Rheological and Technological Aspects in Designing the Properties of Shear Thickening Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Raw Materials
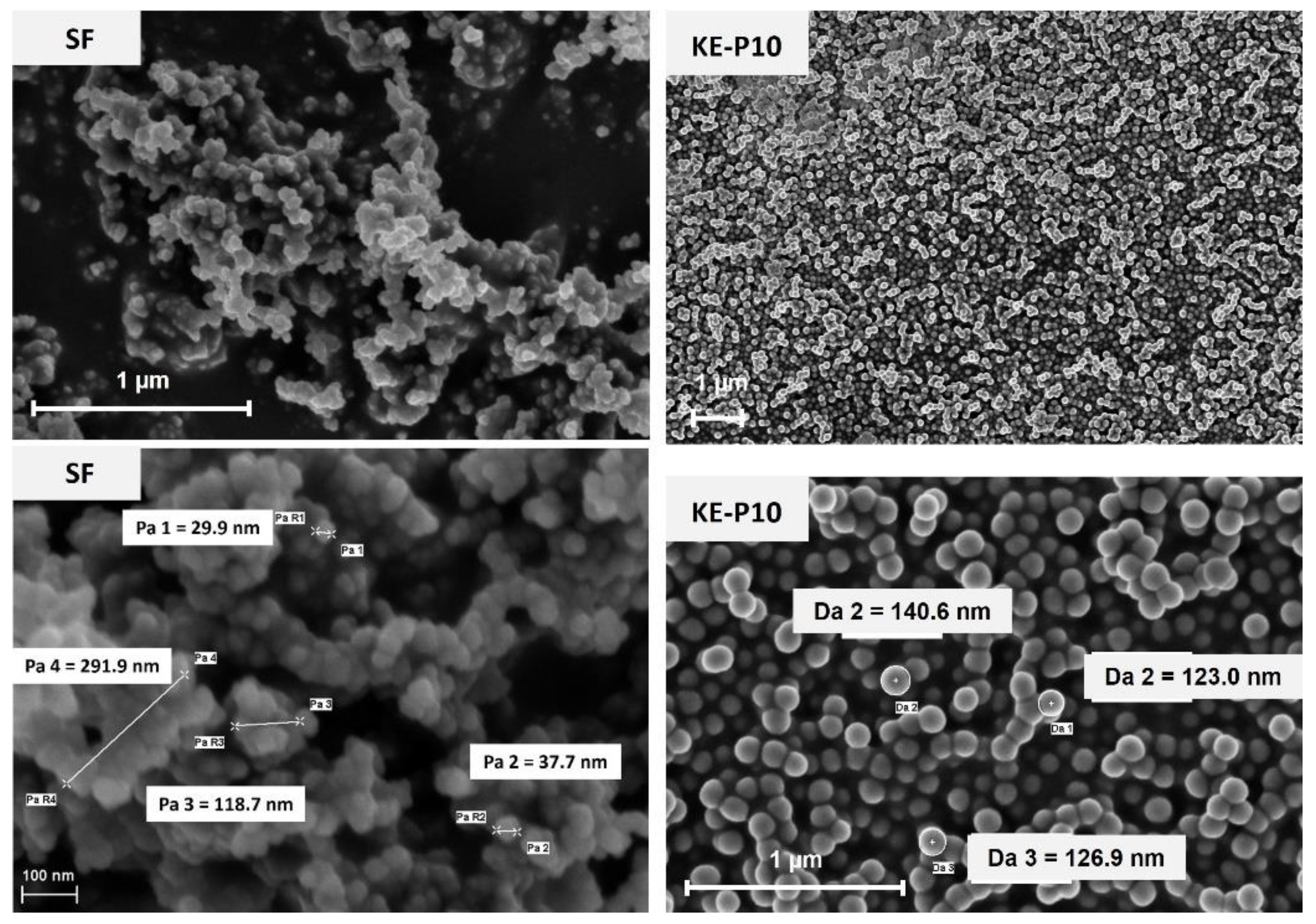
2.2.1. Characterization of the Solid Phase
2.2.2. Characterization of the Continuous Phase
2.3. Preparation of Shear Thickening Fluids
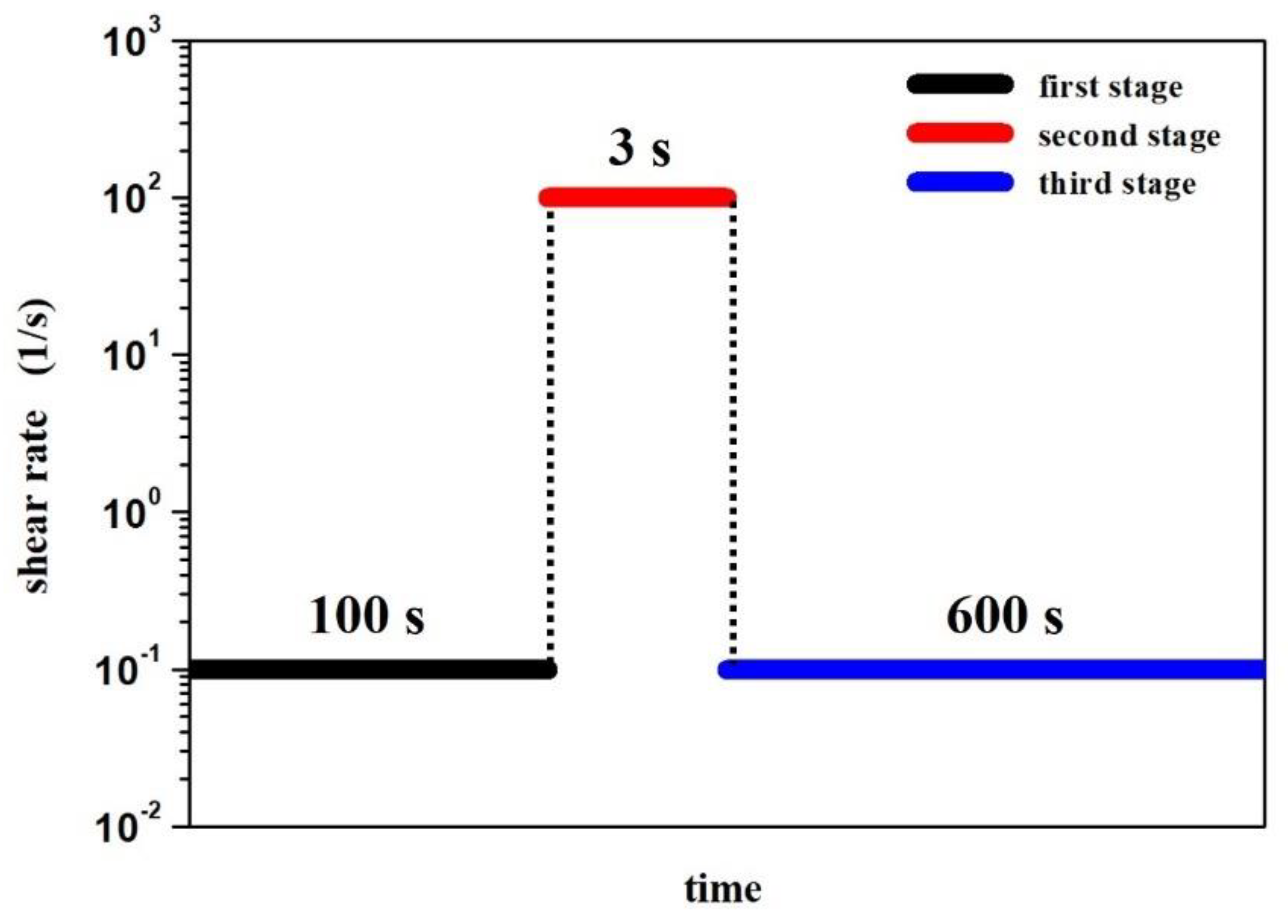
2.4. Characterization of the Rheological Properties of STFs
- A simple shear of the sample with a shear rate of 10−1 s−1 for 100 s;
- A rapid increase in the shear rate to 100 s−1 for 3 s;
- A simple shear of the sample with a shear rate of 10−1 s−1 for 10 min.
