Microwave-Assisted Coal-Derived Few-Layer Graphene as an Anode Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Synthesis of the FLG Composite Materials
2.3. Characterization of the FLG Composite Materials
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Physicochemical Properties of the Composite FLG Materials
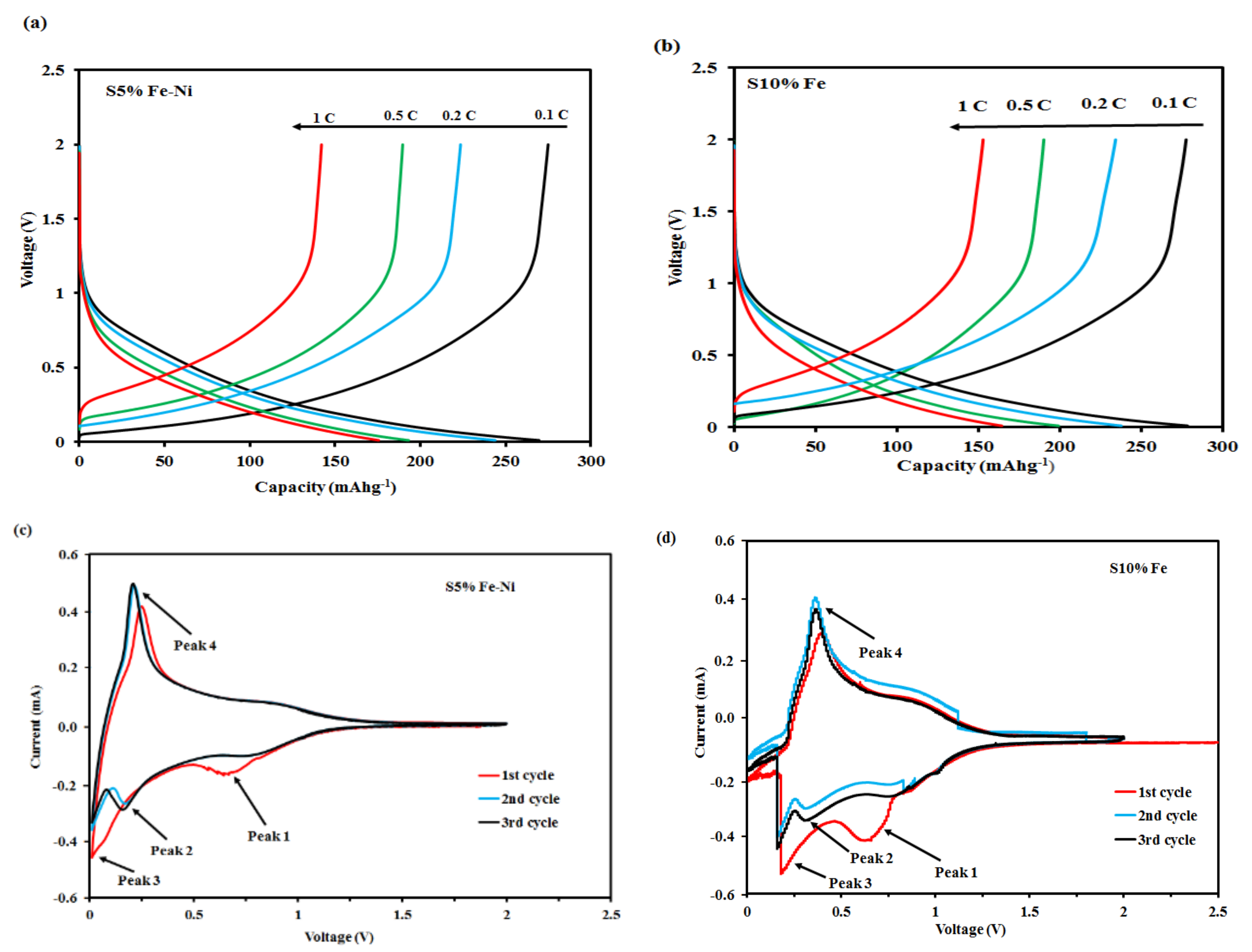
3.2. GCD and CV Analysis of the Composite FLG Materials
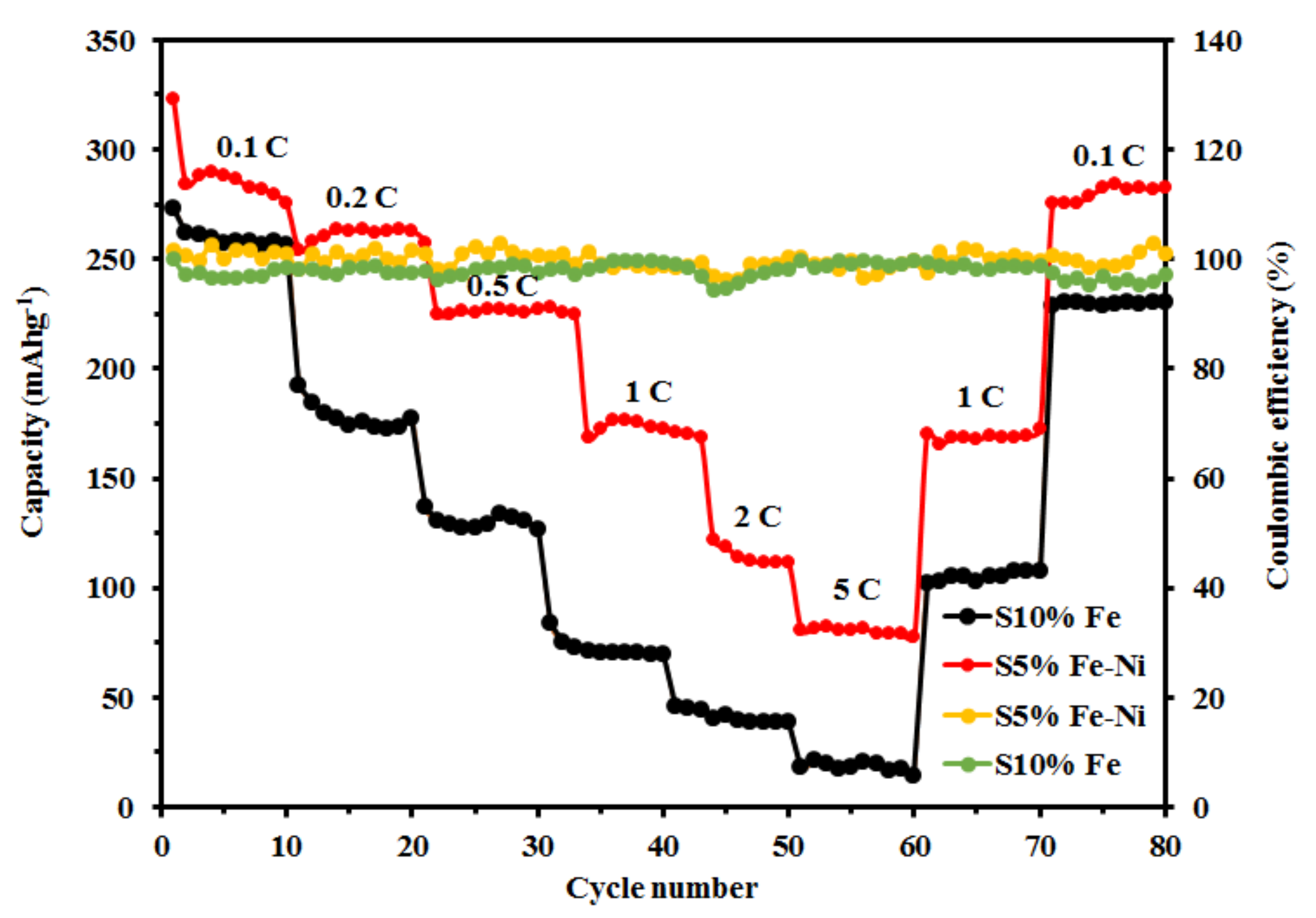
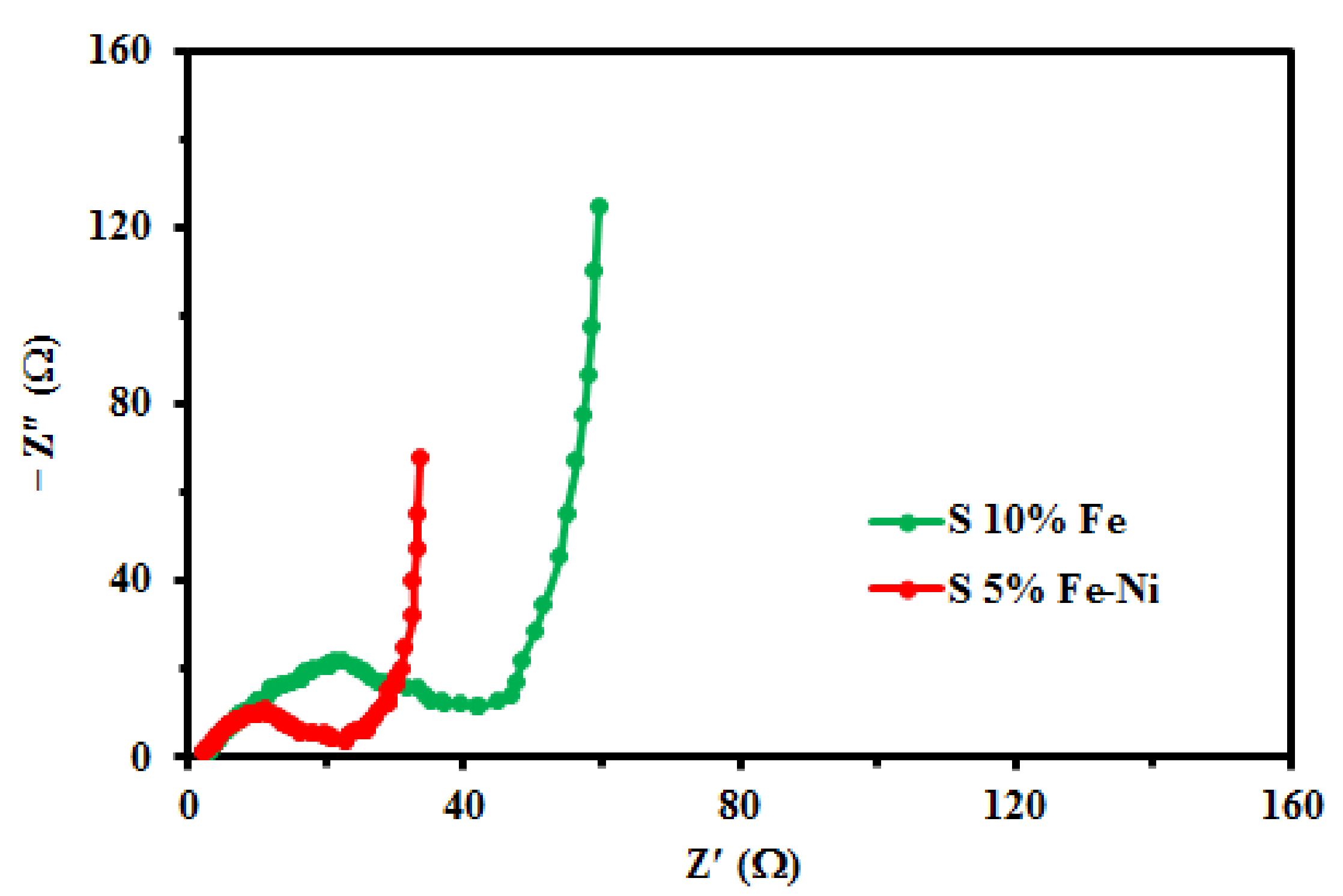
3.3. The Rate Capability, Cycling Stability, and Coulombic Efficiency of the Composite FLG Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Dou, Y.; Wei, Z.; Ma, J.; Deng, Y.; Li, Y.; Liu, H.K.; Dou, S.X. Recent Progress in Graphite Intercalation Compounds for Rechargeable Metal (Li, Na, K, Al)-Ion Batteries. Adv. Sci. 2017, 4, 1700146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Luo, B.; Zhi, L. Design and construction of three dimensional graphene-based composites for lithium ion battery applications. Energy Environ. Sci. 2015, 8, 456–477. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, H.; Tang, X.; Fan, W.; Peng, G.; Qu, M. Graphite/graphene oxide composite as high capacity and binder-free anode material for lithium ion batteries. J. Power Sources 2013, 241, 619–626. [Google Scholar] [CrossRef]
- Huang, S.; Guo, H.; Li, X.; Wang, Z.; Gan, L.; Wang, J.; Xiao, W. Carbonization and graphitization of pitch applied for anode materials of high power lithium ion batteries. J. Solid State Electrochem. 2013, 17, 1401–1408. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Liu, X.; Zhang, Y.; Zhao, W.; Li, Y.; Qin, C.; Bakenov, Z. High specific surface area bimodal porous carbon derived from biomass reed flowers for high performance lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 569, 22–33. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, Y.I.; Wee, J.-H.; Kim, C.H.; Ahn, B.W.; Lee, J.W.; Shu, S.J.; Terrones, M.; Kim, Y.A.; Yang, C.-M. Few-layer graphene coated current collectors for safe and powerful lithium ion batteries. Carbon 2019, 153, 495–503. [Google Scholar] [CrossRef]
- Delhaes, P. Graphite and Precursors; CRC Press: Boca Raton, FL, USA, 2000; Volume 1. [Google Scholar]
