Biomolecular, Histological, Clinical, and Radiological Analyses of Dental Implant Bone Sites Prepared Using Magnetic Mallet Technology: A Pilot Study in Animals
Abstract
:1. Introduction
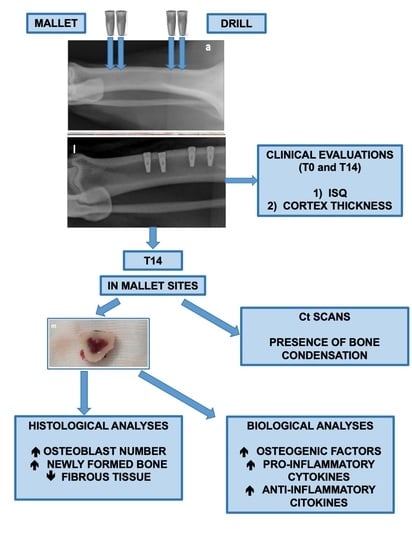
2. Materials and Methods
2.1. Animals
2.2. Focal Point
- ○
- Population: three minipig animals (we performed biomolecular and histological analyses on 3 implants for each technique).
- ○
- Intervention: magneto-dynamic mallet technique for implant site preparation.
- ○
- Comparison: conventional drilling for implant site preparation.
- ○
- Outcomes: improving implant osseointegration.
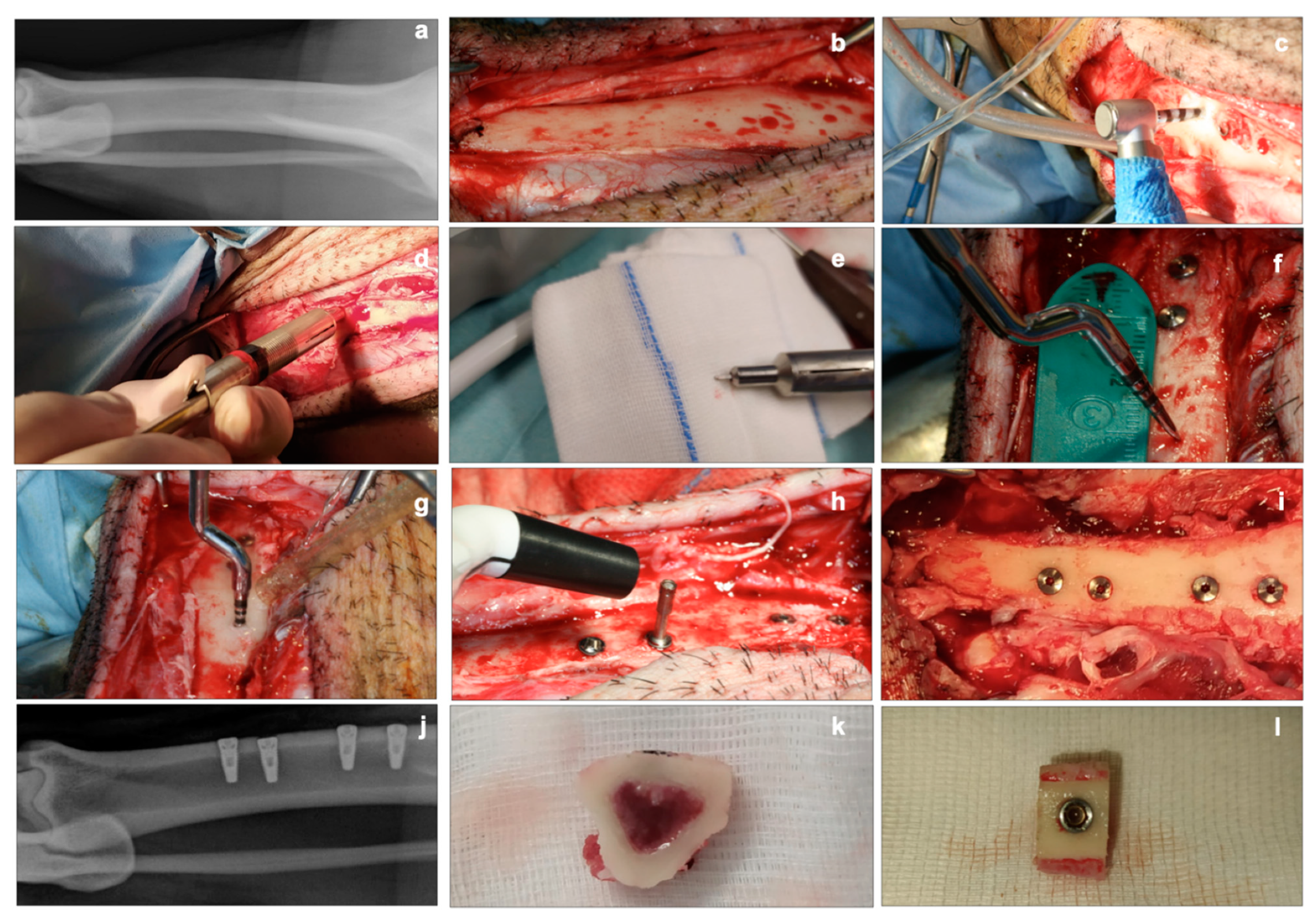
2.3. Implant Insertion and Explantation
2.4. Histological Analyses
2.5. Biomolecular Analyses
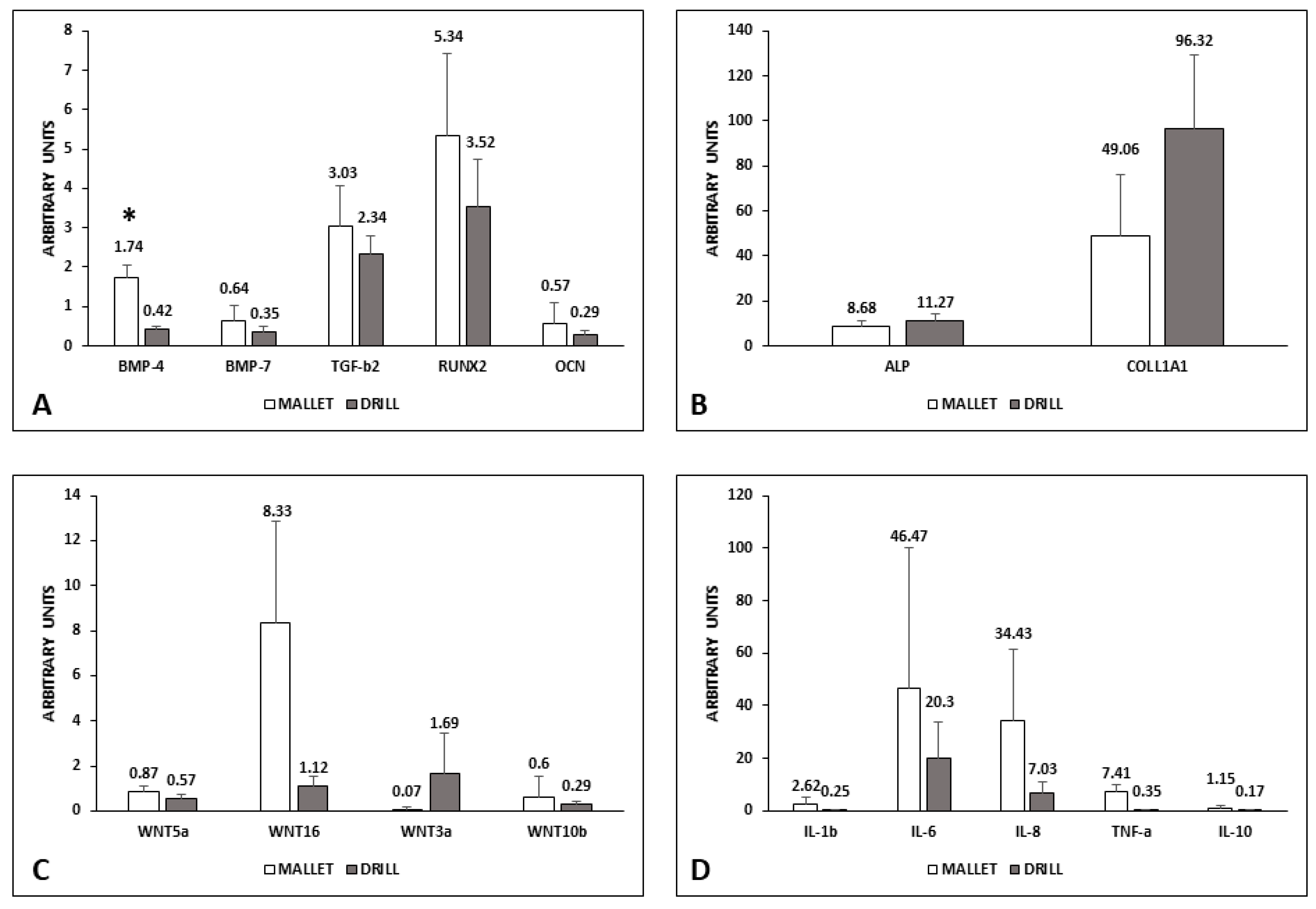
- Osteogenesis: BMP-4; BMP-7; transforming growth factor-beta2 (TGF-β2); RUNX2, alkaline phosphatase (ALP); osteocalcin (OCN); collagen type I α1 (COLL1A1); Wnt3a; Wnt5a; Wnt10b; Wnt16.
