Limitation of Water-Soluble Tetrazolium Salt for the Cytocompatibility Evaluation of Zinc-Based Metals
Abstract
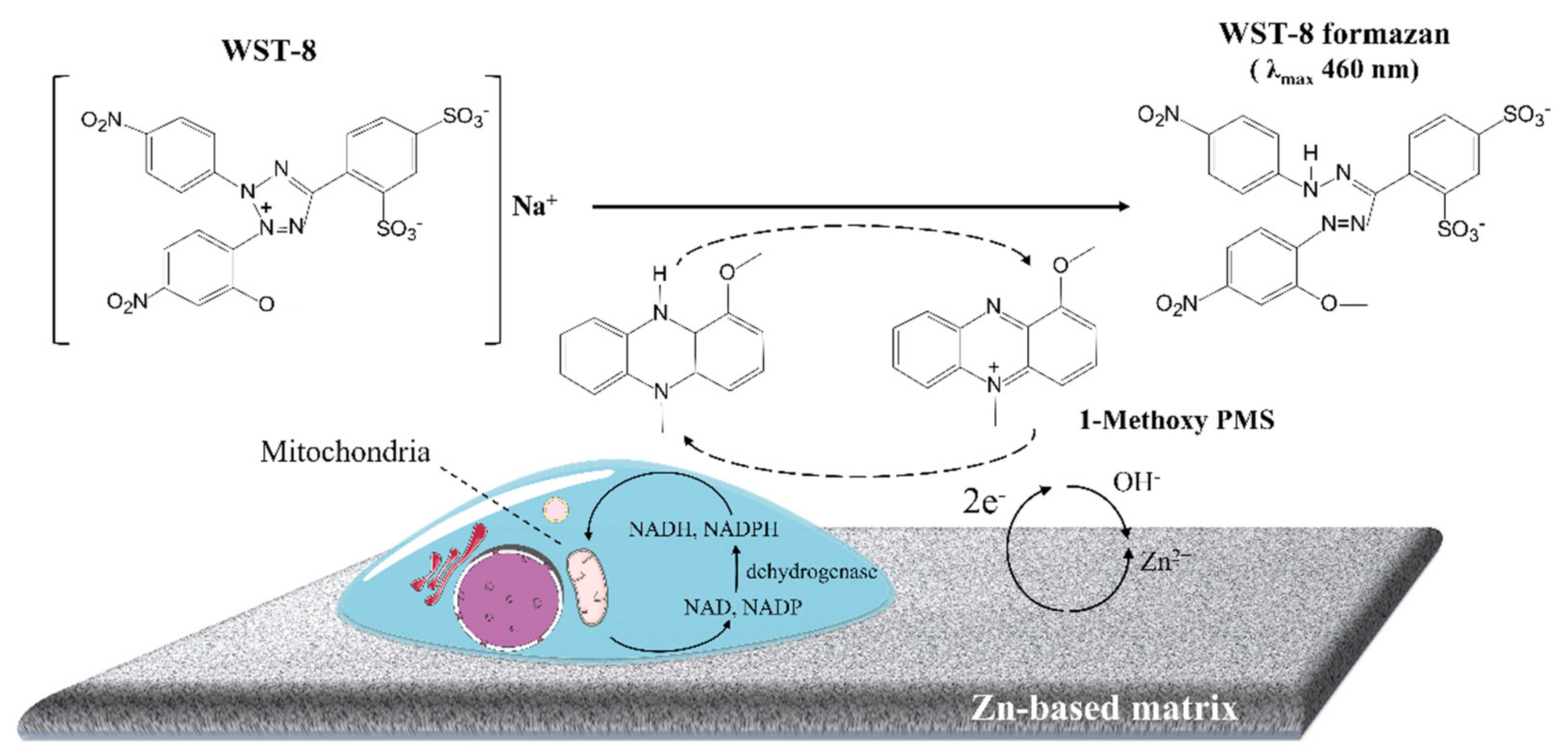
:1. Introduction
2. Materials and Methods
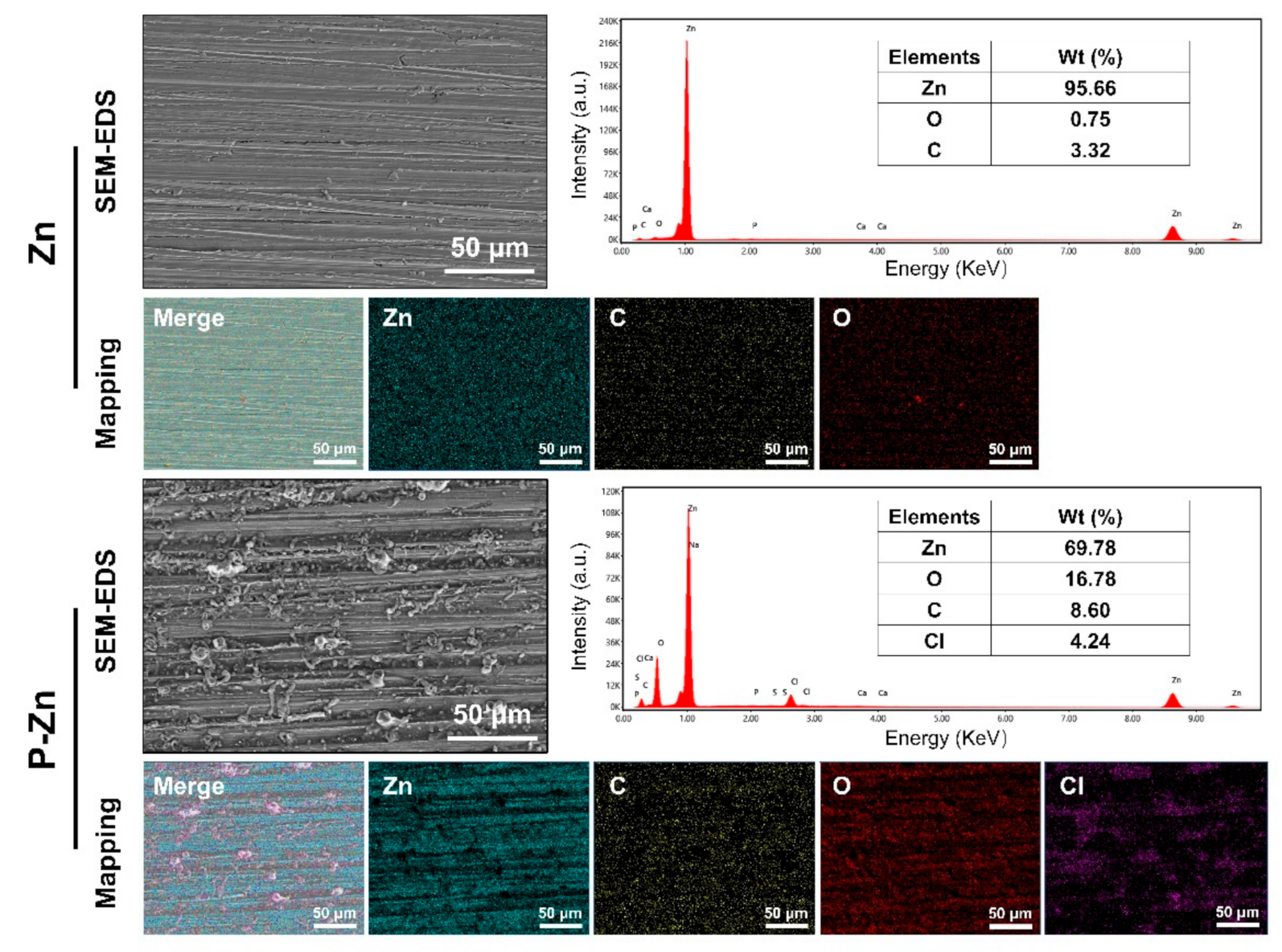
2.1. Specimen Preparation and Surface Characterization
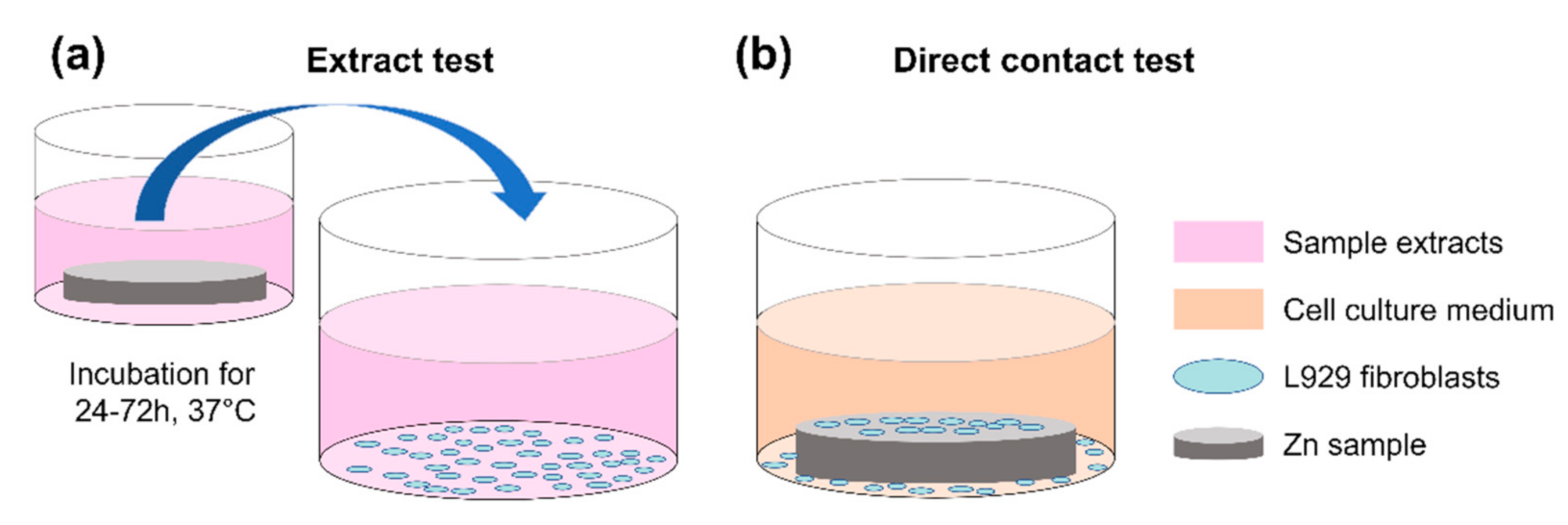
2.2. Direct Contact Test
2.3. Acellular Assay
2.4. Statistical Analysis
3. Results
3.1. Surface Characteristics
3.2. Effect of Zn-Based Metals on Cell Membrane Integrity
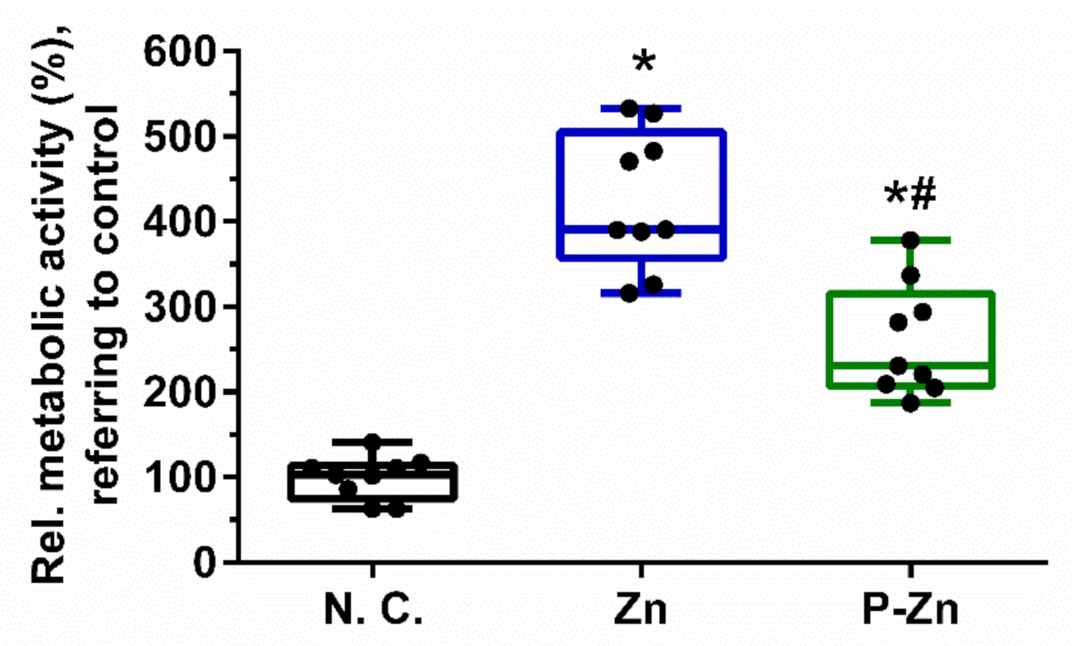
3.3. Influence of Zn-Based Metals on Cell Metabolic Activity
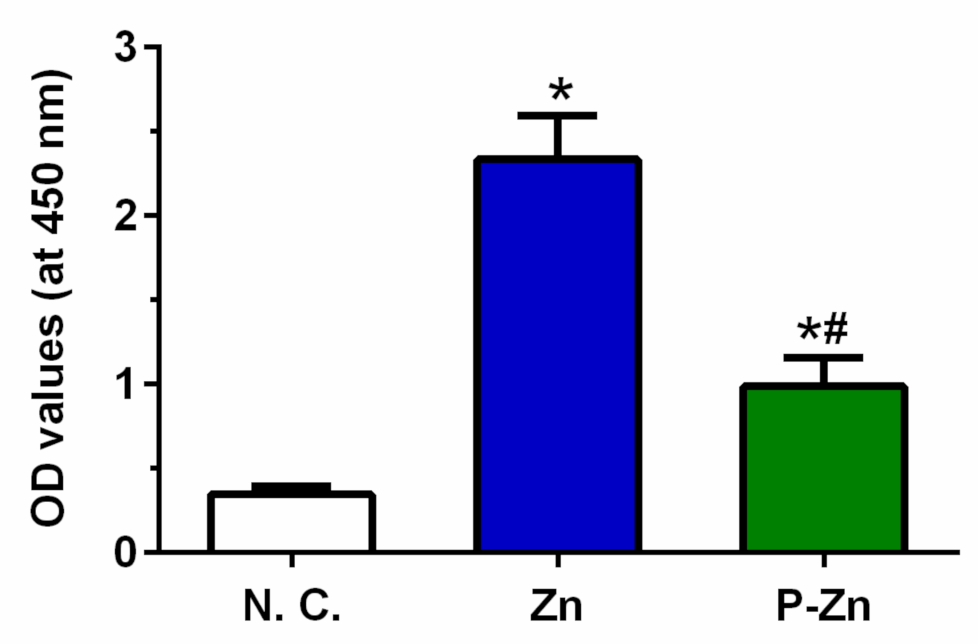
3.4. Role of Zn-Based Metals in Formazan Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental Theory of Biodegradable Metals-Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef]
- Geis-Gerstorfer, J.; Schille, C.; Schweizer, E.; Rupp, F.; Scheideler, L.; Reichel, H.P.; Hort, N.; Nolte, A.; Wendel, H.P. Blood triggered corrosion of magnesium alloys. Mater. Sci. Eng. B 2011, 176, 1761–1766. [Google Scholar] [CrossRef]
- Pierson, D.; Edick, J.; Tauscher, A.; Pokorney, E.; Bowen, P.; Gelbaugh, J.; Stinson, J.; Getty, H.; Lee, C.H.; Drelich, J.; et al. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. Part B 2012, 100, 58–67. [Google Scholar] [CrossRef]
- Drynda, A.; Hassel, T.; Bach, F.W.; Peuster, M. In vitro and in vivo corrosion properties of new iron-manganese alloys designed for cardiovascular applications. J. Biomed. Mater. Res. Part B 2015, 103, 649–660. [Google Scholar] [CrossRef]