- Fan, C.-L.; He, H.; Zhang, K.-H.; Han, S.-C. Structural developments of artificial graphite scraps in further graphitization and its relationships with discharge capacity. Electrochim. Acta 2012, 75, 311–315. [Google Scholar] [CrossRef]
- Mathur, R.B.; Lal, C.; Sharma, D.K. Catalyst-Free Carbon Nanotubes from Coal-Based Material. Energy Sources Part A Recover. Util. Environ. Eff. 2007, 29, 21–27. [Google Scholar] [CrossRef]
- Du, A.-B.; Liu, X.-G.; Fu, D.-J.; Han, P.-D.; Xu, B.-S. Onion-like fullerenes synthesis from coal. Fuel 2007, 86, 294–298. [Google Scholar] [CrossRef]
- Sasikala, S.P.; Henry, L.; Tonga, G.Y.; Huang, K.; Das, R.; Giroire, B.; Marre, S.; Rotello, V.M.; Penicaud, A.; Poulin, P.; et al. High Yield Synthesis of Aspect Ratio Controlled Graphenic Materials from Anthracite Coal in Supercritical Fluids. ACS Nano 2016, 10, 5293–5303. [Google Scholar] [CrossRef] [PubMed]
- Haenel, M.W. Recent progress in coal structure research. Fuel 1992, 71, 1211–1223. [Google Scholar] [CrossRef]
- Xing, B.; Zhang, C.; Cao, Y.; Huang, G.; Liu, Q.; Zhang, C.; Chen, Z.; Yi, G.; Chen, L.; Yu, J. Preparation of synthetic graphite from bituminous coal as anode materials for high performance lithium-ion batteries. Fuel Process. Technol. 2018, 172, 162–171. [Google Scholar] [CrossRef]
- Cameán, I.; LaVela, P.; Tirado, J.L.; Garcia, A. On the electrochemical performance of anthracite-based graphite materials as anodes in lithium-ion batteries. Fuel 2010, 89, 986–991. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Jiang, S.P.; Liang, Y.M.; Shen, P.K. Synthesis and Characterization of Platinum Catalysts on Multiwalled Carbon Nanotubes by Intermittent Microwave Irradiation for Fuel Cell Applications. J. Phys. Chem. B 2006, 110, 5343–5350. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Quan, X.; Bo, L.; Chen, S.; Zhao, Y.; Chang, M. Temperature measurement of GAC and decomposition of PCP loaded on GAC and GAC-supported copper catalyst in microwave irradiation. Appl. Catal. A Gen. 2004, 264, 53–58. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation of oil palm (Elaeis) empty fruit bunch activated carbon by microwave-assisted KOH activation for the adsorption of methylene blue. Desalination 2011, 275, 302–305. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zheng, T.; Li, N.; Wang, P.; Abulikemu, G. Modification of bamboo-based activated carbon using microwave radiation and its effects on the adsorption of methylene blue. Appl. Surf. Sci. 2010, 256, 3309–3315. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Yuan, S.-J.; Dong, B.; Dai, X.-H. Facile and scalable synthesis of high-quality few-layer graphene from biomass by a universal solvent-free approach. Appl. Surf. Sci. 2021, 562, 150203. [Google Scholar] [CrossRef]
- Cai, W.; Zhu, Y.; Li, X.; Piner, R.D.; Ruoff, R.S. Large area few-layer graphene/graphite films as transparent thin conducting electrodes. Appl. Phys. Lett. 2009, 95, 123115. [Google Scholar] [CrossRef]
- Islam, F.; Tahmasebi, A.; Wang, R.; Yu, J. Structure of Coal-Derived Metal-Supported Few-Layer Graphene Composite Materials Synthesized Using a Microwave-Assisted Catalytic Graphitization Process. Nanomaterials 2021, 11, 1672. [Google Scholar] [CrossRef]
- Marsh, H.; Crawford, D.; Taylor, D. Catalytic graphitization by iron of isotropic carbon from polyfurfuryl alcohol, 725–1090 K. A high resolution electron microscope study. Carbon 1983, 21, 81–87. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J.; Moreno-Castilla, C.; Rivera-Utrilla, J.; Hanzawa, Y.; Yamada, Y. Catalytic Graphitization of Carbon Aerogels by Transition Metals. Langmuir 2000, 16, 4367–4373. [Google Scholar] [CrossRef]
- Gutierrez-Pardo, A.; Ramírez-Rico, J.; De Arellano-López, A.R.; Martínez-Fernández, J. Characterization of porous graphitic monoliths from pyrolyzed wood. J. Mater. Sci. 2014, 49, 7688–7696. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Wang, J.; Tian, L.; Zhang, H.; Zhang, S. Preparation of Hierarchically Porous Graphitic Carbon Spheres and Their Applications in Supercapacitors and Dye Adsorption. Nanomaterials 2018, 8, 625. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.-M.; Liu, C.; Yu, W.-J.; Zhang, L.-L.; Hou, P.-X.; Li, J.-C.; Li, F.; Bando, Y.; Golberg, D.; Cheng, H.-M. Structural Changes in Iron Oxide and Gold Catalysts during Nucleation of Carbon Nanotubes Studied by In Situ Transmission Electron Microscopy. ACS Nano 2014, 8, 292–301. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Xin, S.; You, Y.; Yu, L.; Lin, Y.; Xu, D.-W.; Qiao, C.; Huang, Z.-H.; Yang, N.; Yu, S.-H.; et al. Ion-Catalyzed Synthesis of Microporous Hard Carbon Embedded with Expanded Nanographite for Enhanced Lithium/Sodium Storage. J. Am. Chem. Soc. 2016, 138, 14915–14922. [Google Scholar] [CrossRef]