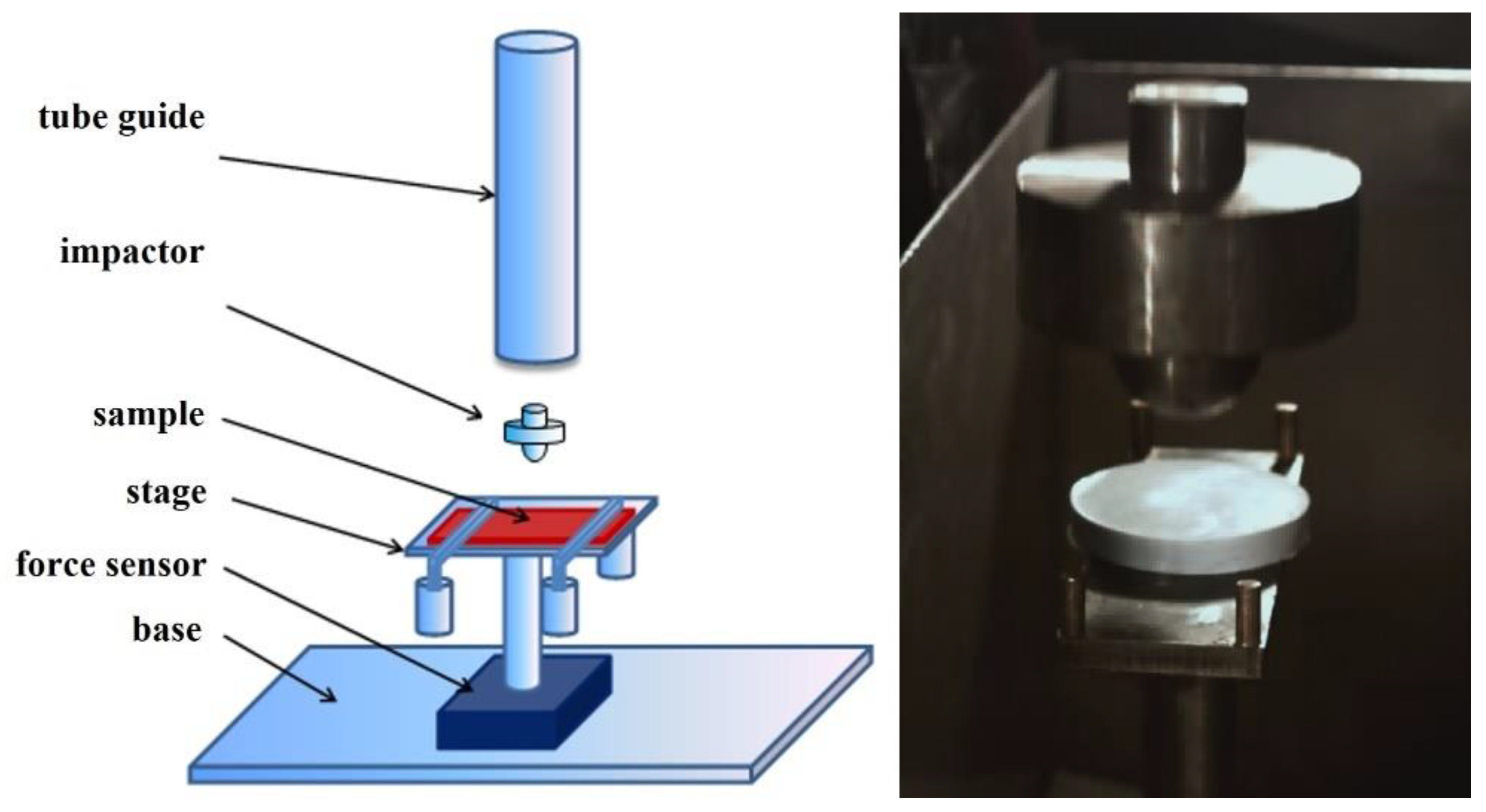
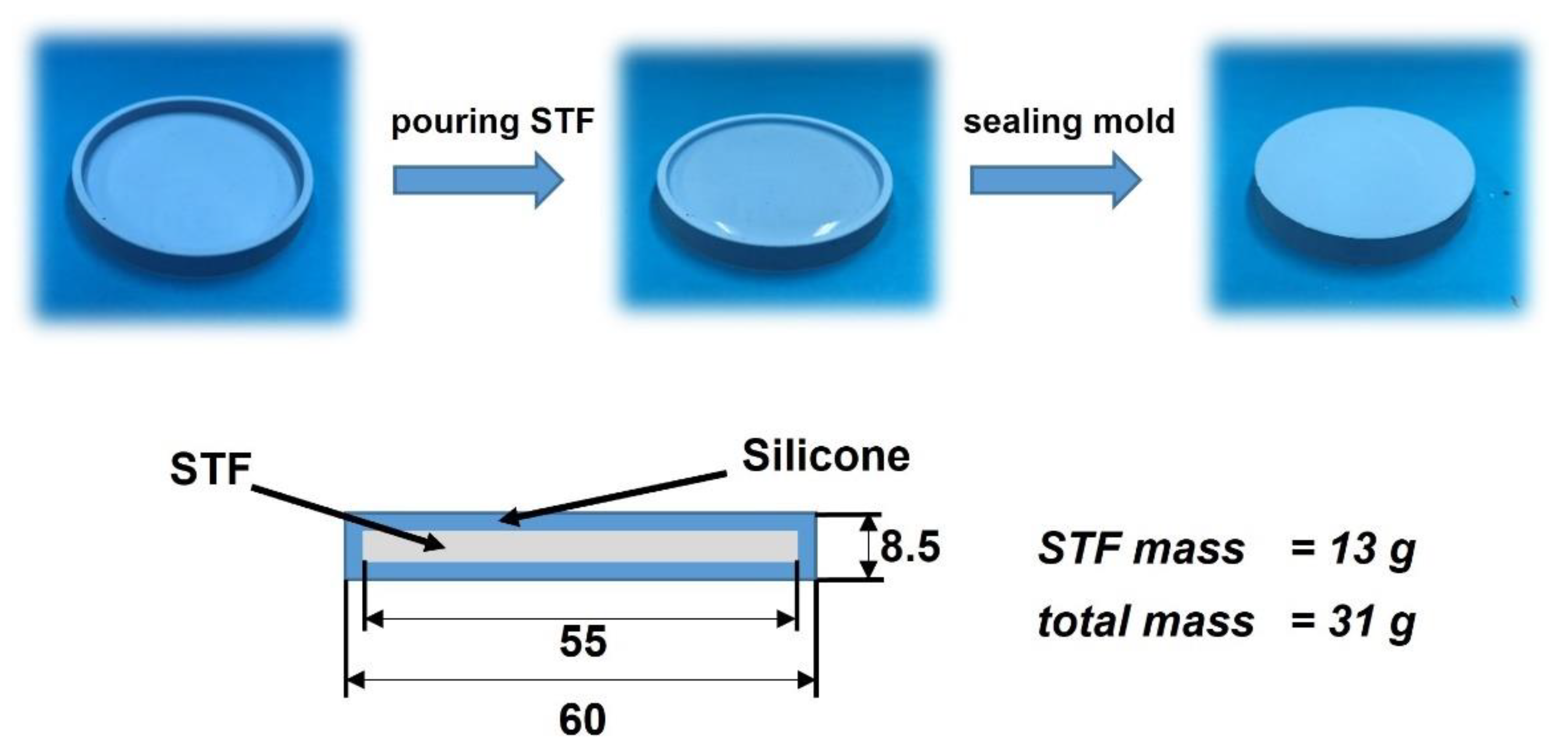
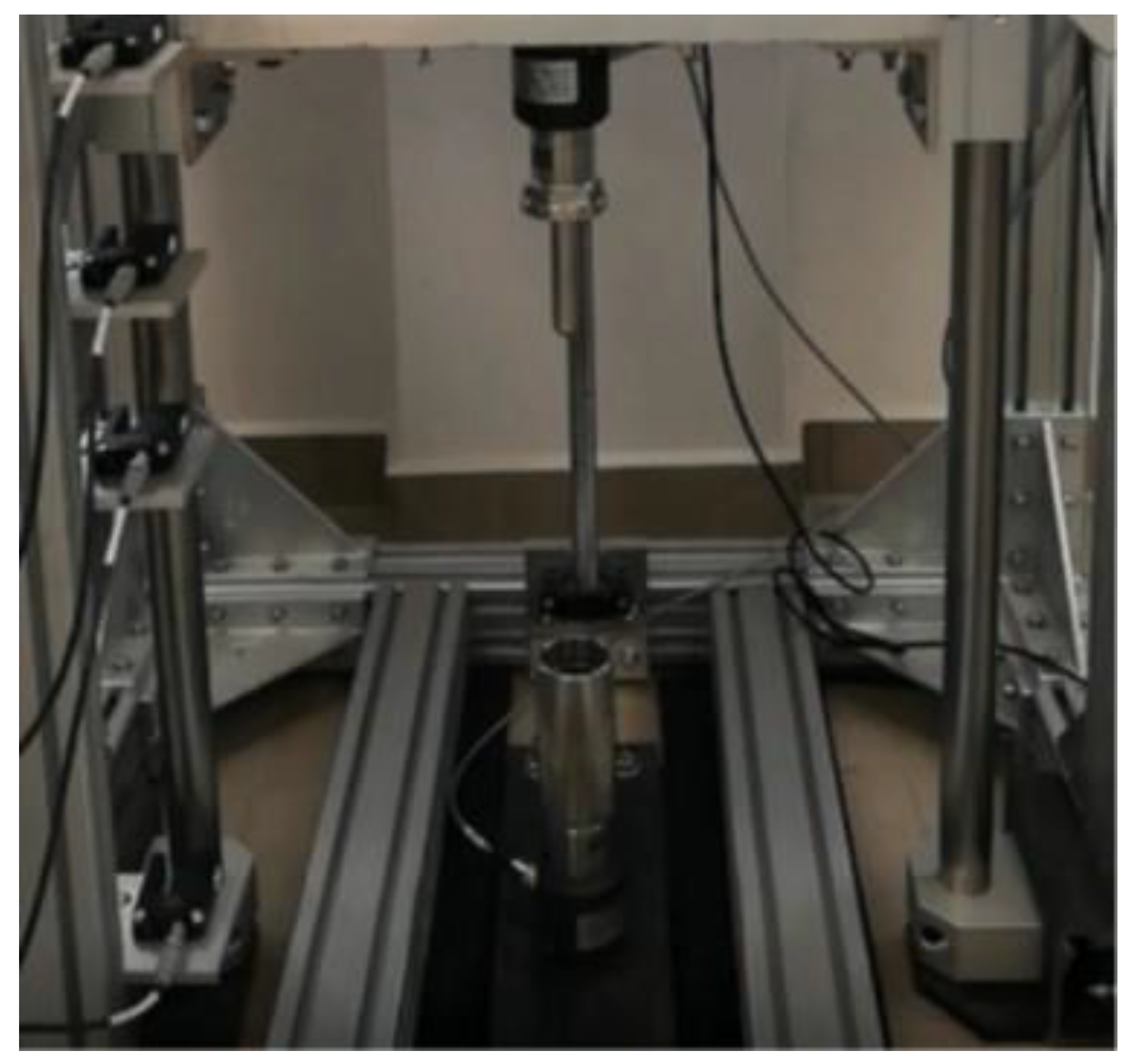
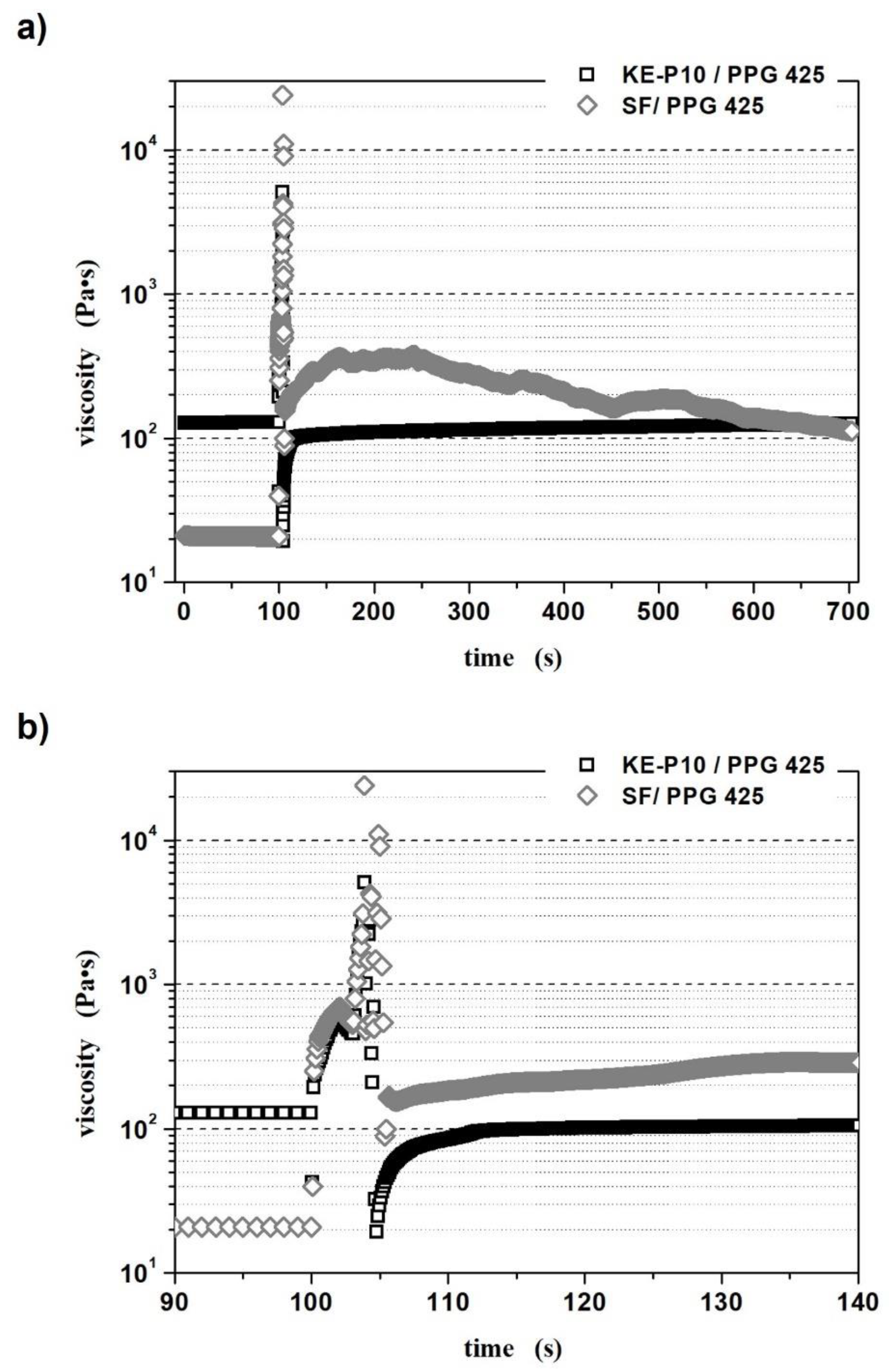
2.5. Kinetic Energy Dissipation Tests
2.5.1. Kinetic Energy Dissipation Tests with Silicone–STF Samples
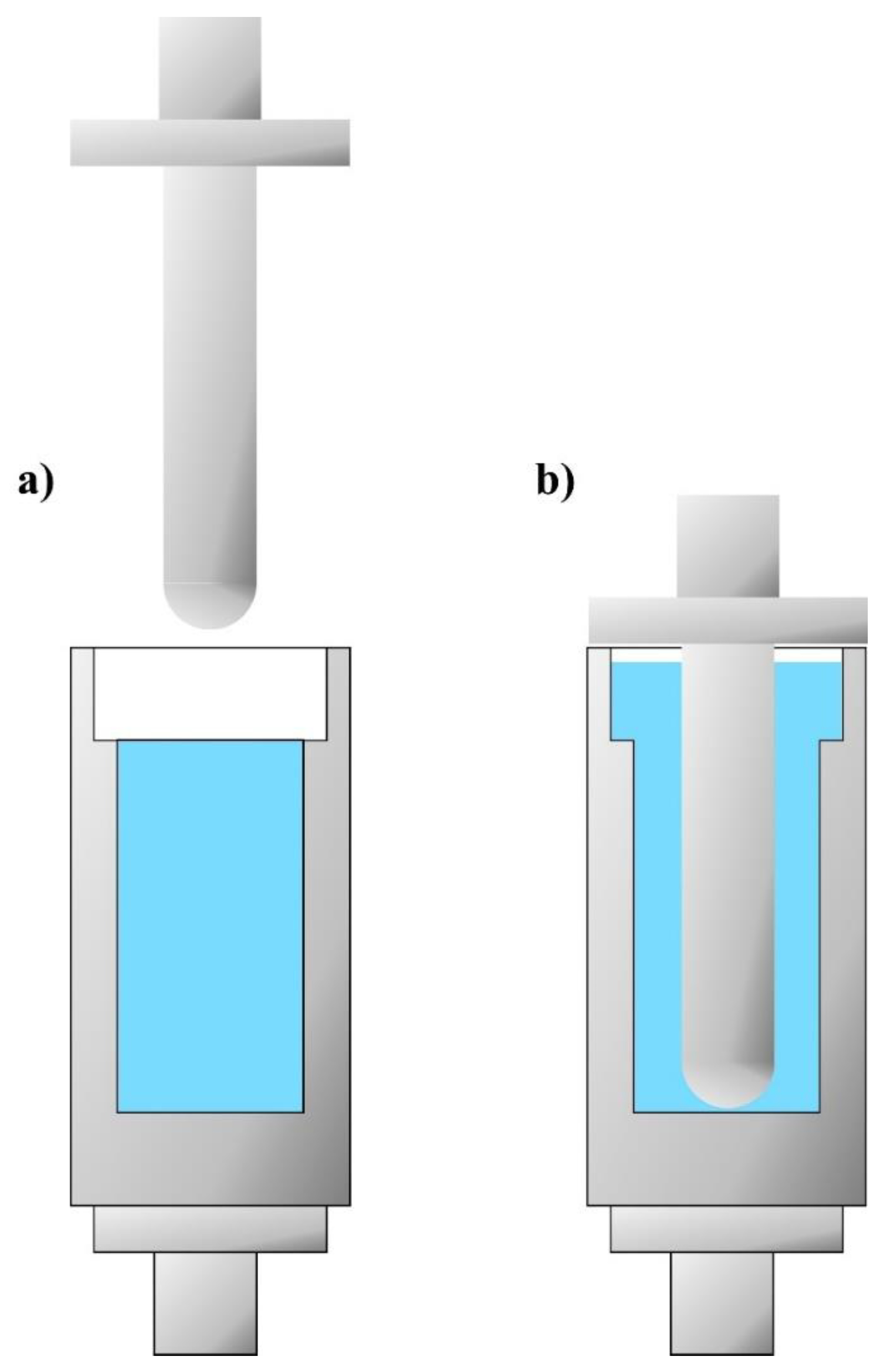
2.5.2. Kinetic Energy Dissipation Tests in the Rod–Cup Measuring System
- A bottom-threaded steel cup was filled with a specific shear thickening fluid. The fluid column height equaled 80 mm and its mass was approximately 145 g;
- The cup filled with STF was screwed into the force sensor attached to the bottom plate;
- The appropriate height of the traverse on the guideway was set;
- The traverse, with the impactor, was dropped onto the STF;
- Sample (fluid) space: h = 80 mm and Ø = 40 mm;
- Space above the sample (fluid): h = 20 mm and Ø = 50 mm.