- Levy, G.K.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants-A Review Paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, P.; Neumann, B.; Haag, H.; Li, M.; Xu, Z.; Zhou, C.; Scheideler, L.; Wendel, H.P.; Zhang, H.; et al. Chandler-Loop surveyed blood compatibility and dynamic blood triggered degradation behavior of Zn-4Cu alloy and Zn. Mater. Sci. Eng. C 2021, 119, 111594. [Google Scholar] [CrossRef]
- Hosova, K.; Pinc, J.; Skolakova, A.; Bartunek, V.; Vertat, P.; Skolakova, T.; Prusa, F.; Vojtech, D.; Capek, J. Influence of Ceramic Particles Character on Resulted Properties of Zinc-Hydroxyapatite/Monetite Composites. Metals 2021, 11, 499. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Kimmerle-Müller, E.; Rupp, F.; Han, X.; Heiss, A.; Richter, A.; Legner, C.; Klotz, U.E.; et al. Evaluation of a Zn-2Ag-1.8Au-0.2V Alloy for Absorbable Biocompatible Materials. Materials 2019, 13, 56. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Avior, O.; Ben Ghedalia-Peled, N.; Ron, T.; Vago, R.; Aghion, E. The Effect of Ca on In Vitro Behavior of Biodegradable Zn-Fe Alloy in Simulated Physiological Environments. Metals 2020, 10, 1624. [Google Scholar] [CrossRef]
- Drelich, A.J.; Zhao, S.; Guillory, R.J., 2nd; Drelich, J.W.; Goldman, J. Long-term surveillance of zinc implant in murine artery: Surprisingly steady biocorrosion rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, W.; Dai, J.; Xepapadeas, A.B.; Schweizer, E.; Alexander, D.; Scheideler, L.; Zhou, C.; Zhang, H.; Wan, G.; et al. Investigation of zinc-copper alloys as potential materials for craniomaxillofacial osteosynthesis implants. Mater. Sci. Eng. C 2019, 103, 109826. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Schille, C.; Schweizer, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Mechanical Characteristics, In Vitro Degradation, Cytotoxicity, and Antibacterial Evaluation of Zn-4.0Ag Alloy as a Biodegradable Material. Int. J. Mol. Sci. 2018, 19, 109826. [Google Scholar] [CrossRef] [Green Version]
- Čapek, J.; Kubásek, J.; Pinc, J.; Fojt, J.; Krajewski, S.; Rupp, F.; Li, P. Microstructural, mechanical, in vitro corrosion and biological characterization of an extruded Zn-0.8Mg-0.2Sr (wt%) as an absorbable material. Mater. Sci. Eng. C 2021, 122, 111924. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Kimmerle-Müller, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Selection of extraction medium influences cytotoxicity of zinc and its alloys. Acta Biomater. 2019, 98, 235–245. [Google Scholar] [CrossRef]
- Loos, A. Biocompatibility Testing and Marketing Authorisation of Degradable Magnesium Implants, Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 331–353. [Google Scholar] [CrossRef]
- Willbold, E.; Weizbauer, A.; Loos, A.; Seitz, J.M.; Angrisani, N.; Windhagen, H.; Reifenrath, J. Magnesium alloys: A stony pathway from intensive research to clinical reality. Different test methods and approval—Related considerations. J. Biomed. Mater. Res. A 2017, 105, 329–347. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Spintzyk, S.; Schweizer, E.; Krajewski, S.; Alexander, D.; Dai, J.; Xu, S.; Wan, G.; Rupp, F. Impact of sterilization treatments on biodegradability and cytocompatibility of zinc-based implant materials. Mater. Sci. Eng. C. 2021, 130, 112430. [Google Scholar] [CrossRef]
- Semisch, A.; Hartwig, A. Copper ions interfere with the reduction of the water-soluble tetrazolium salt-8. Chem. Res. Toxicol. 2014, 27, 169–171. [Google Scholar] [CrossRef]
- Scarcello, E.; Lambremont, A.; Vanbever, R.; Jacques, P.J.; Lison, D. Mind your assays: Misleading cytotoxicity with the WST-1 assay in the presence of manganese. PLoS ONE 2020, 15, e0231634. [Google Scholar] [CrossRef] [Green Version]
- Al Hegy, A.; Smith, R.; Gauthier, E.R.; Gray-Munro, J.E. Investigation of a cyanine dye assay for the evaluation of the biocompatibility of magnesium alloys by direct and indirect methods. Bioact. Mater. 2020, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Su, Y.; Qin, Y.X.; Zheng, Y.; Wang, Y.; Zhu, D. Evolution of metallic cardiovascular stent materials: A comparative study among stainless steel, magnesium and zinc. Biomaterials 2020, 230, 119641. [Google Scholar] [CrossRef] [PubMed]
- Jablonská, E.; Vojtěch, D.; Fousová, M.; Kubásek, J.; Lipov, J.; Fojt, J.; Ruml, T. Influence of surface pre-treatment on the cytocompatibility of a novel biodegradable ZnMg alloy. Mater. Sci. Eng. C 2016, 68, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.K.; Kafri, A.; Ventura, Y.; Leon, A.; Vago, R.; Goldman, J.; Aghion, E. Surface stabilization treatment enhances initial cell viability and adhesion for biodegradable zinc alloys. Mater. Lett. 2019, 248, 130–133. [Google Scholar] [CrossRef]
- ISO 10993-12: 2012. Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- ISO 10993-5: 2009. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Guo, H.; Xia, D.; Zheng, Y.; Zhu, Y.; Liu, Y.; Zhou, Y. A pure zinc membrane with degradability and osteogenesis promotion for guided bone regeneration: In vitro and in vivo studies. Acta Biomater. 2020, 106, 396–409. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yang, H.T.; Li, X.; Zheng, Y.F. In Vitro Evaluation of the Feasibility of Commercial Zn Alloys as Biodegradable Metals. J. Mater. Sci. Technol. 2016, 32, 909–918. [Google Scholar] [CrossRef]
- Shearier, E.R.; Bowen, P.K.; He, W.; Drelich, A.; Drelich, J.; Goldman, J.; Zhao, F. In Vitro Cytotoxicity, Adhesion, and Proliferation of Human Vascular Cells Exposed to Zinc. ACS Biomater. Sci. Eng. 2016, 2, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Wang, K.; Gao, J.; Yang, Y.; Qin, Y.X.; Zheng, Y.; Zhu, D. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Maitz, M.F.; Chen, M.; Zhang, H.; Mao, J.; Zhao, Y.; Huang, N.; Wan, G. Comparative corrosion behavior of Zn with Fe and Mg in the course of immersion degradation in phosphate buffered saline. Corros. Sci. 2016, 111, 541–555. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Held, P. An Absorbance-Based Cytotoxicity Assay Using High Absorptivity, Water-Soluble Tetrazolium Salts, Application Note; BioTek Instruments, Inc.: Winooski, Vermont, 2009. [Google Scholar]
