Microcapsule-Type Self-Healing Protective Coating That Can Maintain Its Healed State upon Crack Expansion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Selection of the Healing Agent
2.4. Microencapsulation
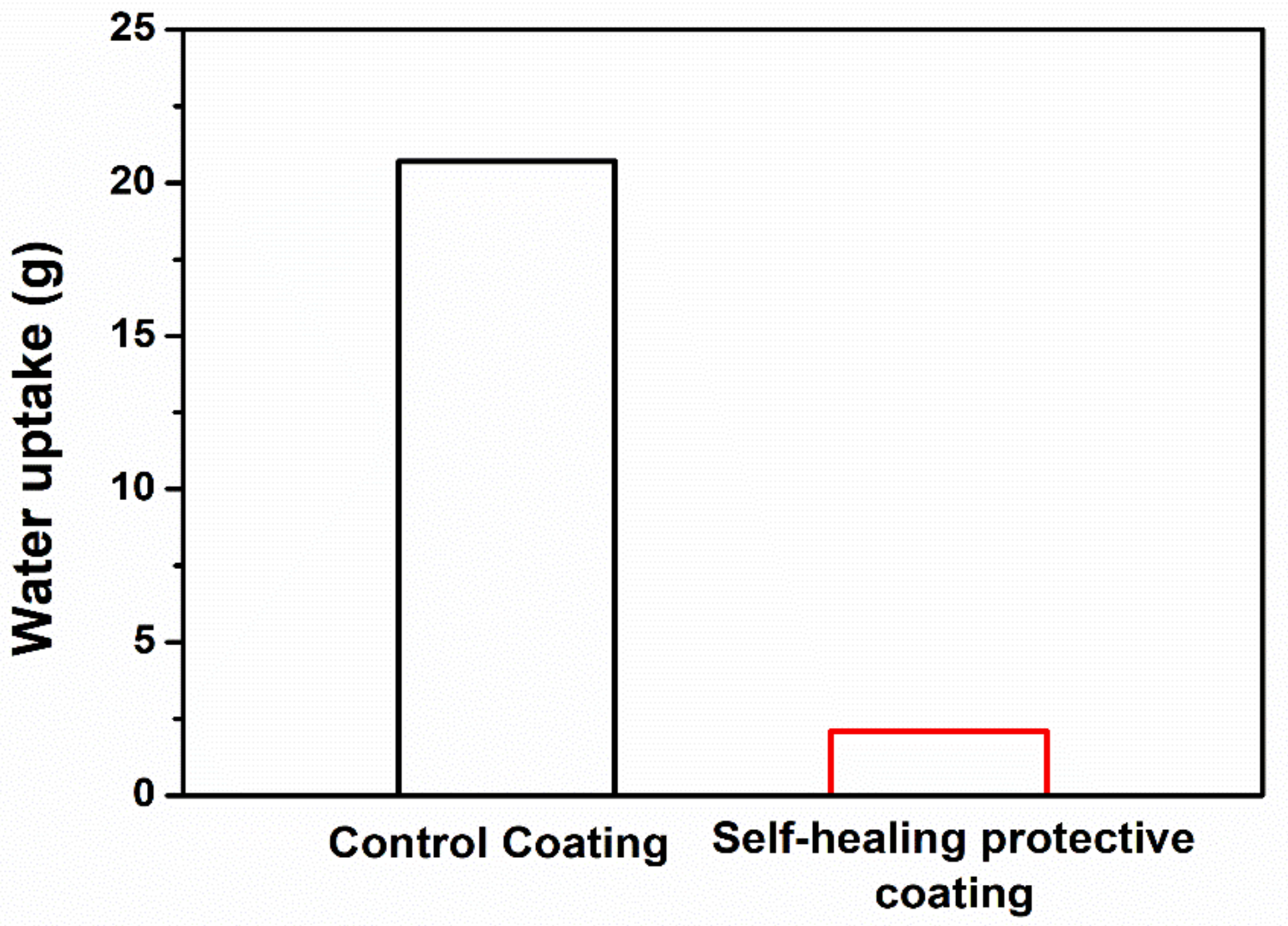
2.5. Evaluation of Primary Self-Healing Function by Water Sorptivity Test
2.6. Evaluation of the Capability to Maintain the Healed State
3. Results and Discussion
3.1. Preparation and Characterization of the Healing Agent
3.2. Microencapsulation
3.3. Primary Self-Healing Function
3.4. Capability of Maintaining the Healed State
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-healing polymers and composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2015, 3, 469–480. [Google Scholar] [CrossRef]
- Saji, V.S. Supramolecular concepts and approaches in corrosion and biofouling prevention. Corros. Rev. 2019, 37, 187–230. [Google Scholar] [CrossRef]
- Song, Y.K.; Jo, Y.H.; Cho, S.Y.; Yu, H.C.; Ryu, B.C.; Lee, S.I.; Chung, C.M. Sunlight-induced self-healing of a microcapsule-type protective coating. ACS Appl. Mater. Interfaces 2013, 5, 1378–1384. [Google Scholar] [CrossRef]
- Song, Y.K.; Lee, T.H.; Kim, J.C.; Lee, K.C.; Lee, S.H.; Noh, S.M.; Park, Y.I. Dual monitoring of cracking and healing in self-healing coatings using microcapsules loaded with two fluorescent dyes. Molecules 2019, 24, 1679. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Cao, K.; Wu, L. Synthesis of UV-responsive self-healing microcapsules and their potential application in aerospace coatings. ACS Appl. Mater. Interfaces 2019, 11, 33314–33322. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Liao, L.P.; Wang, S.J.; Li, W.J. Self-healing coatings containing microcapsule. Appl. Surf. Sci. 2012, 258, 1915–1918. [Google Scholar] [CrossRef]
- Hia, L.L.; Lam, W.H.; Chai, S.P.; Chan, E.S.; Pasbakhsh, P. Surface modified alginate multicore microcapsules and their application in self-healing epoxy coatings for metallic protection. Mater. Chem. Phys. 2018, 215, 69–80. [Google Scholar] [CrossRef]
- Li, H.; Cui, Y.; Li, Z.; Zhu, Y.; Wang, H. Fabrication of microcapsules containing dual-functional tung oil and properties suitable for self-healing and self-lubricating coatings. Prog. Org. Coat. 2018, 115, 164–171. [Google Scholar] [CrossRef]
- Lang, S.; Zhou, Q. Synthesis and characterization of poly(urea-formaldehyde) microcapsules containing linseed oil for self-healing coating development. Prog. Org. Coat. 2017, 105, 99–110. [Google Scholar] [CrossRef]
- Parsaee, S.; Mirabedini, M.; Farnood, R.; Alizadegan, F. Development of self-healing coatings based on urea-formaldehyde/polyurethane microcapsules containing epoxy resin. J. Appl. Polym. Sci. 2020, 137, 49663. [Google Scholar] [CrossRef]
- Yang, H.I.; Kim, D.M.; Yu, H.C.; Chung, C.M. Microcapsule-type organogel-based self-healing system having secondary damage preventing capability. ACS Appl. Mater. Interfaces 2016, 8, 11070–11075. [Google Scholar] [CrossRef]