3. Results and Discussion
3.1. Characteristics of the Materials Used
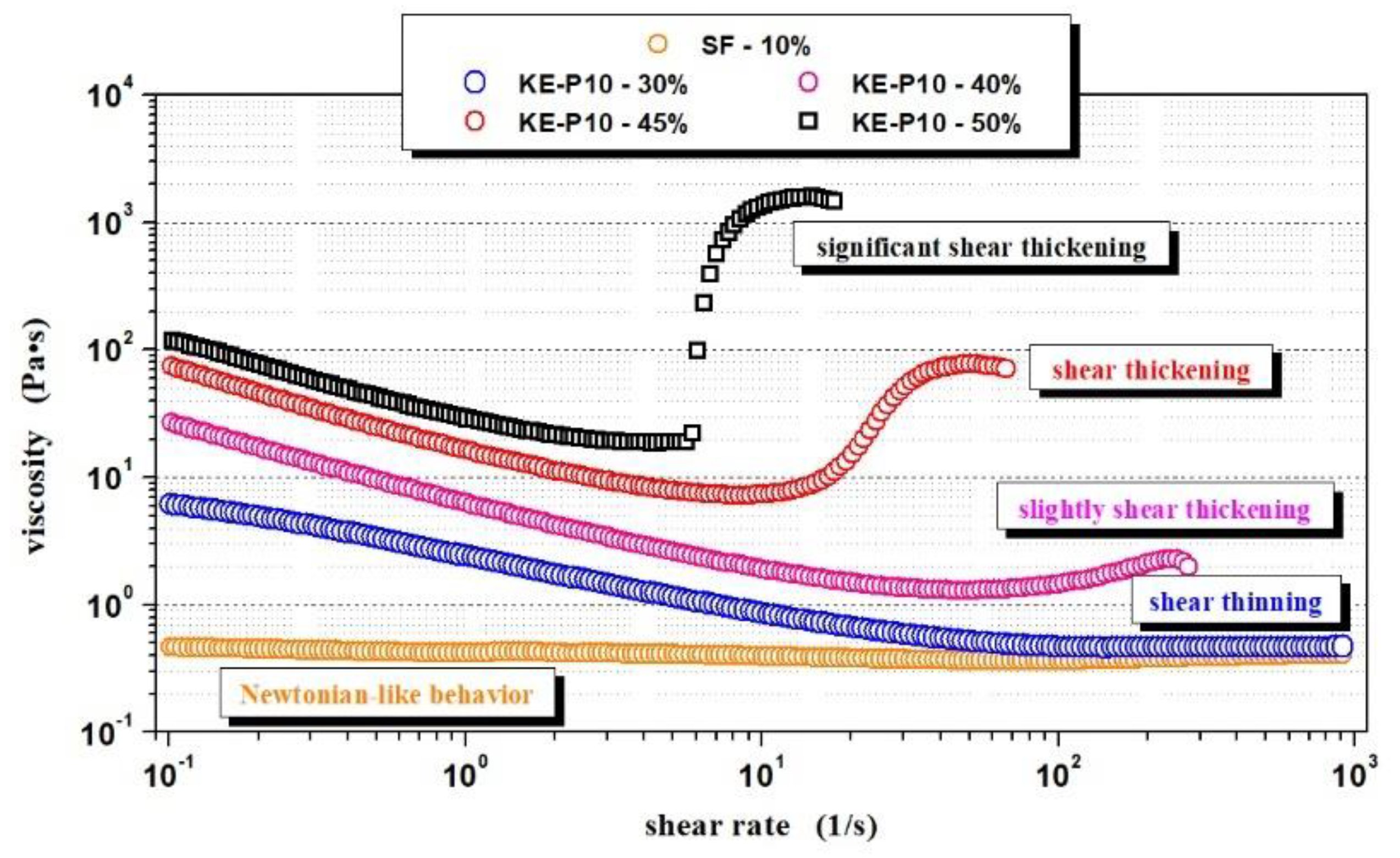
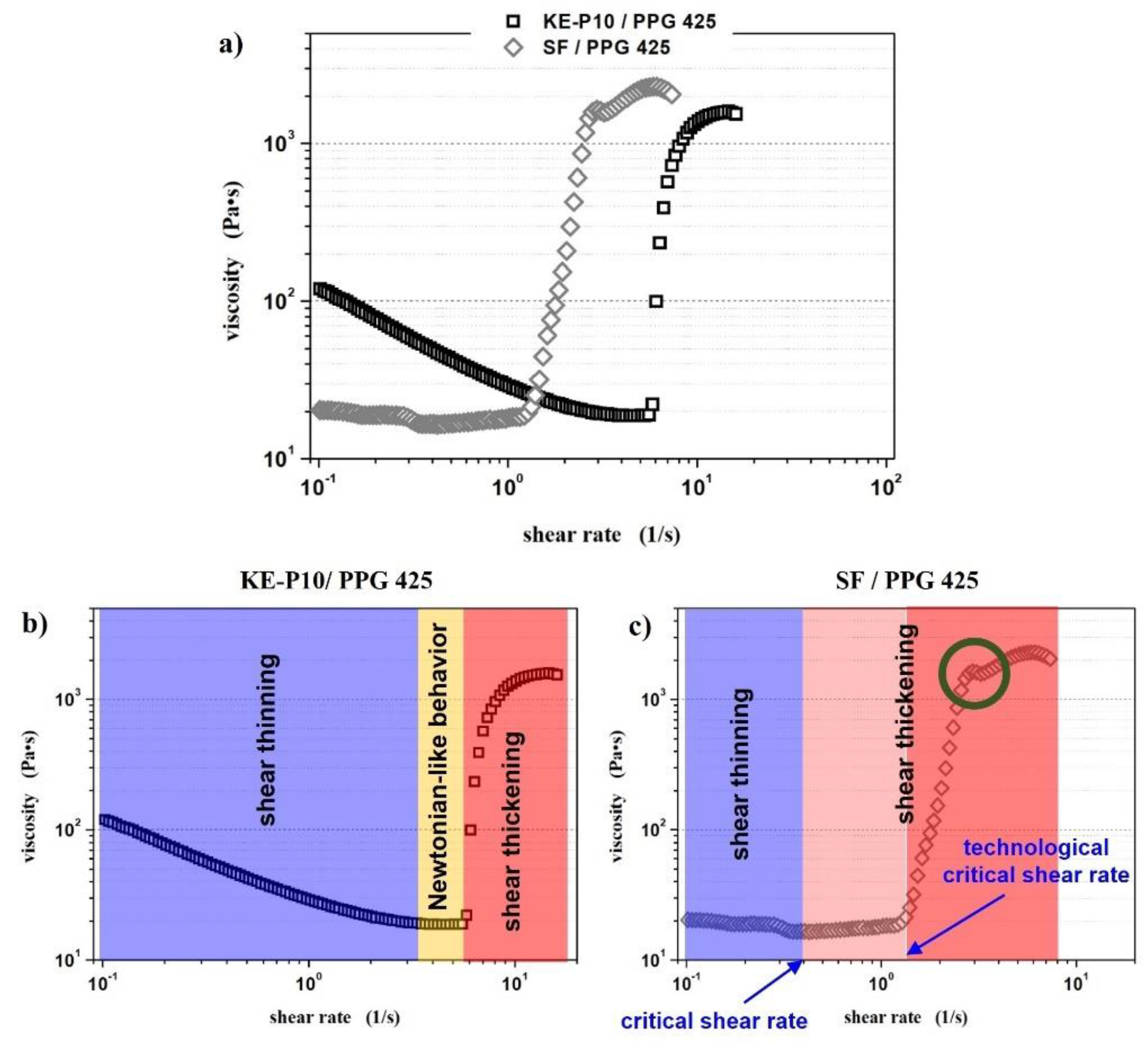
3.2. Effect of Silica Morphology on the Rheological Properties of STFs
- Newtonian-like behavior—the fluid displayed a relatively constant viscosity at a shear rate range of 10−1 to 103 s−1;
- Shear thinning—between a shear rate of 10−1 and 103 s−1, the viscosity of the fluid decreased with increasing shear rate;
- Slight shear thickening—between a shear rate of 10−1 and 103 s−1, a small increase in viscosity was observed; however, the increase in viscosity was no greater than 50 Pa∙s;
- Shear thickening—between a shear rate of 10−1 and 103 s−1, an increase in viscosity was observed and was greater than 500 Pa∙s;
- Significant shear thickening—between a shear rate of 10−1 and 103 s−1, a sudden and sharp increase in fluid viscosity was observed and was greater than 500 Pa∙s;
- Not possible to obtain—the solid phase concentration was too high and it was not possible to prepare such a sample;
- Homogenous fluid—it was possible to obtain the homogenous dispersion of silica in PPG; however, the viscosity was too high for reliable measurements of such fluid with a rotational rheometer;
- X—the fluid with this composition was not prepared.
