. Extrapolation Modelling for Corrosion Rate of Steel Rebar in Chloride Contaminated Various Cement Extracts
4. Conclusions
- Chrono-potential studies showed that the optimum cement replacement level of up to 20% of SSA is sufficiently suitable for better durability of the reinforced concrete structures under marine environments.
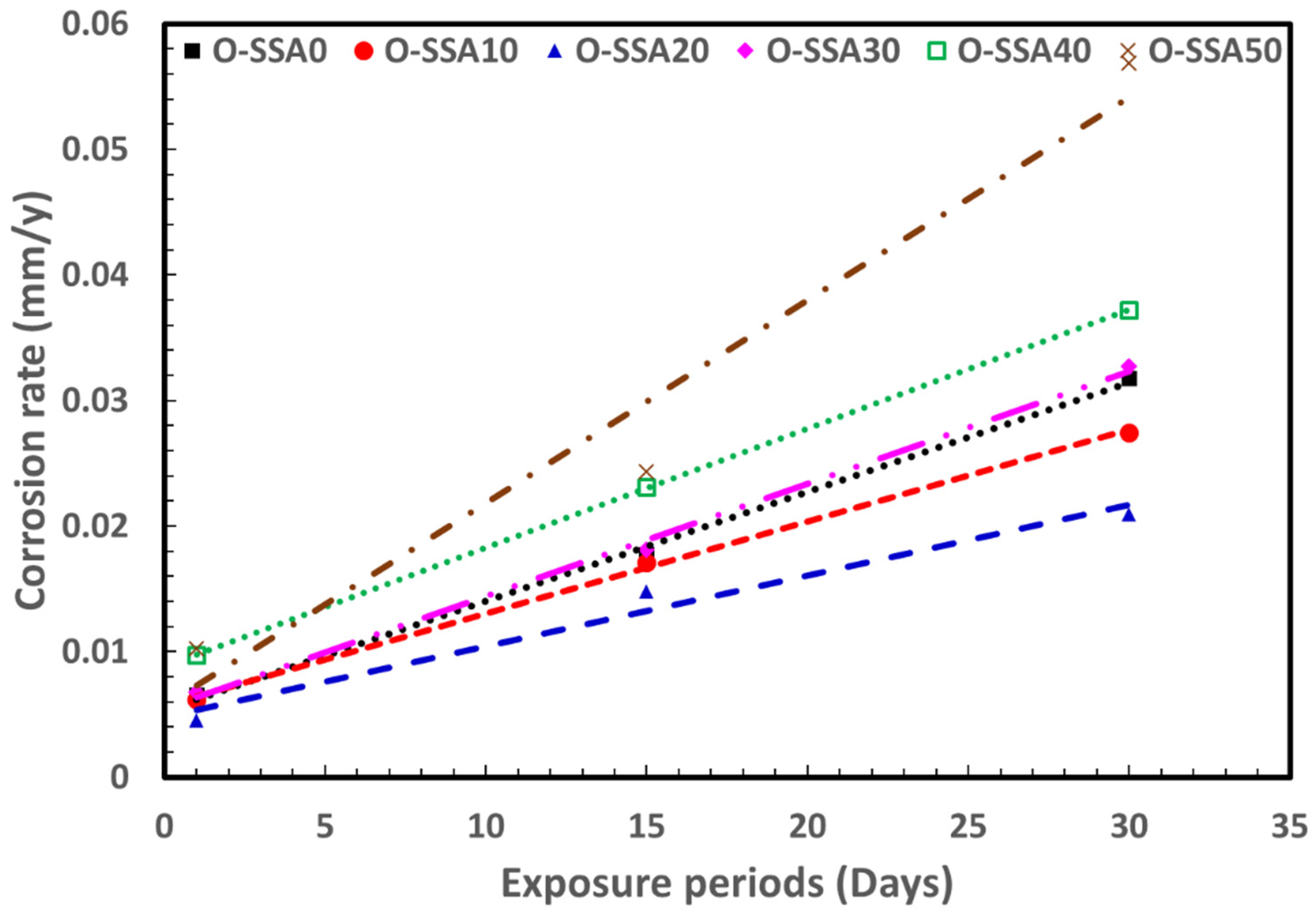
- Potentiodynamic polarization studies revealed that the comparable corrosion rate was obtained up to a 20% replacement level. At 20% replacement level of SSA (O-SSA20) showed lesser corrosion current density (0.3857, 1.2536, and 1.7815 μA/cm2) and corrosion rate values (0.4539, 1.4755, and 2.0969 mm/y) at all the exposure periods (1, 15, and 30 days), which compared to all other systems.
- Weight loss measurements indicated that there was a reduction in the corrosion rate of rebar by 1.408 times at a 20% replacement level and 1.157 times at a 10% replacement level when compared to control. The quantitative data confirmed that up to a critical level of 20% to 30% replacement, SSA improved the corrosion resistance of concrete.
- Mechanical properties of SSA blended concrete, such as compressive strength and split tensile strength showed that up to a 30% replacement level, SSA improved the strength of concrete.
- Comparable corrosion rate with control was noticed up to a 30% replacement level. Hence, the cement replacement level of 30% by SSA is safely used for steel rebar in concrete under marine environments.
- EIS studies revealed that at a 20% replacement level, a gradual increase of film resistance across the steel-concrete interfacial regions was noticed. At higher replacement levels, chloride ions penetrate the iron hydroxide layer on the steel rear surface and it has become diffused/porous, thus the speed of corrosion rate on the steel rebar surface.
- Surface examinations inferred that the formation of iron oxide and iron hydroxide rust products on the steel rebar under aggressive marine environments.
