Silk Powder from Cocoons and Woven Fabric as a Potential Bio-Modifier
Abstract
:1. Introduction
2. Materials and Methods
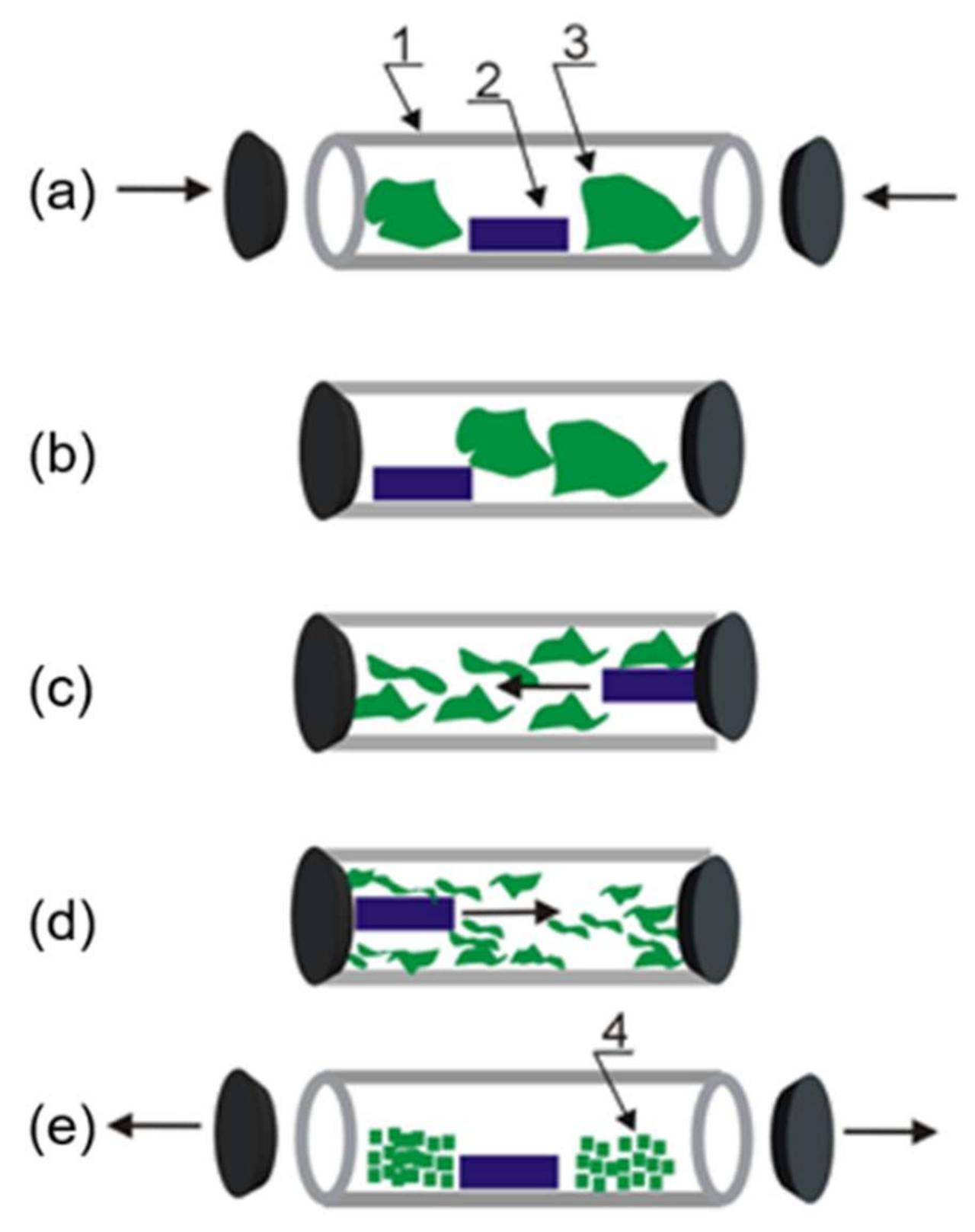
2.1. Silk Cocoon Degumming and Cryogenic Milling
2.2. Characterization of Silk-Based Powders
3. Results and Discussion
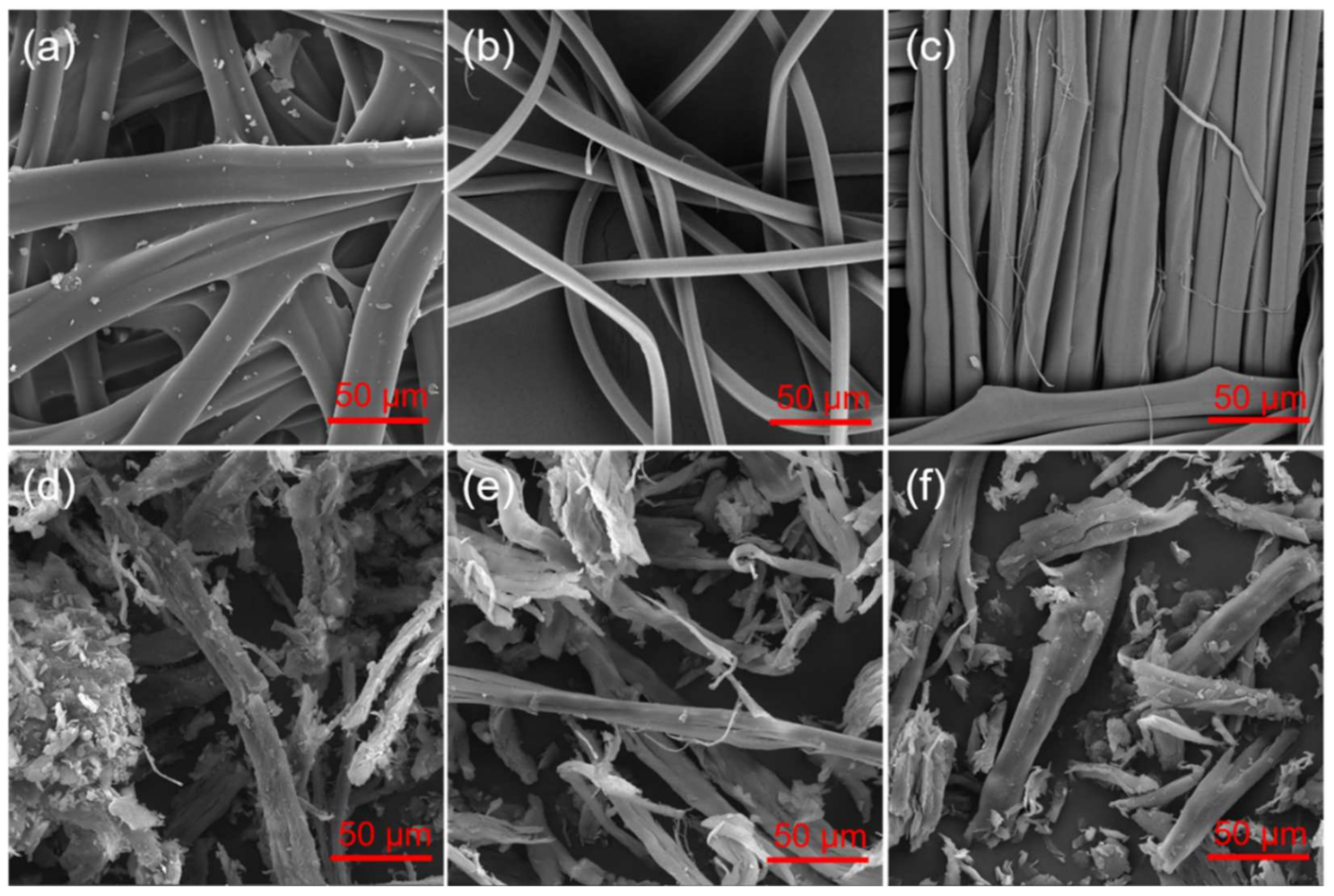
3.1. Powder Formation and SEM/EDS Studies
3.2. Raman Studies
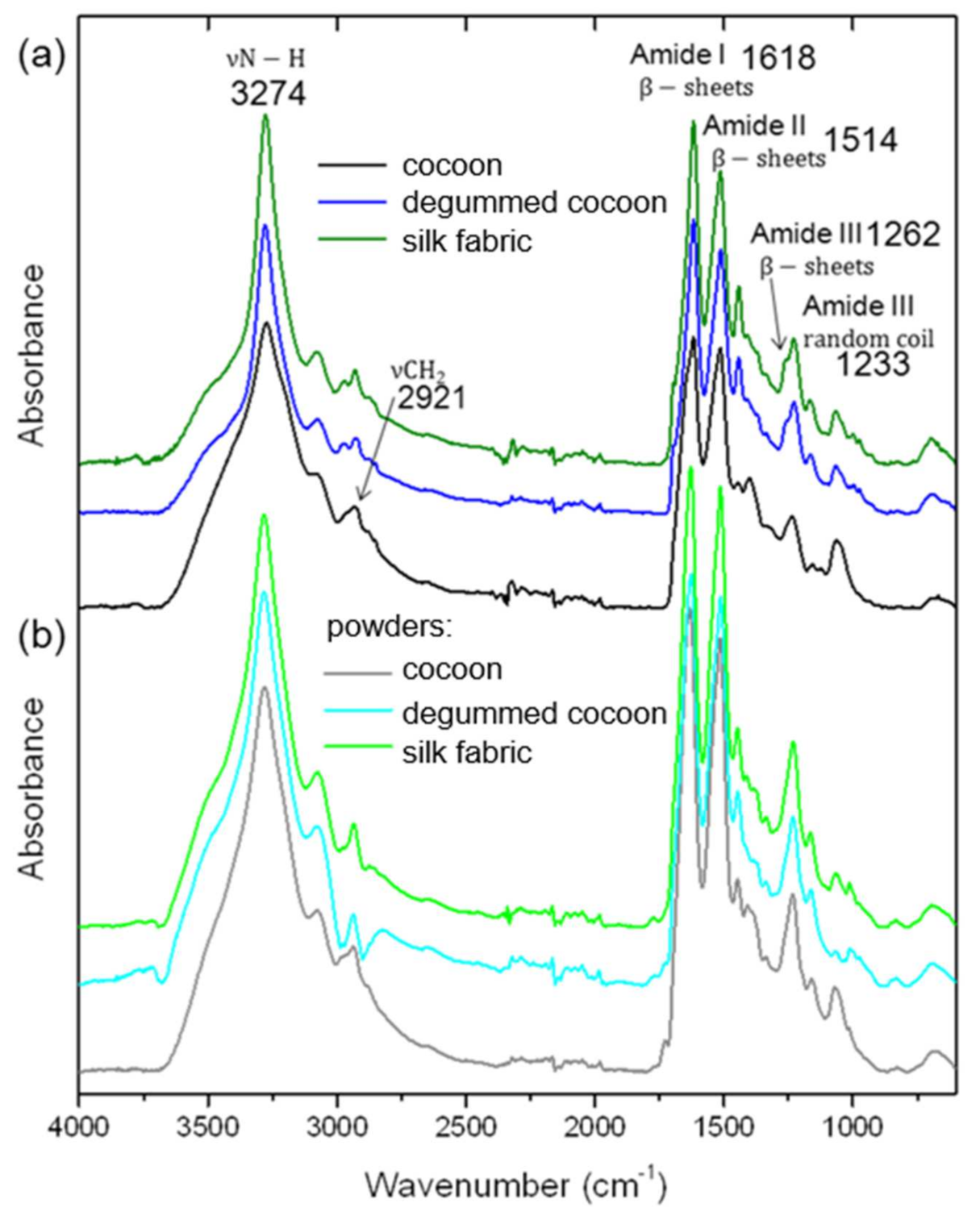
3.3. Fourier Transformed Infrared (FTIR) Analysis
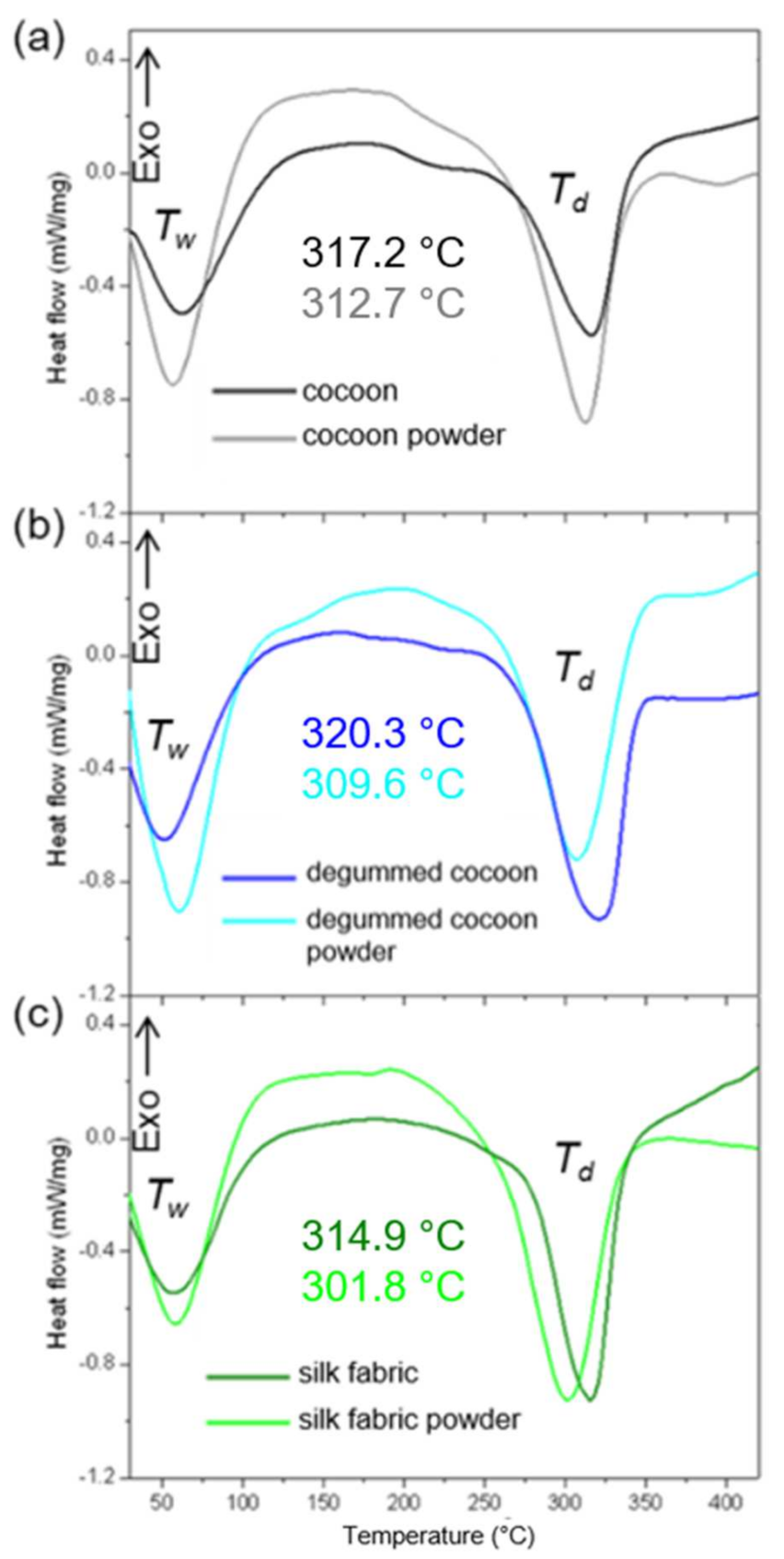
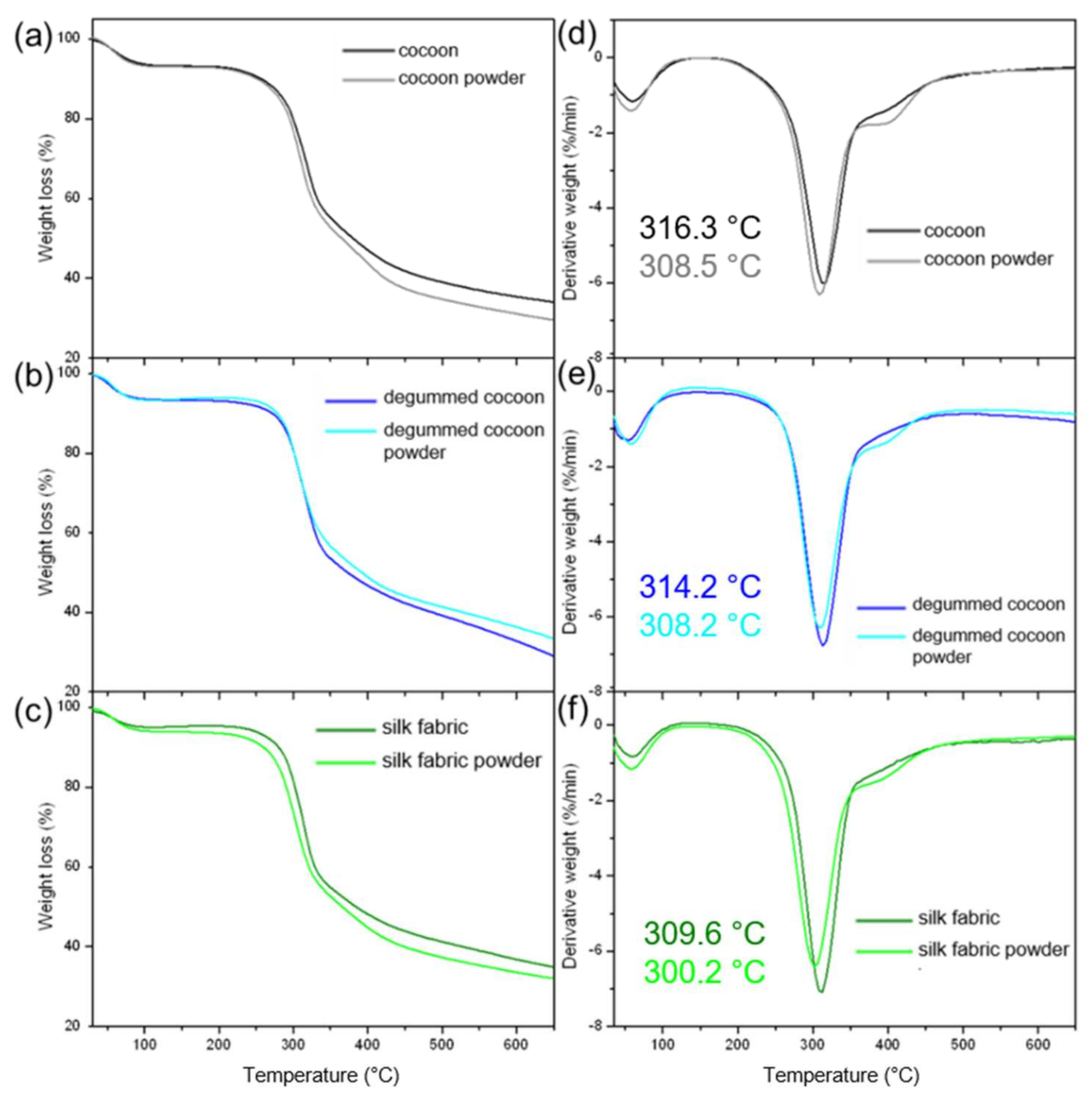
3.4. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badawy, I.M.; Ali, B.A.; Abbas, W.A.; Allam, N.K. Natural silk for energy and sensing applications: A review. Environ. Chem. Lett. 2021, 19, 2141–2155. [Google Scholar] [CrossRef]
- Koh, L.D.; Cheng, Y.; Teng, C.P.; Khin, Y.W.; Loh, X.J.; Tee, S.Y.; Low, M.; Ye, E.; Yu, H.D.; Zhang, Y.W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Zhang, Y.; Kaplan, D.L. Silk-Based Advanced Materials for Soft Electronics. Acc. Chem. Res. 2019, 52, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthcare Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.P.; Alam, S.; Jain, A.K.; Ansari, K.M.; Mandal, B.B. Protective Activity of Silk Sericin against UV Radiation-Induced Skin Damage by Downregulating Oxidative Stress. ACS Appl. Bio Mater. 