MoS2 Nanoparticle Effects on 80 °C Thermally Stable Water-Based Drilling Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization and Analyses Methods
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Viscosity Measurements
2.2.3. Anton Parr Rheometer
2.2.4. API Filter Press
2.2.5. CSM Tribometer
2.2.6. ICP-EOS Filtrate Element Analysis
2.2.7. Thermal Conductivity Analysis
2.2.8. Electrical Conductivity Analysis
2.2.9. Modelling of Viscosity Characterization Method
2.3. Drilling Fluid Formulation
3. Results and Discussion
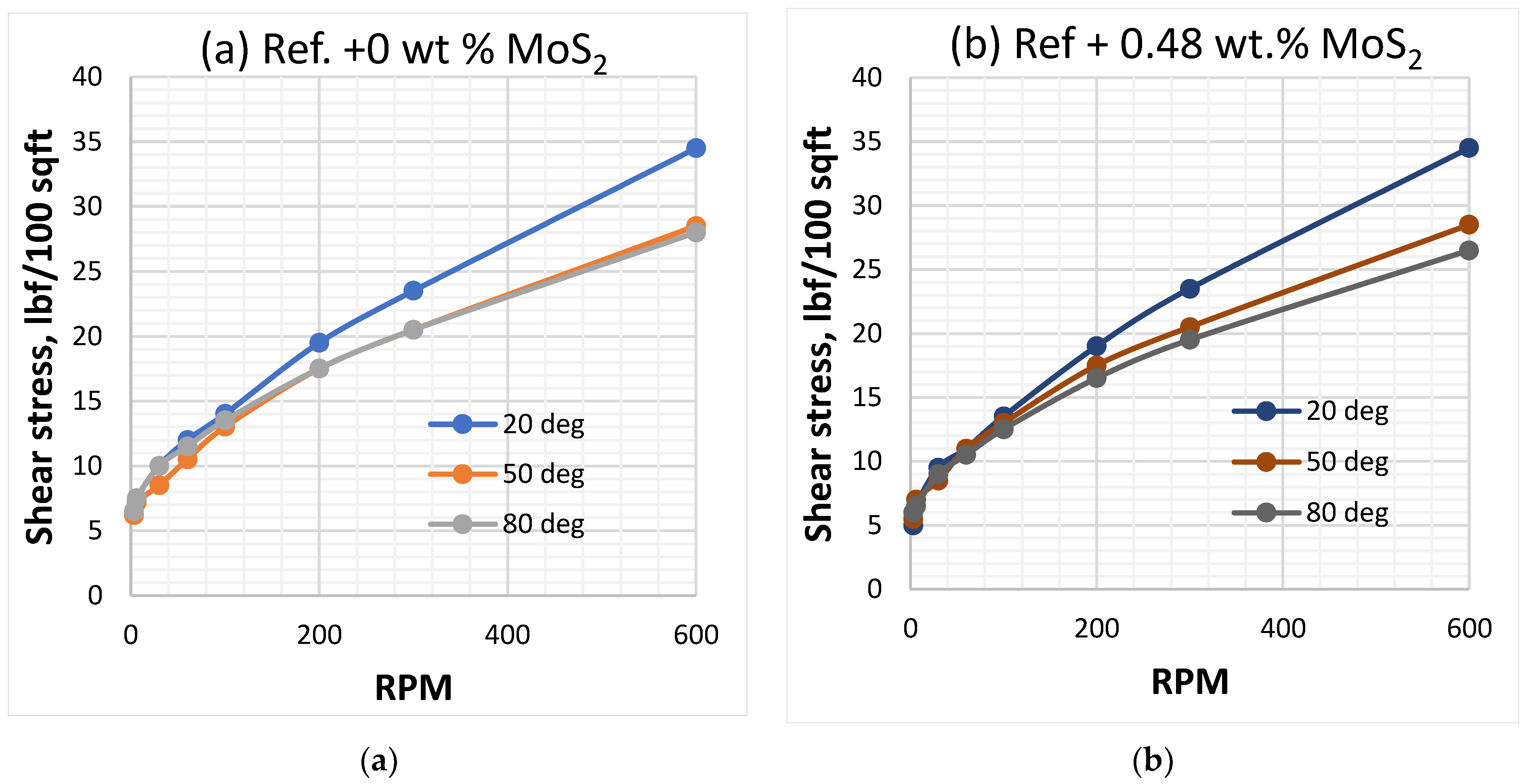
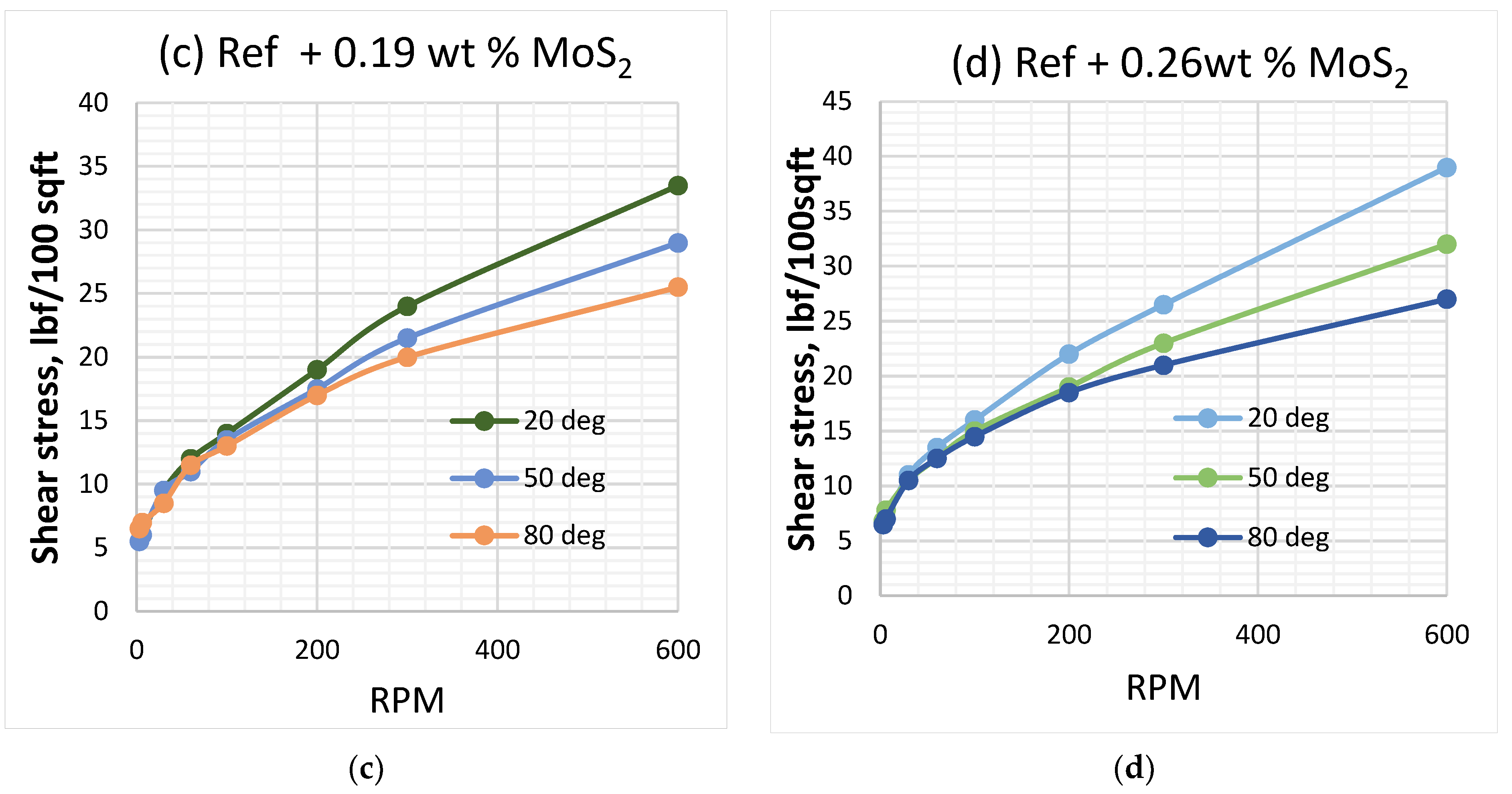
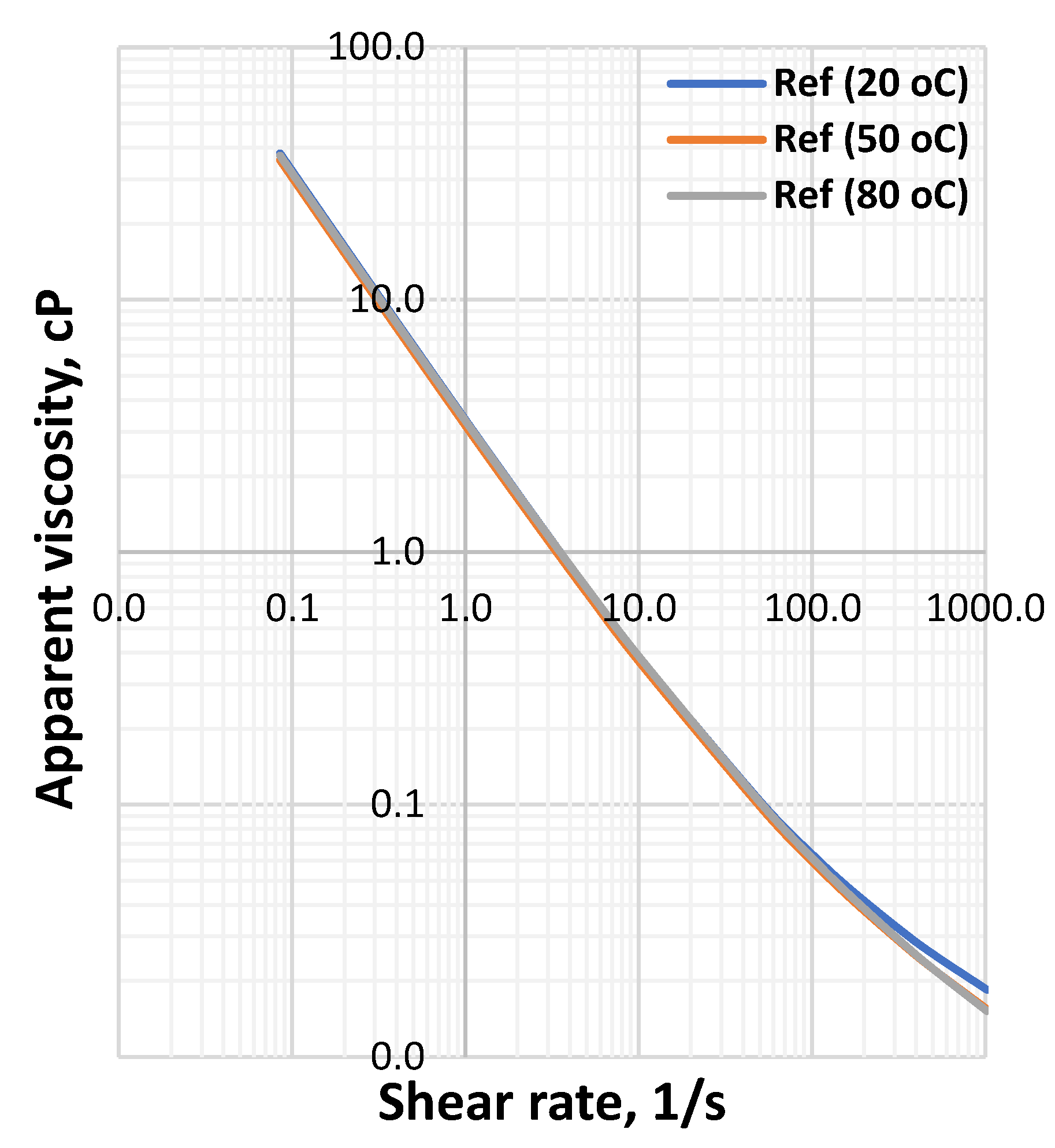
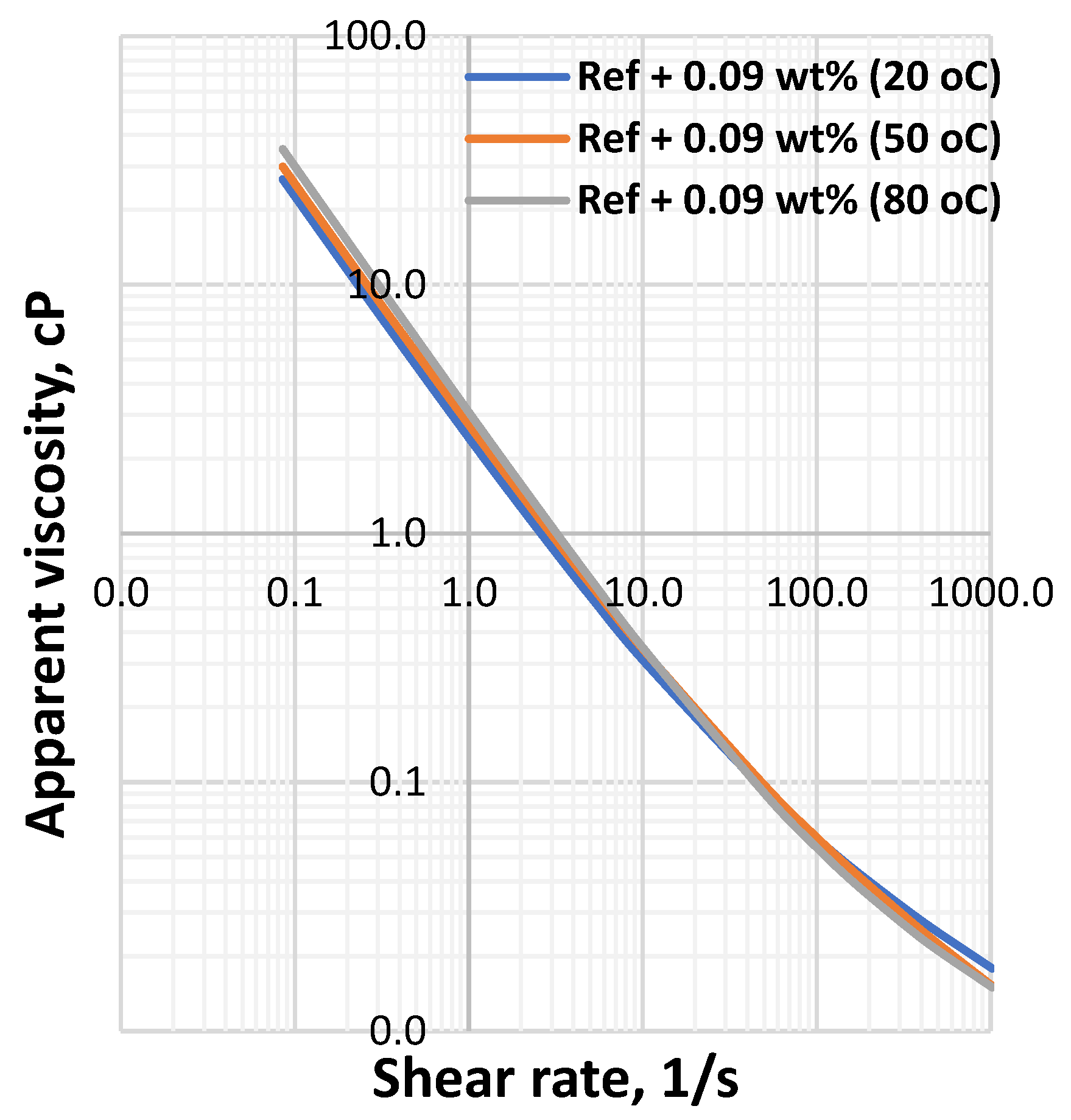
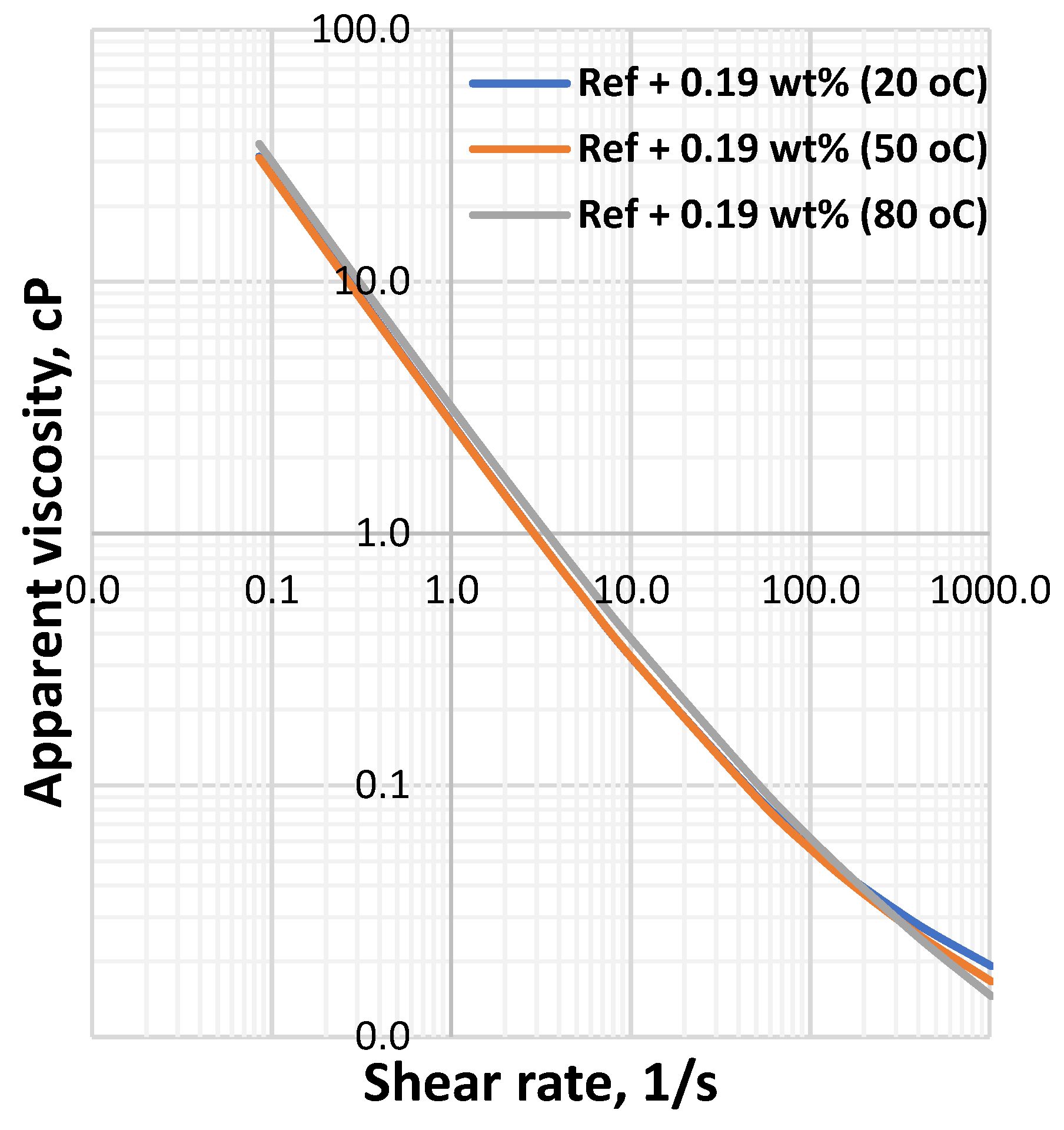
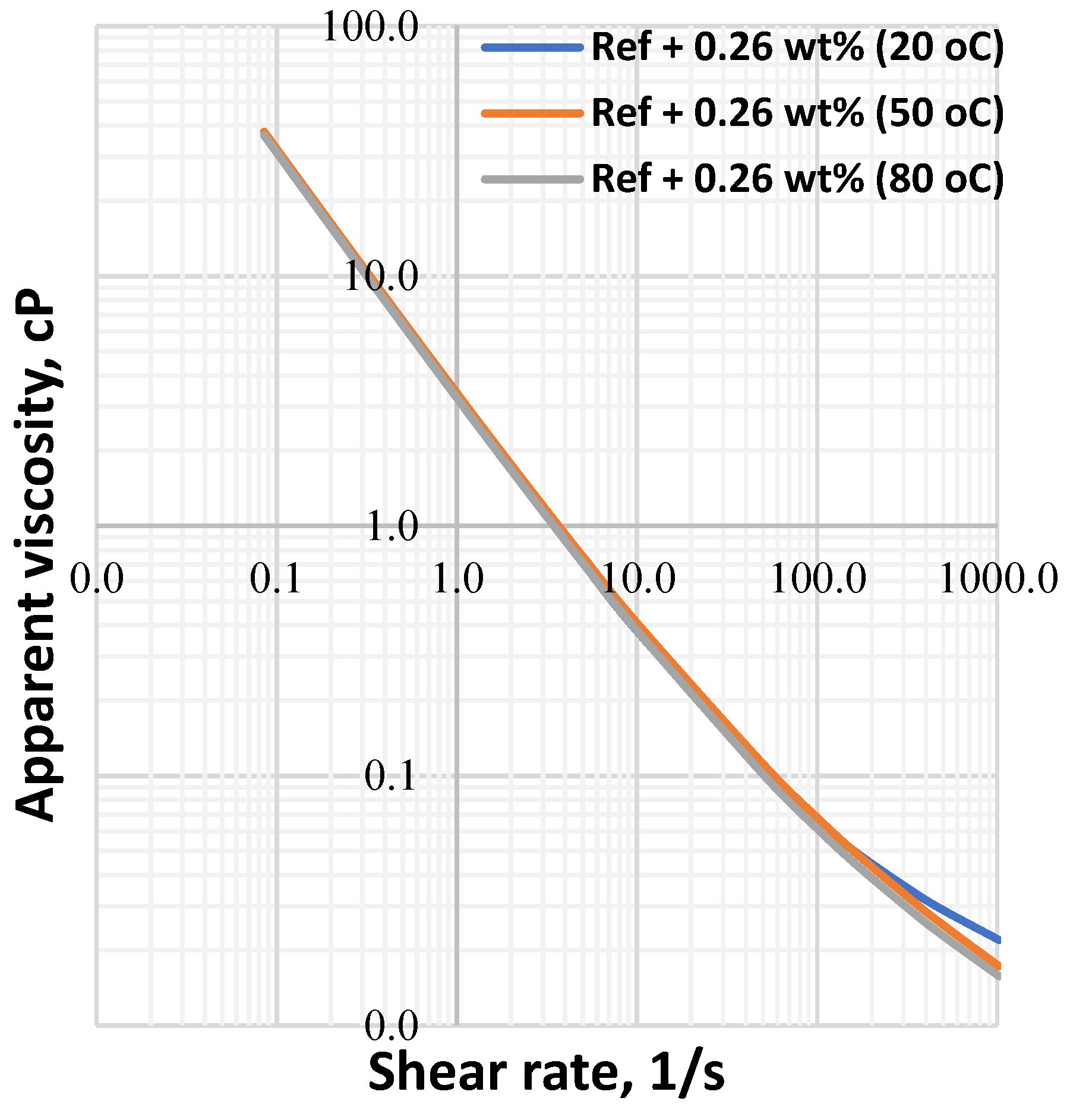
3.1. Effect of MoS2 and Temperature on the Viscosity of the Thermal Stable Drilling Fluid