- It was concluded that the animal origin from the marine was found to be a sustainable engineering material for construction industries and also for the marine environment. SSA is a suitable replacement material for natural limestone in cement productions.
- The fitting equations are used to extrapolate compressive strength up to 365 curing days. The obtained values suggest that the 20% replacement of SSA will give a compressive strength of ~57.8 N/mm2 after 365 days of curing under ideal conditions followed by 30% replacement of SSA, which gives compressive strength of ~56.5 N/mm2.
Supplementary Materials
Author Contributions
Funding
Institutional review Board statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Environment, U.; Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar]
- Lim, C.; Jung, E.; Lee, S.; Jang, C.; Oh, C.; Shin, K.N. Global trend of cement production and utilization of circular resources. J. Energy Eng. 2020, 29, 57–63. [Google Scholar]
- International Energy Agency. Low-Carbon Transition in the Cement Industry. Available online: https://docs.wbcsd.org/2018/04/Cement_RM_Summary.pdf (accessed on 27 October 2021).
- British Geological Survey. Cement Raw Materials; British Geological Survey: Nottingham, UK, 2005; pp. 1–20. [Google Scholar]
- Farfan, J.; Fasihi, M.; Breyer, C. Trends in the global cement industry and opportunities for long-term sustainable CCU potential for Power-to-X. J. Clean. Prod. 2019, 217, 821–835. [Google Scholar] [CrossRef]
- Schmidt, W.; Commeh, M.; Olonade, K.; Schiewer, G.L.; Dodoo-Arhin, D.; Dauda, R.; Fataei, S.; Tawiah, A.T.; Mohamed, F.; Thiedeitz, M. Sustainable circular value chains: From rural waste to feasible urban construction materials solutions. Dev. Built Environ. 2021, 6, 100047. [Google Scholar] [CrossRef]
- Golewski, G.L. The influence of microcrack width on the mechanical parameters in concrete with the addition of fly ash: Consideration of technological and ecological benefits. Constr. Build. Mater. 2019, 197, 849–861. [Google Scholar] [CrossRef]
- Saraswathy, V.; Muralidharan, S.; Thangavel, K.; Srinivasan, S. Activated fly ash cements: Tolerable limit of replacement for durable steel reinforced concrete. Adv. Cem. Res. 2002, 14, 9–16. [Google Scholar] [CrossRef]
- Kathirvel, P.; Saraswathy, V.; Karthik, S.; Sekar, A. Strength and durability properties of quaternary cement concrete made with fly ash, rice husk ash and limestone powder. Arab. J. Sci. Eng. 2013, 38, 589–598. [Google Scholar] [CrossRef]
- Duraman, S.B.; Richardson, I.G. Microstructure & properties of steel-reinforced concrete incorporating Portland cement and ground granulated blast furnace slag hydrated at 20 °C. Cem. Concr. Res. 2020, 137, 106193. [Google Scholar]
