Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Ethical Considerations
2.2. Study Population
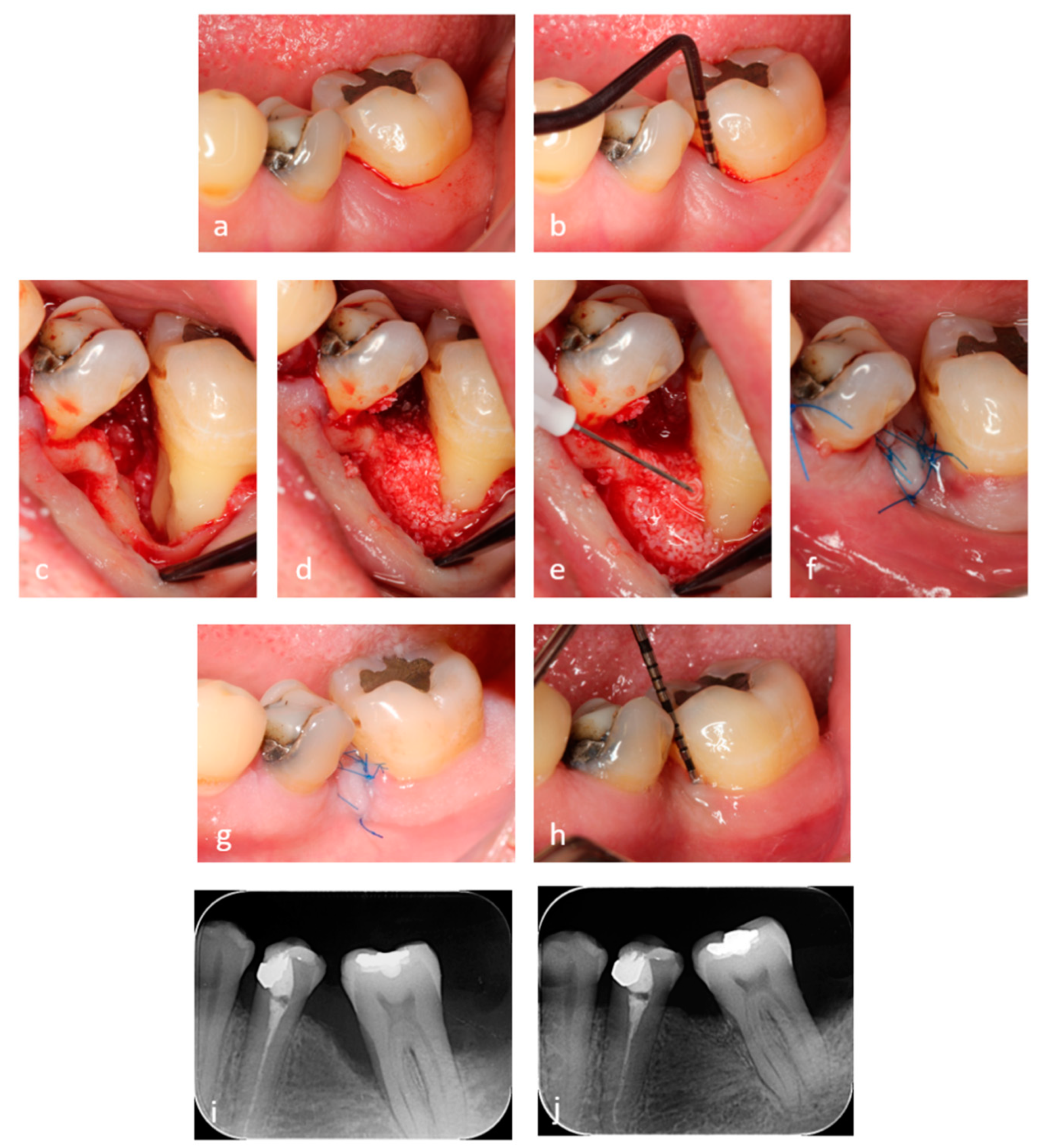
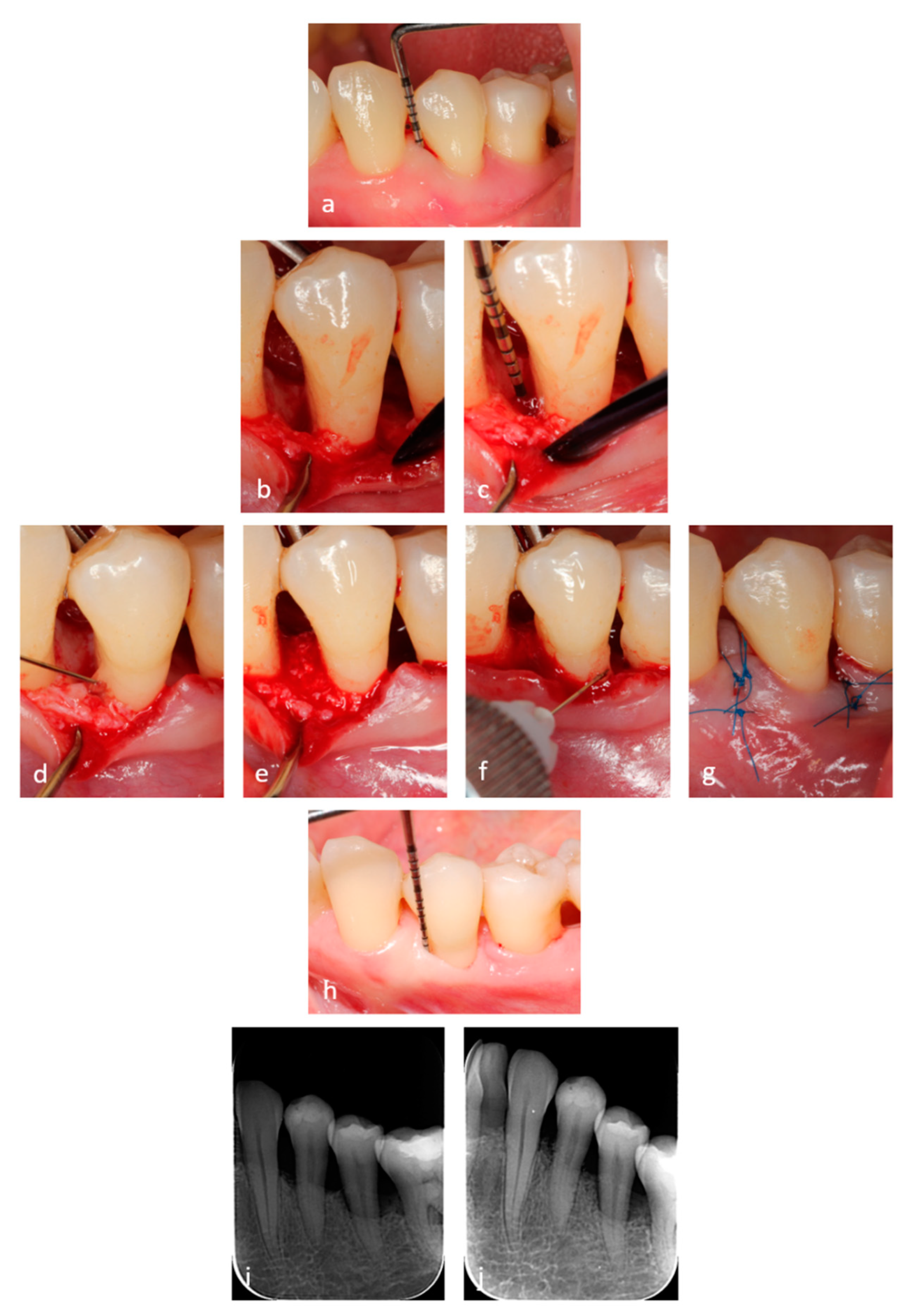
2.3. Surgical Procedures
2.4. Application of the Hyaluronic Acid (HA) and Xenograft
2.5. Post-Surgical Instructions and Plaque Control
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Baseline Data
3.2. Early Wound Healing
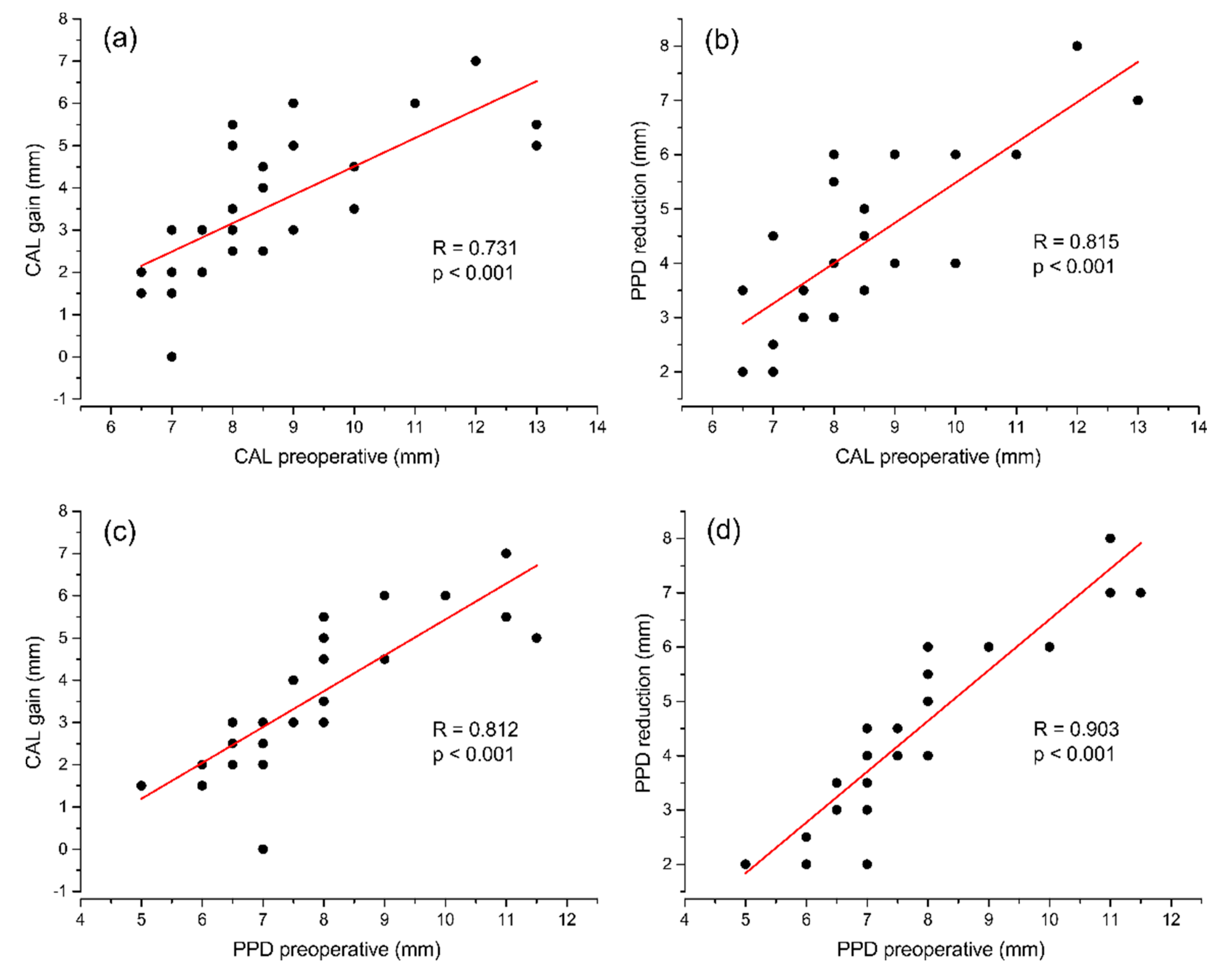
3.3. Clinical Outcomes at 6 Months
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abusleme, L.; Hoare, A.; Hong, B.; Diaz, P.I. Microbial Signatures of Health, Gingivitis, and Periodontitis. Periodontol. 2000 2021, 86, 57–78. [Google Scholar] [CrossRef]
- Darveau, R.P.; Curtis, M.A. Oral Biofilms Revisited: A Novel Host Tissue of Bacteriological Origin. Periodontol. 2000 2021, 86, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The Dental Plaque Biofilm Matrix. Periodontol. 2000 2021, 86, 32–56. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Curtis, M.A. Microbial Transitions from Health to Disease. Periodontol. 2000 2021, 86, 201–209. [Google Scholar] [CrossRef]
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J.L. Global Burden of Oral Conditions in 1990-2010: A Systematic Analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990-2010: A Systematic Review and Meta-Regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Lamster, I.; Greenspan, J.; Pitts, N.; Scully, C.; Warnakulasuriya, S. Global Burden of Oral Diseases: Emerging Concepts, Management and Interplay with Systemic Health. Oral Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Wennstrom, J.L. The Angular Bony Defect as Indicator of Further Alveolar Bone Loss. J. Clin. Periodontol. 1991, 18, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Matuliene, G.; Pjetursson, B.E.; Salvi, G.E.; Schmidlin, K.; Brägger, U.; Zwahlen, M.; Lang, N.P. Influence of Residual Pockets on Progression of Periodontitis and Tooth Loss: Results after 11 Years of Maintenance. J. Clin. Periodontol. 2008, 35, 685–695. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Clinical Concepts for Regenerative Therapy in Intrabony Defects. Periodontol. 