- Inflammation: interleukins (IL-1β; IL-6; IL-8; IL-10), tumor necrosis factor alpha (TNF-α).
2.6. Statistical Analysis
3. Results
3.1. Biomolecular Data
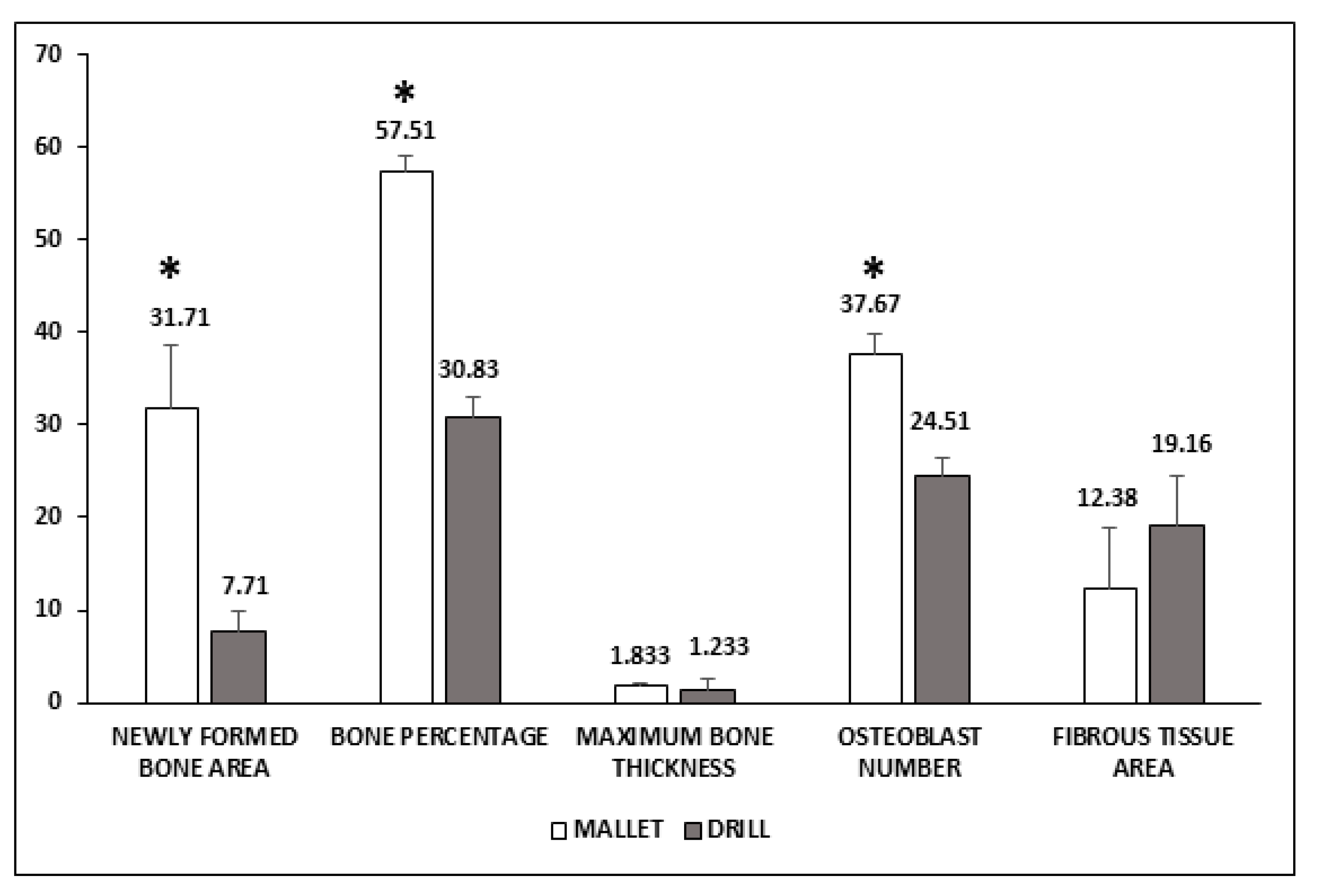
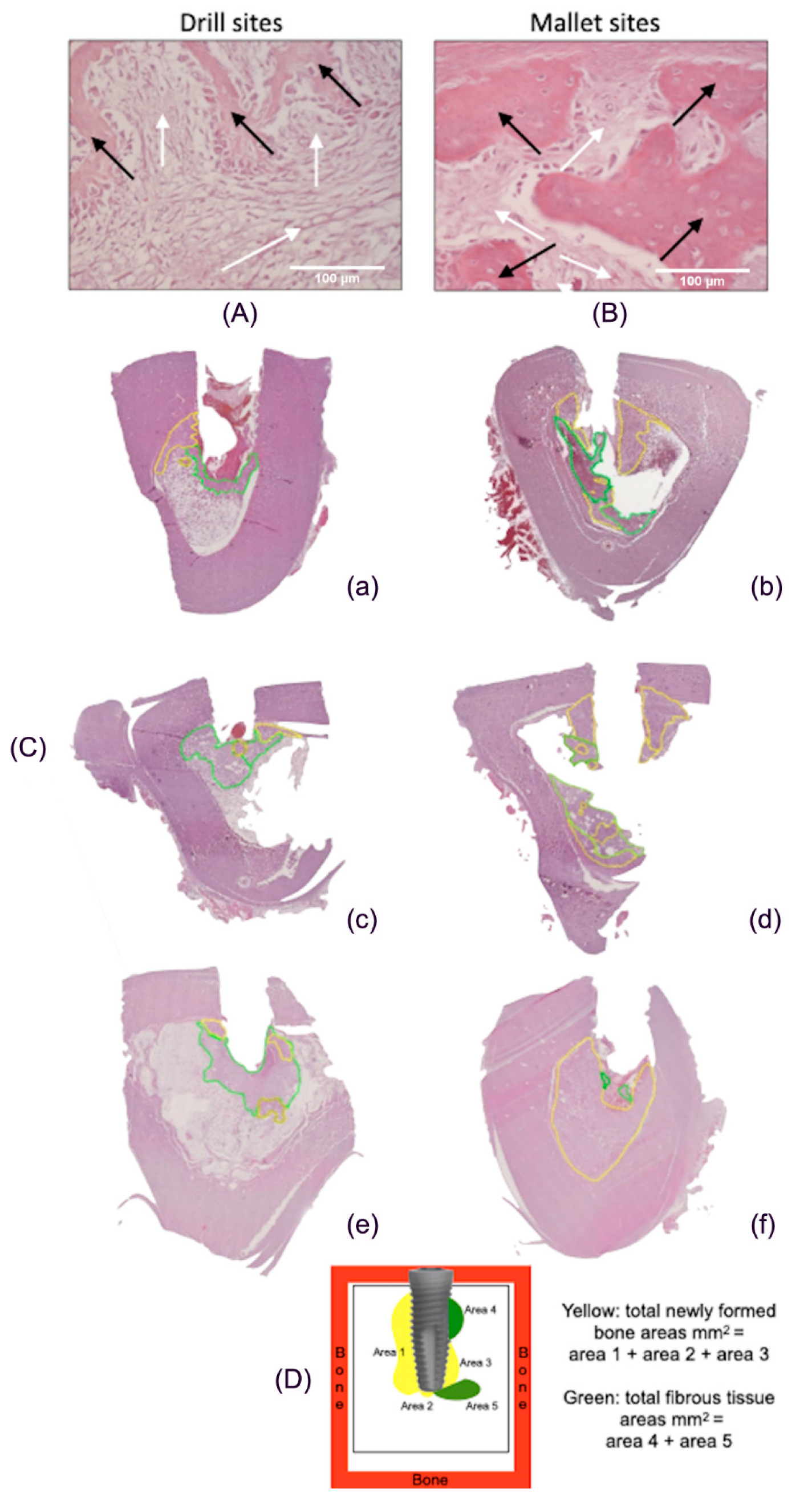
3.2. Histological Data
3.3. Clinical and Radiological Data
4. Discussion
4.1. Biological Factors
4.1.1. Osteogenic Process
4.1.2. Inflammatory Process
4.1.3. Wnt
4.2. Histological Analyses
4.3. Clinical and Radiological Observations
4.4. Limitations of the Study
4.5. Value of the Study
4.6. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vercellotti, T. Piezoelectric surgery in implantology: A case report—A new piezoelectric ridge expansion technique. Int. J. Periodontics Restor. Dent. 2000, 20, 358–365. [Google Scholar]
- Preti, G.; Martinasso, G.; Peirone, B.; Navone, R.; Manzella, C.; Muzio, G.; Russo, C.; Canuto, R.A.; Schierano, G. Cytokines and Growth Factors Involved in the Osseointegration of Oral Titanium Implants Positioned Using Piezoelectric Bone Surgery Versus a Drill Technique: A Pilot Study in Minipigs. J. Periodontol. 2007, 78, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Vercellotti, T.; Stacchi, C.; Russo, C.; Rebaudi, A.; Vincenzi, G.; Pratella, U.; Baldi, D.; Mozzati, M.; Monagheddu, C.; Sentineri, R.; et al. Ultrasonic implant site preparation using piezosurgery: A multicenter case series study analyzing 3579 implants with a 1- to 3-year follow-up. Int. J. Periodontics Restor. Dent. 2014, 34, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassi, F.; Cicciù, M.; Di Lenarda, R.; Galindo Moreno, P.; Galli, F.; Herford, A.S.; Jokstad, A.; Lombardi, T.; Nevins, M.; Sennerby, L.; et al. Piezoelectric bone surgery compared with conventional rotary instruments in oral surgery and implantology: Summary and consensus statements of the International Piezoelectric Surgery Academy Consensus Conference 2019. Int. J. Oral Implantol. 2020, 13, 1–5. [Google Scholar]
- Sagheb, K.; Kumar, V.V.; Azaripour, A.; Walter, C.; Al-Nawas, B.; Kämmerer, P.W. Comparison of conventional twist drill protocol and piezosurgery for implant insertion: Anex vivostudy on different bone types. Clin. Oral Implant. Res. 2017, 28, 207–213. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Pabst, A.M.; Dau, M.; Staedt, H.; Al-Nawas, B.; Heller, M. Immobilization of BMP-2, BMP-7 and alendronic acid on titanium surfaces: Adhesion, proliferation and differentiation of bone marrow-derived stem cells. J. Biomed. Mater. Res. Part A 2019, 108, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Schierano, G.; Canuto, R.A.; von Degerfeld, M.M.; Navone, R.; Peirone, B.; Preti, G.; Muzio, G. Role of rhBMP-7, Fibronectin, And Type I Collagen in Dental Implant Osseointegration Process: An Initial Pilot Study on Minipig Animals. Materials 2021, 14, 2185. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Sinus floor elevation by osteotome: Hand mallet versus electric mallet. A prospective clinical study. Int. J. Oral Maxillofac. Implant. 2012, 27, 1144–1150. [Google Scholar]
- Crespi, R.; Capparè, P.; Gherlone, E. Electrical Mallet in Implants Placed in Fresh Extraction Sockets with Simultaneous Osteotome Sinus Floor Elevation. Int. J. Oral Maxillofac. Implant. 2013, 28, 869–874. [Google Scholar] [CrossRef]
- Crespi, R.; Cappare’, P.; Gherlone, E. Electrical Mallet Provides Essential Advantages in Maxillary Bone Condensing. A Prospective Clinical Study. Clin. Implant. Dent. Relat. Res. 2013, 15, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Bruschi, G.B.; Capparé, P.; Gherlone, E. The Utility of the Electric Mallet. J. Craniofacial Surg. 2014, 25, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparé, P.; Gherlone, E. Electrical mallet provides essential advantages in split-crest and immediate implant placement. Oral Maxillofac. Surg. 2014, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. A comparison of manual and electrical mallet in maxillary bone condensing for immediately loaded implants: A randomized study. Clin. Implant Dent. Relat. Res. 2014, 16, 374–382. [Google Scholar] [CrossRef]
- Crespi, R.; Bruschi, G.B.; Gastaldi, G.; Capparé, P.; Gherlone, E.F. Immediate Loaded Implants in Split-Crest Procedure. Clin. Implant Dent. Relat. Res. 2015, 17, e692–e698. [Google Scholar] [CrossRef]
- Crespi, R.; Capparè, P.; Crespi, G.; Gastaldi, G.; Gherlone, E.F. Delayed Implants Outcome in Maxillary Molar Region. Clin. Implant Dent. Relat. Res. 2016, 19, 261–267. [Google Scholar] [CrossRef]