- Jang, S.-Y.; Karthick, S.; Kwon, S.-J. Investigation on durability performance in early aged high-performance concrete containing GGBFS and FA. Adv. Mater. Sci. Eng. 2017. [Google Scholar] [CrossRef]
- Wu, Z.; Khayat, K.H.; Shi, C. Changes in rheology and mechanical properties of ultra-high performance concrete with silica fume content. Cem. Concr. Res. 2019, 123, 105786. [Google Scholar] [CrossRef]
- Kang, S.-H.; Hong, S.-G.; Moon, J. The use of rice husk ash as reactive filler in ultra-high performance concrete. Cem. Concr. Res. 2019, 115, 389–400. [Google Scholar] [CrossRef]
- Jung, S.-H.; Saraswathy, V.; Karthick, S.; Kathirvel, P.; Kwon, S.-J. Microstructure characteristics of fly ash concrete with rice husk ash and lime stone powder. Int. J. Concr. Struct. Mater. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Quedou, P.G.; Wirquin, E.; Bokhoree, C. Sustainable concrete: Potency of sugarcane bagasse ash as a cementitious material in the construction industry. Case Stud. Constr. Mater. 2021, 14, e00545. [Google Scholar] [CrossRef]
- Hamada, H.M.; Jokhio, G.A.; Yahaya, F.M.; Humada, A.M.; Gul, Y. The present state of the use of palm oil fuel ash (POFA) in concrete. Constr. Build. Mater. 2018, 175, 26–40. [Google Scholar] [CrossRef]
- Hart, A. Mini-review of waste shell-derived materials’ applications. Waste Manag. Res. 2020, 38, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Lertwattanaruk, P.; Makul, N.; Siripattarapravat, C. Utilization of ground waste seashells in cement mortars for masonry and plastering. J. Environ. Manag. 2012, 111, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Leelatawonchai, P.; Laonapakul, T. Preparation and characterization of calcium sources from golden apple snail shell for naturally based biomaterials. Adv. Mater. Res. 2014, 931–932, 370–374. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rajkumari, K.; Gupta, R.; Chatterjee, S.; Paul, B.; Rokhum, L. Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Adv. 2018, 8, 20131–20142. [Google Scholar] [CrossRef]
- Gumus, R.H.; Okpeku, I. Production of activated carbon and characterization from snail shell waste (Helix pomatia). Adv. Chem. Eng. Sci. 2014, 5, 51. [Google Scholar] [CrossRef][Green Version]
- Tamjidi, S.; Ameri, A. A review of the application of sea material shells as low cost and effective bio-adsorbent for removal of heavy metals from wastewater. Environ. Sci. Pollut. Res. 2020, 27, 31105–31119. [Google Scholar] [CrossRef]
- Yang, E.-I.; Yi, S.-T.; Leem, Y.-M. Effect of oyster shell substituted for fine aggregate on concrete characteristics: Part I. Fundamental properties. Cem. Concr. Res. 2005, 35, 2175–2182. [Google Scholar] [CrossRef]