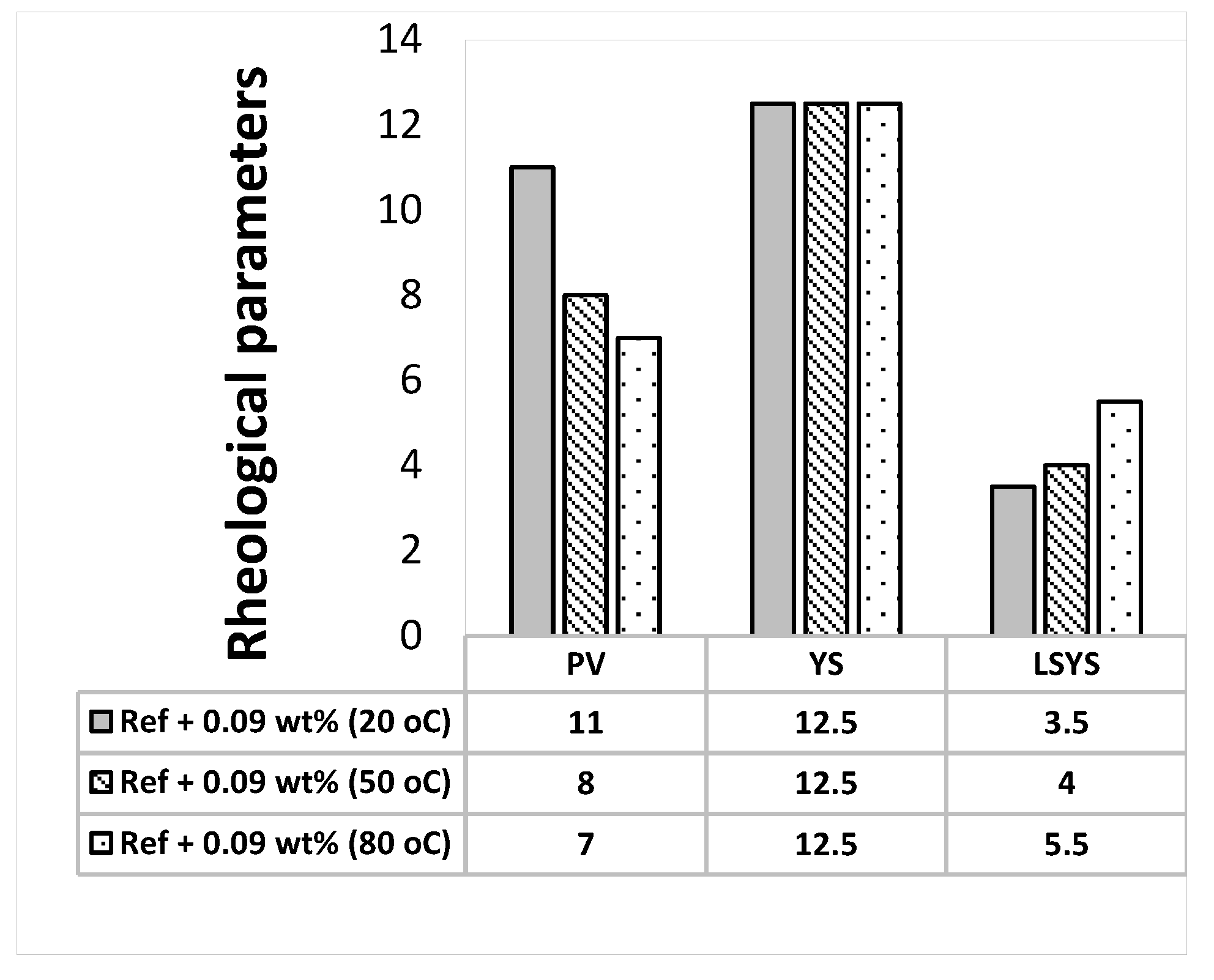
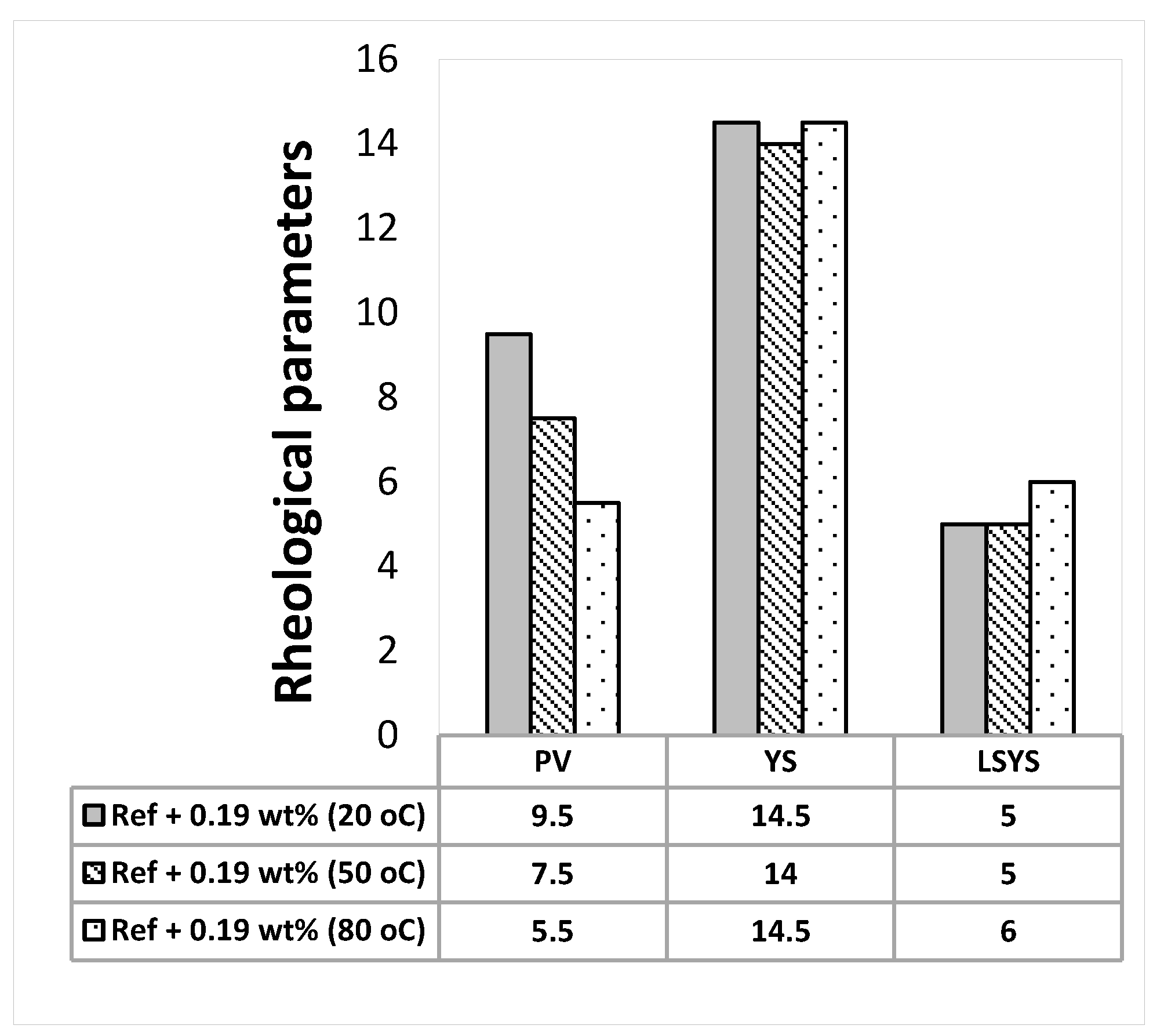
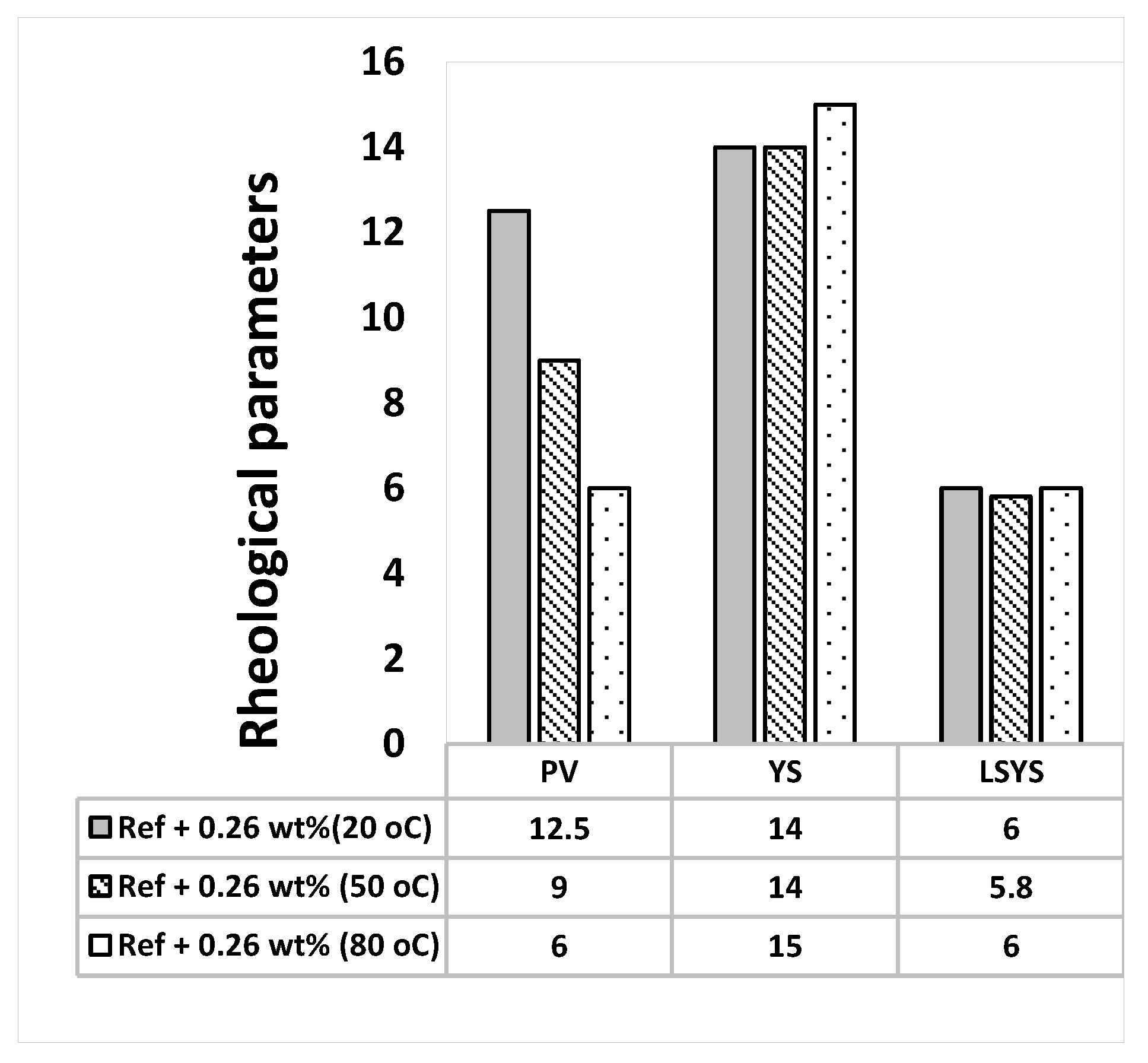
3.2. Effect of MoS2 and Temperatures on the Rheological Properties of the Thermal Stable Drilling Fluid
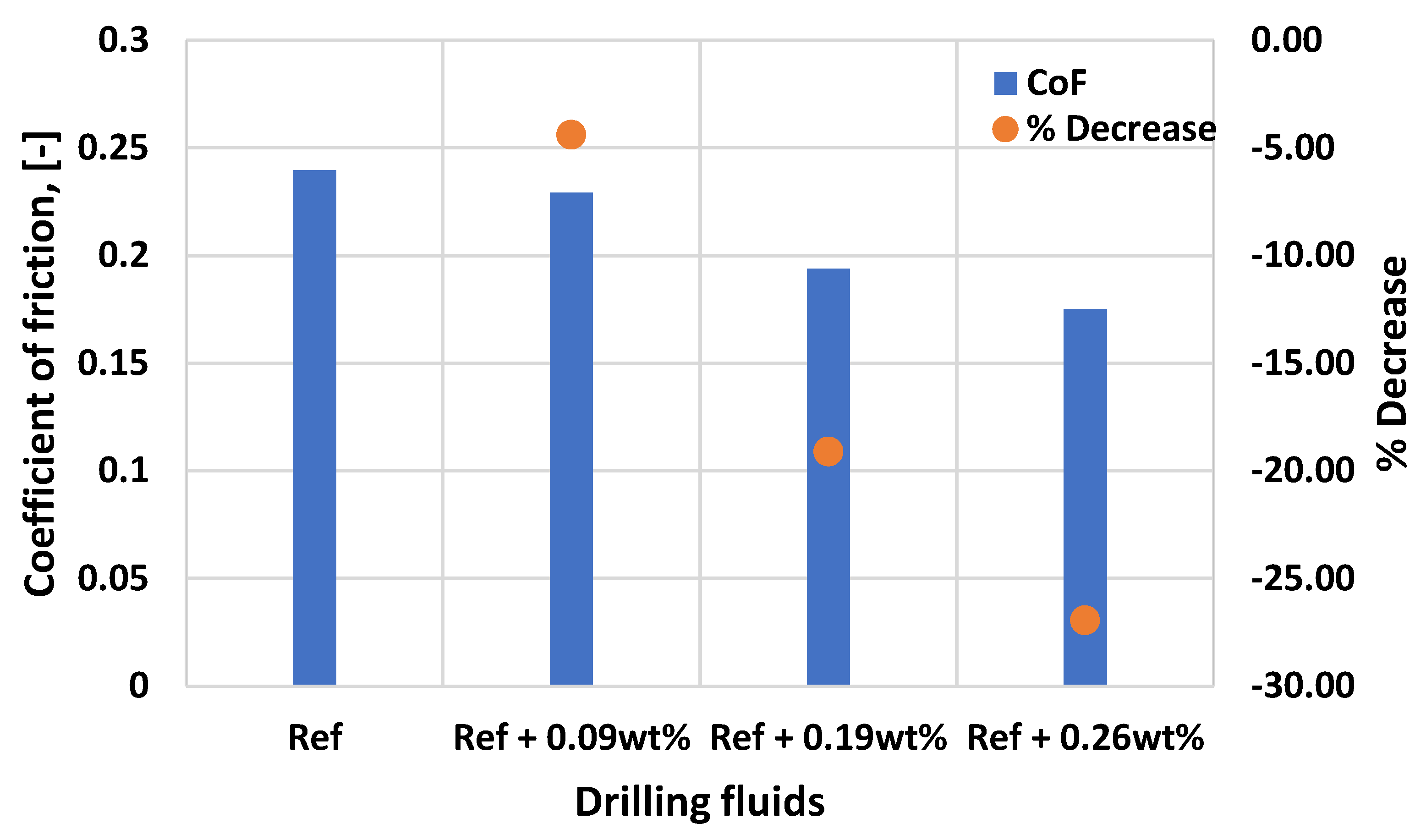
3.3. Effect of MoS2 on the Lubricity of the Thermal Stable Drilling Fluid
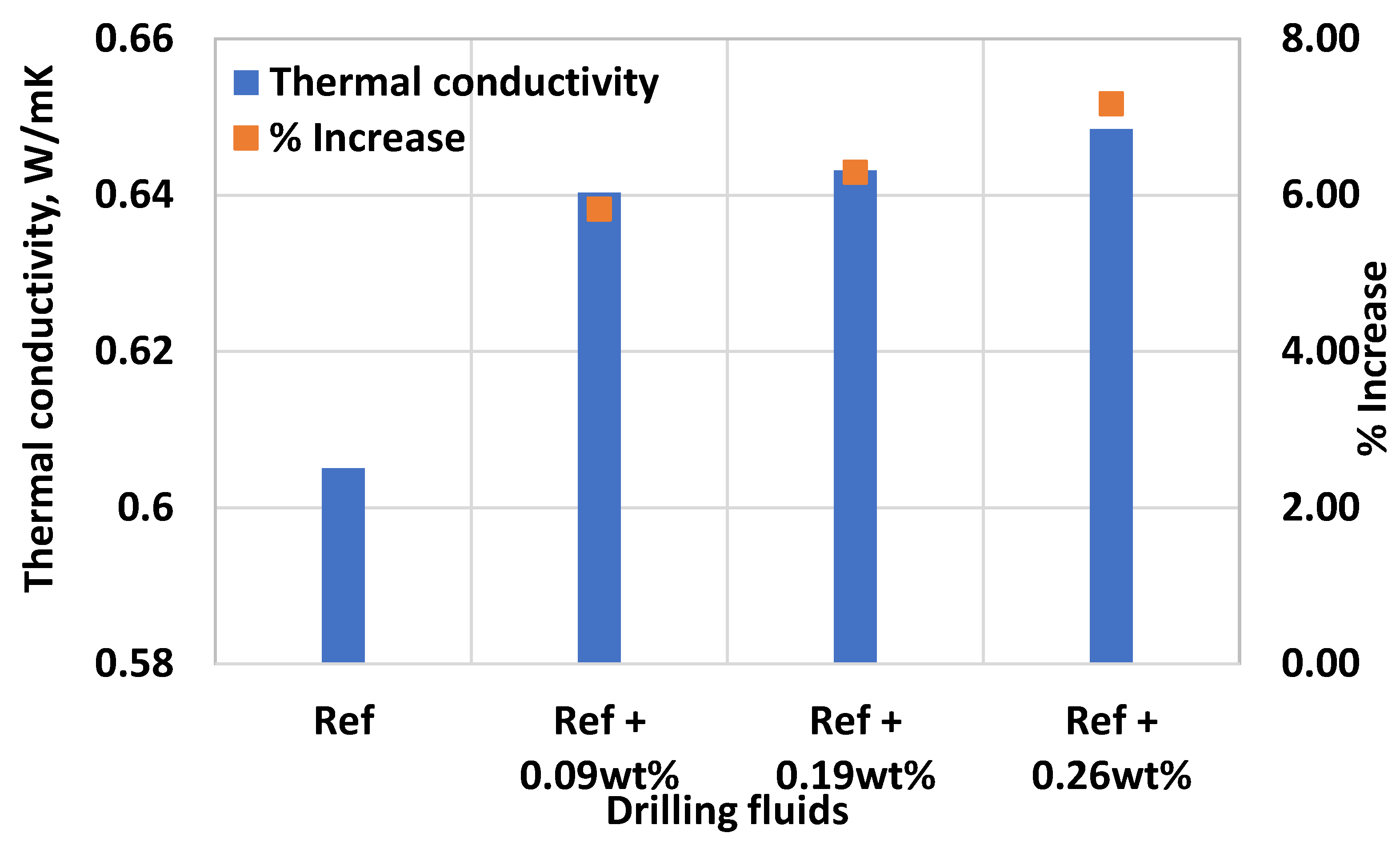
