Recycling Black Tea Waste Biomass as Activated Porous Carbon for Long Life Cycle Supercapacitor Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Generation of Hydrochar from Black Tea Sludge
2.3. Generation of BTC
2.4. Structural Characterization
2.5. Electrochemical Analysis
3. Results
3.1. Physiochemical Characterization
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Lo, C.-Y.; Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef]
- Seow, W.J.; Low, D.Y.; Pan, W.; Gunther, S.H.; Sim, X.; Torta, F.; Herr, D.R.; Kovalik, J.; Ching, J.; Khoo, C.M.; et al. Coffee, Black Tea, and Green Tea Consumption in Relation to Plasma Metabolites in an Asian Population. Mol. Nutr. Food Res. 2020, 64, 2000527. [Google Scholar] [CrossRef]
- Long, H.; Li, X.; Wang, H.; Jia, J. Biomass resources and their bioenergy potential estimation: A review. Renew. Sustain. Energy Rev. 2013, 26, 344–352. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Pleguezuelo, C.R.R.; Zuazo, V.H.D.; Bielders, C.; Bocanegra, J.A.J.; PereaTorres, F.; Martínez, J.R.F. Bioenergy farming using woody crops. A review. Agron. Sustain. Dev. 2014, 35, 95–119. [Google Scholar] [CrossRef] [Green Version]
- Srirangan, K.; Akawi, L.; Moo-Young, M.; Chou, C.P. Towards sustainable production of clean energy carriers from biomass resources. Appl. Energy 2012, 100, 172–186. [Google Scholar] [CrossRef]