2000 2015, 68, 282–307. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for Promoting Periodontal Regeneration in Human Intrabony Defects: A Systematic Review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Buti, J.; Pini Prato, G.; Tonetti, M.S. Periodontal Regeneration Compared with Access Flap Surgery in Human Intra-Bony Defects 20-Year Follow-up of a Randomized Clinical Trial: Tooth Retention, Periodontitis Recurrence and Costs. J. Clin. Periodontol. 2017, 44, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, M.; Rasperini, G.; Milani, S. 120 Infrabony Defects Treated With Regenerative Therapy: Long-Term Results. J. Periodontol. 2011, 82, 668–675. [Google Scholar] [CrossRef] [PubMed]
- De Ry, S.P.; Roccuzzo, A.; Lang, N.P.; Sculean, A.; Salvi, G.E. Long-Term Clinical Outcomes of Periodontal Regeneration with Enamel Matrix Derivative: A Retrospective Cohort Study with a Mean Follow-up of 10 Years. J. Periodontol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Donos, N.; Schwarz, F.; Becker, J.; Brecx, M.; Arweiler, N.B. Five-Year Results Following Treatment of Intrabony Defects with Enamel Matrix Proteins and Guided Tissue Regeneration. J. Clin. Periodontol. 2004, 31, 545–549. [Google Scholar] [CrossRef]
- Sculean, A.; Kiss, A.; Miliauskaite, A.; Schwarz, F.; Arweiler, N.B.; Hannig, M. Ten-Year Results Following Treatment of Intra-Bony Defects with Enamel Matrix Proteins and Guided Tissue Regeneration. J. Clin. Periodontol. 2008, 35, 817–824. [Google Scholar] [CrossRef]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal Regeneration—Intrabony Defects: A Systematic Review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S77–S104. [Google Scholar] [CrossRef]
- Li, F.; Yu, F.; Xu, X.; Li, C.; Huang, D.; Zhou, X.; Ye, L.; Zheng, L. Evaluation of Recombinant Human FGF-2 and PDGF-BB in Periodontal Regeneration: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Nibali, L. Guest Editorial: Time to Reflect on New Evidence about Periodontal Regenerative Surgery of Intrabony Defects. J. Clin. Periodontol. 2021, 48, 557–559. [Google Scholar] [CrossRef]