- Yang, Y.; Cheng, Y.; Peng, S.; Xu, L.; He, C.; Qi, F.; Zhao, M.; Shuai, C. Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold. Bioact. Mater. 2021, 6, 1230–1241. [Google Scholar] [CrossRef]
- Lin, J.; Tong, X.; Shi, Z.; Zhang, D.; Wen, C. A biodegradable Zn-1Cu-0.1Ti alloy with antibacterial properties for orthopedic applications. Acta Biomater. 2020, 106, 410–427. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Chen, J.; Li, P.; Xu, S. Limitation of Water-Soluble Tetrazolium Salt for the Cytocompatibility Evaluation of Zinc-Based Metals. Materials 2021, 14, 6247. https://doi.org/10.3390/ma14216247
Zhu P, Chen J, Li P, Xu S. Limitation of Water-Soluble Tetrazolium Salt for the Cytocompatibility Evaluation of Zinc-Based Metals. Materials. 2021; 14(21):6247. https://doi.org/10.3390/ma14216247
Chicago/Turabian StyleZhu, Peijun, Jiahao Chen, Ping Li, and Shulan Xu. 2021. "Limitation of Water-Soluble Tetrazolium Salt for the Cytocompatibility Evaluation of Zinc-Based Metals" Materials 14, no. 21: 6247. https://doi.org/10.3390/ma14216247
APA StyleZhu, P., Chen, J., Li, P., & Xu, S. (2021). Limitation of Water-Soluble Tetrazolium Salt for the Cytocompatibility Evaluation of Zinc-Based Metals. Materials, 14(21), 6247. https://doi.org/10.3390/ma14216247