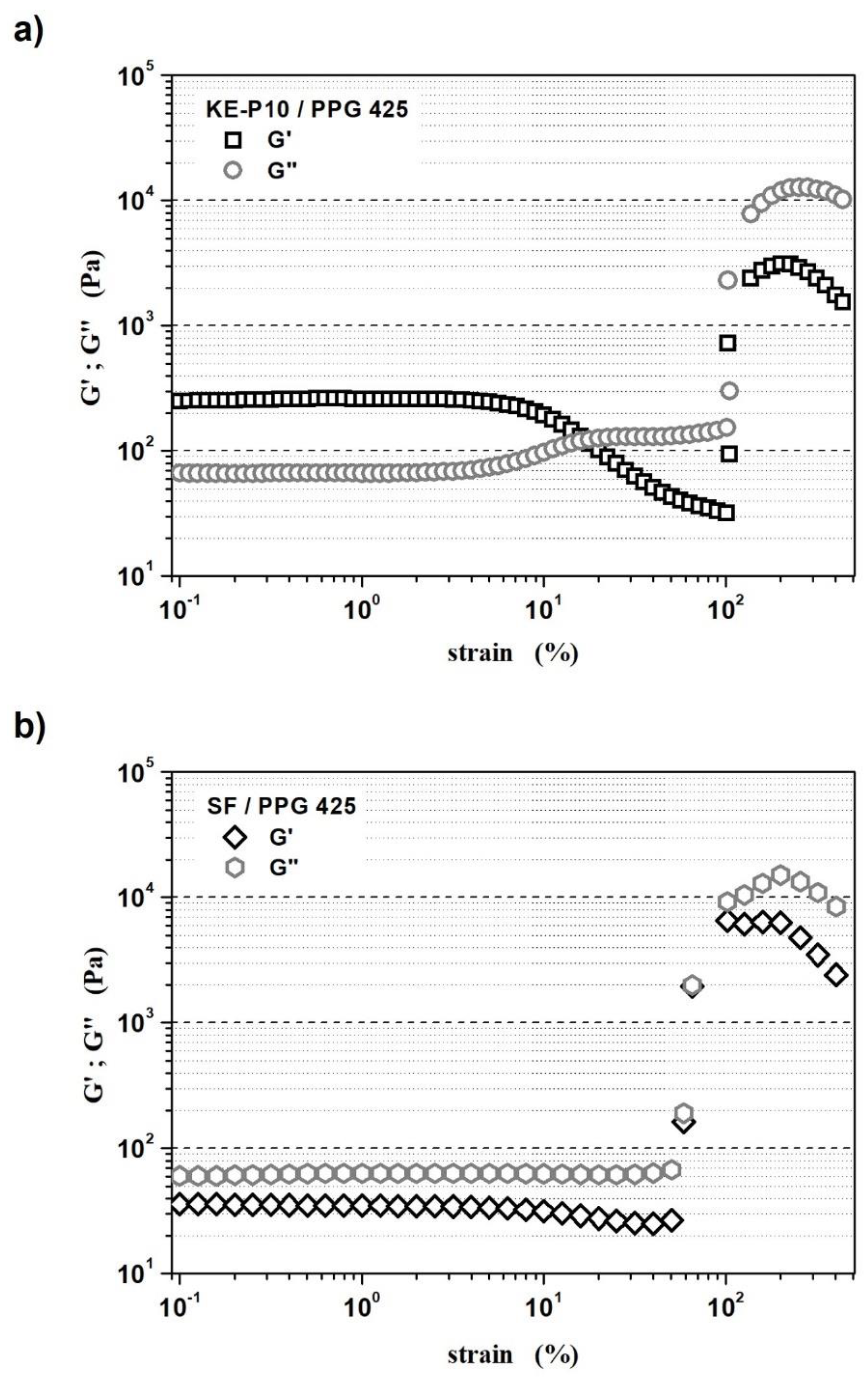
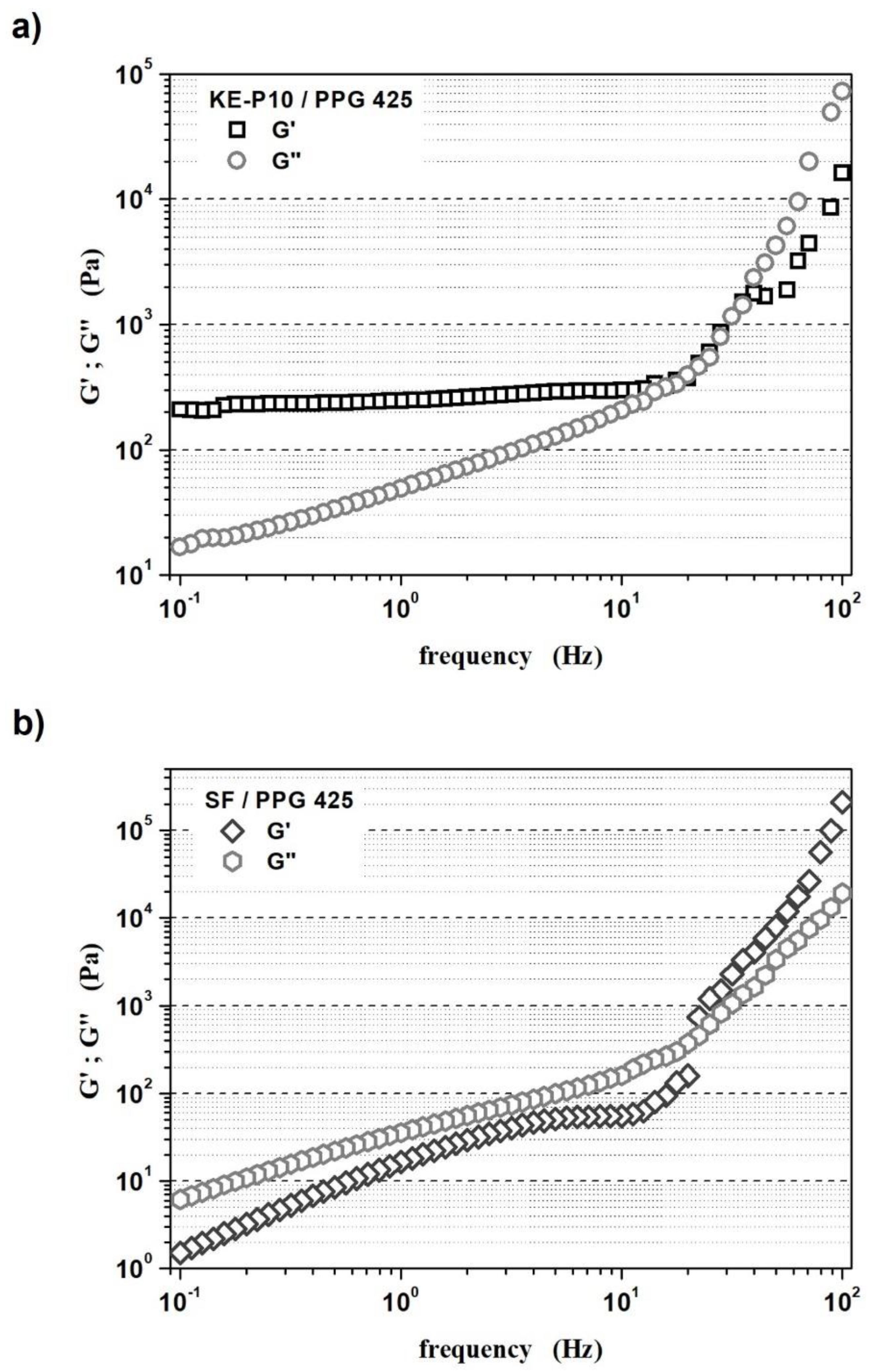
- The LVER of the STF with KE-P10 was from 0.1 to 3.2% of oscillation strain;
- The LVER of the STF with SF was from 0.1 to 4% of oscillation strain.
3.3. Effect of Silica Morphology on the Protective Abilities of STFs
4. Conclusions
- The rheological properties of silica–glycol systems are strongly dependent on the morphology of the particles used as the solid phase:
- -
- Nanosized, but highly agglomerated, silica SF with irregular shapes of particles promotes the earlier occurrence of the shear thickening (dilatant) effect, with 12.5 vol.% of silica being enough to observe the thickening effect. Furthermore, it is hardly possible to prepare an STF with more than 22 vol.% of this silica;
- -
- In order to create a shear thickening effect when spherical and well-dispersed KE-P10 silica is used, it is necessary to add three times more silica (40 vol.%). The maximum concentration of this silica in the fluid may be 53.75 vol%. Due to the high volume of the solid phase, the shear thickening effect is three times greater than in the case of the STF with SF silica and is equal to 29 kPa∙s;
- -
- The application of the irregular SF silica favors higher STR coefficients;
- -
- Fluids with SF silica show a different profile of viscosity curves than fluids with KE-P10;
- -
- The morphology of silica particles influences the reorganization of the internal structure after the shear;
- -
- Spherical silica facilitates reorganization and the fluid more quickly returns to its initial state;
- We propose the distinguishment of a new term—technological critical shear rate . From a technological point of view, should be taken into account, because only after exceeding this value of shear rate does the viscosity of the system increase rapidly. When considering the practical use of shear thickening fluids, only a sudden and abrupt increase in viscosity ensures a proper response to the external stimulus applied;
- The high silica content in shear thickening fluids might enhance the formation of internal networks through hydrogen bonds that cause the domination of elastic properties over viscous properties (G′ > G″);
- The unique measurement system utilized during the kinetic energy dissipation tests allows one to measure the response of pure fluids (without their prior encapsulation or packaging), and thus obtain real and precise results;
- The results of research on the protective properties of the tested STFs show that it is not necessarily the fluids with a greater shear thickening effect as well as higher STR coefficient that can dissipate more energy and protect to a greater extent. This finding questions the legitimacy of obtaining STFs with the highest possible shear thickening effect in the context of their protective properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pampuch, R. An Introduction to Ceramics; Springer International Publishing: Cham, Switzerland, 2014; ISBN 978-3-319-36350-9. [Google Scholar]
- Lee, Y.S.; Wagner, N.J. Dynamic properties of shear thickening colloidal suspensions. Rheol. Acta 2003, 42, 199–208. [Google Scholar] [CrossRef]
- Maranzano, B.J.; Wagner, N.J. The effects of particle size on reversible shear thickening of concentrated colloidal dispersions. J. Chem. Phys. 2001, 114, 10514–10527. [Google Scholar] [CrossRef]
- Barnes, H.A. Shear-Thickening (“Dilatancy”) in Suspensions of Nonaggregating Solid Particles Dispersed in Newtonian Liquids. J. Rheol. 1989, 33, 329–366. [Google Scholar] [CrossRef]
- Soutrenon, M.; Michaud, V.; Manson, J.-A.E. Influence of Processing and Storage on the Shear Thickening Properties of Highly Concentrated Monodisperse Silica Particles in Polyethylene Glycol. Appl. Rheol. 2013, 23, 54865. [Google Scholar] [CrossRef]
- Gürgen, S.; Kuşhan, M.C.; Li, W. Shear thickening fluids in protective applications: A review. Prog. Polym. Sci. 2017, 75, 48–72. [Google Scholar] [CrossRef]
- Pacek, D.; Rutkowski, J. The composite structure for human body impact protection. Compos. Struct. 2021, 265, 113763. [Google Scholar] [CrossRef]
- Lee, Y.S.; Wetzel, E.D.; Wagner, N.J. The ballistic impact characteristics of Kevlar® woven fabrics impregnated with a colloidal shear thickening fluid. J. Mater. Sci. 2003, 38, 2825–2833. [Google Scholar] [CrossRef]
- Majumdar, A.; Butola, B.S.; Srivastava, A. Development of soft composite materials with improved impact resistance using Kevlar fabric and nano-silica based shear thickening fluid. Mater. Des. 2014, 54, 295–300. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.; Zhao, Z.; Liu, X.; Chen, W. The influences of rheological property on the impact performance of kevlar fabrics impregnated with SiO2/PEG shear thickening fluid. Thin-Walled Struct. 2020, 151, 106717. [Google Scholar] [CrossRef]
- Arora, S.; Majumdar, A.; Butola, B.S. Structure induced effectiveness of shear thickening fluid for modulating impact resistance of UHMWPE fabrics. Compos. Struct. 2019, 210, 41–48. [Google Scholar] [CrossRef]
- Asija, N.; Chouhan, H.; Gebremeskel, S.A.; Singh, R.K.; Bhatnagar, N. High strain rate behavior of STF-treated UHMWPE composites. Int. J. Impact Eng. 2017, 110, 359–364. [Google Scholar] [CrossRef]
- Pacek, D.; Gieleta, R. The fluid-based structure for human body impact protection. J. Phys. Conf. Ser. 2020, 1507, 32016. [Google Scholar] [CrossRef]
- Sun, L.; Wei, M.; Zhu, J. Low velocity impact performance of fiber-reinforced polymer impregnated with shear thickening fluid. Polym. Test. 2021, 96, 107095. [Google Scholar] [CrossRef]
- Taş, H.; Soykok, I.F. Investigation of the Low Velocity Impact Behaviour of Shear Thickening Fluid Impregnated Kevlar, Hybrid (Kevlar/Carbon) and Carbon Fabrics. Fibers Polym. 2021, 22, 2626–2634. [Google Scholar] [CrossRef]
- Bajya, M.; Majumdar, A.; Butola, B.S.; Verma, S.K.; Bhattacharjee, D. Design strategy for optimising weight and ballistic performance of soft body armour reinforced with shear thickening fluid. Compos. Part B Eng. 2020, 183, 107721. [Google Scholar] [CrossRef]
- Zarei, M.; Aalaie, J. Application of shear thickening fluids in material development. J. Mater. Res. Technol. 2020, 9, 10411–10433. [Google Scholar] [CrossRef]
- Nakonieczna, P.; Wierzbicki, Ł.; Wróblewski, R.; Płociński, T.; Leonowicz, M. The influence of carbon nanotube addition on the properties of shear thickening fluid. Bull. Mater. Sci. 2019, 42, 162. [Google Scholar] [CrossRef]