- Arrigo, R.; Sasaki, T.; Callison, J.; Gianolio, D.; Schuster, M.E. Monitoring dynamics of defects and single Fe atoms in N-functionalized few-layer graphene by in situ temperature programmed scanning transmission electron microscopy. J. Energy Chem. 2022, 64, 520–530. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Cheng, G.; Ma, C.; Liu, X.; Wang, J.; Qiao, W.; Ling, L. Catalytic Graphitization of Anthracite as an Anode for Lithium-Ion Batteries. Energy Fuels 2020, 34, 8911–8918. [Google Scholar] [CrossRef]
- Bystrzejewski, M. Synthesis of carbon-encapsulated iron nanoparticles via solid state reduction of iron oxide nanoparticles. J. Solid State Chem. 2011, 184, 1492–1498. [Google Scholar] [CrossRef]
- Ohtani, H.; Hasebe, M.; Nishizawa, T. Calculation of Fe-C, Co-C and Ni-C phase diagrams. Trans. Iron Steel Inst. Jpn. 1984, 24, 857–864. [Google Scholar] [CrossRef]
- Yeh, T.-S.; Wu, Y.-S.; Lee, Y.-H. Graphitization of unburned carbon from oil-fired fly ash applied for anode materials of high power lithium ion batteries. Mater. Chem. Phys. 2011, 130, 309–315. [Google Scholar] [CrossRef]
- Badenhorst, H. Microstructure of natural graphite flakes revealed by oxidation: Limitations of XRD and Raman techniques for crystallinity estimates. Carbon 2014, 66, 674–690. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Tang, Y.; Wang, L.; Liu, L.; Jia, D.; Zhao, Z. NiFe nanoalloys in-situ immobilized on coal based activated carbons through one-step pyrolysis as magnetically recoverable catalysts for reduction of 4-nitrophenol. J. Alloys Compd. 2017, 702, 531–537. [Google Scholar] [CrossRef]
- Li, J.; Wen, W.; Xu, G.; Zou, M.; Huang, Z.; Guan, L. Fe-added Fe3C carbon nanofibers as anode for Li ion batteries with excellent low-temperature performance. Electrochim. Acta 2015, 153, 300–305. [Google Scholar] [CrossRef]
- Zhang, J.; Tahmasebi, A.; Omoriyekomwan, J.E.; Yu, J. Microwave-assisted synthesis of biochar-carbon-nanotube-NiO composite as high-performance anode materials for lithium-ion batteries. Fuel Process. Technol. 2021, 213, 106714. [Google Scholar] [CrossRef]
