Corrosion Resistance and Surface Bioactivity of Ti6Al4V Alloy after Finish Turning under Ecological Cutting Conditions
Abstract
:1. Introduction
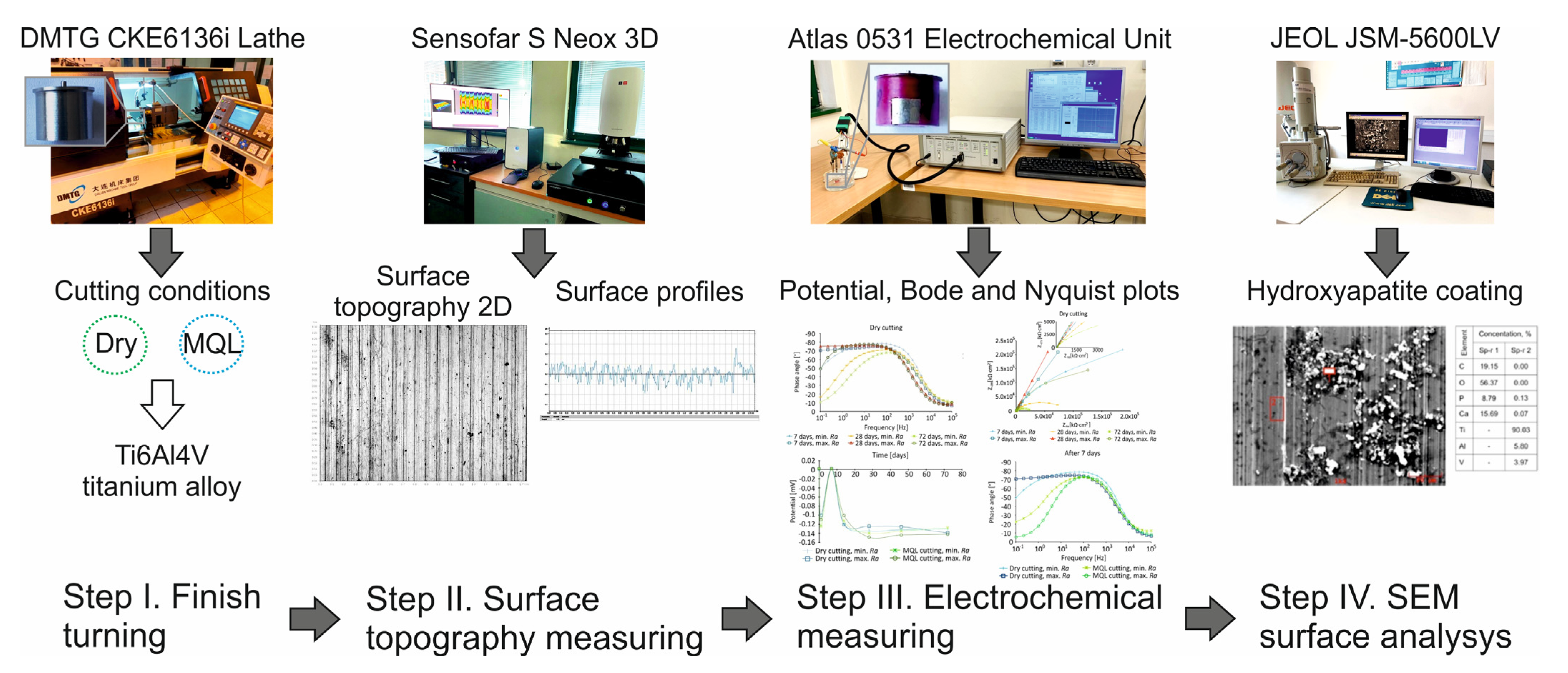
2. Materials and Methods
2.1. Workpiece Details and Preparation
2.2. Measurement of Surface Roughness Metrics
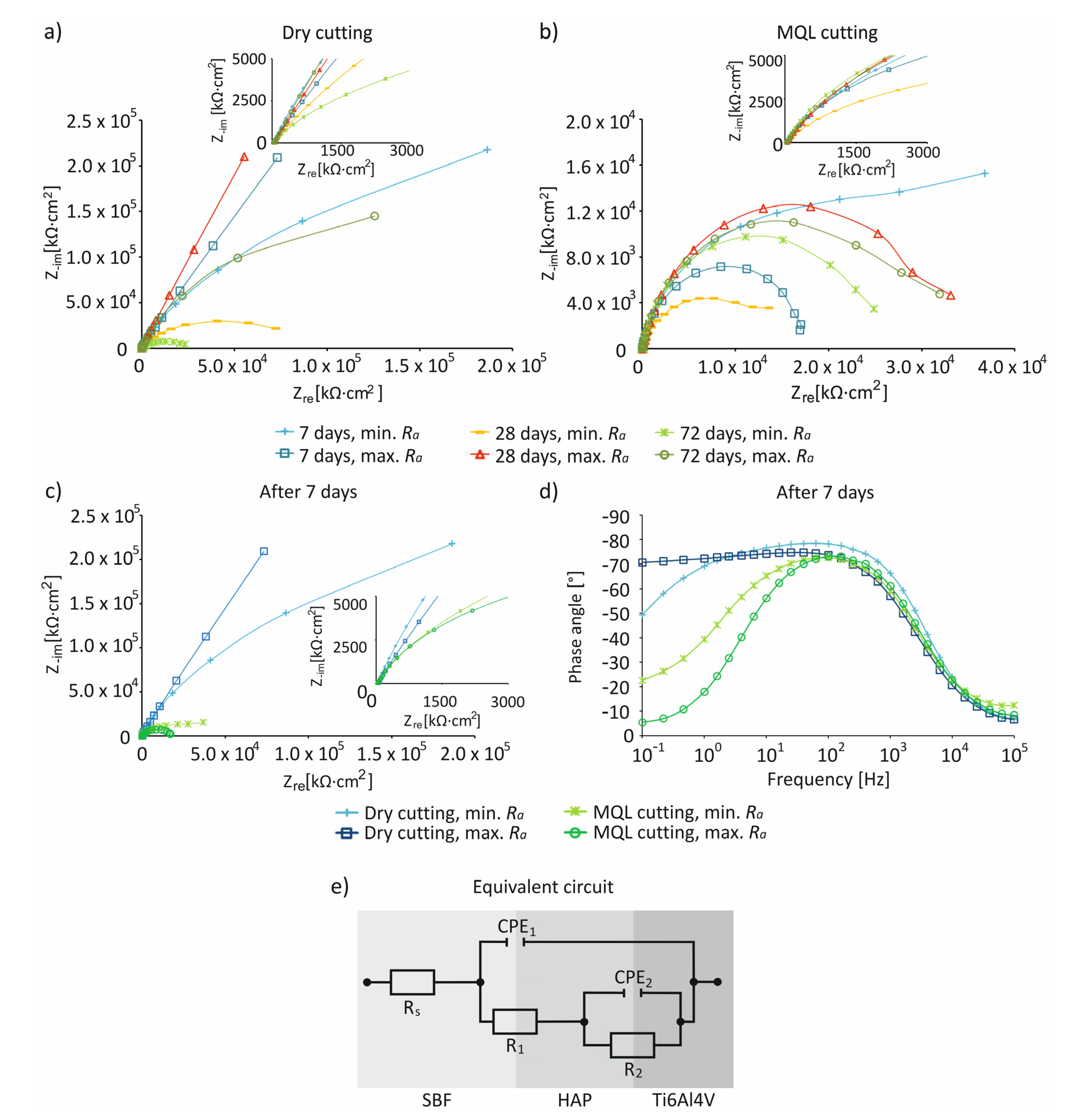
3. Results and Discussion
4. Conclusions
- For samples with Ramin after dry turning, a reduction in surface roughness