3.4. Effect of MoS2 on the Thermal Conductivity of the Thermal Stable Drilling Fluid
3.5. Effect of MoS2 on the Electrical Conductivity of the Thermal Stable Drilling Fluid
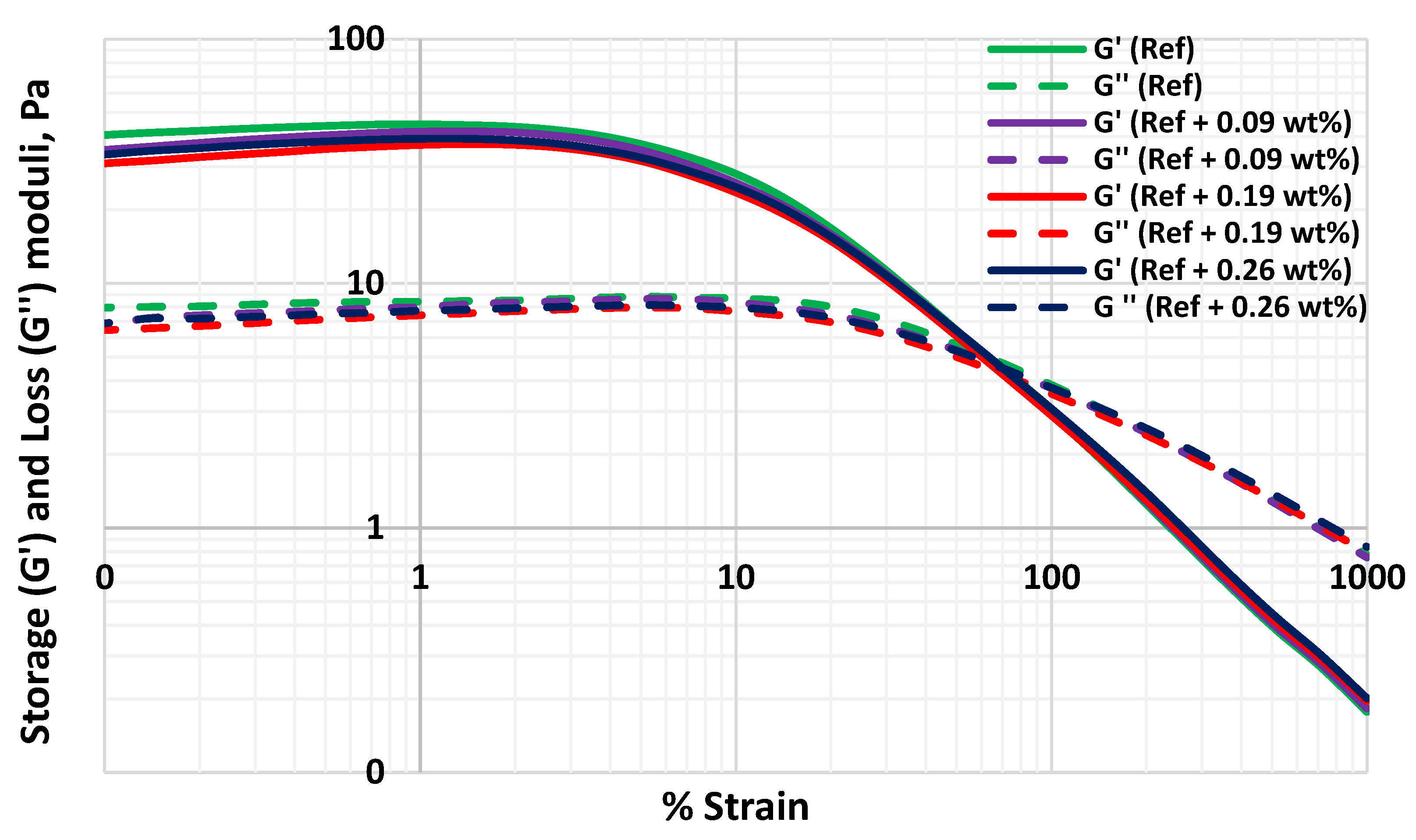
3.6. Effect of MoS2 on the Viscoelasticity of the Thermal Stable Drilling Fluid
3.7. Effect of MoS2 on the Filtrate Loss of the Thermal Stable Drilling Fluid
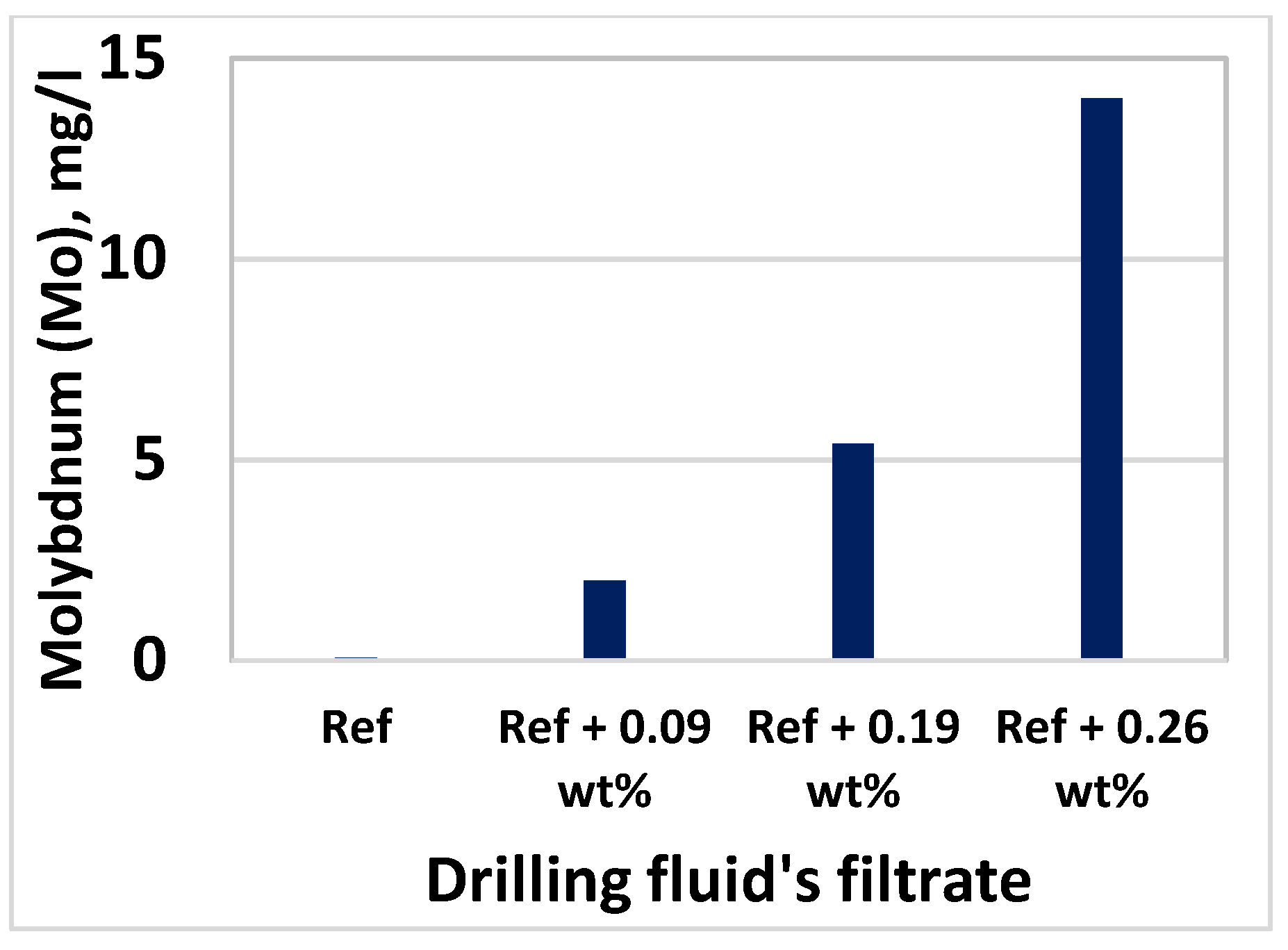
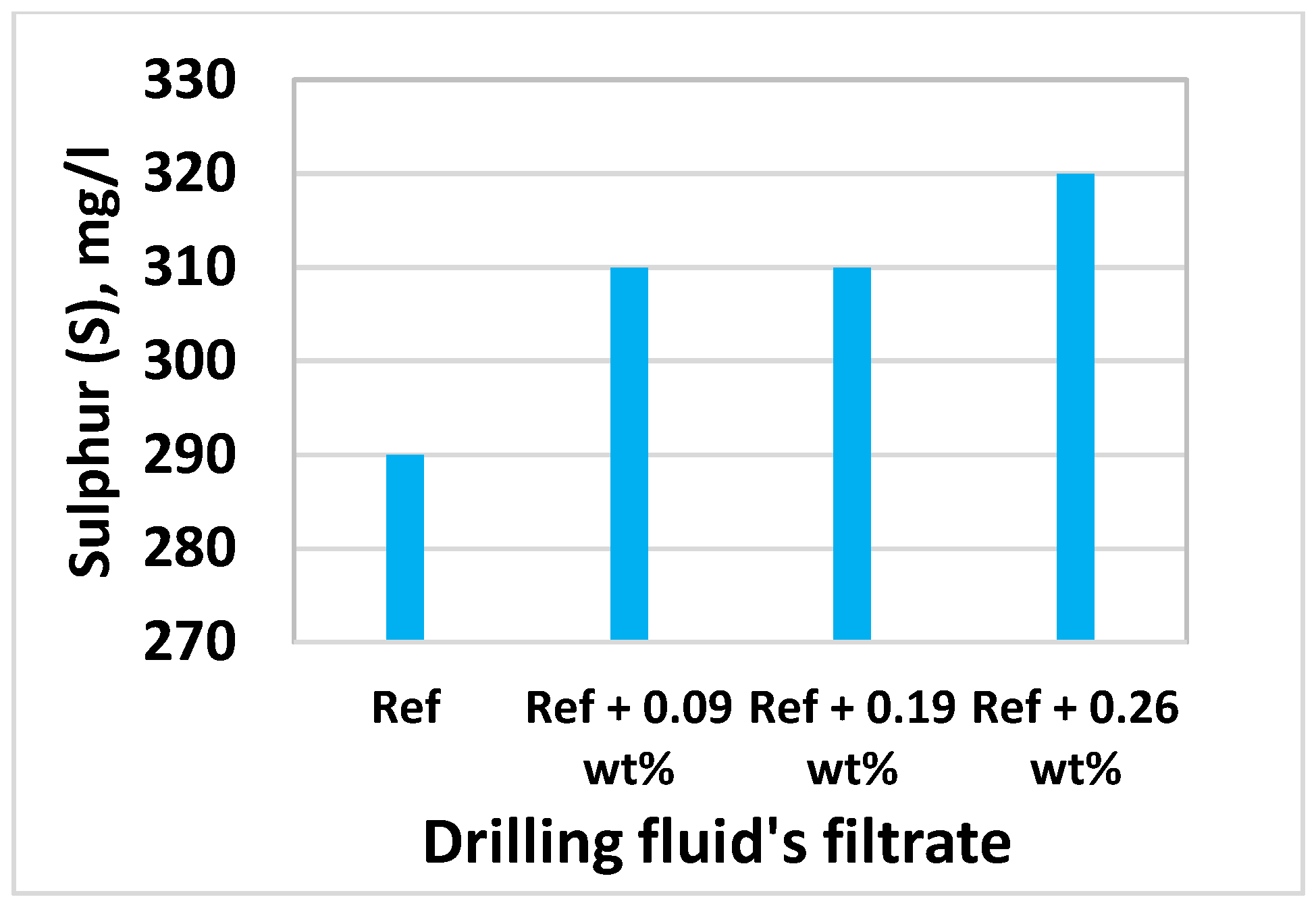
3.8. Effect of MoS2 on Filtrate Loss and Ionic Concentrations
4. Discussion
5. Conclusions
- The addition of 0.17 wt.% lignosulfonate maintains thermally stable H-B and Bingham plastic yield stresses.
- The 0.26 wt.% molybdenum disulphide additive enhanced the lubricity of the thermally stable base fluid by 27%. The nanoparticles maintain the thermal stability of the fluid and did not show a significant impact on viscoelasticity.