- Toti, P.; Barone, A.; Marconcini, S.; Fabris, G.B.M.; Martuscelli, R.; Covani, U. Pose determination of a blade implant in three dimensions from a single two-dimensional radiograph. Dentomaxillofacial Radiol. 2018, 47, 20170258. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.B.; Toti, P.; Crespi, G.; Covani, U.; Crespi, R. Distal Displacement of Maxillary Sinus Anterior Wall Versus Conventional Sinus Lift with Lateral Access: A 3-Year Retrospective Computerized Tomography Study. Int. J. Environ. Res. Public Health 2020, 17, 7199. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Han, J.-M.; Hong, G.; Lin, H.; Shimizu, Y.; Wu, Y.; Zheng, G.; Zhang, H.; Sasaki, K. Biomechanical and histological evaluation of the osseointegration capacity of two types of zirconia Implants. Int. J. Nanomed. 2016, 11, 6507–6516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branemark, P.I.; Zarb, G.A.; Albrektsson, T. Procedure Chirurgiche. In Osteointegrazione Tissutale. Osteointegrazione in Odontoiatria; Special Edition for Nobelpharma; Adell, R.U., Lekholm, U., Branemark, P.I., Eds.; Quintessenz Verlags-Gmbh: Berlin, Germany, 1987; pp. 211–232. ISBN 3-87652-539-X. [Google Scholar]
- Stadlinger, B.; Pilling, E.; Huhle, M.; Mai, R.; Bierbaum, S.; Scharnweber, D.; Kuhlisch, E.; Loukota, R.; Eckelt, U. Evaluation of osseointegration of dental implants coated with collagen, chondroitin sulphate and BMP-4: An animal study. Int. J. Oral Maxillofac. Surg. 2008, 37, 54–59. [Google Scholar] [CrossRef]
- Stacchi, C.; Vercellotti, T.; Torelli, L.; Furlan, F.; Di Lenarda, R. Changes in Implant Stability Using Different Site Preparation Techniques: Twist Drills versus Piezosurgery. A Single-Blinded, Randomized, Controlled Clinical Trial. Clin. Implant Dent. Relat. Res. 2013, 15, 188–197. [Google Scholar] [CrossRef]
- Abe, E.; Yamamoto, M.; Taguchi, Y.; Lecka-Czernik, B.; O’Brien, C.A.; Economides, A.N.; Stahl, N.; Jilka, R.L.; Manolagas, S.C. Essential Requirement of BMPs-2/4 for Both Osteoblast and Osteoclast Formation in Murine Bone Marrow Cultures from Adult Mice: Antagonism by Noggin. J. Bone Miner. Res. 2000, 15, 663–673. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Harris, M.A.; Rossini, G.; Dunstan, C.R.; Dallas, S.L.; Feng, J.Q.; Mundy, G.R.; Harris, S.E. Bone Morphogenetic Protein 2 (BMP-2) Enhances BMP-3, BMP-4, and Bone Cell Differentiation Marker Gene Expression During the Induction of Mineralized Bone Matrix Formation in Culturesof Fetal Rat Calvarial Osteoblasts. Calcif. Tissue Int. 1997, 60, 283–290. [Google Scholar] [CrossRef]