parameters of 6% to 165% was obtained compared to MQL conditions. On the other hand, for samples with Ramax machined under MQL conditions, a reduction was obtained of 25% to 27%.
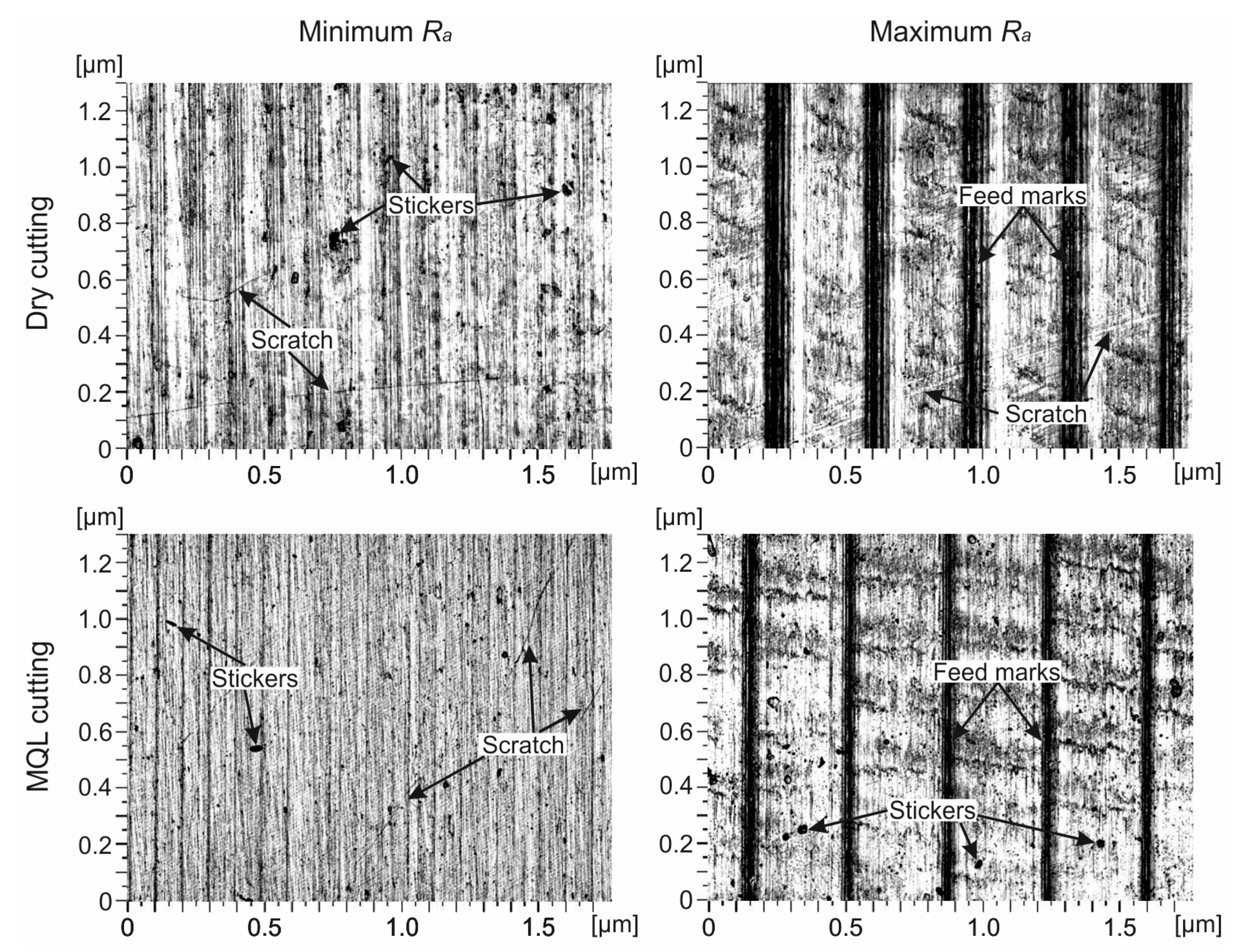
- Regardless of the cutting conditions within the minimum range Ra = 0.25–0.37 μm, flattened peaks were observed on the surface roughness profiles, and, within the range of the maximum Ra = 1.62–2.22 μm, there were visible feed marks.