- Kim, D.M.; Yu, H.C.; Yang, H.I.; Cho, Y.J.; Lee, K.M.; Chung, C.M. Microcapsule-type self-healing protective coating for cementitious composites with secondary crack preventing ability. Materials 2017, 10, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Sun, K.; Xu, Y.; Zhang, S.; Huang, X.; Zeng, S. Concree crack detection method based on optical fiber sensing network and microbending principle. Saf. Sci. 2019, 117, 299–304. [Google Scholar] [CrossRef]
- Su, H.; Wen, Z.; Li, P. Experimental study on PPP-BOTDA-based monitoring approach of concrete structure crack. Opt. Fiber Technol. 2021, 65, 102590. [Google Scholar] [CrossRef]
- He, Z.; Wu, C.; Hua, M.; Wu, S.; Wu, D.; Zhu, X.; Wang, J.; He, X. Bioinspired multifurnctional anti-icing hydrogel. Matter 2020, 2, 723–734. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, J.C.; Tieppo, A.; White, C.J.; Byrne, M.E. Adjusting biomaterial composition to achieve controlled multiple-day release of dexamethasone from an extended-wear silicone hydrogel contact lens. J. Biomater. Sci. Polym. Ed. 2014, 25, 88–100. [Google Scholar] [CrossRef]
- Huh, D.; Choi, H.-J.; Byun, M.; Kim, K.; Lee, H. Long-term analysis of PV module with large-area patterned anti-reflective film. Renew. Energy 2019, 135, 525–528. [Google Scholar] [CrossRef]
- Shin, J.-H.; Han, K.-S.; Lee, H. Anti-reflection and hydrophobic characteristics of M-PDMS based moth-eye nano-patterns on protection glass of photovoltaic systems. Prog. Photovolt. 2010, 19, 339–344. [Google Scholar] [CrossRef]
- Cai, Y.; Li, Y.; Wang, L. A facile synthesis of water-resistant CsPbBr3 perovskite quantum dots loaded poly(methyl methacrylate) composite microspheres based on in situ polymerization. Adv. Opt. Mater. 2019, 7, 1901075. [Google Scholar] [CrossRef]










| Sample Code a | BMT-PDMS | MMT-PDMS | State of Photoreaction Product |
|---|---|---|---|
| B-M-3-1 | 3 | 1 | Semi-solid |
| B-M-2-1 | 2 | 1 | Semi-solid |
| B-M-1.5-1 | 1.5 | 1 | Semi-solid |
| B-M-1-1 | 1 | 1 | Semi-solid |
| B-M-1-1.5 | 1 | 1.5 | High-viscous liquid |
| B-M-1-2 | 1 | 2 | Low-viscous liquid |
| B-M-1-3 | 1 | 3 | Low-viscous liquid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-S.; Kim, H.-W.; Lee, J.-S.; An, H.-S.; Chung, C.-M. Microcapsule-Type Self-Healing Protective Coating That Can Maintain Its Healed State upon Crack Expansion. Materials 2021, 14, 6198. https://doi.org/10.3390/ma14206198
Lee J-S, Kim H-W, Lee J-S, An H-S, Chung C-M. Microcapsule-Type Self-Healing Protective Coating That Can Maintain Its Healed State upon Crack Expansion. Materials. 2021; 14(20):6198. https://doi.org/10.3390/ma14206198
Chicago/Turabian StyleLee, Ji-Sun, Hyun-Woo Kim, Jun-Seo Lee, Hyun-Soo An, and Chan-Moon Chung. 2021. "Microcapsule-Type Self-Healing Protective Coating That Can Maintain Its Healed State upon Crack Expansion" Materials 14, no. 20: 6198. https://doi.org/10.3390/ma14206198
APA StyleLee, J.-S., Kim, H.-W., Lee, J.-S., An, H.-S., & Chung, C.-M. (2021). Microcapsule-Type Self-Healing Protective Coating That Can Maintain Its Healed State upon Crack Expansion. Materials, 14(20), 6198. https://doi.org/10.3390/ma14206198