- Yoon, G.-L.; Kim, B.-T.; Kim, B.-O.; Han, S.-H. Chemical–mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Yoon, H.; Park, S.; Lee, K.; Park, J. Oyster shell as substitute for aggregate in mortar. Waste Manag. Res. 2004, 22, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Dankwah, J.R.; Nkrumah, E. Recycling blends of rice husk ash and snail shells as partial replacement for Portland cement in building block production. Ghana J. Technol. 2016, 1, 67–74. [Google Scholar]
- Mohanalakshmi, V.; Indhu, S.; Hema, P.; Prabha, V. Developing concrete using sea shell as a fine aggregate. IJIRST 2017, 3, 282–286. [Google Scholar]
- Adeala, A.; Olaoye, J. Structural properties of snail shell ash concrete (SSAC). J. Emerg. Technol. Innov. Res. (JETIR) 2019, 6, 24–31. [Google Scholar]
- Ketebu, O.; Farrow, S.T. Comparative study on cementitious content of ground mollusc snail and clam shell and their mixture as an alternative to cement. Int. J. Eng. Trends Technol. (IJETT) 2017, 50, 8–11. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Portland Cement; ASTM: West Conshohocken, PA, USA, 2021. [Google Scholar]
- ASTM. Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM: West Conshohocken, PA, USA, 2003. [Google Scholar]
- ASTM. Standard Guide for Laboratory Immersion Corrosion Testing of Metals; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM. Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Kwon, S.-J.; Lee, H.-S.; Karthick, S.; Saraswathy, V.; Yang, H.-M. Long-term corrosion performance of blended cement concrete in the marine environment–A real-time study. Constr. Build. Mater. 2017, 154, 349–360. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM: West Conshohocken, PA, USA, 2021. [Google Scholar]
- ASTM. Standard Test Method for Splitting Tensile Strength of Cylindrical Concrete Specimens; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Lécuyer, C.; Hutzler, A.; Amiot, R.; Daux, V.; Grosheny, D.; Otero, O.; Martineau, F.; Fourel, F.; Balter, V.; Reynard, B. Carbon and oxygen isotope fractionations between aragonite and calcite of shells from modern molluscs. Chem. Geol. 2012, 332, 92–101. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Han, H. Utilization of waste freshwater mussel shell as an economic catalyst for biodiesel production. Biomass Bioenergy 2011, 35, 3627–3635. [Google Scholar] [CrossRef]
- Seesanong, S.; Laosinwattana, C.; Chaiseeda, K.; Boonchom, B. A simple and rapid transformation of golden apple snail (Pomacea canaliculata) shells to calcium carbonate, monocalcium and tricalcium phosphates. Asian J. Chem. 2019, 31, 2522–2526. [Google Scholar] [CrossRef]