- Zhang, L.; Bin Wu, H.; Lou, X.W.D. Iron-Oxide-Based Advanced Anode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1300958. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, K.-H. Full graphitization of amorphous carbon by microwave heating. RSC Adv. 2016, 6, 24667–24674. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Salunkhe, R.; Zhang, H.; Malgras, V.; Ahamad, T.; AlShehri, S.M.; Kobayashi, N.; Tominaka, S.; Ide, Y.; Kim, J.H.; et al. Bimetallic Metal-Organic Frameworks for Controlled Catalytic Graphitization of Nanoporous Carbons. Sci. Rep. 2016, 6, 30295. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Cameán, I.; García, R.; Garcia, A. Graphitized boron-doped carbon foams: Performance as anodes in lithium-ion batteries. Electrochim. Acta 2011, 56, 5090–5094. [Google Scholar] [CrossRef]
- Robertson, A.W.; Warner, J.H. Hexagonal Single Crystal Domains of Few-Layer Graphene on Copper Foils. Nano Lett. 2011, 11, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Schumann, T.; de Oliveira, M.H., Jr.; Lopes, J.M.J. Controlled synthesis and characterization of multilayer graphene films on the C-face of silicon carbide. Phys. Status Solidi 2017, 214, 1600721. [Google Scholar] [CrossRef]
- Santangelo, S.; Messina, G.; Faggio, G.; Lanza, M.; Milone, C. Evaluation of crystalline perfection degree of multi-walled carbon nanotubes: Correlations between thermal kinetic analysis and micro-Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 593–602. [Google Scholar] [CrossRef]
- Ōya, A.; Ōtani, S. Catalytic graphitization of carbons by various metals. Carbon 1979, 17, 131–137. [Google Scholar] [CrossRef]
- Derbyshire, F.; Presland, A.; Trimm, D. Graphite formation by the dissolution—Precipitation of carbon in cobalt, nickel and iron. Carbon 1975, 13, 111–113. [Google Scholar] [CrossRef]
- Xiong, W.; Zhou, Y.S.; Hou, W.J.; Guillemet, T.; Silvain, J.F.; Gao, Y.; Lahaye, M.; Lebraud, E.; Xu, S.; Wang, X.W.; et al. Solid-state graphene formation via a nickel carbide intermediate phase. RSC Adv. 2015, 5, 99037–99043. [Google Scholar] [CrossRef]