- Szafran, M.; Antosik, A.; Głuszek, M.; Falkowski, P.; Bobryk, E.; Żurowski, R.; Rokicki, G.; Tryznowski, M.; Kaczorowski, M.; Leonowicz, M.; et al. Nagolennik Piłkarski i Sposób Wytwarzania Nagolennika. Polish Patent PL231755B1, 11 September 2017. [Google Scholar]
- Gong, X.; Chen, Q.; Liu, M.; Cao, S.; Xuan, S.; Jiang, W. Squeeze flow behavior of shear thickening fluid under constant volume. Smart Mater. Struct. 2017, 26, 65017. [Google Scholar] [CrossRef]
- Holt, S.E.; Perez, M.P. Impact Resistant, Torsion-Reducing Protective Athletic Gear Using Shear Thickening Fluid. U.S. Patent US8679047B2, 24 November 2015. [Google Scholar]
- Williams, T.H.; Day, J.; Pickard, S. Surgical and Medical Garments and Materials Incorporating Shear Thickening Fluids. U.S. Patent US0255023A1, 15 October 2009. [Google Scholar]
- Allen, S.D. Body Limb Movement Limiter. U.S. Patent US7402147B1, 7 September 2010. [Google Scholar]
- Wei, M.; Hu, G.; Li, L.; Liu, H. Development and theoretically evaluation of an STF–SF isolator for seismic protection of structures. Meccanica 2018, 53, 841–856. [Google Scholar] [CrossRef]
- Tian, T.; Nakano, M. Design and testing of a rotational brake with shear thickening fluids. Smart Mater. Struct. 2017, 26, 35038. [Google Scholar] [CrossRef]
- Zhao, Q.; He, Y.; Yao, H.; Wen, B. Dynamic performance and mechanical model analysis of a shear thickening fluid damper. Smart Mater. Struct. 2018, 27, 75021. [Google Scholar] [CrossRef]
- Yang, J.; Sun, S.; Guo, N.; Ning, D.; Nakano, M.; Li, Z.; Du, H.; Zhang, S.W.; Li, W.H. Development of a smart rubber joint for train using shear thickening fluids. Smart Mater. Struct. 2020, 29, 55036. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, Y.; Zou, J.; Huang, C.; Wu, X.; Liu, Z.; Yang, Z. Impact and usage of the shear thickening fluid (STF) material in damping vibration of bolted flange joints. Smart Mater. Struct. 2019, 28, 95005. [Google Scholar] [CrossRef]
- Cwalina, C.D.; Dombrowski, R.D.; McCutcheon, C.J.; Christiansen, E.L.; Wagner, N.J. MMOD Puncture Resistance of EVA Suits with Shear Thickening Fluid (STF)–Armortm Absorber Layers. Procedia Eng. 2015, 103, 97–104. [Google Scholar] [CrossRef]
- Cohen, D. Shear Thickening Fluid Reinforced Fabrics for Use with and Expandable Spacecraft. U.S. Patent US0296435A1, 4 December 2008. [Google Scholar]
- Ye, Y.; Xiao, H.; Reaves, K.; McCulloch, B.; Mike, J.F.; Lutkenhaus, J.L. Effect of Nanorod Aspect Ratio on Shear Thickening Electrolytes for Safety-Enhanced Batteries. ACS Appl. Nano Mater. 2018, 1, 2774–2784. [Google Scholar] [CrossRef]
- Prabhu, T.A.; Singh, A. Effect of carrier fluid and particle size distribution on the rheology of shear thickening suspensions. Rheol. Acta 2021, 60, 107–118. [Google Scholar] [CrossRef]
- Ghosh, A.; Majumdar, A.; Butola, B.S. Role of surface chemistry of fibres additives on rheological behavior of ceramic particle based Shear Thickening Fluids. Ceram. Int. 2018, 44, 21514–21524. [Google Scholar] [CrossRef]
- Haris, A.; Lee, H.P.; Tay, T.E.; Tan, V.B.C. Shear thickening fluid impregnated ballistic fabric composites for shock wave mitigation. Int. J. Impact Eng. 2015, 80, 143–151. [Google Scholar] [CrossRef]
- Ozturk, D.; Morgan, M.L.; Sandnes, B. Flow-to-fracture transition and pattern formation in a discontinuous shear thickening fluid. Commun. Phys. 2020, 3, 119. [Google Scholar] [CrossRef]
- Wetzel, E.D.; Lee, Y.S.; Egres, R.G.; Kirkwood, K.M.; Kirkwood, J.E.; Wagner, N.J. The Effect of Rheological Parameters on the Ballistic Properties of Shear Thickening Fluid (STF)-Kevlar Composites. AIP Conf. Proc. 2004, 712, 288–293. [Google Scholar] [CrossRef]
- Chadwick, M.D.; Goodwin, J.W.; Vincent, B.; Lawson, E.J.; Mills, P.D.A. Rheological behaviour of titanium dioxide (uncoated anatase) in ethylene glycol. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 196, 235–245. [Google Scholar] [CrossRef]
- Cao, C.; Luo, Y.; Xiao, M. Preparation and characterization of imidazolyl ionic liquid-based shear thickening dispersion system. J. Appl. Polym. Sci. 2021, 138, 49719. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, G.; Shi, X. Study of a shear thickening fluid: The suspensions of monodisperse polystyrene microspheres in polyethylene glycol. J. Dispers. Sci. Technol. 2017, 38, 935–942. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Kurkin, A.; Chen, Q.; Gong, X.; Zhang, Z.; Yang, E.-H.; Yang, J. Mechanical response of shear thickening fluid filled composite subjected to different strain rates. Int. J. Mech. Sci. 2021, 196, 106304. [Google Scholar] [CrossRef]
- Antosik, A.; Gluszek, M.; Zurowski, R.; Szafran, M. Influence of carrier fluid on the electrokinetic and rheological properties of shear thickening fluids. Ceram. Int. 2017, 43, 12293–12301. [Google Scholar] [CrossRef]
- Tian, T.; Peng, G.; Li, W.; Ding, J.; Nakano, M. Experimental and modelling study of the effect of temperature on shear thickening fluids. Korea Aust. Rheol. J. 2015, 27, 17–24. [Google Scholar] [CrossRef]
- Warren, J.; Cole, M.; Offenberger, S.; Kota, K.R.; Lacy, T.E.; Toghiani, H.; Burchell, M.; Kundu, S.; Pittman, C.U. Hypervelocity Impacts on Honeycomb Core Sandwich Panels Filled with Shear Thickening Fluid. Int. J. Impact Eng. 2021, 150, 103803. [Google Scholar] [CrossRef]
- Singh, M.; Verma, S.K.; Biswas, I.; Mehta, R. Effect of molecular weight of polyethylene glycol on the rheological properties of fumed silica-polyethylene glycol shear thickening fluid. Mater. Res. Express 2018, 5, 55704. [Google Scholar] [CrossRef]
- Antosik, A.; Guszek, M.; Zurowski, R.; Szafran, M. Effect of SiO2 particle size and length of poly(propylene glycol) chain on rheological properties of shear thickening fluids. Arch. Metall. Mater. 2016, 61, 1165–1168. [Google Scholar] [CrossRef][Green Version]
- Moriana, A.D.; Tian, T.; Sencadas, V.; Li, W. Comparison of rheological behaviors with fumed silica-based shear thickening fluids. Korea Aust. Rheol. J. 2016, 28, 197–205. [Google Scholar] [CrossRef]
- Kaczorowski, M.; Ronowicz, M.; Rokicki, G. Organogels containing immobilized shear thickening fluid and their composites with polyurethane elastomer. Smart Mater. Struct. 2019, 28, 35034. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, Y.; Xu, Y.; Peng, C.; Gong, X.; Zhang, Z. Shear-thickening behavior of polymethylmethacrylate particles suspensions in glycerine–water mixtures. Rheol. Acta 2010, 49, 1157–1163. [Google Scholar] [CrossRef]