- Gu, K.; Zhang, L.; Jin, T.; Rutherford, R.B. Identification of Potential Modifiers of Runx2/Cbfa1 Activity in C2C12 Cells in Response to Bone Morphogenetic Protein-7. Cells Tissues Organs 2004, 176, 28–40. [Google Scholar] [CrossRef]
- Shen, B.; Wei, A.; Whittaker, S.; Williams, L.A.; Tao, H.; Ma, D.D.; Diwan, A. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J. Cell. Biochem. 2009, 109, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Taşlı, P.N.; Aydın, S.; Yalvac, M.; Şahin, F. Bmp 2 and Bmp 7 Induce Odonto- And Osteogenesis of Human Tooth Germ Stem Cells. Appl. Biochem. Biotechnol. 2014, 172, 3016–3025. [Google Scholar] [CrossRef]
- Ren, Y.; Han, C.; Wang, J.; Jia, Y.; Kong, L.; Eerdun, T.; Wu, L.; Jiang, D. hBMP-7 induces the differentiation of adipose-derived mesenchymal stem cells into osteoblast-like cells. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef]
- Marcelli, C.; Yates, A.J.; Mundy, G.R. In vivo effects of human recombinant transforming growth factor β on bone turnover in normal mice. J. Bone Miner. Res. 2009, 5, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Cassatella, A.M.; Meda, L.; Bonora, S.; Ceska, M.; Constantin, G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J. Exp. Med. 1993, 178, 2207–2211. [Google Scholar] [CrossRef]
- Kim, J.-B.; Leucht, P.; Lam, K.; Luppen, C.; Berge, D.T.; Nusse, R.; Helms, A.J. Bone Regeneration Is Regulated by Wnt Signaling. J. Bone Miner. Res. 2007, 22, 1913–1923. [Google Scholar] [CrossRef]
- Chen, Y.; Alman, B.A. Wnt pathway, an essential role in bone regeneration. J. Cell. Biochem. 2009, 106, 353–362. [Google Scholar] [CrossRef]
- Minear, S.; Leucht, P.; Jiang, J.; Liu, B.; Zeng, A.; Fuerer, C.; Nusse, R.; Helms, J.A. Wnt Proteins Promote Bone Regeneration. Sci. Transl. Med. 2010, 2, 29ra30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, D.G.; McGee-Lawrence, M.E.; Oursler, M.J.; Westendorf, J.J. Update on Wnt signaling in bone cell biology and bone disease. Gene 2012, 492, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Li, H. Tricin enhances osteoblastogenesis through the regulation of Wnt/β-catenin signaling in human mesenchymal stem cells. Mech. Dev. 2018, 152, 38–43. [Google Scholar] [CrossRef]
