Quantum Chemical Analysis of the Corrosion Inhibition Potential by Aliphatic Amines
Abstract
:1. Introduction
2. Theory and Calculation Method
3. Results and Discussion
3.1. Molecular Geometry
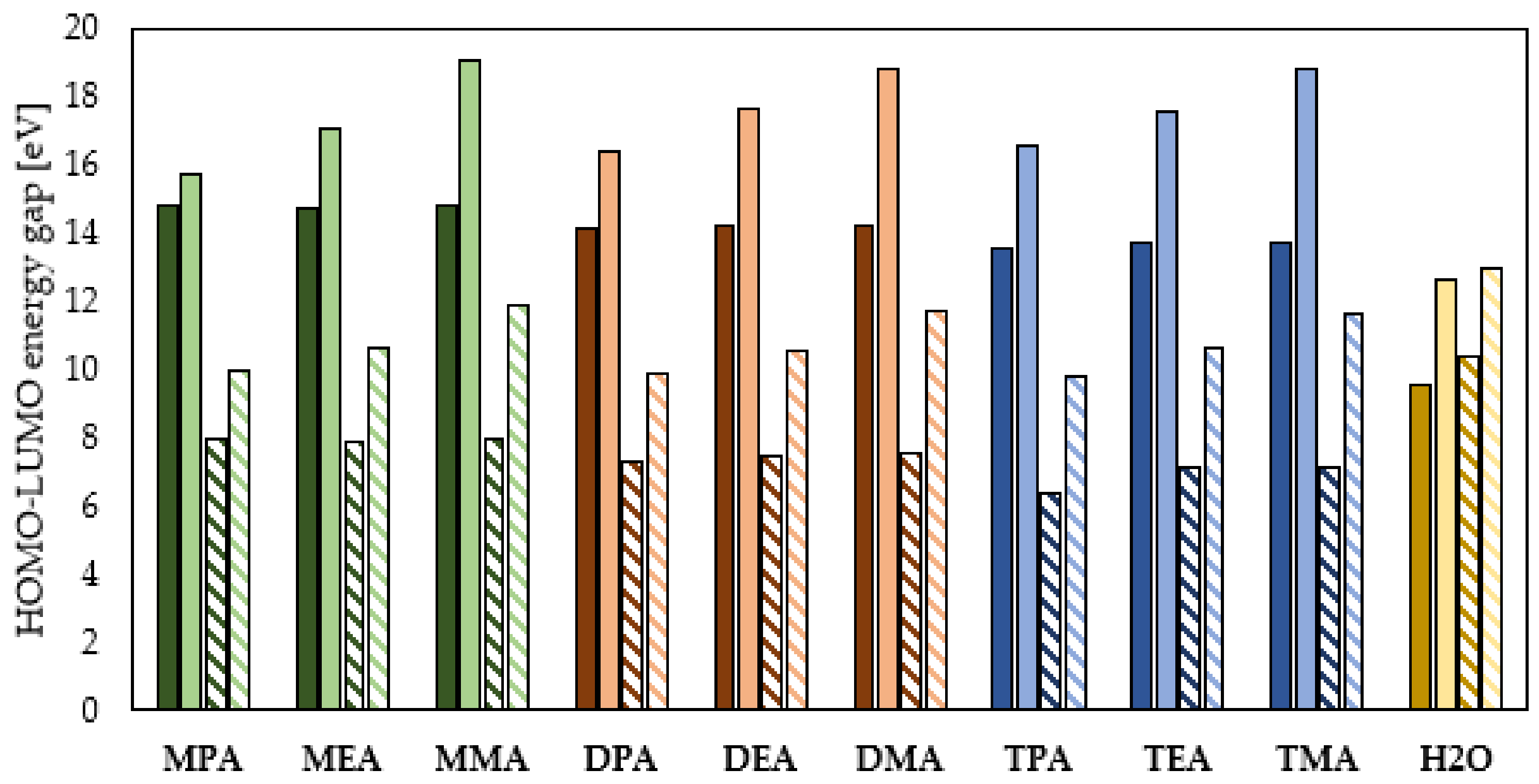
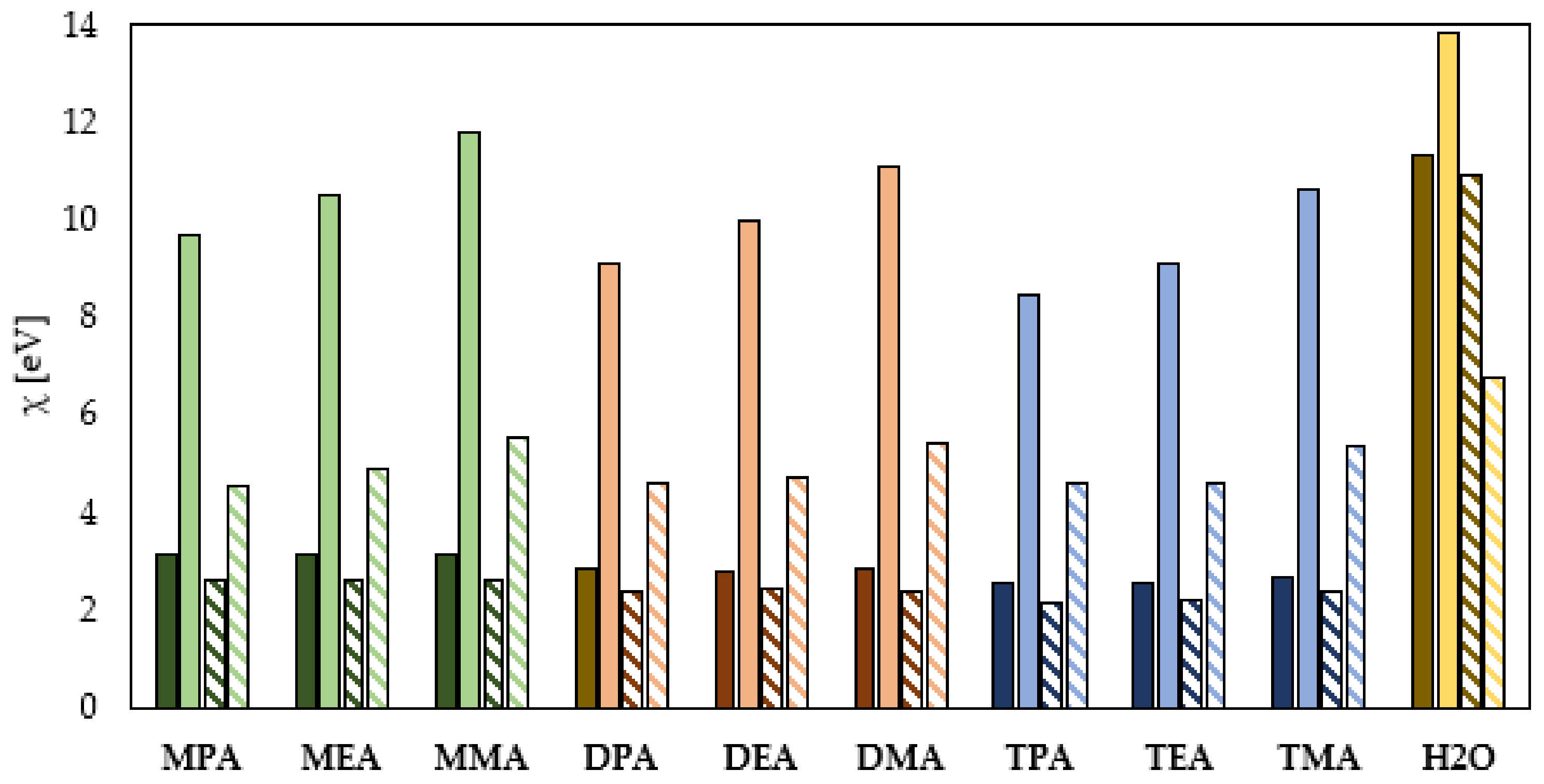
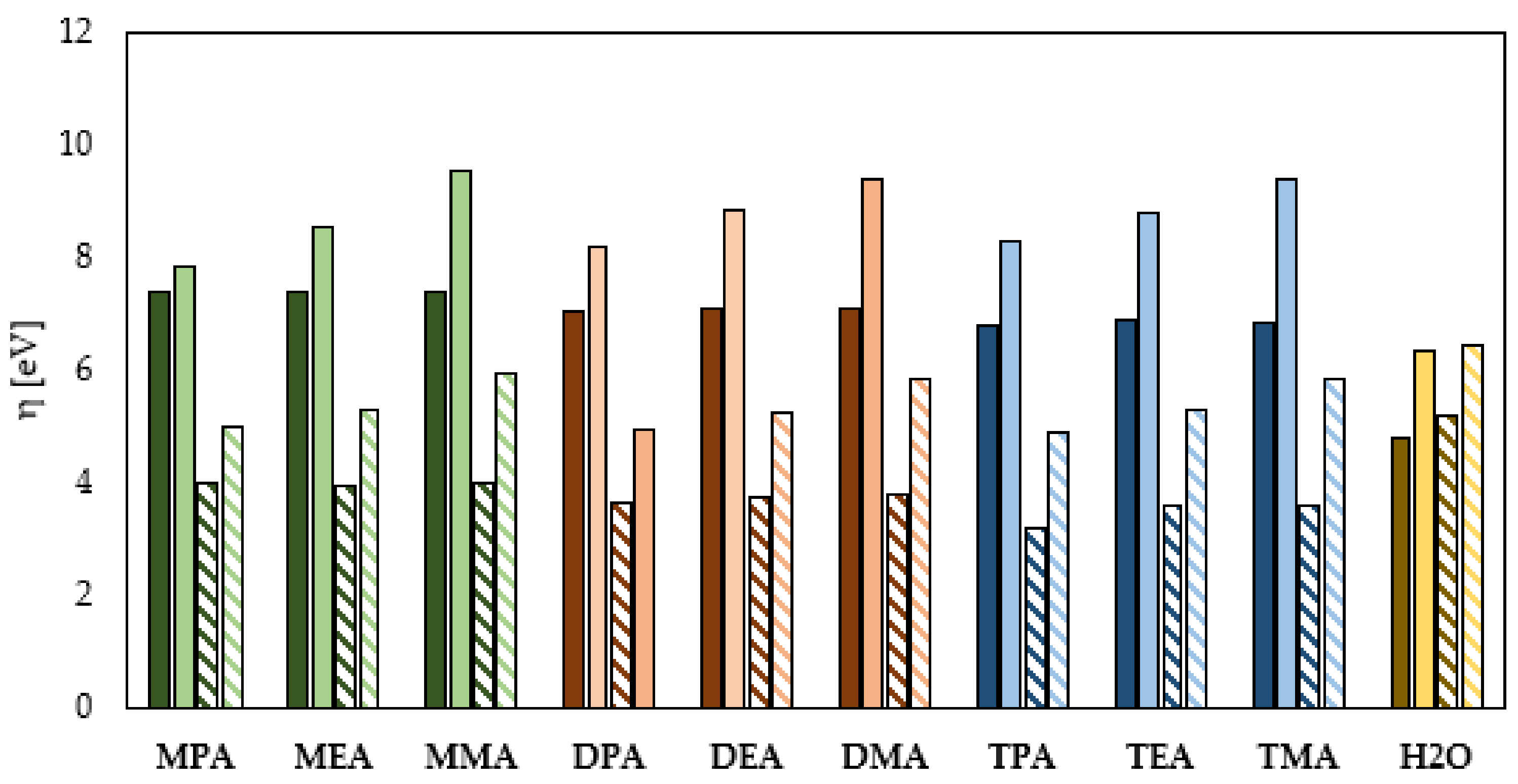
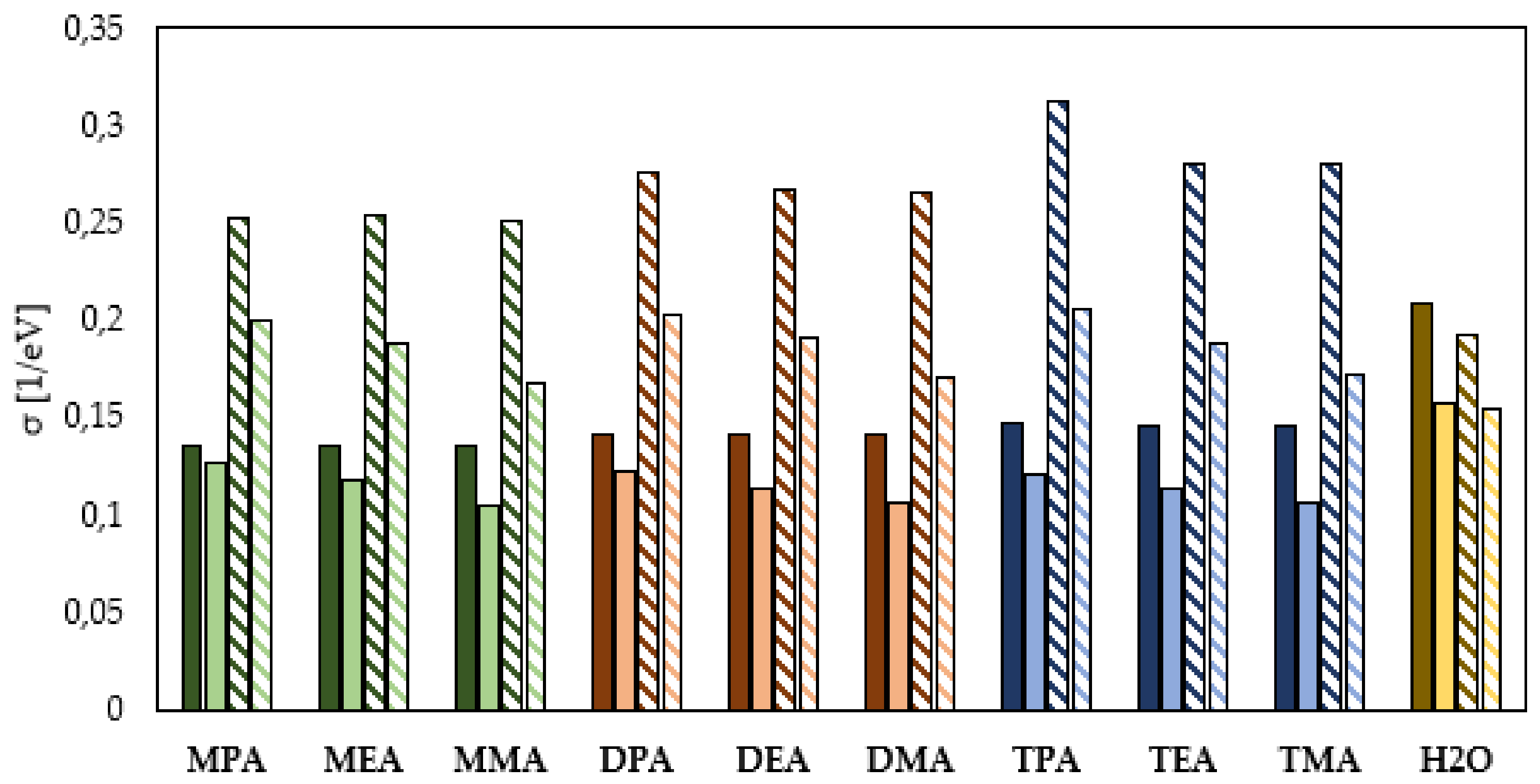
Global Molecular Reactivity
3.2. Electrostatic Interactions
3.3. Number of Transferred Electrons (ΔN) and Back-Donation Energy (ΔEb-d)
4. Comparison of DFT Studies with Experimental Findings
5. Conclusions
- The electron donating ability depends on the form of aliphatic amines in an aqueous solution and their subcategory (primary, secondary, tertiary), but not on the length of the hydrocarbon chains. The efficiency of electron donation to the conduction band of the protected metal is higher for the neutral form of the aliphatic amines and grows with the increase in the number of the hydrocarbon chains attached to the N atom.
- The protonation of amines leads to an increase in their ability to accept the electrons from the conduction band of the protected metal. Among the studied amines, the highest electron accepting ability is characteristic for the primary amines, and this ability decreases with an increasing number of the hydrocarbon chains attached to the nitrogen atom.
- The HOMO–LUMO energy gap indicates that the neutral aliphatic amines have lower kinetic stability and higher chemical reactivity and can therefore more efficiently form a protective film on the surface of the metal to be protected.
- The protonation reaction of the aliphatic amines leads to a deformation of their electron cloud and consequently an improved ability to inhibit the corrosion process. This effect is most evident for the primary amines.
- The electronegativity values determined from EHOMO and ELUMO and the HSAB theory indicate that the protonation reaction of the aliphatic amines negatively affects their corrosion inhibition performance.