- Abd El Haleem, S.; Abd El Wanees, S.; Bahgat, A. Environmental factors affecting the corrosion behaviour of reinforcing steel. V. Role of chloride and sulphate ions in the corrosion of reinforcing steel in saturated Ca(OH)2 solutions. Corros. Sci. 2013, 75, 1–15. [Google Scholar] [CrossRef]
- Rodrigues, R.; Gaboreau, S.; Gance, J.; Ignatiadis, I.; Betelu, S. Reinforced concrete structures: A review of corrosion mechanisms and advances in electrical methods for corrosion monitoring. Constr. Build. Mater. 2020, 121240. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Michel, A.; Gehlen, C.; Wong, H.; Isgor, O.B.; Elsener, B.; Hansson, C.M.; François, R.; Hornbostel, K. The steel–concrete interface. Mater. Struct. 2017, 50, 1–24. [Google Scholar] [CrossRef]
- Subbiah, K.; Velu, S.; Kwon, S.-J.; Lee, H.-S.; Rethinam, N.; Park, D.-J. A novel in-situ corrosion monitoring electrode for reinforced concrete structures. Electrochim. Acta 2018, 259, 1129–1144. [Google Scholar] [CrossRef]
- Miyamoto, S. On the oxidation of ferrous hydroxide by air. Bull. Chem. Soc. Jpn. 1929, 4, 65–68. [Google Scholar] [CrossRef]
- Leidheiser, H., Jr.; Czakó-Nagy, I. A Mössbauer spectroscopic study of rust formed during simulated atmospheric corrosion. Corros. Sci. 1984, 24, 569–577. [Google Scholar] [CrossRef]
- Keiser, J.T.; Brown, C.W.; Heidersbach, R.H. Characterization of the passive film formed on weathering steels. Corros. Sci. 1983, 23, 251–259. [Google Scholar] [CrossRef]
- Dufour, J.; Marrón, J.; Negro, C.; Latorre, R.; Formoso, A.; López-Mateos, F. Mechanism and kinetic control of the oxyprecipitation of sulphuric liquors from steel pickling. Chem. Eng. J. 1997, 68, 173–187. [Google Scholar] [CrossRef]
- Hamzah, E.; Hussain, M.; Ibrahim, Z.; Abdolahi, A. Influence of Pseudomonas aeruginosa bacteria on corrosion resistance of 304 stainless steel. Corros. Eng. Sci. Technol. 2013, 48, 116–120. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Microbially influenced corrosion of steels by Pseudomonas aeruginosa. Corros. Rev. 2014, 32, 129–141. [Google Scholar] [CrossRef]
- Wei, J.; Fu, X.; Dong, J.; Ke, W. Corrosion evolution of reinforcing steel in concrete under dry/wet cyclic conditions contaminated with chloride. J. Mater. Sci. Technol. 2012, 28, 905–912. [Google Scholar] [CrossRef]
- Galai, M.; Rbaa, M.; Ouakki, M.; Abousalem, A.S.; Ech-chihbi, E.; Dahmani, K.; Dkhireche, N.; Lakhrissi, B.; EbnTouhami, M. Chemically functionalized of 8-hydroxyquinoline derivatives as efficient corrosion inhibition for steel in 1.0 M HCl solution: Experimental and theoretical studies. Surf. Interfaces 2020, 21, 100695. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Assis, S.; Wolynec, S.; Costa, I. The electrochemical behaviour of Ti-13Nb-13Zr alloy in various solutions. Mater. Corros. 2008, 59, 739–743. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F.; Monetta, T.; Bellucci, F. Correlation between electrochemical impedance measurements and corrosion rate of magnesium investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar] [CrossRef]