2018, 1, 2120–2132. [Google Scholar] [CrossRef]
- Zhang, X.; Tsukada, M.; Morikawa, H.; Aojima, K.; Zhang, G.; Miura, M. Production of silk sericin/silk fibroin blend nanofibers. Nanoscale Res. Lett. 2011, 6, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkhowa, R.; Wang, L.; Wang, X. Ultra-fine silk powder preparation through rotary and ball milling. Powder Technol. 2008, 185, 87–95. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Wang, L.; Kanwar, J.; Wang, X. Fabrication of ultrafine powder from eri silk through attritor and jet milling. Powder Technol. 2009, 191, 155–163. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, Z.; Ma, H.; Yang, H.; Xu, W. Effective removal of dyes from aqueous solution using ultrafine silk fibroin powder. Adv. Powder Technol. 2014, 25, 574–581. [Google Scholar] [CrossRef]
- Zhao, M.; Qi, Z.; Tao, X.; Newkirk, C.; Hu, X.; Lu, S. Chemical, thermal, time, and enzymatic stability of silk materials with silk i structure. Int. J. Mol. Sci. 2021, 22, 4136. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Martin Scholze, H.; Stolle, A. Temperature progression in a mixer ball mill. Int. J. Ind. Chem. 2016, 7, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Holland, C.; Hawkins, N.; Frydrych, M.; Laity, P.; Porter, D.; Vollrath, F. Differential Scanning Calorimetry of Native Silk Feedstock. Macromol. Biosci. 2019, 19, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, L.K.H.; Favaro, L.I.L.; Rios, A.C.; Silva, E.C.; Silva, W.F.; Stigliani, T.P.; Guilger, M.; Lima, R.; Oliveira, J.M.; Aranha, N.; et al. Sericin from Bombyx mori cocoons. Part I: Extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem. 2017, 61, 163–177. [Google Scholar] [CrossRef]
- Boonyawan, D. Atmospheric Pressure Plasma Jet Induced Graft-Polymerization for Flame Retardant Silk. In Advanced Plasma Spray Applications; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef] [Green Version]
- Tulachan, B.; Meena, S.K.; Rai, R.K.; Mallick, C.; Kusurkar, T.S.; Teotia, A.K.; Sethy, N.K.; Bhargava, K.; Bhattacharya, S.; Kumar, A.; et al. Electricity from the silk cocoon membrane. Sci. Rep. 2014, 4, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puchowicz, D.; Cieslak, M. Raman