- The Mulliken population analysis indicates that the electrostatic interactions of the tested corrosion inhibitors occur via the N atoms. The protonation reaction causes a weakening of the electrostatic interactions between the N atom and the surface of the protected metal. The increase in the partial charge of the N atom depends on the amine subcategory and increases as follows: primary amines > secondary amines > tertiary amines.
- The quantum chemical calculations performed using the COSMO model confirm the conclusions of the gas phase studies regarding the influence of the structure and subcategory of aliphatic amines on their ability to inhibit the corrosion process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamadi, L.; Mansouri, S.; Oulmi, K.; Kareche, A. The use of amino acids as corrosion inhibitors for metals: A review. Egypt. J. Pet. 2018, 27, 1157–1165. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.L.; Ma, T.F.; Gao, L.X.; Zhang, D.Q.; Wei, G.A.; Yan, H.B.; Wei, S.L. Vapor phase assembly of urea–amine compounds and their protection against the atmospheric corrosion of carbon steel. J. Coat. Technol. Res. 2020, 17, 503–515. [Google Scholar] [CrossRef]
- Saha, S.K.; Murmu, M.; Murmu, N.C.; Obot, I.B.; Banerjee, P. Molecular level insights for the corrosion inhibition effectiveness of three amine derivatives on the carbon steel surface in the adverse medium: A combined density functional theory and molecular dynamics simulation study. Surf. Interfaces 2018, 10, 65–73. [Google Scholar] [CrossRef]
- Ali, A.A.I.; El-dougdoug, W.I.A. Green Chemistry Letters and Reviews Preparation and evaluation of amido poly amine surfactant based on Melia azedarach seeds oil as corrosion inhibitor of C-steel in 2.0 M HCl pickling medium. Green Chem. Lett. Rev. 2017, 10, 346–358. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhu, S.; Zhang, S.; He, Q.; Li, W. Theoretical studies of three triazole derivatives as corrosion inhibitors for mild steel in acidic medium. Corros. Sci. 2014, 87, 366–375. [Google Scholar] [CrossRef]
- Topal, E.; Gece, G. Untangling the inhibition effects of aliphatic amines on silver corrosion: A computational study. Chem. J. Mold. 2017, 12, 64–70. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Jafar Mazumder, M.A.; Ali, S.A.; Aljeaban, N.A.; Alharbi, B.G. Design and synthesis of a novel corrosion inhibitor embedded with quaternary ammonium, amide and amine motifs for protection of carbon steel in 1 M HCl. J. Mol. Liq. 2020, 317, 113917. [Google Scholar] [CrossRef]
- Rihan, R.; Shawabkeh, R.; Al-Bakr, N. The effect of two amine-based corrosion inhibitors in improving the corrosion resistance of carbon steel in sea water. J. Mater. Eng. Perform. 2014, 23, 693–699. [Google Scholar] [CrossRef]
- Lai, Y.; Gao, Y.; Jin, Y.; Wen, L. Study of methionine as green corrosion inhibitor for TWIP steel in neutral chloride solution Study of methionine as green corrosion inhibitor for TWIP steel in neutral chloride solution. Mater. Res. Express 2021, 8, 046533. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wu, Y. The inhibition effect and mechanism of l-cysteine on the corrosion of bronze covered with a CuCl patina. Corros. Sci. 2015, 97, 89–99. [Google Scholar] [CrossRef]
- Wang, D.; Gao, L.; Zhang, D.; Yang, D.; Wang, H.; Lin, T. Experimental and theoretical investigation on corrosion inhibition of AA5052 aluminium alloy by l-cysteine in alkaline solution. Mater. Chem. Phys. 2016, 169, 142–151. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, N.; Zhang, W.; Huang, X.; Ruan, L.; Wu, L. Inhibition of carbon steel corrosion in phase-change-materials solution by methionine and proline. Corros. Sci. 2016, 111, 675–689. [Google Scholar] [CrossRef]