- Rsk–Rku topological map showed very high peaks and very deep pits on surfaces with Ramax, as well as high peaks and deep pits on surfaces with Ramin, machined under MQL conditions. Flattened peaks were seen on the surface with Ramax under dry machined conditions. On the 2D images of the surface with Ramax, clear traces of the feed rate were recorded, and, on the surfaces with Ramin, scratches and irregularly spaced stickers were observed under the analyzed cutting conditions.
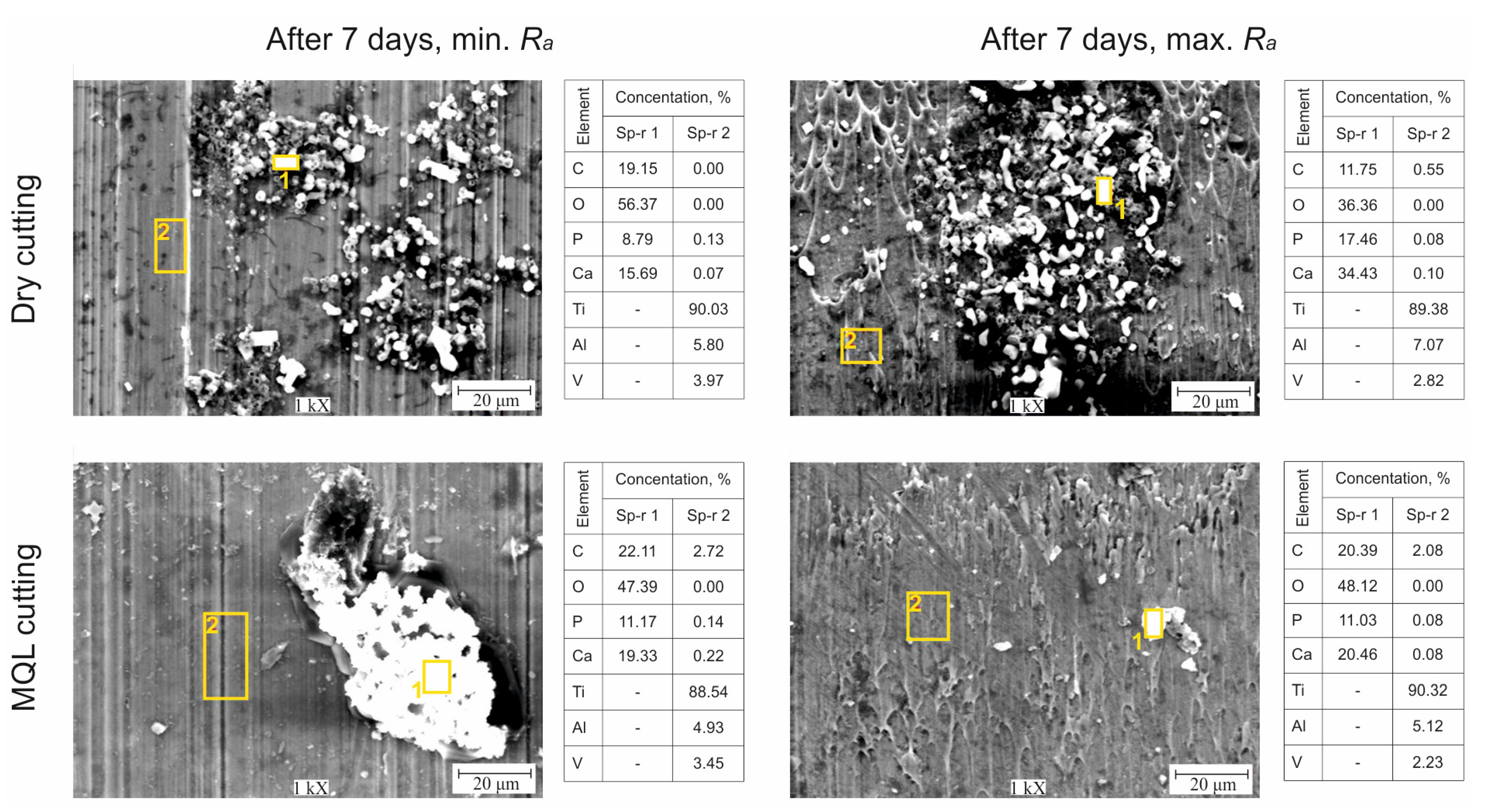
- After turning under dry and MQL conditions, unevenly distributed precipitates of hydroxyapatite compounds were present on the surfaces of the samples. The Ca/P molar ratio for samples with Ramin was within the range 1.73–1.78, whereas that for samples with Ramax was within the range 1.85–1.97.