- Addition of 0.26 wt.% MoS2 nanoparticle solution increased the thermal and electrical conductivity of the base fluid by about 7.2% and by 8.8%, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caenn, R.; Darley, H.C.H.; Gray, G.R. Composition and Properties of Drilling and Completion Fluids, 6th ed.; Gulf Professional Publishing: Houston, TX, USA, 2011; ISBN 978-0-12-383858-2. [Google Scholar]
- Bleler, R. Selecting a drilling fluid. J. Pet. Technol. 1990, 42, 832–834. [Google Scholar] [CrossRef]
- Boyd, P.A.; Whitfill, D.L.; Carter, T.S.; Allamon, J.P. Low-viscosity base fluid for low-toxicity oil-mud systems. SPE Drill. Eng. 1987, 2, 218–228. [Google Scholar] [CrossRef]
- Growcock, F.B.; Frederick, T.P.; Reece, A.R.; Green, G.W. Novel lubricants for water-based drilling fluids, SPE 50710. In Proceedings of the 1999 International Symposium on Oilfield Chemistry, Houston, TX, USA, 16–19 February 1998. [Google Scholar]
- Foxenberg, W.E.; Ali, S.A.; Long, T.P.; Vian, J. Field experience shows that new lubricant reduces friction and improves formation compatibility and environmental impact, SPE 112483. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 13–15 February 2008. [Google Scholar]
- Stjern, G.; Agle, A.; Horsrud, P. Local rock mechanical knowledge improves drilling performance in fractured formations at the Heidrun field. J. Pet. Sci. Eng. 2008, 38, 83–96. [Google Scholar] [CrossRef]
- Ehrhorn, C.; Saasen, A. Barite sag in drilling fluids. Ann. Trans. Nord. Rheol. Soc. 1996, 4, 66. [Google Scholar]
- Skjeggestad, O. Boreslam Teknologi; Alma Mater Forlag AS: Bergen, Norway, 1989; ISBN 82-419-0010-4. [Google Scholar]
- Elward-Berry, J.; Darby, J.B. Rheologically Stable, Nontoxic, High-Temperature Water-Base Drilling Fluid; SPE-24589-PA. SPE Drill. Compl. 1997, 12, 158–162. [Google Scholar] [CrossRef]
- Altun, G.; Osgouei, A.E.; Ozyurtkan, M.H.; Serpen, U. Sepiolite Based Muds as an Alternate Drilling Fluid for Hot Environments. In Proceedings of the World Geothermal Congress 2015, Melbourne, Australia, 19–24 April 2015; p. 10. [Google Scholar]
- Browning, W.C. Lignosulfonate Stabilized Emulsions in Oil Well Drilling Fluids, SPE 393-G. J. Pet. Technol. 1955, 7, 9–15. [Google Scholar] [CrossRef]
- Tehrani, M.A.; Popplestone, A.; Guarneri, A.; Carminati, S. Water base drilling fluids for HP/HT applications. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar]
- Abdo, J.; Haneef, M.D. Nanoparticles: Promising Solution to Overcome Stern Drilling Problems. In Proceedings of the 2010 Clean Technology Conference and Trade Show, Washington, DC, USA, 18–20 October 2010; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4398-3419-0. [Google Scholar]
- Amanullah, M.D.; Al-Tahini, M.A. Nano-Technology—Its Significance in Smart Fluid Development for Oil and Gas Field Application, SPE-126102-MS. In Proceedings of the Saudi Arabia Section Technical Symposium, Al-Khobar, Saudi Arabia, 9 May 2009. [Google Scholar]
- Vryzas, Z.; Zaspalis, V.; Nalbantian, L.; Mahmoud, O.; Nasr-El-Din, H.A.; Kelessidis, V.C. A Comprehensive Approach for the Development of New Magnetite Nanoparticles Giving Smart Drilling Fluids with Superior Properties for HP/HT Applications, IPTC-18731-MS. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 14–16 November 2016. [Google Scholar]
- Sadeghalvaad, M.; Sabbaghi, S. The effect of the TiO2/polyacrylamide nanocomposite on water-based drilling fluid properties. Powder Technol. 2015, 272, 113–119. [Google Scholar] [CrossRef]
- Mohamadian, N.; Ghorbani, H.; Wood, D.A.; Khoshmardan, M.A. A hybrid nanocomposite of poly (styrene-methyl methacrylate-acrylic acid), clay as a novel rheology-improvement additive for drilling fluids. J. Polym. Res. 2019, 26, 33. [Google Scholar] [CrossRef]
- Mohamud, O.; Mady, A.; Aftab, A.S.D.A. Al2O3 and CuO nanoparticles as promising additives to improve the properties of KCl-polymer mud: An experimental investigation. Can. J. Chem. Eng. 2021, 1–14. [Google Scholar] [CrossRef]
- Mohamadian, N.; Ghorbani, H.; Wood, D.A.; Khoshmardan, M.A. Rheological and filtration characteristics of drilling fluids enhanced by nanoparticles with selected additives: An experimental study. Adv. Geo-Energy Res. 2018, 2, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Aftab, A.; Ali, M.; Sahito, M.F.; Mohanty, U.S.; Jha, N.K.; Akhondzadeh, H.; Azhar, M.R.; Ismail, A.R.; Keshavarz, A.; Iglauer, S. Environmental friendliness and high performance of multifunctional tween 80/ZnO-nanoparticles-added water-based drilling fluid: An experimental approach. ACS Sustain. Chem. Eng. 2020, 8, 11224–11243. [Google Scholar] [CrossRef]
- Sharma, M.M.; Zhang, R.; Chenevert, M.E.; Ji, L.; Guo, Q.; Friedheim, J. A new family of nanoparticle-based drilling fluids, SPE-160045-MS. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar]