- Zhao, M.; Song, H.; Chen, X.; Lian, W. Large-scale synthesis of onion-like carbon nanoparticles by carbonization of phenolic resin. Acta Mater. 2007, 55, 6144–6150. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Gu, J.; Kang, D.; Zhou, F.; Zhang, W.; Wu, Y.; Zhang, D. Highly porous graphitic materials prepared by catalytic graphitization. Carbon 2013, 64, 132–140. [Google Scholar] [CrossRef]
- Juang, Z.-Y.; Wu, C.-Y.; Lo, C.-W.; Chen, W.-Y.; Huang, C.-F.; Hwang, J.-C.; Chen, F.-R.; Leou, K.-C.; Tsai, C.-H. Synthesis of graphene on silicon carbide substrates at low temperature. Carbon 2009, 47, 2026–2031. [Google Scholar] [CrossRef]
- Zan, R. Microscopy and Spectroscopy of Graphene: Atomic Scale Structure and Interaction with Foreign Atom Species; The University of Manchester: Manchester, UK, 2013. [Google Scholar]
- Sekar, S.; Lee, Y.; Kim, D.Y.; Lee, S. Substantial LIB Anode Performance of Graphitic Carbon Nanoflakes Derived from Biomass Green-Tea Waste. Nanomaterials 2019, 9, 871. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Zhang, W.; Qian, D.; Cui, C.; Xu, Q.; Li, L.; Li, S.; Li, Y. Solution-processed nickel acetate as hole collection layer for polymer solar cells. Phys. Chem. Chem. Phys. 2012, 14, 14217–14223. [Google Scholar] [CrossRef]
- Graat, P.C.; Somers, M.A.J. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe 2p spectra. Appl. Surf. Sci. 1996, 100–101, 36–40. [Google Scholar] [CrossRef]
- Varghese, B.; Reddy, M.V.; Yanwu, Z.; Lit, C.S.; Hoong, T.C.; Rao, G.V.S.; Chowdari, B.V.R.; Wee, A.; Lim, C.T.; Sow, C.-H. Fabrication of NiO Nanowall Electrodes for High Performance Lithium Ion Battery. Chem. Mater. 2008, 20, 3360–3367. [Google Scholar] [CrossRef]
- Yue, H.; Shi, Z.; Wang, Q.; Cao, Z.; Dong, H.; Qiao, Y.; Yin, Y.; Yang, S. MOF-derived cobalt-doped ZnO@ C composites as a high-performance anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 17067–17074. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhuo, L.; Cheng, H.; Zhang, C.; Zhao, F. Porous carbon nanotubes decorated with nanosized cobalt ferrite as anode materials for high-performance lithium-ion batteries. J. Power Sources 2015, 283, 289–299. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Y.; Wang, X.; Kharel, P.R.; Gibbons, W.; Luo, B.; Gu, Z.; Fan, Q.; Metzger, L. Nickel catalytic graphitized porous carbon as electrode material for high performance supercapacitors. Energy 2016, 101, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yang, X.; Zhang, F.; Cai, L.; Zhang, L.; Wen, Z. Carbon-coated isotropic natural graphite spheres as anode material for lithium-ion batteries. Ceram. Int. 2017, 43, 9458–9464. [Google Scholar] [CrossRef]