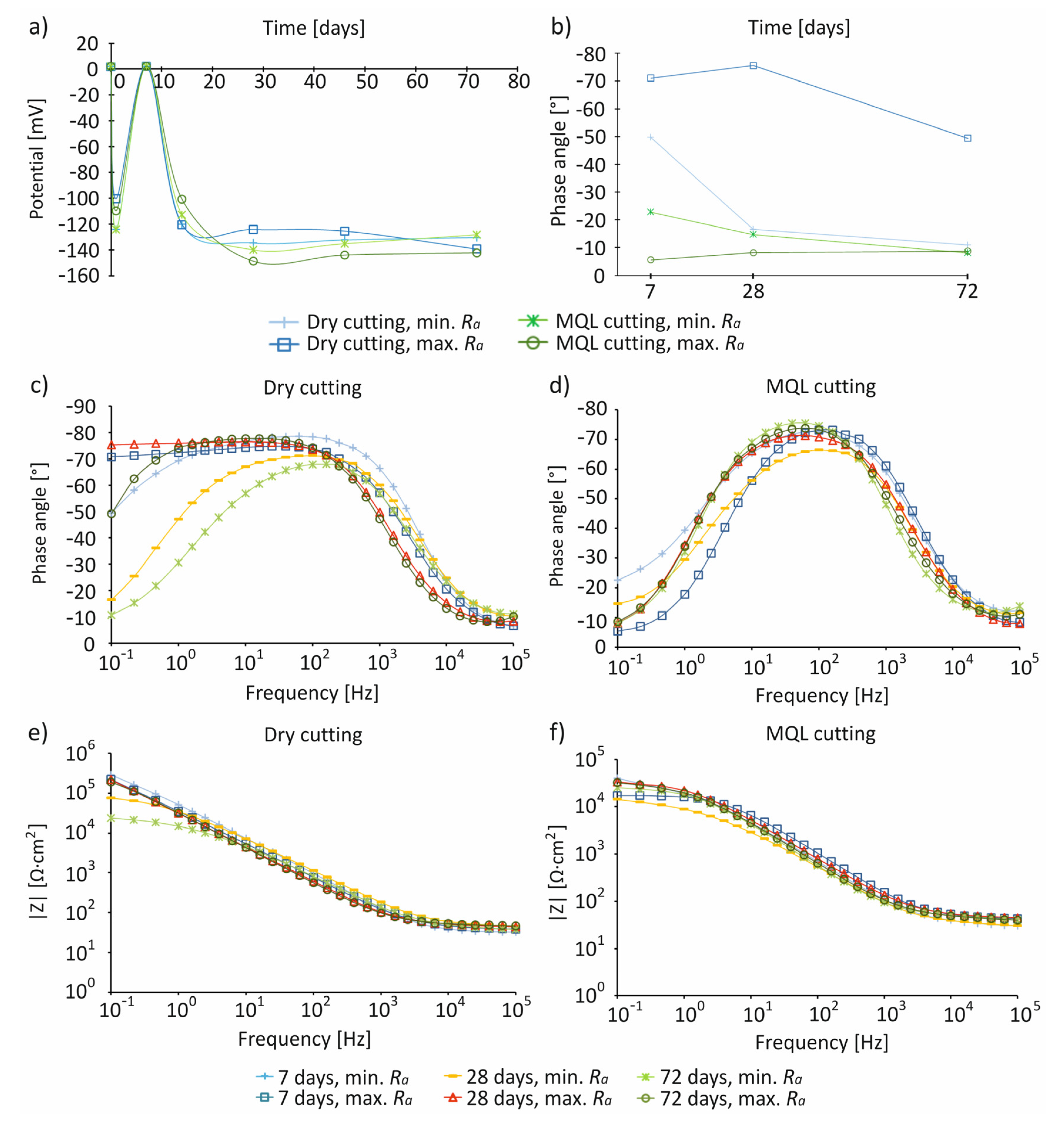
- For the studied cutting conditions and surface roughness, the highest values of Ecorr ~0 mV were recorded on day 7 of immersion in the SBF solution.
- The impedance characteristics indicated that, compared to MQL conditions, under dry conditions, surfaces were characterized by a greater resistance and an almost capacitive response, illustrated by a phase angle close to −80° recorded in a wide frequency range by control systems including in the range of 10−1–103 Hz, indicating the presence of a passive layer on the processed surface.