- Volli, V.; Singh, R. Production of bio-oil from de-oiled cakes by thermal pyrolysis. Fuel 2012, 96, 579–585. [Google Scholar] [CrossRef]
- Bilo, F.; Pandini, S.; Sartore, L.; Depero, L.E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. A sustainable bioplastic obtained from rice straw. J. Clean. Prod. 2018, 200, 357–368. [Google Scholar] [CrossRef]
- Piemonte, V. Bioplastic Wastes: The Best Final Disposition for Energy Saving. J. Polym. Environ. 2011, 19, 988–994. [Google Scholar] [CrossRef]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.-H.; Kwon, E.E.; Jeon, Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, S.; Singh, O. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef]
- Zhang, H.; Pereira, B.; Li, Z.; Stephanopoulos, G. Engineering Escherichia coli coculture systems for the production of biochemical products. Proc. Natl. Acad. Sci. USA 2015, 112, 8266–8271. [Google Scholar] [CrossRef] [Green Version]
- Purnomo, C.W.; Kesuma, E.P.; Perdana, I.; Aziz, M. Lithium recovery from spent Li-ion batteries using coconut shell activated carbon. Waste Manag. 2018, 79, 454–461. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.-W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Pramanik, A.; Chattopadhyay, S.; De, G.; Mahanty, S. Efficient energy storage in mustard husk derived porous spherical carbon nanostructures. Mater. Adv. 2021. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Ma, Y.; Huang, J.; Zhao, J.; Xu, H. Waste activated carbon transformed to electrode of supercapacitor through combining with Co(OH)2. Electrochim. Acta 2021, 367, 137475. [Google Scholar] [CrossRef]
- Urbano, J.L.G.; Moreno-Fernández, G.; Arnaiz, M.; Ajuria, J.; Rojo, T.; Carriazo, D. Graphene-coffee waste derived carbon composites as electrodes for optimized lithium ion capacitors. Carbon 2020, 162, 273–282. [Google Scholar] [CrossRef]
- Chen, Q.; Tan, X.; Liu, Y.; Liu, S.; Li, M.; Gu, Y.; Zhang, P.; Ye, S.; Yang, Z.; Yang, Y. Biomass-derived porous graphitic carbon materials for energy and environmental applications. J. Mater. Chem. A 2020, 8, 5773–5811. [Google Scholar] [CrossRef]
- Li, T.; Ma, R.; Lin, J.; Hu, Y.; Zhang, P.; Sun, S.; Fang, L. The synthesis and performance analysis of various biomass-based carbon materials for electric double-layer capacitors: A review. Int. J. Energy Res. 2019, 44, 2426–2454. [Google Scholar] [CrossRef]
- Ratnaji, T.; Kennedy, L.J. Hierarchical porous carbon derived from tea waste for energy storage applications: Waste to worth. Diam. Relat. Mater. 2020, 110, 108100. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ranaweera, C.K.; Zhang, C.; Morey, T.; Hyatt, M.