- Bartold, P. Proteoglycans of the Periodontium: Structure, Role and Function—PubMed. J. Periodontal Res. 1987, 22, 431–444. [Google Scholar] [CrossRef]
- Yeh, Y.; Yang, Y.; Yuan, K. Importance of CD44 in the Proliferation and Mineralization of Periodontal Ligament Cells. J. Periodontal Res. 2014, 49, 827–835. [Google Scholar] [CrossRef]
- Bozic, D.; Grgurevic, L.; Erjavec, I.; Brkljacic, J.; Orlic, I.; Razdorov, G.; Grgurevic, I.; Vukicevic, S.; Plancak, D. The Proteome and Gene Expression Profile of Cementoblastic Cells Treated by Bone Morphogenetic Protein-7 in Vitro. J. Clin. Periodontol. 2012, 39, 80–90. [Google Scholar] [CrossRef]
- Shimabukuro, Y.; Terashima, H.; Takedachi, M.; Maeda, K.; Nakamura, T.; Sawada, K.; Kobashi, M.; Awata, T.; Oohara, H.; Kawahara, T.; et al. Fibroblast Growth Factor-2 Stimulates Directed Migration of Periodontal Ligament Cells via PI3K/AKT Signaling and CD44/Hyaluronan Interaction. J. Cell. Physiol. 2011, 226, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Al-Rekabi, Z.; Fura, A.M.; Juhlin, I.; Yassin, A.; Popowics, T.E.; Sniadecki, N.J. Hyaluronan-CD44 Interactions Mediate Contractility and Migration in Periodontal Ligament Cells. Cell Adhes. Migr. 2019, 13, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujioka-Kobayashi, M.; Müller, H.-D.; Mueller, A.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. In Vitro Effects of Hyaluronic Acid on Human Periodontal Ligament Cells. BMC Oral Health 2017, 17, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asparuhova, M.B.; Kiryak, D.; Eliezer, M.; Mihov, D.; Sculean, A. Activity of Two Hyaluronan Preparations on Primary Human Oral Fibroblasts. J. Periodontal Res. 2019, 54, 33–45. [Google Scholar] [CrossRef]
- Asparuhova, M.B.; Chappuis, V.; Stähli, A.; Buser, D.; Sculean, A. Role of Hyaluronan in Regulating Self-Renewal and Osteogenic Differentiation of Mesenchymal Stromal Cells and Pre-Osteoblasts. Clin. Oral Investig. 2020, 24, 3923–3937. [Google Scholar] [CrossRef] [Green Version]