- The obtained research results have practical significance. They can be used by engineers during the development of technological processes for medical devices made of Ti6Al4V alloy, to obtain favorable functional properties of these devices, i.e., corrosion resistance and bioactivity of the surface after finish turning. Therefore, a lower surface roughness under dry conditions is recommended to achieve this success.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| ap | Depth of cut (mm) |
| Ecorr | Corrosion potential (mV) |
| EDS | Energy dispersive spectroscopy |
| EIS | Electrochemical impedance spectroscopy |
| f | Feed rate (mm/rev) |
| HA | Hydroxyapatite |
| MQL | Minimum quantity lubrication |
| Ra | Arithmetic mean deviation of the roughness profile (μm) |
| Ramin | The minimum value of the surface roughness parameter Ra (μm) |
| Ramax | The maximum value of the surface roughness parameter Ra (μm) |
| Rku | Kurtosis of the roughness profile |
| Rp | Maximum peak height of the roughness profile (μm) |
| Rsk | Skewness of the roughness profile |
| Rv | Maximum valley depth of the roughness profile (μm) |
| Rz | Maximum height of the roughness profile (μm) |
| SBF | Simulated body fluid |
| SEM | Scanning electron microscope |
| vc | Cutting speed (m/min) |
References
- Uo, M.; Watari, F.; Yokoyama, A.; Matsuno, H.; Kawasaki, T. Visualization and detectability of elements rarely contained in soft tissue by X-ray Canning analytical microskopy and elektron-probe micro analysis. Biomaterials 2001, 22, 1787–1794. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef] [Green Version]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Pimenov, D.Y.; Mia, M.; Gupta, M.K.; Machado, A.R.; Tomaz, Í.V.; Sarikaya, M.; Wojciechowski, S.; Mikolajczyk, T.; Kaplonek, W. Improvement of machinability of Ti and its alloys using cooling-lubrication techniques: A review and future prospect. J. Mater. Res. Technol. 2021, 11, 719–753. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Pessoa, J.C. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In Vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Schenk, R. The Corrosion Properties of Titanium and Titanium Alloys. In Titanium in Medicine. Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł.; Belcarz, A.; Przekora, A.; Ginalska, G.; Strugała, G.; Cieślik, B.; Pałubicka, A.; Zieliński, A. The Chemical and Biological Properties of Nanohydroxyapatite Coatings with Antibacterial Nanometals, Obtained in the Electrophoretic Process on the Ti13Zr13Nb Alloy. Int. J. Mol. Sci. 2021, 22, 3172. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Bartmański, M.; Mielewczyk-Gryń, A.; Cieślik, B.; Gajowiec, G.; Zieliński, A. Electrophoretically Deposited Chitosan/Eudragit E 100/AgNPs Composite Coatings on Titanium Substrate as a Silver Release System. Materials 2021, 14, 4533. [Google Scholar] [CrossRef] [PubMed]
- Laboulaisa, J.N.; Mata, J.A.A.; Borras, V.A.; Munoz, A.I. Electrochemical characterization and passivation behaviour of new beta-titanium alloys (Ti35Nb10Ta-xFe). Electrochim. Acta 2017, 227, 410–418. [Google Scholar] [CrossRef]
- Czarnowska, E.; Wierzchoń, T.; Maranda-Niedbala, A.; Karczmarewicz, E. Improvement of titanium alloy for biomedical applications by nitriding and carbonitriding processes under glow discharge conditions. J. Mater. Sci. Mater. Med. 2000, 11, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Gotz, H.E.; Muller, M.; Emmel, A.; Holzwarth, U.; Erben, R.G.; Stangl, R. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef]
- Chi, G.; Yi, D.; Liu, H. Effect of roughness on electrochemical and pitting corrosion of Ti-6Al-4V alloy in 12 wt. % HCl solution at 35 °C. J. Mater. Res. Technol. 2020, 9, 1162–1174. [Google Scholar] [CrossRef]
- Childs, T.H.C.; Arrazola, P.J.; Aristimuno, P.; Garay, A.; Sacristan, I. Ti6Al4V metal cutting chip formation experiments and modeling over a wide range of cutting speeds. J. Mater. Process. Technol. 2018, 255, 898–913. [Google Scholar] [CrossRef] [Green Version]
- Sarıkaya, M.; Gupta, M.K.; Tomaz, I.; Pimenov, D.Y.; Kuntoğlu, M.; Khanna, N.; Yıldırım, Ç.V.; Krolczyk, G.M. A state-of-the-art review on tool wear and surface integrity characteristics in machining of superalloys. CIRP J. Manuf. Sci. Technol. 2021, 35, 624–658. [Google Scholar] [CrossRef]
- Michailidis, N. Variations in the cutting performance of PVD-coated tools in milling Ti6Al4V, explained through temperature-dependent coating properties. Surf. Coat. Technol. 2016, 304, 325–329. [Google Scholar] [CrossRef]
- Gupta, M.K.; Song, Q.; Liu, Z.; Sarikaya, M.; Mia, M.; Jamil, M.; Singla, A.K.; Bansal, A.; Pimenov, D.Y.; Kuntoğlu, M. Tribological performance based machinability investigations in cryogenic cooling assisted turning of α-β titanium Alloy. Tribol. Int. 2021, 160, 107032. [Google Scholar] [CrossRef]