- Liu, T.; Luo, R.; Qiao, W.; Yoon, S.-H.; Mochida, I. Microstructure of carbon derived from mangrove charcoal and its application in Li-ion batteries. Electrochim. Acta 2010, 55, 1696–1700. [Google Scholar] [CrossRef]
- Zhu, C.; Akiyama, T. Cotton derived porous carbon via an MgO template method for high performance lithium ion battery anodes. Green Chem. 2016, 18, 2106–2114. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yang, L.; Wang, P.; Wang, C.-Y.; Cheng, J.; Zhang, G.; Gu, J.-J.; Cao, F.-F. Porous nitrogen-doped carbon derived from peanut shell as anode material for lithium ion battery. Int. J. Electrochem. Sci. 2017, 42673, 9844–9854. [Google Scholar] [CrossRef]
- Gao, F.; Geng, C.; Xiao, N.; Qu, J.; Qiu, J. Hierarchical porous carbon sheets derived from biomass containing an activation agent and in-built template for lithium ion batteries. Carbon 2018, 139, 1085–1092. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, H.; Wang, H.; Zhao, X.; Sun, S.; Shi, J.; Huang, M.; Liu, W.; Zheng, Y.; Li, P. Dual-doped hierarchical porous carbon derived from biomass for advanced supercapacitors and lithium ion batteries. RSC Adv. 2019, 9, 32382–32394. [Google Scholar] [CrossRef] [Green Version]
- Ru, H.; Xiang, K.; Zhou, W.; Zhu, Y.; Zhao, X.S.; Chen, H. Bean-dreg-derived carbon materials used as superior anode material for lithium-ion batteries. Electrochim. Acta 2016, 222, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Naji, A.; Ghanbaja, J.; Willmann, P.; Billaud, D. Electrochemical reduction of graphite in LiClO4-propylene carbonate electrolyte: Influence of the nature of the surface protective layer. Carbon 1997, 35, 845–852. [Google Scholar] [CrossRef]
- Yue, X.; Sun, W.; Zhang, J.; Wang, F.; Yang, Y.; Lu, C.; Wang, Z.; Rooney, D.; Sun, K. Macro-mesoporous hollow carbon spheres as anodes for lithium-ion batteries with high rate capability and excellent cycling performance. J. Power Sources 2016, 331, 10–15. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Z.-J.; Cheng, S.; Liu, M. Fabrication of 3-Dimensional Porous Graphene Materials for Lithium Ion Batteries. Electrochim. Acta 2014, 146, 437–446. [Google Scholar] [CrossRef]