- Fujioka-Kobayashi, M.; Schaller, B.; Kobayashi, E.; Hernandez, M.; Zhang, Y.; Miron, R. Hyaluronic Acid Gel-Based Scaffolds as Potential Carrier for Growth Factors: An In Vitro Bioassay on Its Osteogenic Potential. J. Clin. Med. 2016, 5, 112. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Zou, X.; Chen, L.; Li, H.; Mygind, T.; Kassem, M.; Bünger, C. Effect of Hyaluronan on Osteogenic Differentiation of Porcine Bone Marrow Stromal Cells in Vitro. J. Orthop. Res. 2008, 26, 713–720. [Google Scholar] [CrossRef]
- Kim, J.-J.; Song, H.Y.; Ben Amara, H.; Kyung-Rim, K.; Koo, K.-T. Hyaluronic Acid Improves Bone Formation in Extraction Sockets With Chronic Pathology: A Pilot Study in Dogs. J. Periodontol. 2016, 87, 790–795. [Google Scholar] [CrossRef]
- Kim, J.; Ben Amara, H.; Park, J.; Kim, S.; Kim, T.; Seol, Y.; Lee, Y.; Ku, Y.; Rhyu, I.; Koo, K. Biomodification of Compromised Extraction Sockets Using Hyaluronic Acid and RhBMP-2: An Experimental Study in Dogs. J. Periodontol. 2019, 90, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Nakamura, T.; Kawakami, Y.; Imafuji, T.; Shinohara, Y.; Noguchi, K.; Sculean, A. Healing of Buccal Gingival Recessions Following Treatment with Coronally Advanced Flap Alone or Combined with a Cross-linked Hyaluronic Acid Gel. An Experimental Study in Dogs. J. Clin. Periodontol. 2021, 48, 570–580. [Google Scholar] [CrossRef]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Kawakami, Y.; Shinohara, Y.; Noguchi, K.; Pilloni, A.; Sculean, A. Periodontal Wound Healing/Regeneration of Two-Wall Intrabony Defects Following Reconstructive Surgery with Cross-Linked Hyaluronic Acid-Gel with or without a Collagen Matrix: A Preclinical Study in Dogs. Quintessence Int. Berl. Ger. 1985 2021, 52, 308–316. [Google Scholar] [CrossRef]
- Bertl, K.; Bruckmann, C.; Isberg, P.-E.; Klinge, B.; Gotfredsen, K.; Stavropoulos, A. Hyaluronan in Non-Surgical and Surgical Periodontal Therapy: A Systematic Review. J. Clin. Periodontol. 2015, 42, 236–246. [Google Scholar] [CrossRef]
- Eliezer, M.; Imber, J.-C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic Acid as Adjunctive to Non-Surgical and Surgical Periodontal Therapy: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef]