- Denn, M.M.; Morris, J.F.; Bonn, D. Shear thickening in concentrated suspensions of smooth spheres in Newtonian suspending fluids. Soft Matter 2018, 14, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Gvaramia, M.; Mangiapia, G.; Pipich, V.; Appavou, M.-S.; Jaksch, S.; Holderer, O.; Rukhadze, M.D.; Frielinghaus, H. Tunable viscosity modification with diluted particles: When particles decrease the viscosity of complex fluids. Colloid Polym. Sci. 2019, 297, 1507–1517. [Google Scholar] [CrossRef]
- Passey, P.; Singh, M.; Verma, S.K.; Bhattacharya, D.; Mehta, R. Steady shear and dynamic strain thickening of halloysite nanotubes and fumed silica shear thickening composite. J. Polym. Eng. 2018, 38, 915–923. [Google Scholar] [CrossRef]
- Gürgen, S.; Li, W.; Kuşhan, M.C. The rheology of shear thickening fluids with various ceramic particle additives. Mater. Des. 2016, 104, 312–319. [Google Scholar] [CrossRef]
- Islam, E.; Kaur, G.; Bhattacharjee, D.; Singh, S.; Biswas, I.; Verma, S.K. Effect of cellulose beads on shear-thickening behavior in concentrated polymer dispersions. Colloid Polym. Sci. 2018, 296, 883–893. [Google Scholar] [CrossRef]
- Mawkhlieng, U.; Majumdar, A.; Bhattacharjee, D. Graphene Reinforced Multiphase Shear Thickening Fluid for Augmenting Low Velocity Ballistic Resistance. Fibers Polym. 2021, 22, 213–221. [Google Scholar] [CrossRef]
- Nakonieczna-Dąbrowska, P.; Wróblewski, R.; Płocińska, M.; Leonowicz, M. Impact of the Carbon Nanofillers Addition on Rheology and Absorption Ability of Composite Shear Thickening Fluids. Materials 2020, 13, 3870. [Google Scholar] [CrossRef]
- Głuszek, M.; Kubiś, M.; Żurowski, R.; Wiśniewski, T.; Szafran, M. Enhancement of thermo-rheological properties of smart materials based on SiO2 and PPG modificated with expanded graphite. Int. J. Appl. Ceram. Technol. 2018, 15, 538–545. [Google Scholar] [CrossRef]
- Głuszek, M.; Żurowski, R.; Kubiś, M.; Wiśniewski, T.; Szafran, M. Shear thickening behavior and thermal properties of nanofluids with graphite fillers. Mater. Res. Express 2018, 6, 15701. [Google Scholar] [CrossRef]
- Orawiec, M.; Kaczorowski, M.; Rokicki, G. Dilatant effect enhancers for silica dispersions in poly(propylene glycols). J. Colloid Interface Sci. 2018, 528, 301–308. [Google Scholar] [CrossRef]
- Zhou, R.; Dou, X.; Lang, X.; He, L.; Liu, J.; Mu, S. Foaming ability and stability of silica nanoparticle-based triple-phase foam for oil fire extinguishing: Experimental. Soft Mater. 2018, 16, 327–338. [Google Scholar] [CrossRef]
- Warren, J.; Offenberger, S.; Toghiani, H.; Pittman, C.U.; Lacy, T.E.; Kundu, S. Effect of Temperature on the Shear-Thickening Behavior of Fumed Silica Suspensions. ACS Appl. Mater. Interfaces 2015, 7, 18650–18661. [Google Scholar] [CrossRef]
- Heinze, D.A.; Carastan, D.J. The influence of fumed silica content, dispersion energy, and humidity on the stability of shear thickening fluids. Rheol. Acta 2020, 59, 455–468. [Google Scholar] [CrossRef]
- Nakamura, H.; Makino, S.; Ishii, M. Continuous shear thickening and discontinuous shear thickening of concentrated monodispersed silica slurry. Adv. Powder Technol. 2020, 31, 1659–1664. [Google Scholar] [CrossRef]
- Khodadadi, A.; Liaghat, G.; Vahid, S.; Sabet, A.R.; Hadavinia, H. Ballistic performance of Kevlar fabric impregnated with nanosilica/PEG shear thickening fluid. Compos. Part B Eng. 2019, 162, 643–652. [Google Scholar] [CrossRef]
- Lee, B.-W.; Kim, I.-J.; Kim, C.-G. The Influence of the Particle Size of Silica on the Ballistic Performance of Fabrics Impregnated with Silica Colloidal Suspension. J. Compos. Mater. 2009, 43, 2679–2698. [Google Scholar] [CrossRef]
- Asija, N.; Chouhan, H.; Gebremeskel, S.A.; Bhatnagar, N. Influence of particle size on the low and high strain rate behavior of dense colloidal dispersions of nanosilica. J. Nanopart. Res. 2017, 19, 21. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Zhang, H.; Xu, L.; Wang, P.; Gou, C. The influence of graphene nanoplatelets (GNPs) on the semi-blunt puncture behavior of woven fabrics impregnated with shear thickening fluid (STF). RSC Adv. 2018, 8, 5268–5279. [Google Scholar] [CrossRef]
- Kalman, D.P.; Merrill, R.L.; Wagner, N.J.; Wetzel, E.D. Effect of Particle Hardness on the Penetration Behavior of Fabrics Intercalated with Dry Particles and Concentrated Particle−Fluid Suspensions. ACS Appl. Mater. Interfaces 2009, 1, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, R.; Liu, X.; Zhang, S.; Fang, S.; Yan, R. Effect of dispersing media and temperature on inter-yarn frictional properties of Kevlar fabrics impregnated with shear thickening fluid. Compos. Struct. 2020, 249, 112557. [Google Scholar] [CrossRef]
- Świderska, A.; Parzuchowski, P.G.; Żurowski, R.; Więcław-Midor, A.; Wołosz, D. Energy dissipating poly(hydroxyurethane) elastomers––Synthesis, characterization and comparison with shear thickening fluid materials. Polymer 2021, 230, 124084. [Google Scholar] [CrossRef]
- Hoffman, R.L. Discontinuous and Dilatant Viscosity Behavior in Concentrated Suspensions. I. Observation of a Flow Instability. Trans. Soc. Rheol. 1972, 16, 155–173. [Google Scholar] [CrossRef]
- Brady, J.F.; Bossis, G. The rheology of concentrated suspensions of spheres in simple shear flow by numerical simulation. J. Fluid Mech. 1985, 155, 105–129. [Google Scholar] [CrossRef]
- Foss, D.R.; Brady, J.F. Structure, diffusion and rheology of Brownian suspensions by Stokesian Dynamics simulation. J. Fluid Mech. 2000, 407, 167–200. [Google Scholar] [CrossRef]
- Kamibayashi, M.; Ogura, H.; Otsubo, Y. Shear-thickening flow of nanoparticle suspensions flocculated by polymer bridging. J. Colloid Interface Sci. 2008, 321, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Küçüksönmez, E.; Servantie, J. Shear thinning and thickening in dispersions of spherical nanoparticles. Phys. Rev. E 2020, 102, 12604. [Google Scholar] [CrossRef] [PubMed]
- Peters, I.R.; Majumdar, S.; Jaeger, H.M. Direct observation of dynamic shear jamming in dense suspensions. Nature 2016, 532, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qin, Z.; Yan, R.; Wei, S.; Zhang, W.; Lu, S.; Jia, L. Processing technology and ballistic-resistant mechanism of shear thickening fluid/high-performance fiber-reinforced composites: A review. Compos. Struct. 2021, 266, 113806. [Google Scholar] [CrossRef]
- Available online: https://www.stf-technologies.com/ (accessed on 31 October 2021).