- Bertolini, R.; Bruschi, S.; Bordin, A.; Ghiotti, A.; Pezzato, L.; Dabala, M. Fretting Corrosion Behavior of Additive Manufactured and Cryogenic-Machined Ti6Al4V for Biomedical Applications. Adv. Eng. Mater. 2017, 19, 1500629. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, R.; Bruschi, S.; Ghiotti, A.; Pezzato, L.; Dabala, M. The effect of cooling strategies and machining feed rate on the corrosion behavior and wettability of AZ31 alloy for biomedical applications. Procedia Cirp 2017, 65, 7–12. [Google Scholar] [CrossRef]
- Pu, Z.; Dillon, O.W.; Puelo, D.A.; Jawahir, I.S. Cryogenic machining and burnishing of magnesium alloys to improve In Vivo corrosion resistance. In Surface Modification of Magnesium and its Alloys for Biomedical Applications. Volume II: Modification and Coating Techniques Wood head Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2015; pp. 103–133. [Google Scholar] [CrossRef]
- Bruschi, S.; Pezzato, L.; Ghiotti, A.; Dabala, M.; Bertolini, R. Effectiveness of using low-temperature coolants in machining to enhance durability of AISI 316L stainless steel for reusable biomedical devices. J. Manuf. Process. 2019, 39, 295–304. [Google Scholar] [CrossRef]
- Bertolini, R.; Bruschi, S.; Ghiotti, A. Enhancement of corrosion resistance to sterilization stages of a biomedical grade AISI 316L stainless steel by means of low-temperature machining. Mater. Today 2019, 7, 552–559. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z. Modeling and prediction for 3D surface topography in finish turning with conventional and wiper inserts. Measurement 2016, 94, 37–45. [Google Scholar] [CrossRef]
- Sartori, S.; Bordin, A.; Ghiotti, A.; Bruschi, S. Analysis of the surface integrity in cryogenic turning of Ti6Al4V produced by Direct Melting Laser Sintering. Procedia Cirp 2016, 45, 123–126. [Google Scholar] [CrossRef]
- Mia, M.; Gupta, M.K.; Lozano, J.A.; Carou, D.; Pimenov, D.Y.; Królczyk, G.; Khan, A.M.; Dhar, N.R. Multi-objective optimization and life cycle assessment of eco-friendly cryogenic N2 assisted turning of Ti-6Al-4V. J. Clean. Prod. 2019, 210, 121–133. [Google Scholar] [CrossRef]
- Deiab, I.; Raza, S.W.; Pervaiz, S. Analysis of Lubrication Strategies for Sustainable Machining during Turning of Titanium Ti-6Al-4V Alloy. Procedia CIRP 2014, 17, 766–771. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Huang, B.; Puleo, D.A.; Jawahir, I.S. Enhanced machinability of Ti-5553 alloy from cryogenic machining: Comparison with MQL and flood-cooled machining and modeling. Procedia CIRP 2015, 31, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Huang, S.; Wang, T.; Chen, W. Research on surface integrity of turning titanium alloy TB6. Procedia Cirp 2018, 71, 484–489. [Google Scholar] [CrossRef]
- Gupta, M.K.; Song, Q.; Liu, Z.; Pruncu, C.I.; Mia, M.; Singh, G.; Lozano, J.A.; Carou, D.; Khan, A.M.; Jamil, M.; et al. Machining characteristics based life cycle assessment in eco-benign turning of pure titanium alloy. J. Clean. Prod. 2020, 251, 119598. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, Z.; Shi, Z.; Song, Q.; Wan, Y. Influence of machined surface roughness on thrust performance of micro-nozzle manufactured by micro-milling. Exp. Therm. Fluid Sci. 2016, 77, 295–305. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Kang, M. Finite element simulation of surface roughness in diamond turning of spherical surfaces. J. Manuf. Process. 2018, 31, 768–775. [Google Scholar] [CrossRef]
- Park, K.-Y.; Olortegui-Yume, J.; Yoon, M.-C.; Kwon, P. A study on droplets and their distribution for minimum quantity lubrication (MQL). Int. J. Mach. Tools Manuf. 2010, 50, 824–833. [Google Scholar] [CrossRef]
- Krolczyk, G.M.; Maruda, R.W.; Krolczyk, J.B.; Wojciechowski, S.; Mia, M.; Nieslony, P.; Budzik, G. Ecological trends in machining as a key factor in sustainable production—A review. J. Clean. Prod. 2019, 218, 601–615. [Google Scholar] [CrossRef]
- Ravelingien, M.; Hervent, A.S.; Mullens, S.; Luyten, J.; Vervaet, C.; Remon, P.J. Influence of surface topography and pore architecture of alkali-treated titanium on In Vitro apatite deposition. Appl. Surf. Sci. 2010, 256, 3693–3697. [Google Scholar] [CrossRef]
- Demirci, S.; Dalmis, R.; Dikici, T.; Tuncay, M.M.; Kaya, N.; Gulluoglua, A.N. Effect of surface modifications of additively manufactured Ti-6Al-4V alloys on apatite formation ability for biomedical applications. J. Alloy. Compd. 2021, 887, 161445. [Google Scholar] [CrossRef]
- Constantinescu, F.; Ciocoiu, R.; Trante, O.; Ciuca, I. Hydroxyapatite coating influenced by Ti6Al4V substrate roughness. Key Eng. Mater. 2015, 638, 62–66. [Google Scholar] [CrossRef]
- Kaczmarek-Pawelska, A.; Krasicka-Cydzik, E. Functionalized nanotubular oxide layer on Ti6Al4V as a regulator and biosensor of bone tissue remodelling. Arch. Mater. Sci. Eng. 2015, 73, 53–61. [Google Scholar]
- Yigit, O.; Dikici, B.; Senocak, T.C.; Ozdemir, N. One-step synthesis of nano-hydroxyapatite/graphene nanosheet hybrid coatings on Ti6Al4V alloys by hydrothermal method and their in-vitro corrosion responses. Surf. Coat. Technol. 2020, 394, 125858. [Google Scholar] [CrossRef]