- Pilloni, A.; Rojas, M.A.; Marini, L.; Russo, P.; Shirakata, Y.; Sculean, A.; Iacono, R. Healing of Intrabony Defects Following Regenerative Surgery by Means of Single-Flap Approach in Conjunction with Either Hyaluronic Acid or an Enamel Matrix Derivative: A 24-Month Randomized Controlled Clinical Trial. Clin. Oral Investig. 2021, 25, 5095–5107. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty Years of Enamel Matrix Derivative: The Past, the Present and the Future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The Plaque Control Record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. The Simplified Papilla Preservation Flap. A Novel Surgical Approach for the Management of Soft Tissues in Regenerative Procedures. Int. J. Periodontics Restor. Dent. 1999, 19, 589–599. [Google Scholar]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. The Modified Papilla Preservation Technique. A New Surgical Approach for Interproximal Regenerative Procedures. J. Periodontol. 1995, 66, 261–266. [Google Scholar] [CrossRef]
- Wachtel, H.; Schenk, G.; Böhm, S.; Weng, D.; Zuhr, O.; Hürzeler, M.B. Microsurgical Access Flap and Enamel Matrix Derivative for the Treatment of Periodontal Intrabony Defects: A Controlled Clinical Study: Microsurgical Access Flap and EMD. J. Clin. Periodontol. 2003, 30, 496–504. [Google Scholar] [CrossRef]
- Raines, A.L.; Sunwoo, M.; Gertzman, A.A.; Thacker, K.; Guldberg, R.E.; Schwartz, Z.; Boyan, B.D. Hyaluronic Acid Stimulates Neovascularization during the Regeneration of Bone Marrow after Ablation. J. Biomed. Mater. Res. A 2011, 96A, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Sakai, N.; Shiba, H.; Nagahara, T.; Fujita, T.; Kajiya, M.; Iwata, T.; Matsuda, S.; Kawahara, K.; Kawaguchi, H.; et al. Characteristics of High-Molecular-Weight Hyaluronic Acid as a Brain-Derived Neurotrophic Factor Scaffold in Periodontal Tissue Regeneration. Tissue Eng. Part A 2011, 17, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Giannobile, W.V.; McGuire, M.K.; Kao, R.T.; Mellonig, J.T.; Hinrichs, J.E.; McAllister, B.S.; Murphy, K.S.; McClain, P.K.; Nevins, M.L.; et al. Platelet-Derived Growth Factor Stimulates Bone Fill and Rate of Attachment Level Gain: Results of a Large Multicenter Randomized Controlled Trial. J. Periodontol. 