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Srivastava, A.; Majumdar, A.; Butola, B.S. Improving the Impact Resistance of Textile Structures by using Shear Thickening Fluids: A Review. Crit. Rev. Solid State Mater. Sci. 2012, 37, 115–129. [Google Scholar] [CrossRef]
- Genovese, D.B. Shear rheology of hard-sphere, dispersed, and aggregated suspensions, and filler-matrix composites. Adv. Colloid Interface Sci. 2012, 171–172, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.R.; Walls, H.J.; Khan, S.A. Rheology of Silica Dispersions in Organic Liquids: New Evidence for Solvation Forces Dictated by Hydrogen Bonding. Langmuir 2000, 16, 7920–7930. [Google Scholar] [CrossRef]
- Isobe, T.; Hotta, Y.; Watari, K. Dispersion of nano- and submicron-sized Al2O3 particles by wet-jet milling method. Mater. Sci. Eng. B 2008, 148, 192–195. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Chen, C.-N.; Tseng, W.J. Rheology, structure, and sintering of zirconia suspensions with pyrogallol-poly(ethylene glycol) as polymeric surfactant. J. Eur. Ceram. Soc. 2013, 33, 3177–3184. [Google Scholar] [CrossRef]
- Caglayan, C.; Osken, I.; Ataalp, A.; Turkmen, H.S.; Cebeci, H. Impact response of shear thickening fluid filled polyurethane foam core sandwich composites. Compos. Struct. 2020, 243, 112171. [Google Scholar] [CrossRef]
| Factor | Influence on the Shear Thickening Phenomenon |
|---|---|
| Solid-phase content | The increase in the volume fraction of the solid phase reduces the critical shear rate and enhances the shear thickening effect. The reason for this is the reduction in the distance between solid-phase particles, and hence an increase in hydrodynamic forces [4,10,18,36,46,62,63]. |
| Particle shape of solid phase | The increase in the shape factor enhances the shear thickening effect and reduces the critical shear rate. When using particles of irregular shape, the phenomenon of shear thickening occurs at a lower volume fraction in the system [4,36]. |
| Particle size of solid phase | The decrease in the particle size of the solid phase usually increases the critical shear rate. The reason for this is the domination of Brownian forces for nanometric particle suspensions [4,64,65]. The application of smaller particles often results in a higher viscosity of the STF and a greater shear thickening effect [45,66]. |
| Particle hardness of solid phase | The increase in hardness of the solid phase particles is advantageous in achieving an enhanced shear thickening effect at high stress. Particles with lower hardness (e.g., PMMA) can deform under such conditions, leading to a reduction in the shear thickening phenomenon [67]. |
| Molar mass of carrier fluid | The increase in the molar mass of the carrier fluid increases the viscosity of the STF, enhances the shear thickening effect, and reduces the critical shear rate [32,36,41,44,68,69]. |
| Symbol | Producer | Based on the Specification Provided by the Producer | Based on Own Research | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Specific Density [g/cm3] | Bulk Density [g/cm3] | Particle Size [nm] | Specific Surface Area [m2/g] | Specific Density [g/cm3] | Bulk Density [g/cm3] | Average Particle Size (DLS Method) [nm] | Average Particle Size (SEM Method) [nm] | Specific Surface Area [m2/g] | ||
| KE–P10 | Nippon Shokubai, Japan | 2.0 | 0.2 | 100–200 | no data | 1.96 | 0.33 | 127 | 126 | 114 |
| SF | Sigma-Aldrich, USA | no data | 0.04 | 200–300 agglomerates | 175–225 | 1.53 | 0.04 | 218 | - | 179 |
| Carrier Fluid | Symbol | Producer | Based on the Specification Provided by the Producer | Based on Own Research | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number Average Molecular Weight Mn | Specific Density [g/cm3] | Viscosity [Pa∙s] | Hydroxyl Value [mg KOH/g] | Number Average Molecular Weight of the Major Series Mn | Weight Average Molecular Weight of the Major Series Mw | Dispersity | Viscosity [Pa∙s] | |||
| poly(propylene glycol) | PPG 425 | Sigma-Aldrich, USA | 425 | 1.004 | 0.08 | 263 | 498 | 517 | 1.04 | 0.08 |
| Silica | KE-P10 | SF | |
|---|---|---|---|
| Solid Loading (vol.%) | |||
| 10.0 | X | Newtonian-like behavior | |
| 12.5 | X | slight shear thickening | |
| 15.0 | X | slight shear thickening | |
| 17.5 | X | significant shear thickening | |
| 20.0 | shear thinning | significant shear thickening | |
| 22.0 | shear thinning | significant shear thickening | |
| 23.0 | shear thinning | not possible to obtain | |
| 25.0 | shear thinning | not possible to obtain | |
| 30.0 | shear thinning | X | |
| 35.0 | shear thinning | X | |
| 40.0 | slight shear thickening | X | |
| 45.0 | shear thickening | X | |
| 47.5 | shear thickening | X | |
| 50.0 | significant shear thickening | X | |
| 52.5 | significant shear thickening | X | |
| 53.75 | significant shear thickening | X | |
| 55.0 | homogeneus fluid | X | |
| 56.25 | not possible to obtain | X | |
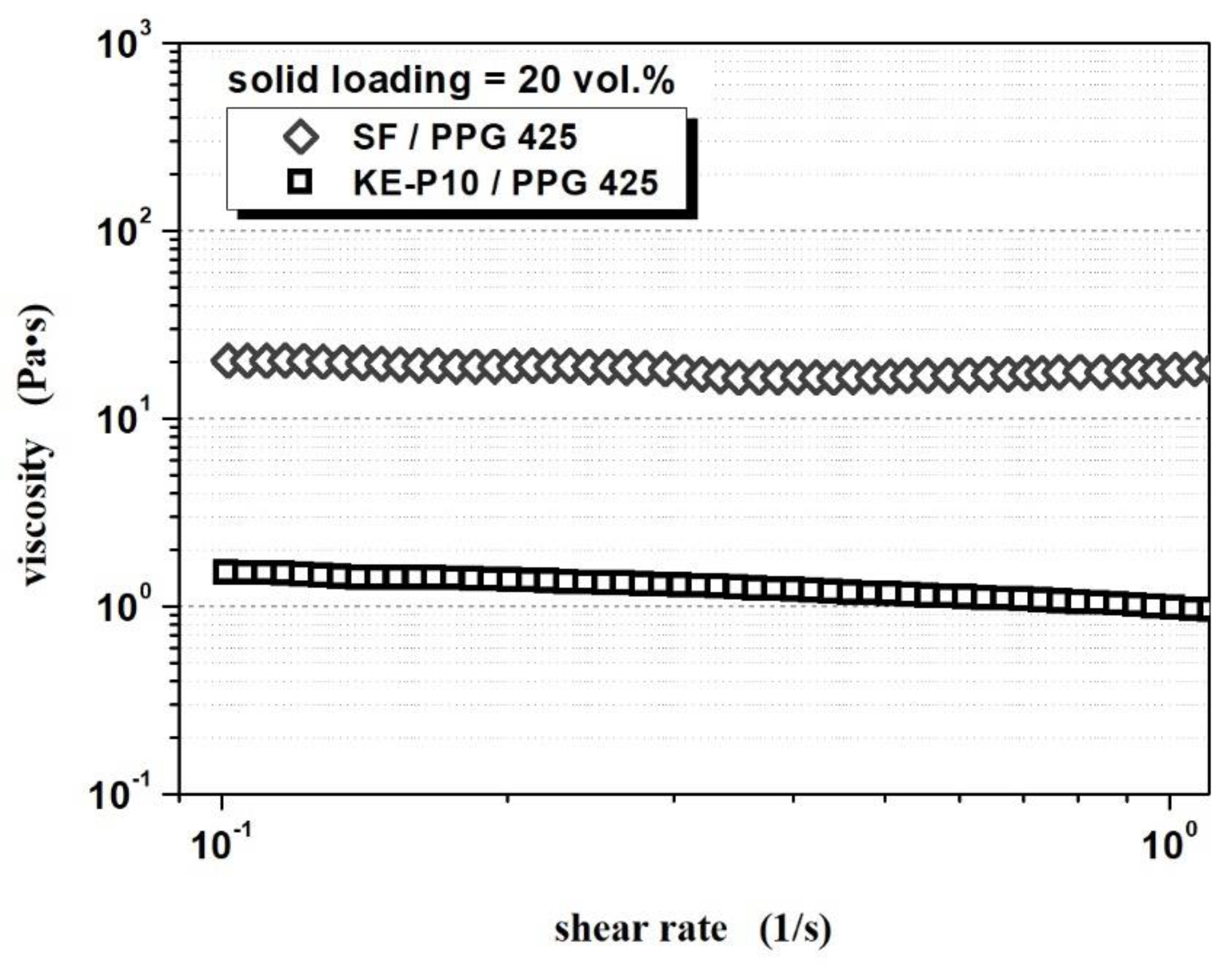
| Composition | Solid Loading (vol.%) | Initial Viscosity (Pa∙s) | Beginning of Shear Thickening | Maximum Viscosity | Shear Thickening Effect (Pa∙s) | Shear Thickening Ratio | ||
|---|---|---|---|---|---|---|---|---|
| Shear Rate (s−1) | Viscosity (Pa∙s) | Shear Rate (s−1) | Viscosity (Pa∙s) | |||||
| KE-P10/PPG 425 | 50.0 | 119.5 | 5.8 | 22.1 | 14.7 | 1590 | 1568 | 71.9 |
| SF/ PPG 425 | 20.0 | 20.4 | 0.4/1.3 * | 21.5 | 5.9 | 2296 | 2275 | 106.8 |
| Composition | Solid Loading (%) | Impact Energy (J) | STF Temperature (°C) | Force (kN) |
|---|---|---|---|---|
| Water (reference) | - | 50 | 20.0 | 40.27 (±1.16) |
| KE-P10/PPG 425 | 50.0 | 50 | 20.1 | 1.27 (±0.02) |
| SF/PPG 425 | 20.0 | 50 | 20.0 | 1.37 (±0.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żurowski, R.; Falkowski, P.; Zygmuntowicz, J.; Szafran, M. Rheological and Technological Aspects in Designing the Properties of Shear Thickening Fluids. Materials 2021, 14, 6585. https://doi.org/10.3390/ma14216585
Żurowski R, Falkowski P, Zygmuntowicz J, Szafran M. Rheological and Technological Aspects in Designing the Properties of Shear Thickening Fluids. Materials. 2021; 14(21):6585. https://doi.org/10.3390/ma14216585
Chicago/Turabian StyleŻurowski, Radosław, Paweł Falkowski, Justyna Zygmuntowicz, and Mikołaj Szafran. 2021. "Rheological and Technological Aspects in Designing the Properties of Shear Thickening Fluids" Materials 14, no. 21: 6585. https://doi.org/10.3390/ma14216585
APA StyleŻurowski, R., Falkowski, P., Zygmuntowicz, J., & Szafran, M. (2021). Rheological and Technological Aspects in Designing the Properties of Shear Thickening Fluids. Materials, 14(21), 6585. https://doi.org/10.3390/ma14216585