- Oskouei, R.H.; Fallahnezhad, K.; Kuppusami, S. An Investigation on the Wear Resistance and Fatigue Behaviour of Ti-6Al-4V Notched Members Coated with Hydroxyapatite Coatings. Materials 2016, 9, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.H.; Ho, S.C.; Chang, C.H.; Chen, C.C.; Say, W.C. Influence of roughness on in-vivo properties of titanium implant surface and their electrochemical behavior. Surf. Coat. Technol. 2016, 302, 215–226. [Google Scholar] [CrossRef]
- Sari, M.; Kristiano, N.A.; Ana, I.D.; Yusuf, Y. Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis. Coatings 2021, 11, 941. [Google Scholar] [CrossRef]
- Kierzkowska, A.; Krasicka-Cydzik, E. Behaviour of Ti6Al4V implant alloy In Vitro after plastic deformation by bending. Surf. Interface Anal. 2008, 40, 507–512. [Google Scholar] [CrossRef]
- Saruta, J.; Sato, N.; Ishijma, M.; Okubo, T.; Hirota, M.; Ogawa, T. Disproportionate Effect of Sub-Micron Topography on Osteoconductive Capability of Titanium. Int. J. Mol. Sci. 2019, 20, 4027. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.-D.; Kim, W.-J.; Kim, S.; Ku, Y.; Ryoo, H.-M. Surface Topography of Titanium Affects Their Osteogenic Potential through DNA Methylation. Int. J. Mol. Sci. 2021, 22, 2406. [Google Scholar] [CrossRef]
- Ball, M.; Grant, D.M.; Lo, W.-J.; Scotchford, C.A. The effect of different surface morphology and roughness on osteoblast-like cells. J. Biomed. Mater. Res. A 2007, 86, 637–647. [Google Scholar] [CrossRef] [PubMed]
- ISO. 5832-3:2016. Implants for Surgery—Metallic Materials—Part 3: Wrought Titanium 6-Aluminium 4-Vanadium Alloy; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Iqbal, A.; Zhao, G.; Zaini, J.; Gupta, M.K.; Jamil, M.; He, N.; Nauman, M.M.; Mikolajczyk, T.; Pimenov, D.Y. Between-the-Holes Cryogenic Cooling of the Tool in Hole-Making of Ti-6Al-4V and CFRP. Materials 2021, 14, 795. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pruncu, C.I.; Gupta, M.K.; Mia, M.; Khan, A.M.; Jamil, M.; Pimenov, D.Y.; Sen, B.; Sharma, V.S. Investigations of Machining Characteristics in the Upgraded MQL-Assisted Turning of Pure Titanium Alloys Using Evolutionary Algorithms. Materials 2019, 12, 999. [Google Scholar] [CrossRef] [Green Version]
- Mia, M.; Gupta, M.K.; Singh, G.; Królczyk, G.; Pimenov, D.Y. An approach to cleaner production for machining hardened steel using different cooling-lubrication conditions. J. Clean. Prod. 2018, 187, 1069–1081. [Google Scholar] [CrossRef]
- Salur, E.; Kuntoğlu, M.; Aslan, A.; Pimenov, D.Y. The Effects of MQL and Dry Environments on Tool Wear, Cutting Temperature, and Power Consumption during End Milling of AISI 1040 Steel. Metals 2021, 11, 1674. [Google Scholar] [CrossRef]
- Gupta, M.K.; Khan, A.M.; Song, Q.; Liu, Z.; Khalid, Q.S.; Jamil, M.; Kuntoğlu, M.; Usca, Ü.A.; Sarıkaya, M.; Pimenov, D.Y. A review on conventional and advanced minimum quantity lubrication approaches on performance measures of grinding process. Int. J. Adv. Manuf. Syst. 2021, 117, 729–750. [Google Scholar] [CrossRef]
- Maruda, R.W.; Krolczyk, G.M.; Feldshtein, E.; Pusavec, F.; Szydlowski, M.; Legutko, S.; Sobczak-Kupiec, A. A study on droplets sizes, their distribution and heat exchange for minimum quantity cooling lubrication (MQCL). Int. J. Mach. Tools Manuf. 2016, 100, 81–92. [Google Scholar] [CrossRef]
- ISO. ISO 5436-1:2000(en). Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters; ISO: Geneva, Switzerland, 2000. [Google Scholar]
- Horvath, R.; Czifra, A.; Dregelyi-Kiss, A. Effect of conventional and non-conventional tool geometries to skewness and kurtosis of surface roughness in case of fine turning of aluminium alloys with diamond tools. Int. J. Adv. Manuf. Technol. 2014, 78, 297–304. [Google Scholar] [CrossRef]
- Sánchez Egea, A.J.; Martynenko, V.; Simoncelli, A.; Serrancoli, G.; Krahmer, D.M. Sliding abrasive wear when combining WEDM conditions and polishing treatment on H13 disks over 1045 carbon steel pins. Int. J. Adv. Manuf. Technol. 2021. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting In Vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- ISO. 16773-2:2016. Electrochemical Impedance Spectroscopy (EIS) on Coated and Uncoated Metallic Specimens—Part 2: Collection of Data; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- ISO. 16773-3:2016. Electrochemical Impedance Spectroscopy (EIS) on Coated and Uncoated Metallic Specimens—Part 3: Processing and Analysis of Data from Dummy Cells; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Feldshtein, E.; Dyachkova, L.N.; Królczyk, G.M. On the evaluation of certain strength characteristics and fracture features of iron-based sintered MMCs with nanooxide additives. Mater. Sci. Eng. A 2019, 756, 455–463. [Google Scholar] [CrossRef]
- Gacia-Martinez, E.; Miguel, V.; Martinez-Martinez, A.; Manjabacas, M.C.; Coello, J. Sustainable lubrication methods for the machining of titanium alloys: An overview. Materials 2019, 12, 3852. [Google Scholar] [CrossRef] [Green Version]
- Alla, R.K.; Ginjupalli, K.; Upadhya, N.; Shammas, M.; Ravi, R.K.; Sekhar, R. Surface roughness of implant: A review. Trends Biomater. Artif. Organs 2011, 25, 112–118. [Google Scholar]