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Eco-Friendly and High Performance Supercapacitors for Elevated Temperature Applications Using Recycled Tea Leaves. Glob. Chall. 2017, 1, 1700063. [Google Scholar] [CrossRef]
- Peng, C.; Yan, X.B.; Wang, R.T.; Lang, J.-W.; Ou, Y.-J.; Xue, Q.-J. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electrochim. Acta 2013, 87, 401–408. [Google Scholar] [CrossRef]
- Jaruwat, D.; Udomsap, P.; Chollacoop, N.; Fuji, M.; Eiad-ua, A. Effects of hydrothermal temperature and time of hydrochar from Cattail leaves. AIP Conf. Proc. 2018, 2010, 020016. [Google Scholar] [CrossRef]
- Bejtka, K.; Milano, G.; Ricciardi, C.; Pirri, C.F.; Porro, S. TEM Nanostructural Investigation of Ag-Conductive Filaments in Polycrystalline ZnO-Based Resistive Switching Devices. ACS Appl. Mater. Interfaces 2020, 12, 29451–29460. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Lu, Y.; Zhao, F.; Chao, C.; Zhang, B.; Zhang, H. Preparing high surface area porous carbon from biomass by carbonization in a molten salt medium. RSC Adv. 2015, 5, 75728–75734. [Google Scholar] [CrossRef]
- Tran, H.; You, S.-J.; Chao, H.-P. Effect of pyrolysis temperatures and times on the adsorption of cadmium onto orange peel derived biochar. Waste Manag. Res. 2016, 34, 129–138. [Google Scholar] [CrossRef]