- Dhaiveegan, P.; Elangovan, N.; Nishimura, T.; Rajendran, N. Weathering steel in industrial-marine-urban environment: Field study. Mater. Trans. 2016, 57, 148–155. [Google Scholar] [CrossRef]
- Repo, E.; Mäkinen, M.; Rengaraj, S.; Natarajan, G.; Bhatnagar, A.; Sillanpää, M. Lepidocrocite and its heat-treated forms as effective arsenic adsorbents in aqueous medium. Chem. Eng. J. 2012, 180, 159–169. [Google Scholar] [CrossRef]
- Guo, Y.; Meng, T.; Wang, D.; Tan, H.; He, R. Experimental research on the corrosion of X series pipeline steels under alternating current interference. Eng. Fail. Anal. 2017, 78, 87–98. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.-l.; Niu, G. The study on corrosion resistance of high-strength spring steel. Corros. Eng. Sci. Technol. 2018, 53, 54–64. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Yu, X.-Y.; Jin, Z.; Jia, Y.; Xu, W.-H.; Luo, T.; Zhu, B.-J.; Liu, J.-H.; Huang, X.-J. Ultra high adsorption capacity of fried egg jellyfish-like γ-AlOOH (Boehmite)@ SiO2/Fe3O4 porous magnetic microspheres for aqueous Pb (II) removal. J. Mater. Chem. 2011, 21, 16550–16557. [Google Scholar] [CrossRef]
- Xue, F.; Wei, X.; Dong, J.; Etim, I.-I.N.; Wang, C.; Ke, W. Effect of residual dissolved oxygen on the corrosion behavior of low carbon steel in 0.1 M NaHCO3 solution. J. Mater. Sci. Technol. 2018, 34, 1349–1358. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Shao, Y.; Ghoshal, S. Mathematical modeling of CO2 uptake by concrete during accelerated carbonation curing. Cem. Concr. Res. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Phoo-ngernkham, T.; Hanjitsuwan, S.; Horpibulsuk, S.; Poowancum, A.; Injorhor, B. Effect of calcium-rich compounds on setting time and strength development of alkali-activated fly ash cured at ambient temperature. Case Stud. Constr. Mater. 2018, 9, e00198. [Google Scholar] [CrossRef]


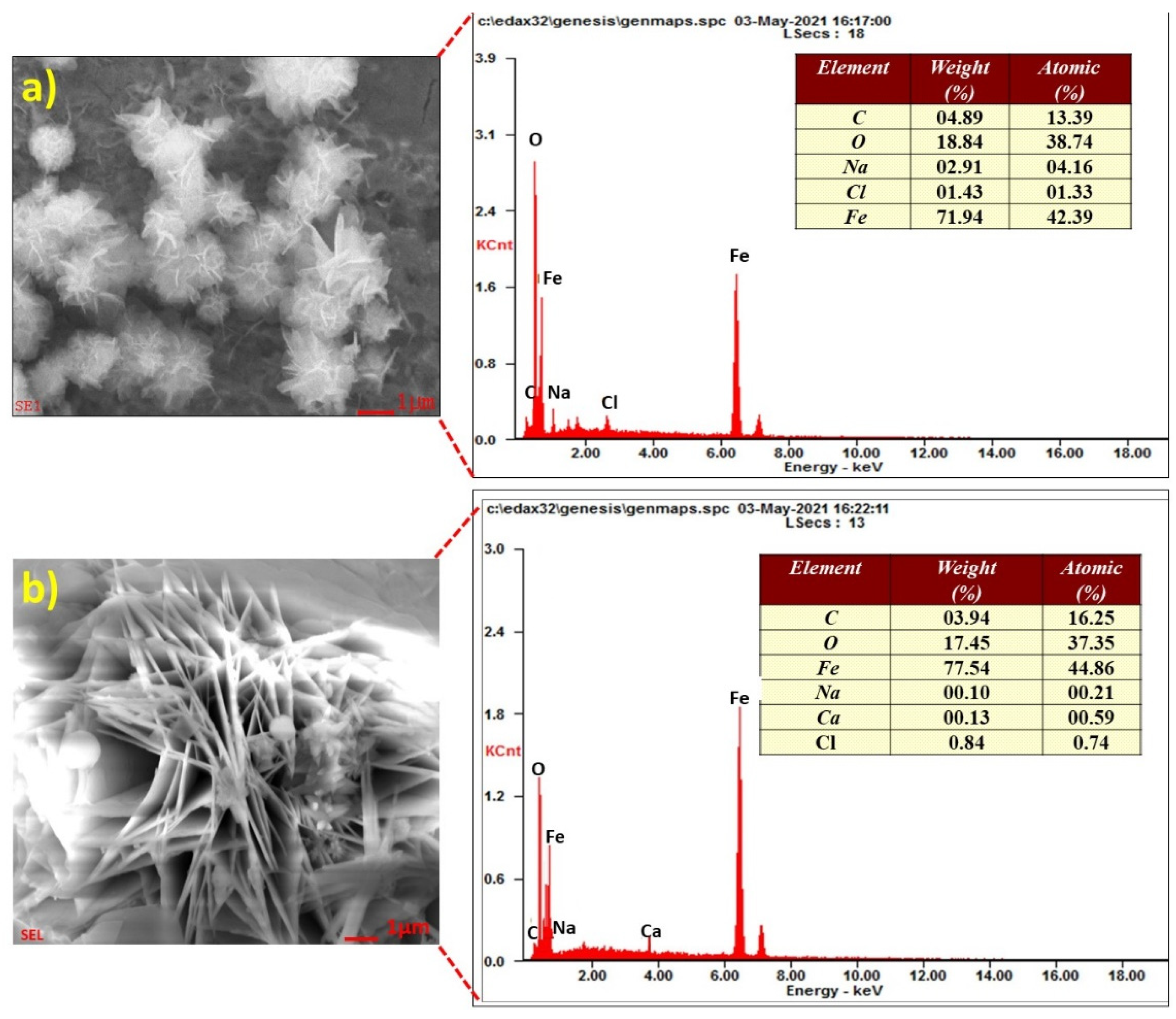
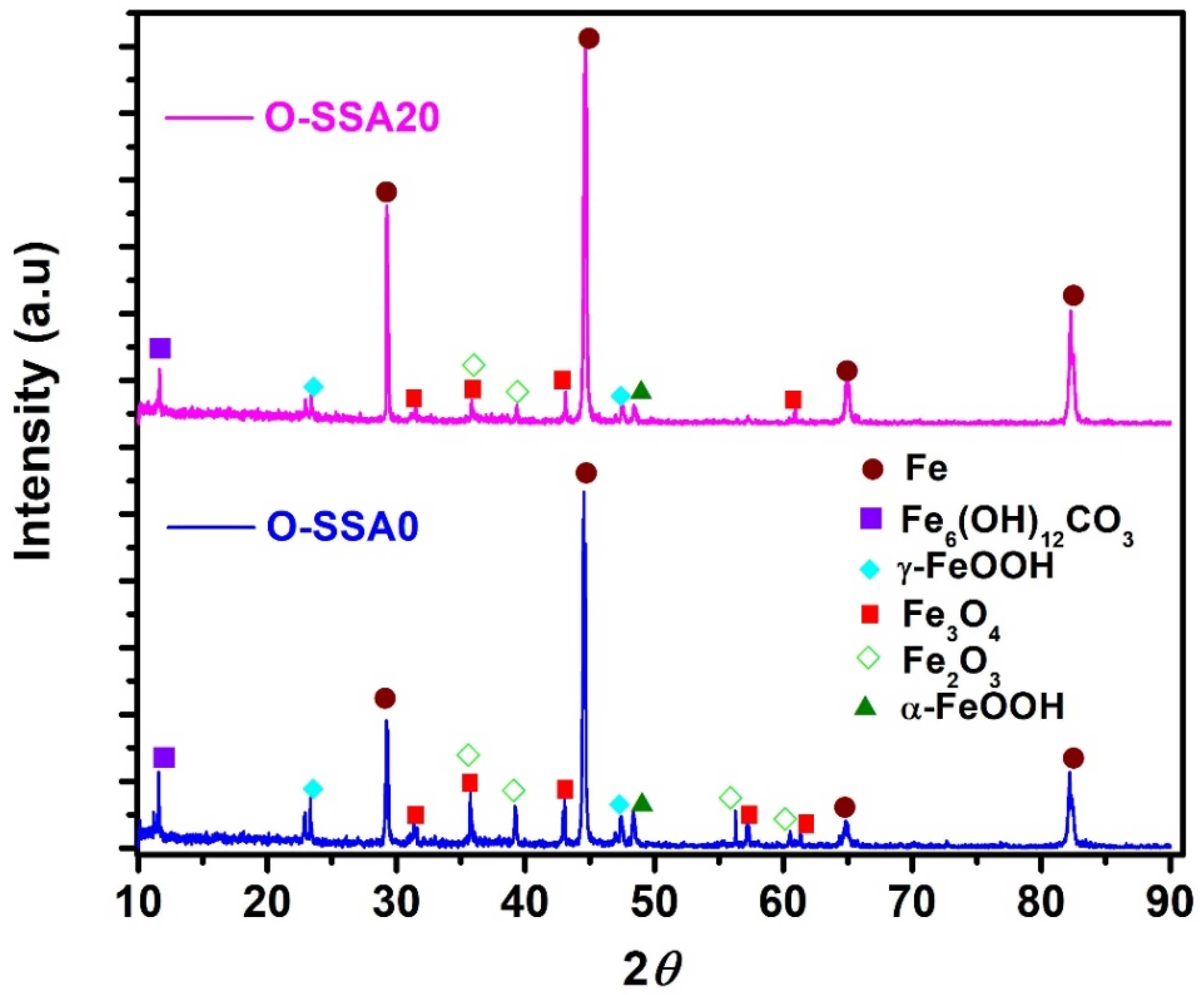
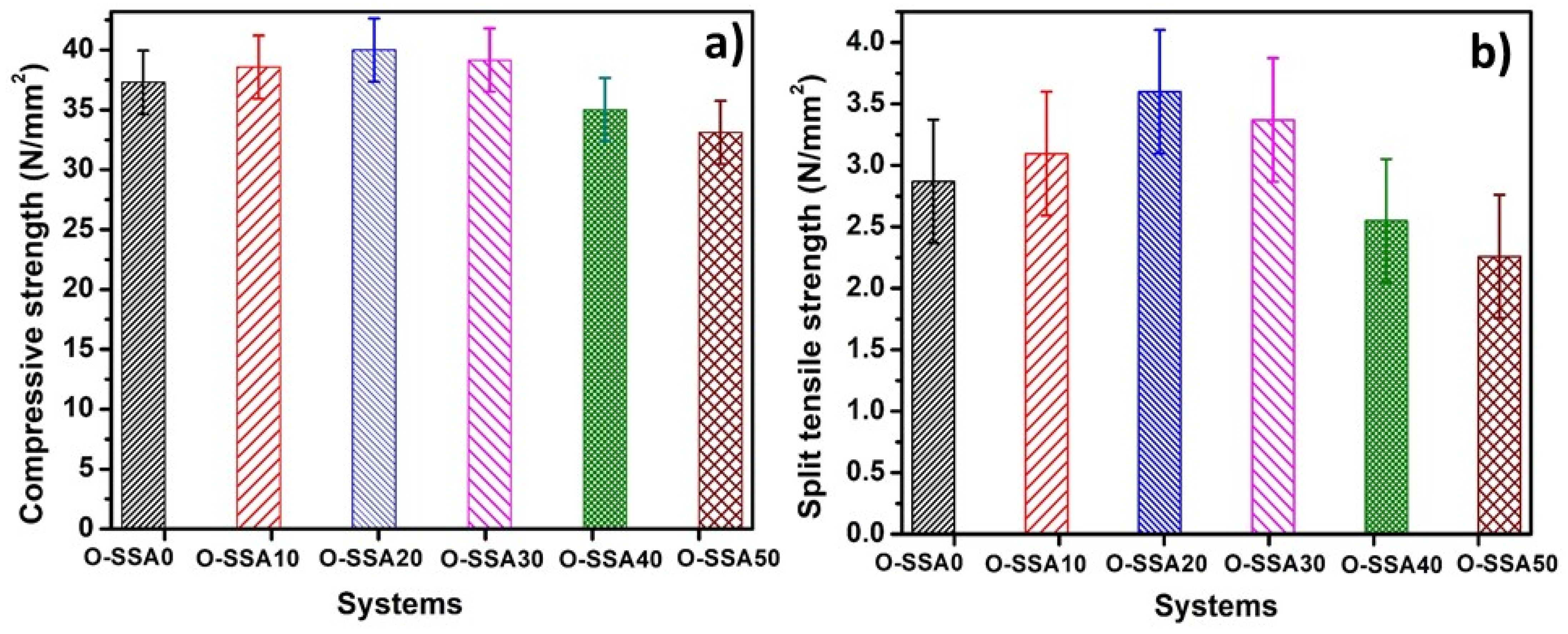
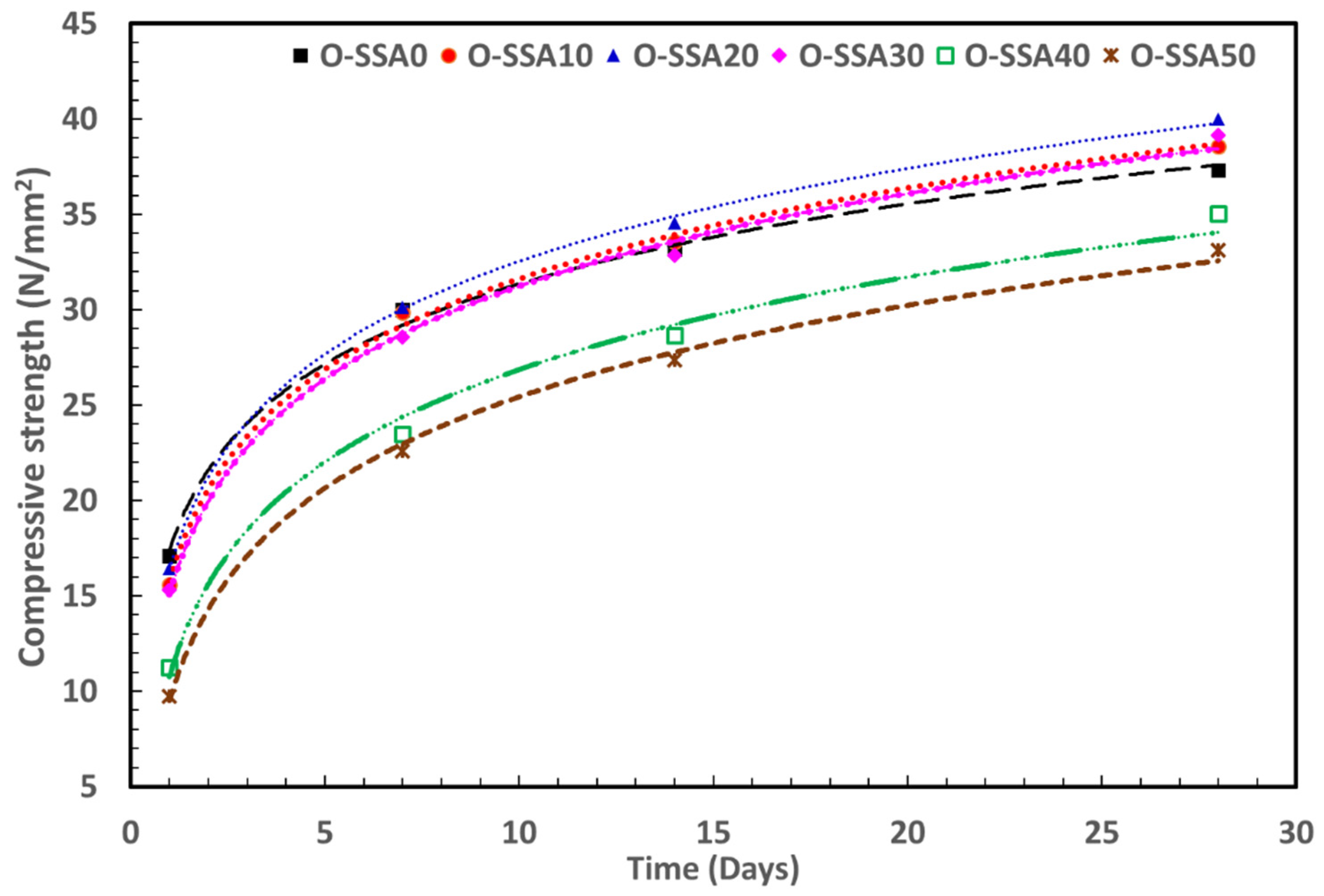
| OPC | Steel Rebar | ||
|---|---|---|---|
| Constituents | wt (%) | Constituents | wt (%) |
| CaO | 63.41 | Iron (Fe) | 98.498 |
| SiO2 | 21.96 | Carbon (C) | 0.236 |
| Al2O3 | 5.27 | Silica (Si) | 0.249 |
| Fe2O3 | 3.44 | Chromium (Cr) | 0.037 |
| MgO | 2.13 | Nickel (Ni) | 0.028 |
| Na2O | 0.12 | Manganese (Mn) | 0.90 |
| K2O | 0.43 | Molybdenum (Mo) | 0.009 |