- Krolczyk, G.M.; Maruda, R.W.; Krolczyk, J.B.; Nieslony, P.; Wojciechowski, S.; Legutko, S. Parametric and nonparametric description of the surface topography in the dry and MQCL cutting conditions. Measurement 2018, 121, 225–239. [Google Scholar] [CrossRef]
- Leksycki, K.; Feldshtein, E.; Lisowicz, J.; Chudy, R.; Mrugalski, R. Cutting Forces and Chip Shaping When Finish Turning of 17-4 PH Stainless Steel under Dry, Wet, and MQL Machining Conditions. Metals 2020, 10, 1187. [Google Scholar] [CrossRef]
- Leksycki, K.; Feldshtein, E.; Królczyk, G.M.; Legutko, S. On the Chip Shaping and Surface Topography When Finish Cutting 17-4 PH Precipitation-Hardening Stainless Steel under Near-Dry Cutting Conditions. Materials 2020, 13, 2188. [Google Scholar] [CrossRef] [PubMed]
- Leksycki, K.; Feldshtein, E. The surface texture of Ti6Al4V titanium alloy under wet and dry finish turning conditions. In Industrial Measurements in Machining, Lecture Notes in Mechanical Engineering; Królczyk, G., Niesłony, P., Królczyk, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 33–44. [Google Scholar] [CrossRef]
- Stango, S.A.X.; Karthick, D.; Swaroop, S.; Mudali, U.K.; Vijayalakshimi, U. Development of hydroxyapatite coatings on laser textured 316 LSS and Ti-6Al-4V and its electrochemical behavior in SBF solution for orthopaedic applications. Ceram. Int. 2018, 44, 3149–3160. [Google Scholar] [CrossRef]
- Krasicka-Cydzik, E. Formowanie Cienkich warstw anodowych na tytanie i jego implantowych stopach w środowisku kwasu fosforowego; Oficyna Wydawnicza Uniwersytetu Zielonogórskiego: Zielona Góra, Poland, 2003. (In Polish) [Google Scholar]


| Chemical Composition (%) | |||||||
|---|---|---|---|---|---|---|---|
| O | V | Al | Fe | H | C | N | Ti |
| <0.20 | 3.5 | 5.5 | <0.30 | <0.0015 | <0.08 | <0.05 | rest |
| Mechanical Properties | |||||||
| Modulus of Elastic (MPa) | Tensile Strength (MPa) | Yield Strength (MPa) | Fatigue Strength (MPa) | ||||
| 110–114 | 960–970 | 850–900 | 620–725 | ||||
| Cutting Conditions | Surface Roughness Range | Ra (μm) | Rz (μm) | Rp (μm) | Rv (μm) | Rsk | Rku |
|---|---|---|---|---|---|---|---|
| Dry | Ramin | 0.29 | 2.18 | 1.16 | 1.01 | −0.208 | 2.68 |
| MQL | 0.37 | 2.33 | 1.27 | 1.07 | 0.135 | 2.49 | |
| Dry | Ramax | 2.22 | 11.8 | 7.23 | 4.62 | 0.287 | 2.58 |
| MQL | 1.62 | 8.80 | 5.43 | 3.37 | 0.311 | 2.64 |
| Cutting Conditions | Surface Roughness Range | Surface Roughness Profiles | Surface Roughness Parameters | Rku–Rsk Topological Maps | 2D Surface Images |
|---|---|---|---|---|---|
| Dry | Ramin | Flattened peaks | Dry compared to MQL: decrease Ra, Rz, Rp, Rv, and Rsk of 6–165% | Flattened peaks, regular shapes | Scratch, stickers |
| MQL | High peaks and deep pits, regular shapes | ||||
| Dry | Ramax | Feed marks | MQL compared to dry: decrease Ra, Rz, Rp, and Rv of 25–27% | Very high peaks and very deep pits, regular shapes | Feed marks, scratch |
| MQL | Feed marks, stickers |
| Cutting Conditions | Surface Roughness Range | Precipitation of Hydroxyapatite, after 7 Days | Stoichiometric Composition (Ca/P), after 7 Days | Highest Values of Ecorr | Impedance Characteristics |
|---|---|---|---|---|---|
| Dry | Ramin | Irregular, spherical | 1.78 | ~0 mV, after 7 days | Higher resistance, an almost capacitive response, presence of a passive layer |
| MQL | More irregular, spherical | 1.73 | Low resistance, lack of presence of a passive layer | ||
| Dry | Ramax | Irregular, spherical | 1.97 | High resistance, an almost capacitive response, presence of a passive layer | |
| MQL | More irregular, spherical | 1.85 | Low resistance, lack of presence of a passive layer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leksycki, K.; Kaczmarek-Pawelska, A.; Ochał, K.; Gradzik, A.; Pimenov, D.Y.; Giasin, K.; Chuchala, D.; Wojciechowski, S. Corrosion Resistance and Surface Bioactivity of Ti6Al4V Alloy after Finish Turning under Ecological Cutting Conditions. Materials 2021, 14, 6917. https://doi.org/10.3390/ma14226917
Leksycki K, Kaczmarek-Pawelska A, Ochał K, Gradzik A, Pimenov DY, Giasin K, Chuchala D, Wojciechowski S. Corrosion Resistance and Surface Bioactivity of Ti6Al4V Alloy after Finish Turning under Ecological Cutting Conditions. Materials. 2021; 14(22):6917. https://doi.org/10.3390/ma14226917
Chicago/Turabian StyleLeksycki, Kamil, Agnieszka Kaczmarek-Pawelska, Kamil Ochał, Andrzej Gradzik, Danil Yurievich Pimenov, Khaled Giasin, Daniel Chuchala, and Szymon Wojciechowski. 2021. "Corrosion Resistance and Surface Bioactivity of Ti6Al4V Alloy after Finish Turning under Ecological Cutting Conditions" Materials 14, no. 22: 6917. https://doi.org/10.3390/ma14226917
APA StyleLeksycki, K., Kaczmarek-Pawelska, A., Ochał, K., Gradzik, A., Pimenov, D. Y., Giasin, K., Chuchala, D., & Wojciechowski, S. (2021). Corrosion Resistance and Surface Bioactivity of Ti6Al4V Alloy after Finish Turning under Ecological Cutting Conditions. Materials, 14(22), 6917. https://doi.org/10.3390/ma14226917