- Salahinejad, M.; Aflaki, F. Toxic and Essential Mineral Elements Content of Black Tea Leaves and Their Tea Infusions Consumed in Iran. Biol. Trace Element Res. 2010, 134, 109–117. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Ghosh, M.; Krajewski, M.; Kurungot, S.; Michalska, M. Biomass-derived activated carbon material from native European deciduous trees as an inexpensive and sustainable energy material for supercapacitor application. J. Energy Storage 2021, 34, 102178. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Rodehorst, U.; Streipert, B.; Wiemers-Meyer, S.; Jakelski, R.; Wagner, R.; Laskovic, I.C.; Winter, M. Learning from Overpotentials in Lithium Ion Batteries: A Case Study on the LiNi1/3Co1/3Mn1/3O2(NCM) Cathode. J. Electrochem. Soc. 2016, 163, A2943–A2950. [Google Scholar] [CrossRef]
- Wang, W.; Wei, X.; Choi, D.; Lu, X.; Yang, G.; Sun, C. Chapter 1—Electrochemical cells for medium- and large-scale energy storage: Fundamentals. In Advances in Batteries for Medium and Large-Scale Energy Storage; Menictas, C., Skyllas-Kazacos, M., Lim, T.M., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 3–28. [Google Scholar]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Yang, I.; Kim, S.-G.; Kwon, S.H.; Kim, M.-S.; Jung, J.C. Relationships between pore size and charge transfer resistance of carbon aerogels for organic electric double-layer capacitor electrodes. Electrochim. Acta 2017, 223, 21–30. [Google Scholar] [CrossRef]