| SO3 | 2.45 | Copper (Cu) | 0.018 |
| LOI | 0.79 | Phosphorous (P) | 0.014 |
| Tin (Sn) | 0.002 | ||
| Sulphur (S) | 0.009 | ||
| Physical properties | |||
| Specific gravity (g/cm3) | 3.15 | Density (g/cm3) | 7.85 |
| System | Cement (g) | SSA (g) | Distilled Water (mL) | NaCl (g) | pH |
|---|---|---|---|---|---|
| O-SSA0 | 100 | 0 | 100 | 3.5 | 12.73 |
| O-SSA10 | 90 | 10 | 12.75 | ||
| O-SSA20 | 80 | 20 | 12.78 | ||
| O-SSA30 | 70 | 30 | 12.76 | ||
| O-SSA40 | 60 | 40 | 12.77 | ||
| O-SSA50 | 50 | 50 | 12.76 |
| System | Cement (kg/m3) | SSA (kg/m3) | Fine Aggregates (kg/m3) | Coarse Aggregates (kg/m3) | Water/Cement Ratio |
|---|---|---|---|---|---|
| O-SSA0 | 415.0 | 0 | 710 | 1287 | 0.5 |
| O-SSA10 | 373.5 | 41.5 | 710 | 1287 | |
| O-SSA20 | 332.0 | 83.0 | 710 | 1287 | |
| O-SSA30 | 290.5 | 124.5 | 710 | 1287 | |
| O-SSA40 | 249.0 | 166.0 | 710 | 1287 | |
| O-SSA50 | 207.5 | 207.5 | 710 | 1287 |
| System | Exposure Period (Days) | Ecorr (mV) vs. SCE | ba (mV/dec) | bc (mV/dec) | Icorr (µA/cm2) | Corrosion Rate (mm/y) ×10−2 |
|---|---|---|---|---|---|---|
| O-SSA0 (OPC) | 1 | −611 | 114 | 102 | 0.5572 | 0.6558 |
| 15 | −680 | 124 | 369 | 1.5062 | 1.7728 | |
| 30 | −751 | 76 | 161 | 2.6981 | 3.1757 | |
| O-SSA10 | 1 | −597 | 115 | 131 | 0.5239 | 0.6167 |
| 15 | −677 | 112 | 364 | 1.4549 | 1.7125 | |
| 30 | −731 | 137 | 127 | 2.3321 | 2.7449 | |
| O-SSA20 | 1 | −593 | 104 | 117 | 0.3857 | 0.4539 |
| 15 | −668 | 111 | 272 | 1.2536 | 1.4755 | |
| 30 | −706 | 103 | 168 | 1.7815 | 2.0969 | |
| O-SSA30 | 1 | −619 | 72 | 223 | 0.5736 | 0.6751 |
| 15 | −690 | 111 | 311 | 1.5382 | 1.8105 | |
| 30 | −753 | 116 | 110 | 2.6915 | 3.1679 | |
| O-SSA40 | 1 | −629 | 145 | 149 | 0.8255 | 0.9716 |
| 15 | −698 | 124 | 395 | 1.9648 | 2.3126 | |
| 30 | −762 | 128 | 100 | 3.1637 | 3.723 | |
| O-SSA50 | 1 | −660 | 130 | 125 | 0.8719 | 1.0263 |
| 15 | −702 | 145 | 482 | 2.0630 | 2.4281 | |
| 30 | −785 | 195 | 122 | 4.8319 | 5.6873 |
| System | Exposure Period (Days) | RS (Ω·cm2) | R1 (Ω·cm2) | CPE1 | Rct | CPEct | ||
|---|---|---|---|---|---|---|---|---|
| Y0 (Ω−1·cm2 S−n) × 10−3 | N | (Ω·cm2) | Y0 (Ω−1·cm2 S−n) ×10−2 | N | ||||
| O-SSA0 (OPC) | 1 | 1.58 | – | – | – | 2097 | 0.131 | 0.74 |
| 15 | 1.99 | 969.6 | 1.598 | 0.66 | 1063 | 1.082 | 0.77 | |
| 30 | 2.85 | 157.9 | 7.307 | 0.63 | 176 | 1.942 | 0.74 | |
| O-SSA10 | 1 | 2.201 | – | – | – | 2201 | 0.125 | 0.75 |
| 15 | 1.59 | 975.8 | 2.148 | 0.65 | 1142 | 1.064 | 0.83 | |
| 30 | 3.03 | 180.7 | 3.061 | 0.62 | 289 | 1.224 | 0.70 | |
| O-SSA20 | 1 | 1.335 | – | – | – | 2281 | 0.123 | 0.74 |
| 15 | 2.48 | 989.8 | 1.523 | 0.65 | 1239 | 0.929 | 0.76 | |
| 30 | 2.53 | 243.9 | 2.606 | 0.65 | 385 | 1.150 | 0.72 | |