Spectroscopy in the Analysis of Textile Structures. In Recent Developments in Atomic Force Microscopy and Raman Spectroscopy for Materials Characterization; IntechOpen: Rijeka, Croatia, 2021; pp. 1–21. [Google Scholar] [CrossRef]
- Narita, C.; Okahisa, Y.; Wataoka, I.; Yamada, K. Characterization of ground silk fibroin through comparison of nanofibroin and higher order structures. ACS Omega 2020, 5, 22786–22792. [Google Scholar] [CrossRef]
- Lefèvre, T.; Paquet-Mercier, F.; Rioux-Dubé, J.F.; Pézolet, M. Review: Structure of silk by Raman spectromicroscopy: From the spinning glands to the fibers. Biopolymers 2012, 97, 322–336. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, H.; Zheng, N.; Gao, L.; Liu, F.; Guan, G.; Zhang, G. Simple fabrication of sericin/graphene nanocomposites for application in articular cartilage repair in knee joints in nursing care. Appl. Nanosci. 2020, 10, 695–702. [Google Scholar] [CrossRef]
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and classification of silks using infrared spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef] [Green Version]
- Koperska, M.A.; Pawcenis, D.; Bagniuk, J.; Zaitz, M.M.; Missori, M.; Łojewski, T.; Łojewska, J. Degradation markers of fibroin in silk through infrared spectroscopy. Polym. Degrad. Stab. 2014, 105, 185–196. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wyeth, P. Using FTIR spectroscopy to detect sericin on historic silk. Sci. China Chem. 2010, 53, 626–631. [Google Scholar] [CrossRef]
- Bawazeer, T.M.; Alsoufi, M.S. Surface Characterization and Properties of Raw and Degummed (Bombyx mori) Silk Fibroin Fiber toward High Performance Applications of “Kisswa Al-Kabba”. Int. J. Curr. Res. 2017, 9, 48335–48343. [Google Scholar]
- Kim, H.J.; Chung, D.E.; Um, I.C. Effect of Processing Conditions on the Homogeneity of Partially Degummed Silk Evaluated by FTIR Spectroscopy. Int. J. Ind. Entomol. 2013, 26, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Mazzi, S.; Zulker, E.; Buchicchio, J.; Anderson, B.; Hu, X. Comparative thermal analysis of Eri, Mori, Muga, and Tussar silk cocoons and fibroin fibers. J. Therm. Anal. Calorim. 2014, 116, 1337–1343. [Google Scholar] [CrossRef]