- Portet, C.; Taberna, P.; Simon, P.; Laberty-Robert, C. Modification of Al current collector surface by sol–gel deposit for carbon–carbon supercapacitor applications. Electrochim. Acta 2004, 49, 905–912. [Google Scholar] [CrossRef]
- Huang, J. Diffusion impedance of electroactive materials, electrolytic solutions and porous electrodes: Warburg impedance and beyond. Electrochim. Acta 2018, 281, 170–188. [Google Scholar] [CrossRef]
- Jung, H.; Kang, J.; Nam, I.; Bae, S. Graphitic Porous Carbon Derived from Waste Coffee Sludge for Energy Storage. Materials 2020, 13, 3972. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Bin Im, Y.; Lee, Y.; Kim, D.Y.; Lee, S. Biomass-derived ultrathin mesoporous graphitic carbon nanoflakes as stable electrode material for high-performance supercapacitors. Mater. Des. 2019, 169, 107688. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Sankar, A.B.; Rohita, D.S.; Rao, T.N.; Karthik, M. Conversion of Biomass Waste into High Performance Supercapacitor Electrodes for Real-Time Supercapacitor Applications. ACS Sustain. Chem. Eng. 2019, 7, 17175–17185. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, N.; Chong, S.; Li, D.; Chen, Y.; Sun, C. Three-dimensional porous graphene-like sheets synthesized from biocarbon via low-temperature graphitization for a supercapacitor. Green Chem. 2018, 20, 694–700. [Google Scholar] [CrossRef]