- Nady, H. Tricine [N-(Tri(hydroxymethyl)methyl)glycine]—A novel green inhibitor for the corrosion inhibition of zinc in neutral aerated sodium chloride solution. Egypt. J. Pet. 2017, 26, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.A.; Khaled, K.F. Copper corrosion inhibition in O2-saturated H2SO4 solutions. Corros. Sci. 2010, 52, 1194–1204. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Bazzi, L.; El Issami, S. The role of pH in corrosion inhibition of tin using the proline amino acid: Theoretical and experimental investigations. RSC Adv. 2020, 10, 29696–29704. [Google Scholar] [CrossRef]
- Caldona, E.B.; Wipf, D.O.; Smith, D.W. Characterization of a tetrafunctional epoxy-amine coating for corrosion protection of mild steel. Prog. Org. Coat. 2021, 151, 106045. [Google Scholar] [CrossRef]
- Malinowski, S.; Jaroszyńska-Wolińska, J.; Herbert, T. Theoretical predictions of anti-corrosive properties of THAM and its derivatives. J. Mol. Model. 2018, 24, 1. [Google Scholar] [CrossRef]
- Farahati, R.; Behzadi, H.; Mousavi-Khoshdel, S.M.; Ghaffarinejad, A. Evaluation of corrosion inhibition of 4-(pyridin-3-yl) thiazol-2-amine for copper in HCl by experimental and theoretical studies. J. Mol. Struct. 2020, 1205, 127658. [Google Scholar] [CrossRef]
- Sığırcık, G.; Yildirim, D.; Tüken, T. Synthesis and inhibitory effect of N,N’-bis(1-phenylethanol)ethylenediamine against steel corrosion in HCl Media. Corros. Sci. 2017, 120, 184–193. [Google Scholar] [CrossRef]
- Shahraki, M.; Dehdab, M.; Elmi, S. Theoretical studies on the corrosion inhibition performance of three amine derivatives on carbon steel: Molecular dynamics simulation and density functional theory approaches. J. Taiwan Inst. Chem. Eng. 2016, 62, 313–321. [Google Scholar] [CrossRef]
- Asegbeloyin, J.N.; Ejikeme, P.M.; Olasunkanmi, L.O.; Adekunle, A.S.; Ebenso, E.E. A novel schiffbase of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran- 2-one and 2,2′-(ethylenedioxy)diethylamine as potential corrosion inhibitor for mild steel in acidic medium. Materials 2015, 8, 2918–2934. [Google Scholar] [CrossRef] [Green Version]
- Al-Sabagh, A.M.; Nasser, N.M.; Farag, A.A.; Migahed, M.A.; Eissa, A.M.F.; Mahmoud, T. Structure effect of some amine derivatives on corrosion inhibition efficiency for carbon steel in acidic media using electrochemical and Quantum Theory Methods. Egypt. J. Pet. 2013, 22, 101–116. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Pearson, P.; Yang, Q.; Puxty, G.; Feron, P.; Xiao, D. A study of designer amine 4-amino-1-propyl-piperidine against the corrosion of carbon steel for application in CO2 capture. Int. J. Greenh. Gas Control 2020, 94, 102929. [Google Scholar] [CrossRef]
- Campbell, K.L.S.; Zhao, Y.; Hall, J.J.; Williams, D.R. The effect of CO2-loaded amine solvents on the corrosion of a carbon steel stripper. Int. J. Greenh. Gas Control 2016, 47, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Iroha, N.B.; Dueke-Eze, C.U.; James, A.O.; Fasina, T.M. Newly synthesized N-(5-nitro-2-hydroxybenzylidene)pyridine-4-amine as a high-potential inhibitor for pipeline steel corrosion in hydrochloric acid medium. Egypt. J. Pet. 2021, 30, 55–61. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, S.; Sharma, U.; Yadav, P.N. Substituted amines as corrosion inhibitors for N80 steel in 15% HCl. J. Mater. Environ. Sci. 2013, 4, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Boughoues, Y.; Benamira, M.; Messaadia, L.; Bouider, N.; Abdelaziz, S. Experimental and theoretical investigations of four amine derivatives as effective corrosion inhibitors for mild steel in HCl medium. RSC Adv. 2020, 10, 24145–24158. [Google Scholar] [CrossRef]