2005, 76, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Oh, T.-J.; Mills, M.P.; Clem, D.S.; McClain, P.K.; Schallhorn, R.A.; McGuire, M.K.; Scheyer, E.T.; Giannobile, W.V.; Reddy, M.S.; et al. A Randomized Clinical Trial Evaluating Rh-FGF-2/β-TCP in Periodontal Defects. J. Dent. Res. 2016, 95, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, M.; Iorio-Siciliano, V.; Blasi, A.; Ramaglia, L.; Salvi, G.E.; Sculean, A. Enamel Matrix Derivative and Bone Grafts for Periodontal Regeneration of Intrabony Defects. A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2015, 19, 1581–1593. [Google Scholar] [CrossRef] [Green Version]
- Briguglio, F.; Briguglio, E.; Briguglio, R.; Cafiero, C.; Isola, G. Treatment of Infrabony Periodontal Defects Using a Resorbable Biopolymer of Hyaluronic Acid: A Randomized Clinical Trial. Quintessence Int. Berl. Ger. 1985 2013, 44, 231–240. [Google Scholar] [CrossRef]
- Vanden Bogaerde, L. Treatment of Infrabony Periodontal Defects with Esterified Hyaluronic Acid: Clinical Report of 19 Consecutive Lesions. Int. J. Periodontics Restor. Dent. 2009, 29, 315–323. [Google Scholar]
- Leonardis, D.D.; Paolantonio, M. Enamel Matrix Derivative, Alone or Associated With a Synthetic Bone Substitute, in the Treatment of 1- to 2-Wall Periodontal Defects. J. Periodontol. 2013, 84, 444–455. [Google Scholar] [CrossRef]
- Nibali, L.; Sultan, D.; Arena, C.; Pelekos, G.; Lin, G.; Tonetti, M. Periodontal Infrabony Defects: Systematic Review of Healing by Defect Morphology Following Regenerative Surgery. J. Clin. Periodontol. 2021, 48, 101–114. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Vecchiatini, R.; Maietti, E.; Simonelli, A. A Simplified Composite Outcome Measure to Assess the Effect of Periodontal Regenerative Treatment in Intraosseous Defects. J. Periodontol. 2020, 91, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Fratini, A.; Manavella, V.; Giraudi, M.; Citterio, F.; Ferrarotti, F.; Mariani, G.M.; Cairo, F.; Baima, G.; Romano, F. Pocket Resolution in Regenerative Treatment of Intrabony Defects with Papilla Preservation Techniques: A Systematic Review and Meta-analysis of Randomized Clinical Trials. J. Clin. Periodontol. 2021, 48, 843–858. [Google Scholar] [CrossRef] [PubMed]