- Ramos, A.; Cameán, I.; Cuesta, N.; García, A.B. Graphitized stacked-cup carbon nanofibers as anode materials for lithium-ion batteries. Electrochim. Acta 2014, 146, 769–775. [Google Scholar] [CrossRef]
- Kim, C.; Jung, J.-W.; Yoon, K.R.; Youn, D.-Y.; Park, S.; Kim, I.-D. A High-Capacity and Long-Cycle-Life Lithium-Ion Battery Anode Architecture: Silver Nanoparticle-Decorated SnO2/NiO Nanotubes. ACS Nano 2016, 10, 11317–11326. [Google Scholar] [CrossRef] [PubMed]
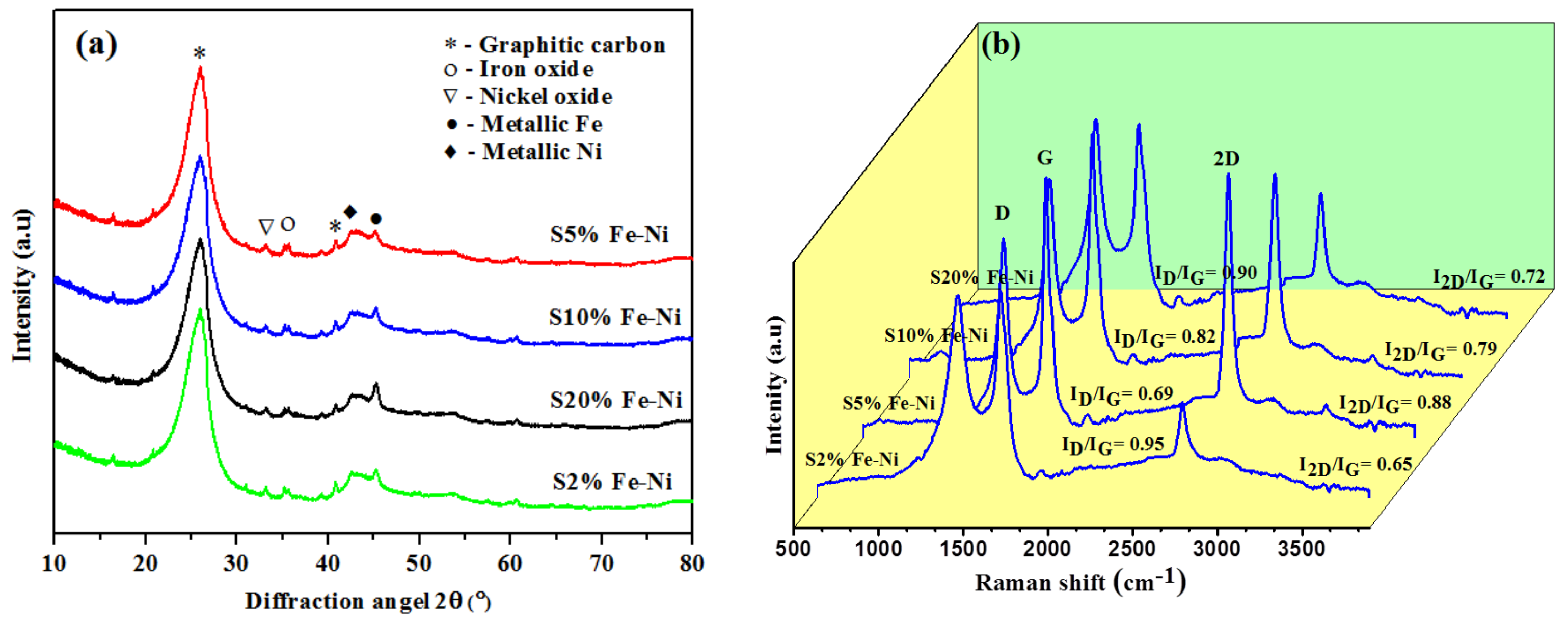




| Fe–Ni Loading (%)/Parameters | S2 | S5 | S10 | S20 |
|---|---|---|---|---|
| Interlayer spacing (d002) (nm) 1 | 0.3368 | 0.3355 | 0.3361 | 0.3366 |
| Crystallite size (Lc) (nm) 1 | 2.01 | 2.30 | 2.12 | 2.07 |
| Crystallite thickness (La) (nm) 1 | 4.34 | 4.95 | 4.57 | 4.45 |
| ID/IG | 0.95 | 0.69 | 0.82 | 0.90 |
| I2D/IG | 0.65 | 0.88 | 0.79 | 0.72 |
| I2D/ID | 0.68 | 1.27 | 0.96 | 0.80 |
| Surface area (m2g−1) | 81.92 | 175.61 | 136.23 | 97.47 |
| “g” factor (%) 2 | 83.4 | 97.9 | 92.3 | 86.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, F.; Wang, J.; Tahmasebi, A.; Wang, R.; Moghtaderi, B.; Yu, J. Microwave-Assisted Coal-Derived Few-Layer Graphene as an Anode Material for Lithium-Ion Batteries. Materials 2021, 14, 6468. https://doi.org/10.3390/ma14216468
Islam F, Wang J, Tahmasebi A, Wang R, Moghtaderi B, Yu J. Microwave-Assisted Coal-Derived Few-Layer Graphene as an Anode Material for Lithium-Ion Batteries. Materials. 2021; 14(21):6468. https://doi.org/10.3390/ma14216468
Chicago/Turabian StyleIslam, Faridul, Jialong Wang, Arash Tahmasebi, Rou Wang, Behdad Moghtaderi, and Jianglong Yu. 2021. "Microwave-Assisted Coal-Derived Few-Layer Graphene as an Anode Material for Lithium-Ion Batteries" Materials 14, no. 21: 6468. https://doi.org/10.3390/ma14216468
APA StyleIslam, F., Wang, J., Tahmasebi, A., Wang, R., Moghtaderi, B., & Yu, J. (2021). Microwave-Assisted Coal-Derived Few-Layer Graphene as an Anode Material for Lithium-Ion Batteries. Materials, 14(21), 6468. https://doi.org/10.3390/ma14216468