- Malay, A.D.; Sato, R.; Yazawa, K.; Watanabe, H.; Ifuku, N.; Masunaga, H.; Hikima, T.; Guan, J.; Mandal, B.B.; Damrongsakkul, S.; et al. Relationships between physical properties and sequence in silkworm silks. Sci. Rep. 2016, 6, 27573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; De Shen, W.; Xiang, R.L.; Zhuge, L.J.; Gao, W.J.; Wang, W.B. Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J. Nanopart. Res. 2007, 9, 885–900. [Google Scholar] [CrossRef]
- Samie, M.; Muhammad, N.; Yameen, M.A.; Chaudhry, A.A.; Khalid, H.; Khan, A.F. Aqueous Solution of a Basic Ionic Liquid: A Perspective Solvent for Extraction and Regeneration of Silk Powder from Bombyx mori Silk Cocoons. J. Polym. Environ. 2020, 28, 657–667. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, H.; Zeng, J.; Liu, Z.; Tong, X.; Li, Z.; Zhao, H.; Dai, F. Effect of different additives in diets on secondary structure, thermal and mechanical properties of silkworm silk. Materials 2018, 12, 14. [Google Scholar] [CrossRef] [Green Version]



| C (wt.%) | N (wt.%) | O (wt.%) | Ca (wt.%) | S (wt.%) | K (wt.%) | Na (wt.%) | |
|---|---|---|---|---|---|---|---|
| raw cocoons | 44.5 ± 0.3 | 16.9 ± 0.3 | 37.6 ± 0.5 | 0.6 ± 0.1 | ~0.2 | ~0.2 | |
| raw cocoons-powder | 46.3 ± 0.6 | 17.5 ± 0.5 | 35.4 ± 0.5 | ~0.3 | ~0.1 | ~0.3 | |