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mouraret, S.; Hunter, D.J.; Bardet, C.; Popelut, A.; Brunski, J.B.; Chaussain, C.; Bouchard, P.; Helms, J.A. Improving oral implant osseointegration in a murine model via Wnt signal amplification. J. Clin. Periodontol. 2014, 41, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-E.; Takanche, J.S.; Jang, S.; Yi, H.-K. Mussel adhesive protein blended with gelatin loaded into nanotube titanium dental implants enhances osseointegration. Drug Deliv. Transl. Res. 2021, 11, 956–965. [Google Scholar] [CrossRef]
- Bilkovski, R.; Schulte, D.M.; Oberhauser, F.; Gomolka, M.; Udelhoven, M.; Hettich, M.M.; Roth, B.; Heidenreich, A.; Gutschow, C.; Krone, W.; et al. Role of Wnt-5a in the Determination of Human Mesenchymal Stem Cells into Preadipocytes. J. Biol. Chem. 2010, 285, 6170–6178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares-Navarrete, R.; Hyzy, S.; Wieland, M.; Boyan, B.D.; Schwartz, Z. The roles of Wnt signaling modulators Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state in osteogenesis on microstructured titanium surfaces. Biomaterials 2010, 31, 2015–2024. [Google Scholar] [CrossRef] [Green Version]
- Olivares-Navarrete, R.; Hyzy, S.L.; Hutton, D.L.; Dunn, G.R.; Appert, C.; Boyan, B.D.; Schwartz, Z. Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta Biomater. 2011, 7, 2740–2750. [Google Scholar] [CrossRef] [Green Version]
- Movérare-Skrtic, S.; Henning, P.; Liu, X.; Nagano, K.; Saito, H.; Börjesson, E.A.; Sjögren, K.; Windahl, S.H.; Farman, H.; Kindlund, B.; et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat. Med. 2014, 20, 1279–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, C.N.; Longo, K.A.; Wright, W.S.; Suva, L.J.; Lane, T.F.; Hankenson, K.D.; MacDougald, O.A. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. USA 2005, 102, 3324–3329. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Bennett, C.N.; Gerin, I.; Rapp, L.A.; Hankenson, K.D.; Macdougald, O.A. Wnt signaling stimulates osteoblastogenesis of mesenchy-malprecursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007, 282, 14515–14524. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Ma, L.; Bai, N.; Xu, H. Wnt5a is involved in LOX-1 and TLR4 induced host inflammatory response in peri-implantitis. J. Periodontal Res. 2019, 55, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, Q.; Guo, S.; Wu, Y. Role of Wnt5a in periodontal tissue development, maintenance, and periodontitis: Implications for periodontal regeneration (Review). Mol. Med. Rep. 2021, 23, 167. [Google Scholar] [CrossRef] [PubMed]