- Hoelscher, K.P.; de Stefano, G.; Riley, M.; Young, S. Application of Nanotechnology in Drilling Fluids, SPE-157031-MS. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Gao, C.; Miska, S.Z.; Yu, M.; Ozbayoglu, E.M.; Takach, N.E. Effective Enhancement of Wellbore Stability in Shales with New Families of Nanoparticles, SPE-180330-MS. In Proceedings of the SPE Deepwater Drilling and Completions Conference, Galveston, TX, USA, 14–15 September 2016. [Google Scholar]
- Taha, N.M.; Lee, S. Nano Graphene Application Improving Drilling Fluids Performance, IPTC-18539-M. In Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 6–9 December 2015. [Google Scholar]
- Awais, M.; Belayneh, M.; Saasen, A.; Fjelde, K.K.; Bernt; Aadnøy, S. Effect of MWCNT and MWCNT Functionalized –OH and -COOH Nanoparticles in Laboratory Water Based Drilling Fluid. In Proceedings of the ASME 2017 37th International Conference on Ocean, Offshore and Arctic Engineering, Madrid, Spain, 17–22 June 2018. [Google Scholar]
- Sabah, A.; Alswasiti, A.; Salam, M. Improving Drilling Fluid Properties at High Pressure Conditions Using Selected Nanomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 579, 012004. [Google Scholar] [CrossRef]
- Nwaoii, C.O.; Hareland, G.; Husein, M.; Nygaard, R.; Zakaria, M.E. Wellbore strengthening-nano-particle drilling fluid. Experimental design using hydraulic fracture apparatus, SPE-163434. In Proceedings of the SPE/IADC Drilling Conference, Amsterdam, The Netherlands, 5–7 March 2013. [Google Scholar]
- Halali, M.A.; Ghotbi, C.; Tahmasbi, K.; Ghazanfari, M.H. The Role of Carbon Nanotubes in Improving Thermal Stability of Polymeric Fluids: Experimental and Modeling. Ind. Eng. Chem. Res. 2016, 55, 7514–7534. [Google Scholar] [CrossRef]
- William, J.K.M.; Ponmani, S.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical, and high-pressure rheology of water-based drilling fluids. J. Pet. Sci. Eng. 2014, 117, 15–27. [Google Scholar] [CrossRef]
- Hassani, S.S.; Amrollahi, A.; Rashidi, A.; Soleymani, M.; Rayatdoost, S. The effect of nanoparticles on the heat transfer properties of drilling fluids. J. Pet. Sci. Eng. 2016, 146, 183–190. [Google Scholar] [CrossRef]
- Fazelabdolabadi, B.; Khodadadi, A.A.; Sedaghatzadeh, M. Thermal and rheological properties improvement of drilling fluids using functionalized carbon nanotubes. Appl. Nanosci. 2015, 5, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Ponmani, S.; Nagarajan, R.; Sangwai, J.S. Effect of nanofluids of CuO and ZnO in polyethylene glycol and polyvinylpyrrolidone on the thermal, electrical, and filtration-loss properties of water-based drilling fluids. SPE J. 2016, 21, 405–415. [Google Scholar] [CrossRef]
- Boul, P.J.; Reddy, B.R.; Zhang, J.; Thaemlitz, C. Functionalized nanosilicas as shale inhibitors in water-based drilling fluids. SPE Drill. Completion 2017, 32, 121–130. [Google Scholar] [CrossRef]
- Kang, Y.; She, J.; Zhang, H.; You, L.; Song, M. Strengthening shale wellbore with silica nanoparticles drilling fluid. Petroleum 2016, 2, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Lansdown, A.R. Molybdenum Disulphide Lubrication; Elsevier Science: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Sandlin, M.S.; Butt, D.P.; Taylor, T.N.; Petrovic, J.J. Aqueous zeta potentials of molybdenum disilicide. J. Mater. Sci. Lett. 1997, 16, 1336–1338. [Google Scholar] [CrossRef]
- Herschel, W.H.; Bulkley, R. Konsistenzmessungen von gummi-benzöllösungen. Kolloid-Z 1926, 39, 291. [Google Scholar] [CrossRef]
- API RP 13-B1. Recommend Practice for Field Testing Water-Based Drilling Fluids, 3rd ed.; API: Washington, DC, USA, 2003. [Google Scholar]
- Zamora, M.; Power, D. Making a Case for AADE Hydraulics, and the Unified Rheological Model, AADE-02-DFWM-HO-13. In Proceedings of the 2002 AADE Technical Conference, Houston, TX, USA, 2–3 April 2002. [Google Scholar]
- Xu, L.; Xu, M.; Zhao, L.; Wen, S.; Liu, W.; Xu, J.; You, F.; Gong, C. Experimental Investigations into the Performance of a Flat-Rheology Water-Based Drilling Fluid, SPE-163107-PA. SPE J. 2014, 19, 69–77. [Google Scholar] [CrossRef]
- van Oort, E.; Lee, J.; Friedheim, J.; Toups, B. New Flat-Rheology Synthetic-Based Mud for Improved Deepwater Drilling, SPE-90987-MS. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. [Google Scholar]
- Friedheim, J.; Lee, J.; Young, S.; Cullum, D. New Thermally Independent Rheology Invert Drilling Fluid for Multiple Applications, OMC-2011-085. In Proceedings of the Offshore Mediterranean Conference and Exhibition, Ravenna, Italy, 23–25 March 2011. [Google Scholar]
- Torsvik, A.; Myrseth, V.; Opedal, N.; Lund, B.; Saasen, A.; Ytrehus, J.D. Rheological Comparison of Bentonite Based and KCl/Polymer Based Drilling Fluids. Annu. Trans. Nord. Rheol. Soc. 2014, 22, 219–224. [Google Scholar]
- Scott, P.D.; Zamora, M. Barite-Sag Management: Challenges, Strategies, Opportunities, SPE-87136-MS IADC. In Proceedings of the SPE Drilling Conference, Dallas, TX, USA, 2–4 March 2004. [Google Scholar]
- Tributsch, H.; Bennett, J.C. Electrochemistry and photochemistry of MoS2 layer crystals. J. Electroanal. Chem. 1977, 81, 97–111. [Google Scholar] [CrossRef]