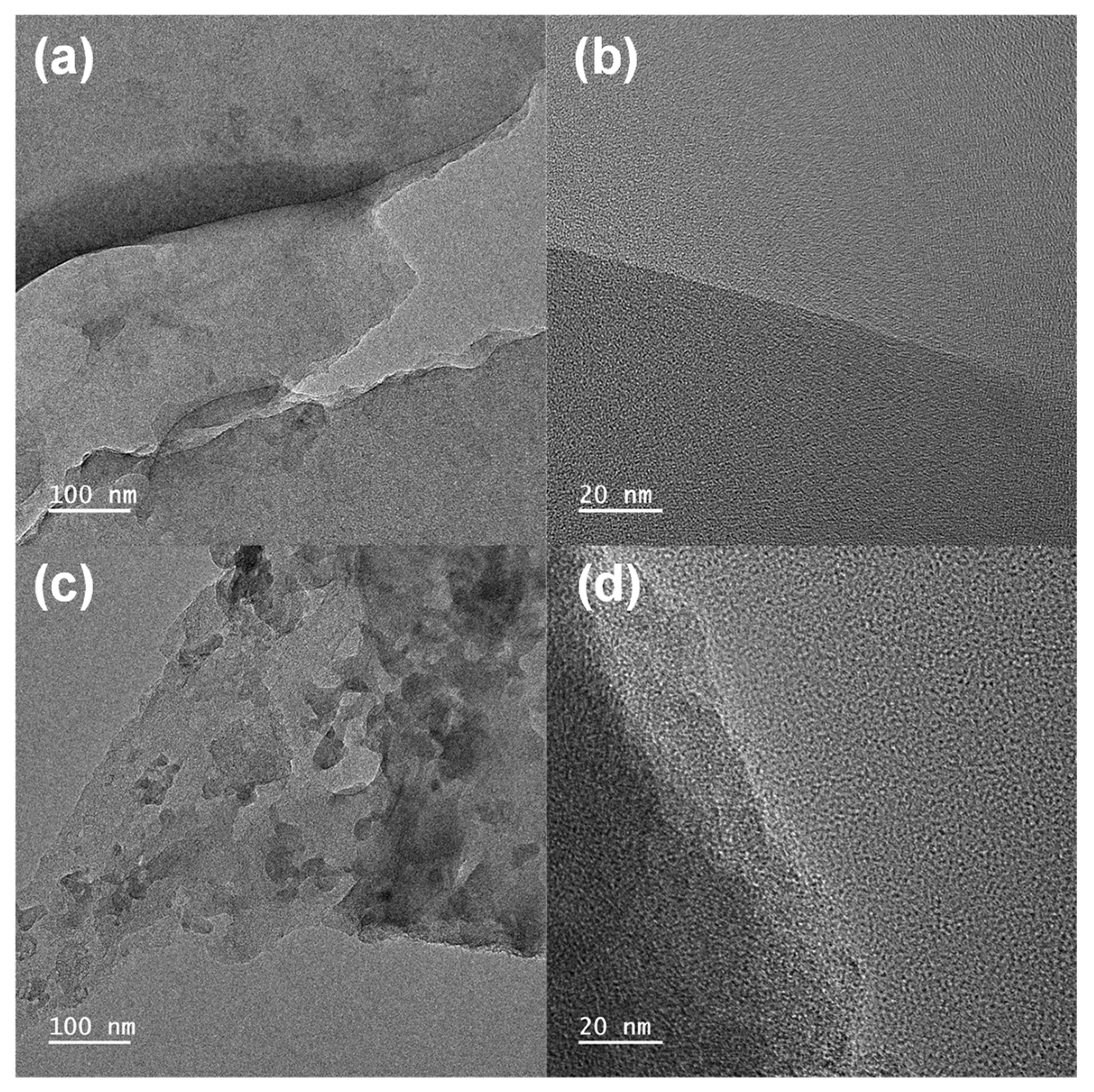
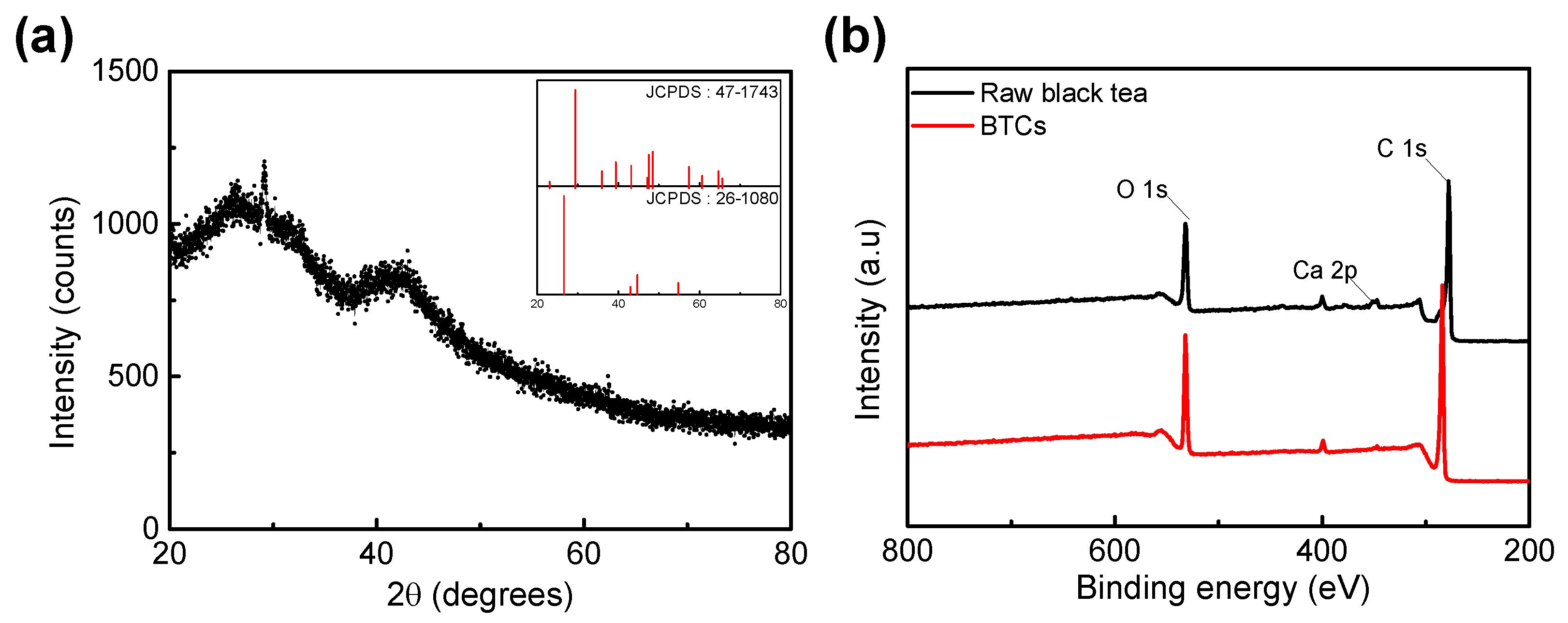
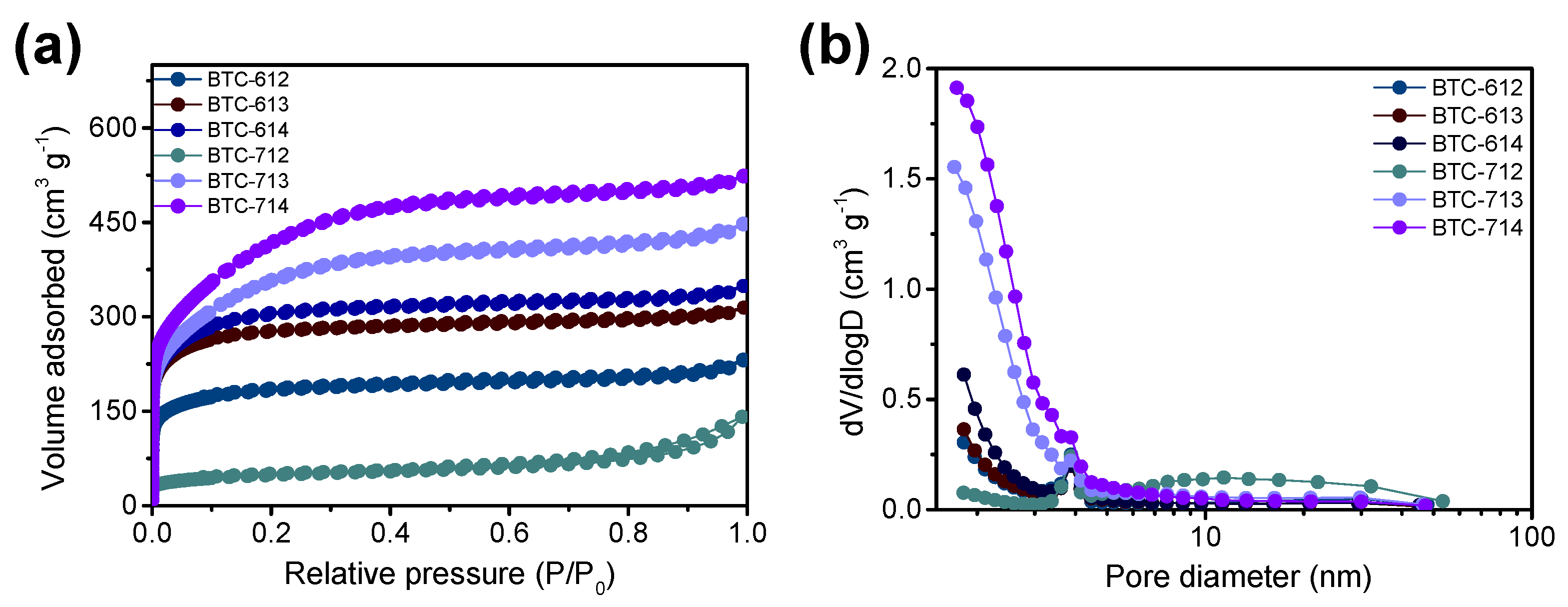
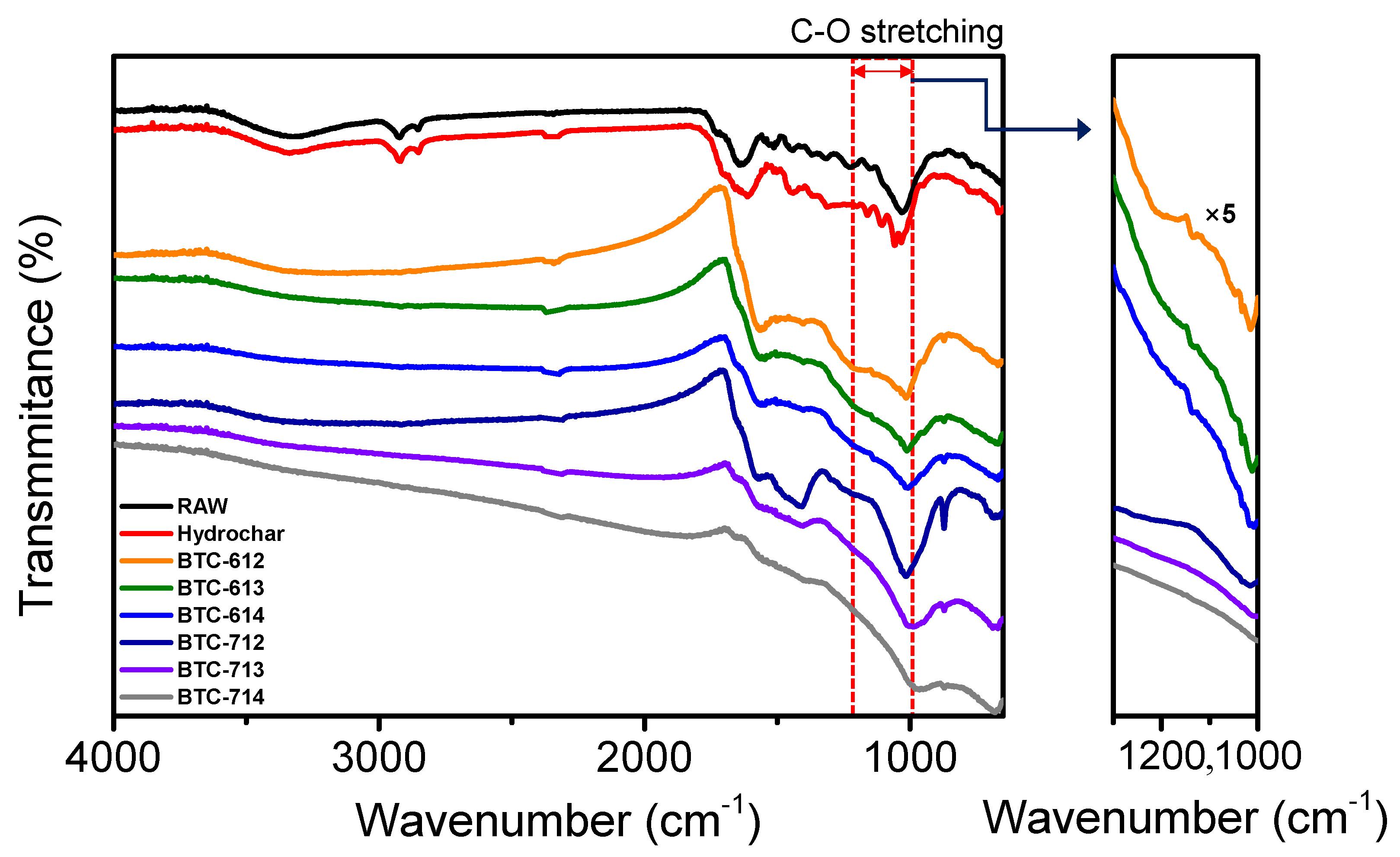
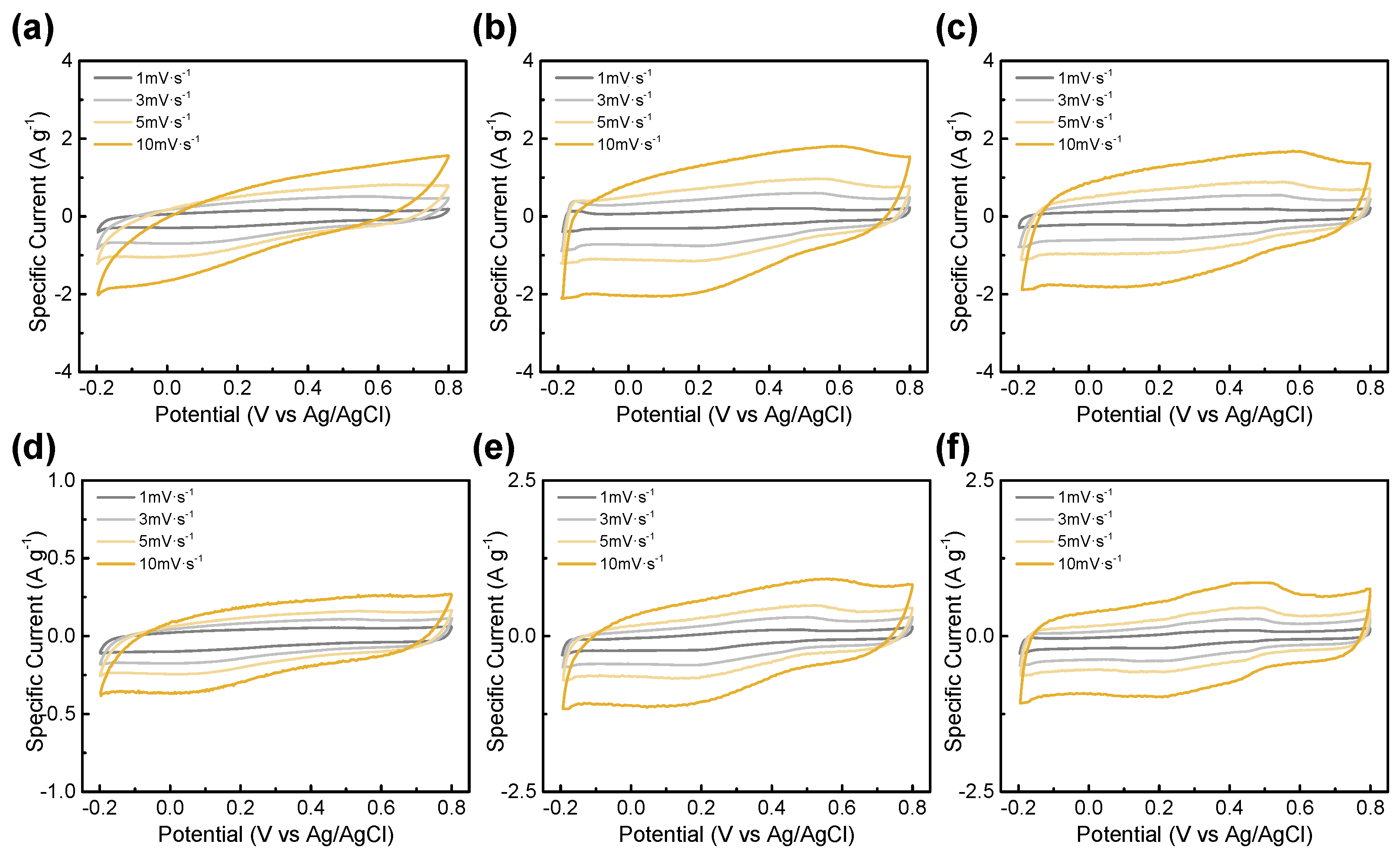
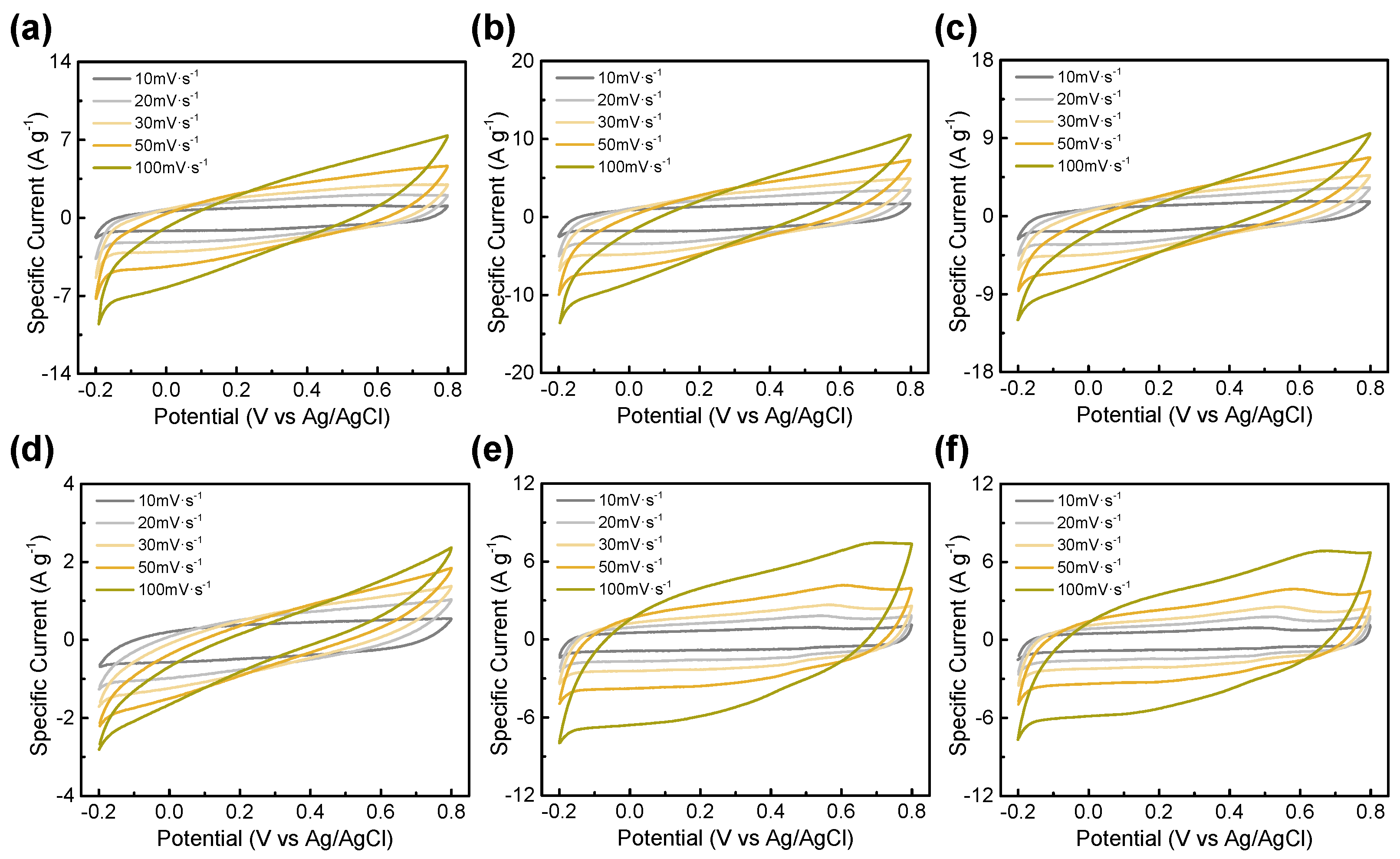
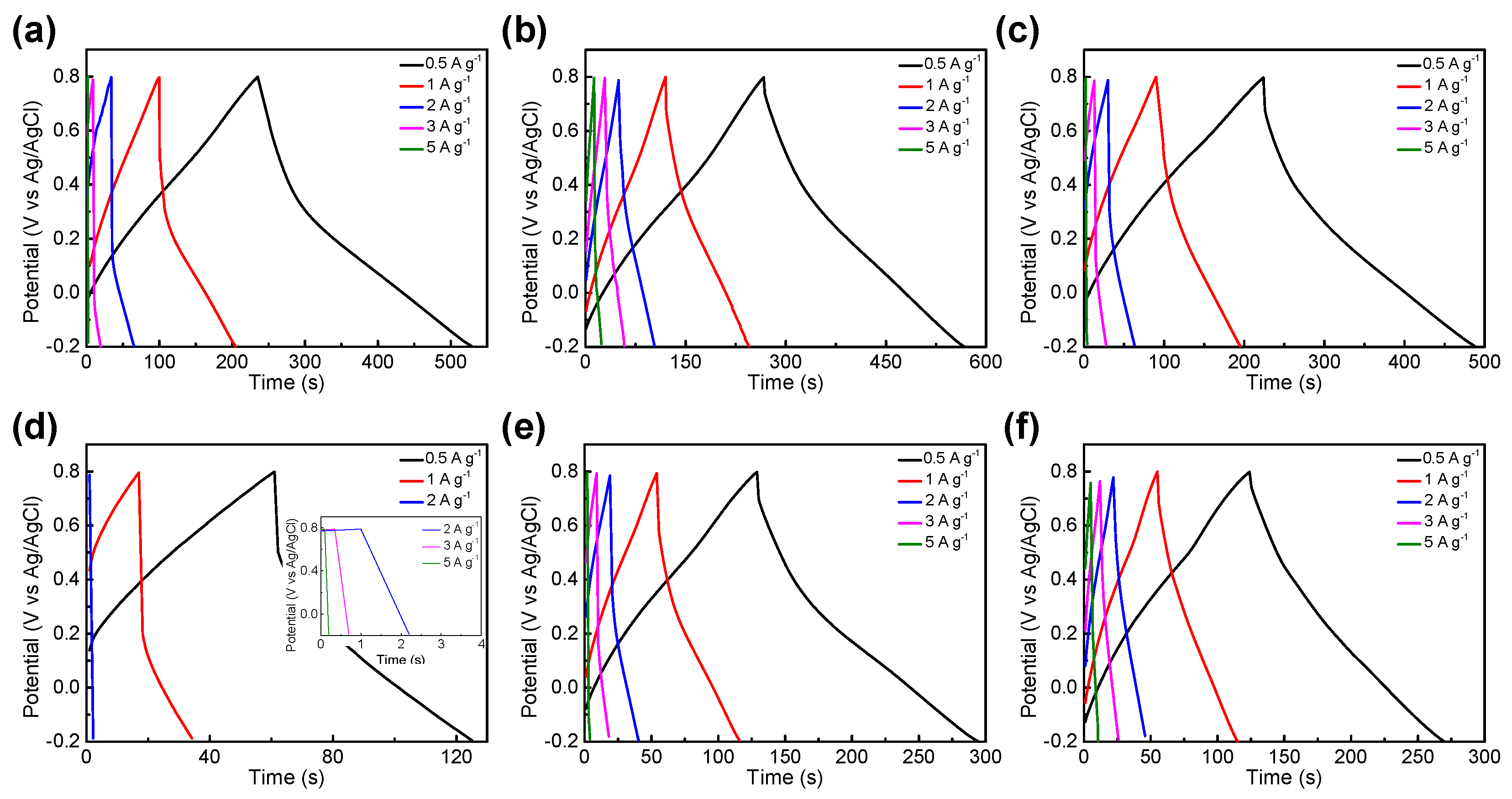
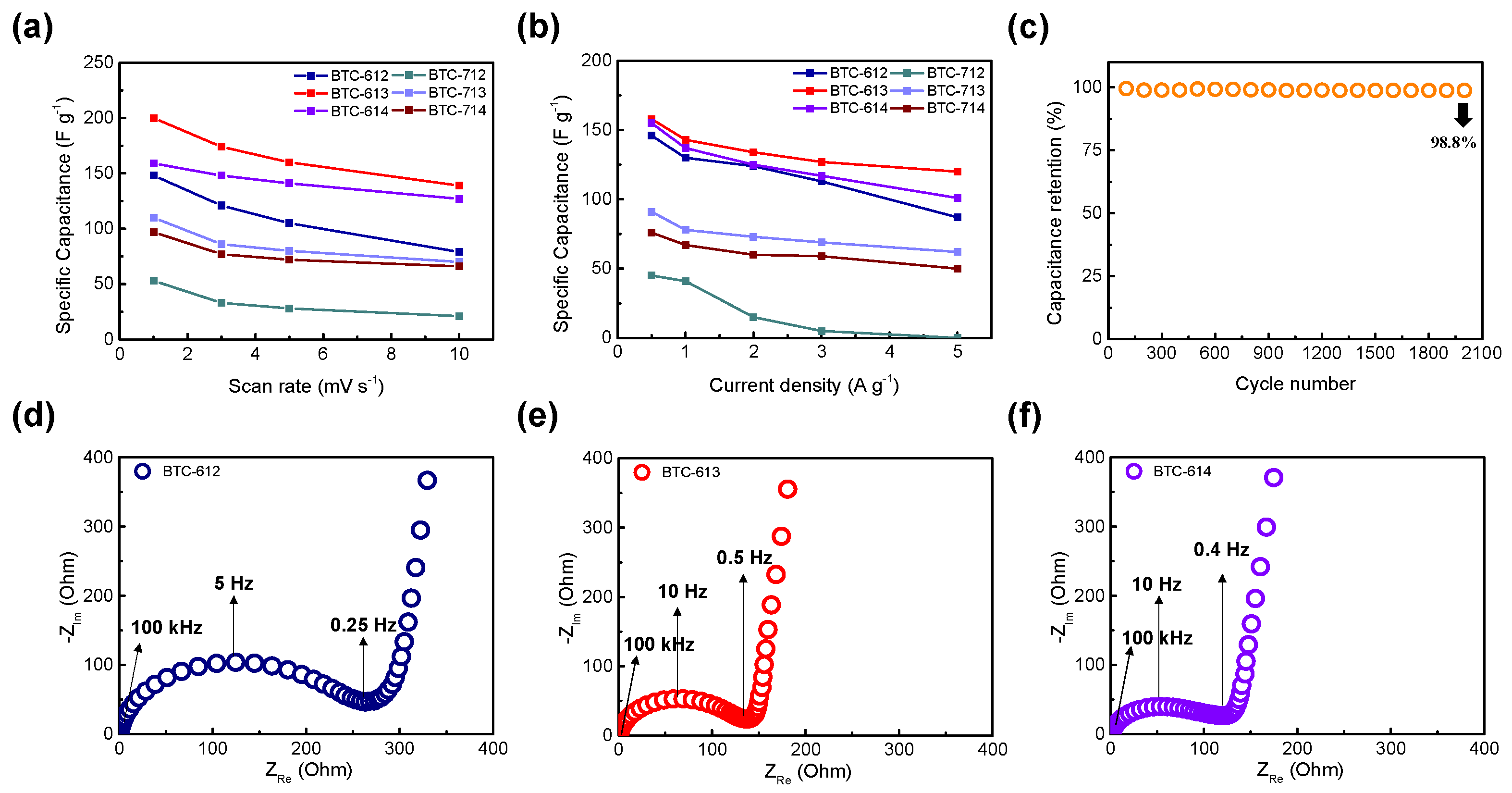
| BTC-XY | BET Surface Area [m2∙g−1] | Total Pore Volume [cm3∙g−1] | Micropore Volume [cm3∙g−1] |
|---|---|---|---|
| BTC-612 | 693 | 0.3404 | 0.2783 |
| BTC-613 | 1062 | 0.4755 | 0.4244 |
| BTC-614 | 1137 | 0.5251 | 0.4641 |
| BTC-712 | 174 | 0.1909 | 0.0516 |
| BTC-713 | 1227 | 0.6754 | 0.5539 |
| BTC-714 | 1398 | 0.7967 | 0.6893 |
| BTC-XY | ESR [Ω] | Specific Capacitance [F·g−1] | Energy Density [Wh·kg−1] | Power Density [W·kg−1] |
|---|---|---|---|---|
| BTC-612 | 0.166 | 146 | 20.3 | 300 |
| BTC-613 | 0.060 | 159 | 22.1 | 266 |
| BTC-614 | 0.122 | 156 | 21.7 | 286 |
| BTC-712 | 0.297 | 45 | 6.27 | 358 |
| BTC-713 | 0.102 | 91 | 12.7 | 279 |
| BTC-714 | 0.056 | 76 | 10.6 | 265 |
| Materials | Specific Capacitance [F·g−1] | Measurement Condition | Reference |
|---|---|---|---|
| Black tea | 200 | 1 mV·s−1/1 M H2SO4 | This paper |