| ISQ Values | |||
| Mallet | Drill | ||
| T0 | T14 | T0 | T14 |
| 77.531 ± 0.542 a | 80.979 ± 0.441 b | 76.250 ± 0.479 a | 81.062 ± 0.455 b |
| ISQ Percent Increase | |||
| Mallet | Drill | ||
| 4.592 ± 0.325 | 6.467 ± 0.525 * | ||
| Cortex Thickness (mm) | |||
| Mallet | Drill | ||
| 3.625 ± 0.311 | 3.475 ± 0.204 | ||
| Correlation between Cortex Thickness and ISQ (r) | |||
| Mallet | Drill | ||
| T0 | T14 | T0 | T14 |
| 0.51 | 0.36 | 0.65 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schierano, G.; Baldi, D.; Peirone, B.; Mauthe von Degerfeld, M.; Navone, R.; Bragoni, A.; Colombo, J.; Autelli, R.; Muzio, G. Biomolecular, Histological, Clinical, and Radiological Analyses of Dental Implant Bone Sites Prepared Using Magnetic Mallet Technology: A Pilot Study in Animals. Materials 2021, 14, 6945. https://doi.org/10.3390/ma14226945
Schierano G, Baldi D, Peirone B, Mauthe von Degerfeld M, Navone R, Bragoni A, Colombo J, Autelli R, Muzio G. Biomolecular, Histological, Clinical, and Radiological Analyses of Dental Implant Bone Sites Prepared Using Magnetic Mallet Technology: A Pilot Study in Animals. Materials. 2021; 14(22):6945. https://doi.org/10.3390/ma14226945
Chicago/Turabian StyleSchierano, Gianmario, Domenico Baldi, Bruno Peirone, Mitzy Mauthe von Degerfeld, Roberto Navone, Alberto Bragoni, Jacopo Colombo, Riccardo Autelli, and Giuliana Muzio. 2021. "Biomolecular, Histological, Clinical, and Radiological Analyses of Dental Implant Bone Sites Prepared Using Magnetic Mallet Technology: A Pilot Study in Animals" Materials 14, no. 22: 6945. https://doi.org/10.3390/ma14226945
APA StyleSchierano, G., Baldi, D., Peirone, B., Mauthe von Degerfeld, M., Navone, R., Bragoni, A., Colombo, J., Autelli, R., & Muzio, G. (2021). Biomolecular, Histological, Clinical, and Radiological Analyses of Dental Implant Bone Sites Prepared Using Magnetic Mallet Technology: A Pilot Study in Animals. Materials, 14(22), 6945. https://doi.org/10.3390/ma14226945