- Spalvins, T. A review of recent advances in solid film lubrication. J. Vac. Sci. Technol. A Vac. Surf. Film. 1987, 5, 212. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.; Patel, H.E. Heat transfer in nanofluids—A review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Su, Y.; Gong, L.; Li, B.; Chen, D. An experimental investigation on thermal properties of molybdenum di Shao Sulfide nanofluids. In Proceedings of the 2015 International Conference on Materials, Environmental and Biological Engineering, Guilin, China, 28–30 March 2015; ISBN 978-94-62520-57-8. [Google Scholar]
- Anu, K.; Hemalatha, J. Magnetic and electrical conductivity studies of zinc doped cobalt ferrite nanofluids. J. Mol. Liq. 2019, 284, 445–453. [Google Scholar] [CrossRef]
- Ganguly, S.; Sikdar, S.; Basu, S. Experimental investigation of the effective electrical conductivity of aluminum oxide nanofluids. Powder Technol. 2009, 196, 326–330. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Khattab, R.M.; Girgis, L.G.; El Daidamony, H.; Abdel Aziz Rehab, E. Stability and electrical conductivity of water based Al2O3 nanofluids for di_erent applications. HBRC J. 2016, 12, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Shen, L.P.; Wang, H.; Wang, H.B.; Miao, J. Investigation on the Electrical Conductivity of Transformer Oil-Based AIN Nanofluid. J. Nanomater. 2013, 2013, 842963. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Hao, Q.; Yan, Z.; Dong, R.; Yang, R.; Zhu, D.; Chao, J.; Zhou, Y.; Wang, L. A molybdenum disulfide@Methylene Blue nanohybrid for electrochemical determination of microRNA-21, dopamine, and uric acid. Microchim. Acta 2019, 186, 607. [Google Scholar] [CrossRef]
- Shen, L.P.; Wang, H.; Dong, M.; Ma, Z.C.; Wang, H.B. Solvothermal synthesis and electrical conductivity model for the zinc oxide-insulated oil nanofluid. Phys. Lett. A 2012, 376, 1053. [Google Scholar] [CrossRef]
- Bui, B.; Saasen, A.; Maxey, J.; Ozbayoglu, M.E.; Miska, S.Z.; Yu, M.; Takach, N.E. Viscoelastic Properties of Oil-Based Drilling Fluids. Annu. Trans. Nord. Rheol. Soc. 2012, 20, 33–47. [Google Scholar]
- Saasen, A.; Dawei, L.; Marken, C. Prediction of Barite Sag Potential of Drilling Fluids from Rheologicial Measurements, SPE 29410. In Proceedings of the SPE/IADC Drilling Conference, Amsterdam, The Netherlands, 28 February–2 March 1995. [Google Scholar]
- Barnes, H.A.; Nguyen, Q.D. Rotating vane rheometry—A review. J. Nonnewton. Fluid Mech. 2001, 98, 1–14. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Andrade, D.E.V.; Franco, A.T.; Negrão, C.O.R. Correlation between the gel-liquid transition stress and the storage modulus of an oil-based drilling fluid. J. Non-Newton. Fluid Mech. 2016, 231, 6–10. [Google Scholar] [CrossRef]
- Hovda, S.; Wolter, H.; Kaasa, G.; Olberg, T.S. Potential of Ultra-High-Speed Drill String Telemetry in Future Improvements of the Drilling Process Control, SPE-115196-MS. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference and Exhibition, Jakarta, Indonesia, 25–27 August 2008. [Google Scholar]
- Reid, D.; Rosenberg, S.; Montgomery, M.; Sutherland, M.; York, P. Drilling Hazard Mitigation-Reducing Non-Productive Time by Application of Common Sense and High Value Well Construction Techniques, Paper OTC 18084. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 1–4 May 2006. [Google Scholar]
- Pilisi, N.; Wei, Y.; Holditch, S.A. Selecting Drilling Technologies and Methods for Tight Gas Sand Reservoirs, IADC/SPE 128191. In Proceedings of the 2010 IADC/SPE Drilling Conference, New Orleans, LA, USA, 2–4 February 2010. [Google Scholar]



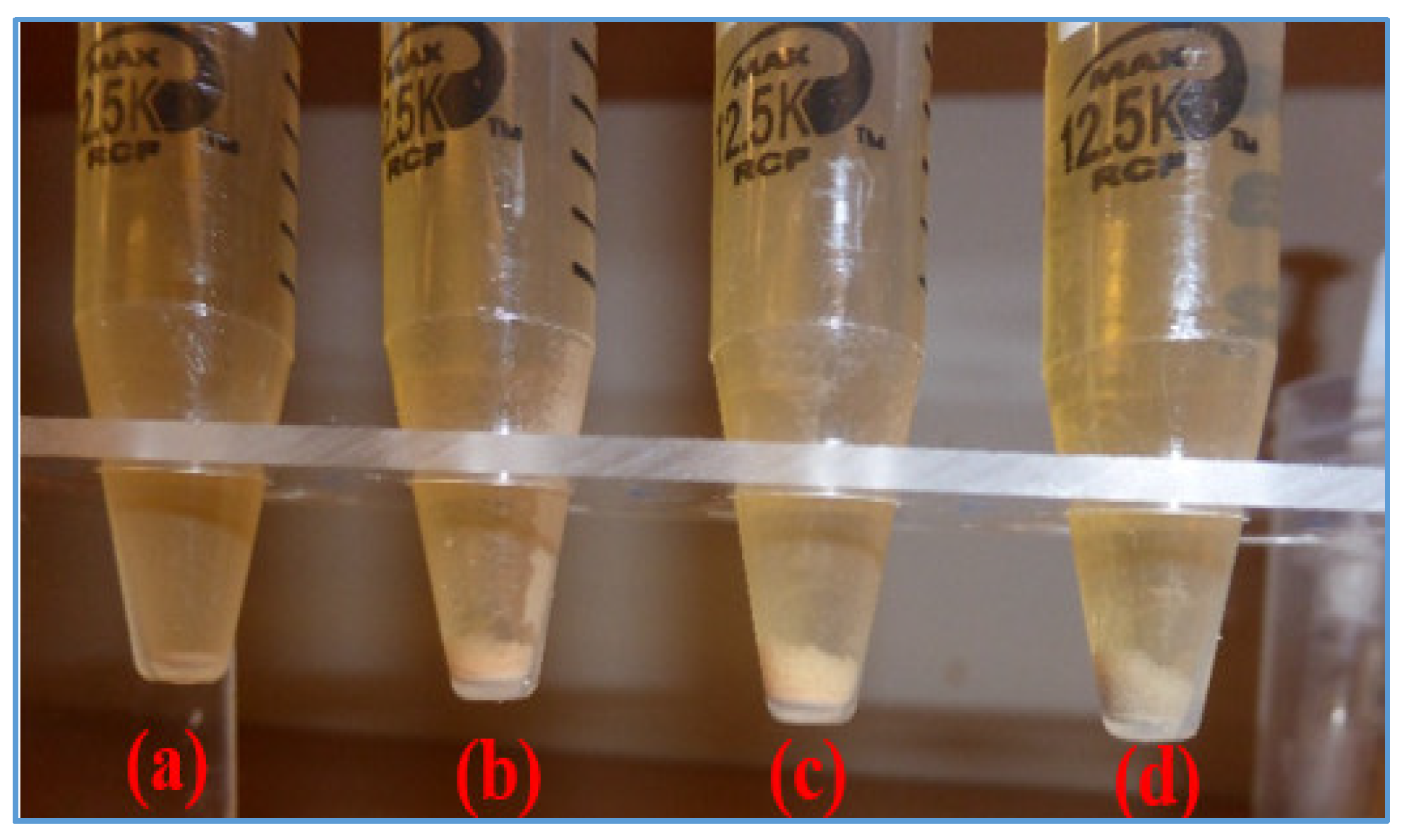
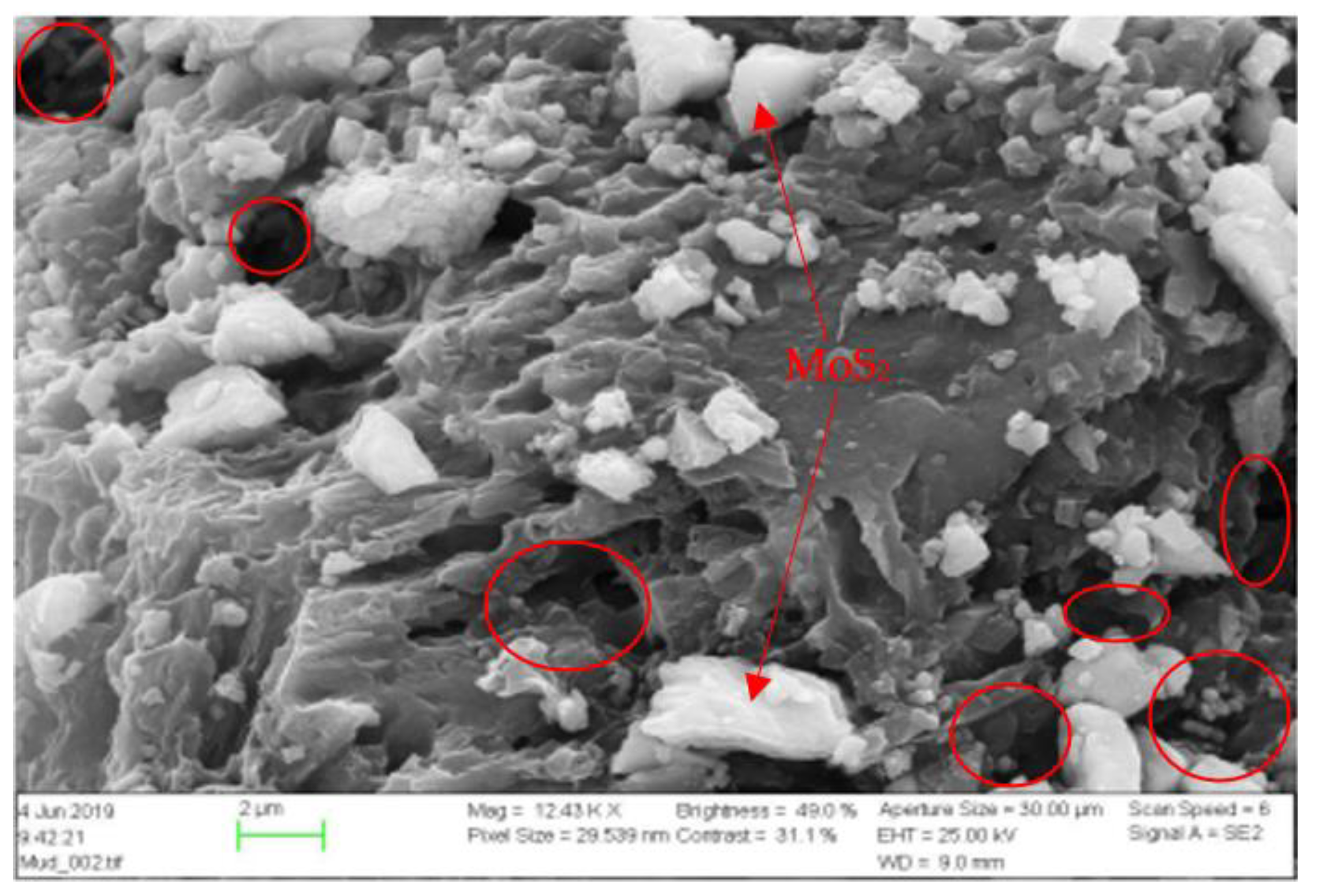
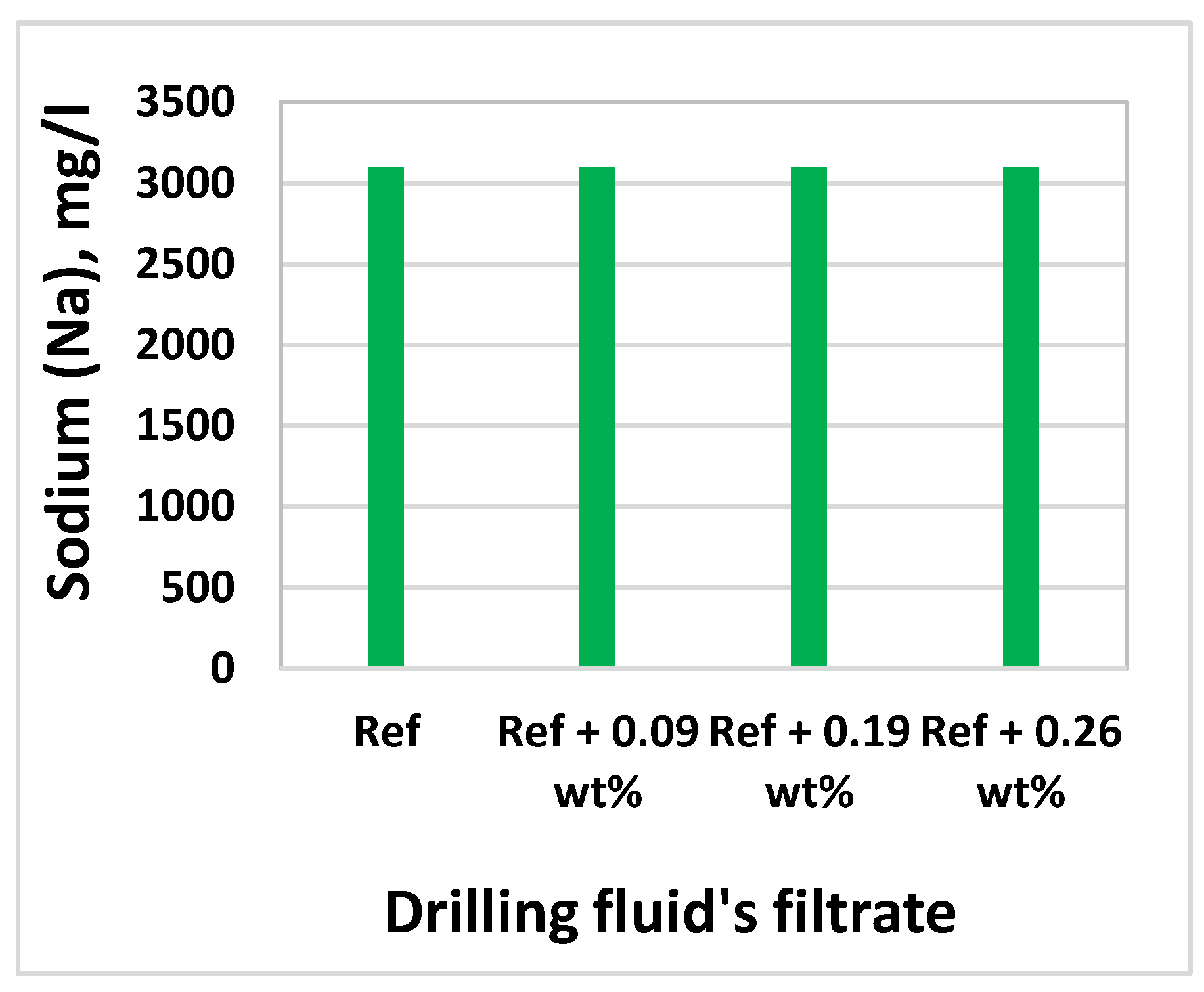
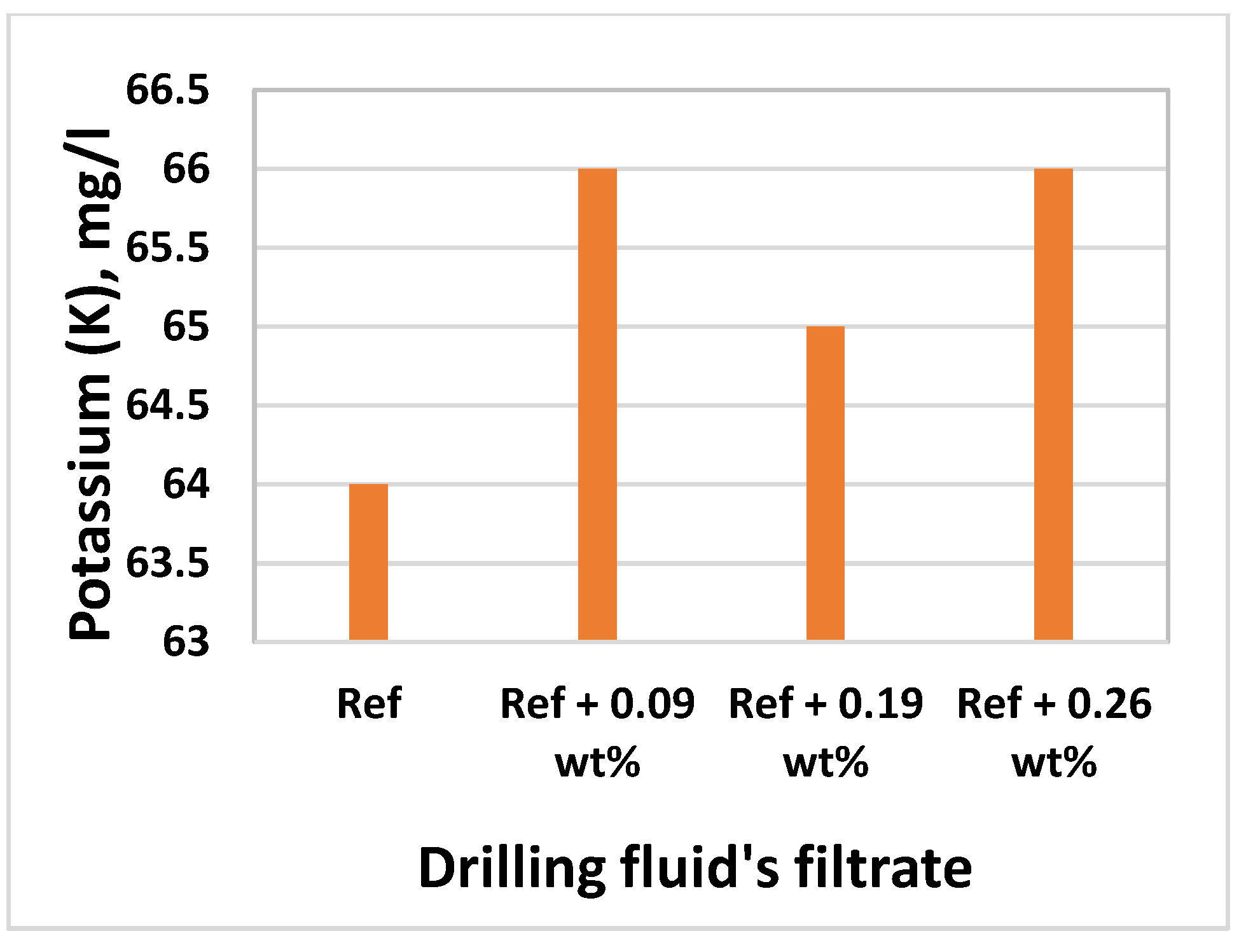
| Chemical | Base Fluid Ref. | Nanofluid Ref. + MoS2 |
|---|---|---|
| Water, [g] | 350 | 350 |
| Bentonite, [g] | 10 | 10 |
| DUO-VIS biopolymer, [g] | 0.6 | 0.6 |
| Anhydrous Soda Ash, [g] | 3.2 | 3.2 |
| Barite, [g] | 150 | 150 |
| Lignosulfonates, [g] | 0.9 | 0,9 |
| Nanoparticle (MoS2) [wt.%] | - | 0.09, 0.19, 0.26 |
| Anti-foam | 6 drops | 6 drops |
| Fluids | Yield Stress, Pa | Flow Point, Pa |
|---|---|---|
| Ref. | 0.9 | 4.4 |
| Ref. + 0.09 wt.% MoS2 | 1.0 | 4.5 |
| Ref. + 0.19 wt.% MoS2 | 1.0 | 4.5 |
| Ref. + 0.26 wt.% MoS2 | 1.0 | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belayneh, M.; Aadnøy, B.; Strømø, S.M. MoS2 Nanoparticle Effects on 80 °C Thermally Stable Water-Based Drilling Fluid. Materials 2021, 14, 7195. https://doi.org/10.3390/ma14237195
Belayneh M, Aadnøy B, Strømø SM. MoS2 Nanoparticle Effects on 80 °C Thermally Stable Water-Based Drilling Fluid. Materials. 2021; 14(23):7195. https://doi.org/10.3390/ma14237195
Chicago/Turabian StyleBelayneh, Mesfin, Bernt Aadnøy, and Simen Moe Strømø. 2021. "MoS2 Nanoparticle Effects on 80 °C Thermally Stable Water-Based Drilling Fluid" Materials 14, no. 23: 7195. https://doi.org/10.3390/ma14237195
APA StyleBelayneh, M., Aadnøy, B., & Strømø, S. M. (2021). MoS2 Nanoparticle Effects on 80 °C Thermally Stable Water-Based Drilling Fluid. Materials, 14(23), 7195. https://doi.org/10.3390/ma14237195