| Coffee | 51 | 0.3 A·g−1/1 M Li2SO4 | [38] |
| Green tea | 135 | 0.5 A·g−1/1 M H2SO4 | [39] |
| Tea leave | 148 | 0.5 A·g−1/3 M LiOH | [21] |
| Tea waste | 220 | 0.1 mV·s−1/1 M Na2SO4 | [20] |
| Tea (Mao feng) | 275 | 1 A·g−1/2 M KOH | [22] |
| Cotton | 118 | 1 A·g−1/1 M TEABF4/AN | [40] |
| Coconut | 91 | 0.2 A·g−1/1 M TEMABF4/PC | [41] |
| Eggshell | 55 | 0.15 A·g−1/2 M NaOH | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, H.; Kim, J.; Nam, I.; Bae, S. Recycling Black Tea Waste Biomass as Activated Porous Carbon for Long Life Cycle Supercapacitor Electrodes. Materials 2021, 14, 6592. https://doi.org/10.3390/ma14216592
Eom H, Kim J, Nam I, Bae S. Recycling Black Tea Waste Biomass as Activated Porous Carbon for Long Life Cycle Supercapacitor Electrodes. Materials. 2021; 14(21):6592. https://doi.org/10.3390/ma14216592
Chicago/Turabian StyleEom, Hojong, Jooyoung Kim, Inho Nam, and Sunyoung Bae. 2021. "Recycling Black Tea Waste Biomass as Activated Porous Carbon for Long Life Cycle Supercapacitor Electrodes" Materials 14, no. 21: 6592. https://doi.org/10.3390/ma14216592
APA StyleEom, H., Kim, J., Nam, I., & Bae, S. (2021). Recycling Black Tea Waste Biomass as Activated Porous Carbon for Long Life Cycle Supercapacitor Electrodes. Materials, 14(21), 6592. https://doi.org/10.3390/ma14216592