| degummed cocoons | 52.4 ± 0.6 | 19.7 ± 0.3 | 27.6 ± 0.5 | ~0.2 | |||
| degummed cocoons-powder | 48.8 ± 0.1 | 20.6 ± 0.2 | 30.5 ± 0.1 | ~0.1 | |||
| silk woven fabric | 48.8 ± 0.4 | 20.9 ± 0.4 | 30.2 ± 0.3 | ~0.3 | |||
| silk woven fabric-powder | 51.9 ± 0.7 | 19.0 ± 0.4 | 28.9 ± 0.3 | ~0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowska-Korczyc, A.; Hudecki, A.; Kamińska, I.; Cieślak, M. Silk Powder from Cocoons and Woven Fabric as a Potential Bio-Modifier. Materials 2021, 14, 6919. https://doi.org/10.3390/ma14226919
Baranowska-Korczyc A, Hudecki A, Kamińska I, Cieślak M. Silk Powder from Cocoons and Woven Fabric as a Potential Bio-Modifier. Materials. 2021; 14(22):6919. https://doi.org/10.3390/ma14226919
Chicago/Turabian StyleBaranowska-Korczyc, Anna, Andrzej Hudecki, Irena Kamińska, and Małgorzata Cieślak. 2021. "Silk Powder from Cocoons and Woven Fabric as a Potential Bio-Modifier" Materials 14, no. 22: 6919. https://doi.org/10.3390/ma14226919
APA StyleBaranowska-Korczyc, A., Hudecki, A., Kamińska, I., & Cieślak, M. (2021). Silk Powder from Cocoons and Woven Fabric as a Potential Bio-Modifier. Materials, 14(22), 6919. https://doi.org/10.3390/ma14226919