| Study population | |
| Age (years, mean ± SD) | 54.59 ± 10.24 |
| Gender (male/female) | 7/16 |
| Smoking (yes/no) | 4/19 |
| Defect characteristics | |
| Dental arch (maxillary/mandibular) | 17/10 |
| Tooth type (incisor/canine/premolar/molar) | 8/3/9/7 |
| CEJ-defect bottom (mean ± SD, mm) | 10.54 ± 3.23 |
| Intrabony component (mean ± SD, mm) | 7.24 ± 2.46 |
| Intrabony width (mean ± SD, mm) | 3.44 ± 0.96 |
| X-ray angle (mean ± SD, degree) | 26.4 ± 8.43 |
| Defect configuration | |
| 1-wall | 7 |
| 2-wall | 13 |
| 3-wall | 6 |
| Crater | 1 |
| Variable | Baseline | 6 Months | Change | Significance, p a |
|---|---|---|---|---|
| PPD (mm) | 7.89 ± 1.60 | 3.35 ± 0.72 | 4.54 ± 1.65 | <0.001 |
| CAL (mm) | 8.72 ± 1.82 | 5.07 ± 1.28 | 3.65 ± 1.67 | <0.001 |
| REC (mm) | 0.83 ± 0.67 | 1.72 ± 0.90 | 0.89 ± 0.59 | <0.001 |
| CAL Gain | Residual PPD | |||
|---|---|---|---|---|
| n | % | n | % | |
| 0–1 mm | 1 | 3.7 | 0 | 0 |
| 2–3 mm | 12 | 44.4 | 14 | 51.9 |
| 4 mm | 3 | 11.1 | 11 | 40.7 |
| 5 mm | 6 | 22.2 | 2 | 7.4 |
| ≥6 mm | 5 | 18.5 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božić, D.; Ćatović, I.; Badovinac, A.; Musić, L.; Par, M.; Sculean, A. Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral. Materials 2021, 14, 6795. https://doi.org/10.3390/ma14226795
Božić D, Ćatović I, Badovinac A, Musić L, Par M, Sculean A. Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral. Materials. 2021; 14(22):6795. https://doi.org/10.3390/ma14226795
Chicago/Turabian StyleBožić, Darko, Ivan Ćatović, Ana Badovinac, Larisa Musić, Matej Par, and Anton Sculean. 2021. "Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral" Materials 14, no. 22: 6795. https://doi.org/10.3390/ma14226795
APA StyleBožić, D., Ćatović, I., Badovinac, A., Musić, L., Par, M., & Sculean, A. (2021). Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral. Materials, 14(22), 6795. https://doi.org/10.3390/ma14226795