| O-SSA30 | 1 | 1.414 | – | – | – | 1924 | 0.134 | 0.74 |
| 15 | 2.04 | 966.9 | 1.924 | 0.60 | 971 | 1.115 | 0.76 | |
| 30 | 3.17 | 159.5 | 6.6344 | 0.72 | 183 | 1.313 | 0.84 | |
| O-SSA40 | 1 | 0.955 | – | – | – | 1875 | 0.161 | 0.70 |
| 15 | 2.32 | 946.7 | 1.527 | 0.69 | 894 | 1.353 | 0.86 | |
| 30 | 2.39 | 157.1 | 8.007 | 0.69 | 175 | 2.015 | 0.68 | |
| O-SSA50 | 1 | 2.61 | – | – | – | 1410 | 0.164 | 0.73 |
| 15 | 2.81 | 920.2 | 1.792 | 0.67 | 799 | 1.966 | 0.92 | |
| 30 | 2.64 | 100.8 | 5.894 | 0.68 | 158 | 2.835 | 0.63 | |
| System | Number of Specimens | Weight Loss (g) | Corrosion Rate (mm/y) | Average Corrosion Rate (mm/y) | pH | |
|---|---|---|---|---|---|---|
| Initial | Final | |||||
| O-SSA0 (OPC) | 1 | 0.7753 | 0.7973 | 0.7998 | 12.73 | 10.14 |
| 2 | 0.7801 | 0.8022 | 12.75 | 10.43 | ||
| O-SSA10 | 1 | 0.6746 | 0.6937 | 0.6913 | 12.75 | 10.85 |
| 2 | 0.6698 | 0.6888 | 12.74 | 10.29 | ||
| O-SSA20 | 1 | 0.5499 | 0.5655 | 0.5679 | 12.78 | 11.21 |
| 2 | 0.5546 | 0.5703 | 12.76 | 11.35 | ||
| O-SSA30 | 1 | 0.7747 | 0.7966 | 0.7983 | 12.76 | 10.37 |
| 2 | 0.7779 | 0.7999 | 12.77 | 10.41 | ||
| O-SSA40 | 1 | 0.8301 | 0.8536 | 0.8586 | 12.77 | 10.08 |
| 2 | 0.8398 | 0.8636 | 12.77 | 10.12 | ||
| O-SSA50 | 1 | 0.8662 | 0.8907 | 0.8869 | 12.76 | 9.92 |
| 2 | 0.8589 | 0.8832 | 12.77 | 10.07 | ||
| Replacement Level (%) | A | B | R2 | Compressive Strength at 365th Day (N/mm2) |
|---|---|---|---|---|
| O-SSA0 | 6.0585 | 17.403 | 0.9964 | 53.15 |
| O-SSA10 | 6.8661 | 15.818 | 0.9977 | 56.33 |
| O-SSA20 | 7.0229 | 16.372 | 0.9994 | 57.81 |
| O-SSA30 | 7.0118 | 15.092 | 0.9963 | 56.46 |
| O-SSA40 | 6.9946 | 10.763 | 0.9926 | 52.03 |
| O-SSA50 | 6.9208 | 9.5042 | 0.9977 | 50.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raghav, M.; Karthick, S.; Park, T.; Lee, H.-S. Assessment of Corrosion Performance of Steel Rebar in Snail Shell Ash Blended Cements under Marine Environments. Materials 2021, 14, 7286. https://doi.org/10.3390/ma14237286
Raghav M, Karthick S, Park T, Lee H-S. Assessment of Corrosion Performance of Steel Rebar in Snail Shell Ash Blended Cements under Marine Environments. Materials. 2021; 14(23):7286. https://doi.org/10.3390/ma14237286
Chicago/Turabian StyleRaghav, Muralidharan, Subbiah Karthick, Taejoon Park, and Han-Seung Lee. 2021. "Assessment of Corrosion Performance of Steel Rebar in Snail Shell Ash Blended Cements under Marine Environments" Materials 14, no. 23: 7286. https://doi.org/10.3390/ma14237286
APA StyleRaghav, M., Karthick, S., Park, T., & Lee, H.-S. (2021). Assessment of Corrosion Performance of Steel Rebar in Snail Shell Ash Blended Cements under Marine Environments. Materials, 14(23), 7286. https://doi.org/10.3390/ma14237286