- Shihab, M.S.; Al-Doori, H.H. Experimental and theoretical study of [N-substituted] p-aminoazobenzene derivatives as corrosion inhibitors for mild steel in sulfuric acid solution. J. Mol. Struct. 2014, 1076, 658–663. [Google Scholar] [CrossRef]
- Khadom, A.A. Quantum chemical calculations of some amines corrosion inhibitors/ copper alloy interaction in hydrochloric acid. J. Mater. Environ. Sci. 2017, 8, 1153–1160. [Google Scholar]
- Kumar, H.; Kumari, M. Experimental and theoretical investigation of 3,3′-diamino dipropyl amine: Highly efficient corrosion inhibitor for carbon steel in 2 N HCl at normal and elevated temperatures. J. Mol. Struct. 2021, 1229, 129598. [Google Scholar] [CrossRef]
- Tuzun, B.; Bhawsar, J. Quantum chemical study of thiaozole derivatives as corrosion inhibitors based on density functional theory. Arab. J. Chem. 2021, 14, 102927. [Google Scholar] [CrossRef]
- Sait, N.; Aliouane, N.; Toukal, L.; Hammache, H.; Al-Noaimi, M.; Helesbeux, J.J.; Duval, O. Synthesis of ethylene bis [(2-hydroxy-5,1,3-phenylene) bis methylene] tetraphosphonic acid and their anticorrosive effect on carbon steel in 3% NaCl solution. J. Mol. Liq. 2021, 326, 115316. [Google Scholar] [CrossRef]
- Khnifira, M.; Mahsoune, A.; Belghiti, M.E.; Khamar, L.; Sadiq, M.; Abdennouri, M.; Barka, N. HF and SiF4 adsorption on carbon graphite (1 1 1) surface in aqueous medium: A combined DFT and MD simulation approach. Mater. Today Proc. 2020, 37, 3987–3993. [Google Scholar] [CrossRef]
- Wichmann, K.A.; Boyd, P.D.W.; Söhnel, T.; Allen, G.R.; Phillips, A.R.J.; Cooper, G.J.S. Characterization of dicarboxylic salts of protonated triethylenetetramine useful for the treatment of copper-related pathologies. Cryst. Growth Des. 2007, 7, 1844–1850. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Ding, K.; Zhang, Y.; Chen, W.; Lin, W. A boron-decorated melon-based carbon nitride as a metal-free photocatalyst for N2 fixation: A DFT study. Phys. Chem. Chem. Phys. 2020, 22, 21872–21880. [Google Scholar] [CrossRef]
- El Adnani, Z.; Mcharfi, M.; Sfaira, M.; Benzakour, M.; Benjelloun, A.T.; Ebn Touhami, M.; Hammouti, B.; Taleb, M. DFT study of 7-R-3methylquinoxalin-2(1H)-ones (R=H; CH 3; Cl) as corrosion inhibitors in hydrochloric acid. Int. J. Electrochem. Sci. 2012, 7, 6738–6751. [Google Scholar]
- Khadiri, A.; Saddik, R.; Bekkouche, K.; Aouniti, A.; Hammouti, B.; Benchat, N.; Bouachrine, M.; Solmaz, R. Gravimetric, electrochemical and quantum chemical studies of some pyridazine derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. J. Taiwan Inst. Chem. Eng. 2016, 58, 552–564. [Google Scholar] [CrossRef]
- Wazzan, N.A. DFT calculations of thiosemicarbazide, arylisothiocynates, and 1-aryl-2,5-dithiohydrazodicarbonamides as corrosion inhibitors of copper in an aqueous chloride solution. J. Ind. Eng. Chem. 2015, 26, 291–308. [Google Scholar] [CrossRef]
- Diki, N.Y.S.; Gbassi, G.K.; Ouedraogo, A.; Berte, M.; Trokourey, A. Aluminum corrosion inhibition by cefixime drug: Experimental and DFT studies. J. Electrochem. Sci. Eng. 2018, 8. [Google Scholar] [CrossRef]
- Migahed, M.A.; Attia, A.A.; Habib, R.E. Study on the efficiency of some amine derivatives as corrosion and scale inhibitors in cooling water systems. RSC Adv. 2015, 5, 57254–57262. [Google Scholar] [CrossRef]
- Zor, S.; Kandemirli, F.; Bingul, M. Inhibition effects of methionine and tyrosine on corrosion of iron in HCl solution: Electrochemical, FTIR, and quantum-chemical study. Prot. Met. Phys. Chem. Surf. 2009, 45, 46–53. [Google Scholar] [CrossRef]
- Paul, P.K.; Yadav, M. Investigation on corrosion inhibition and adsorption mechanism of triazine-thiourea derivatives at mild steel / HCl solution interface: Electrochemical, XPS, DFT and Monte Carlo simulation approach. J. Electroanal. Chem. 2020, 877, 114599. [Google Scholar] [CrossRef]

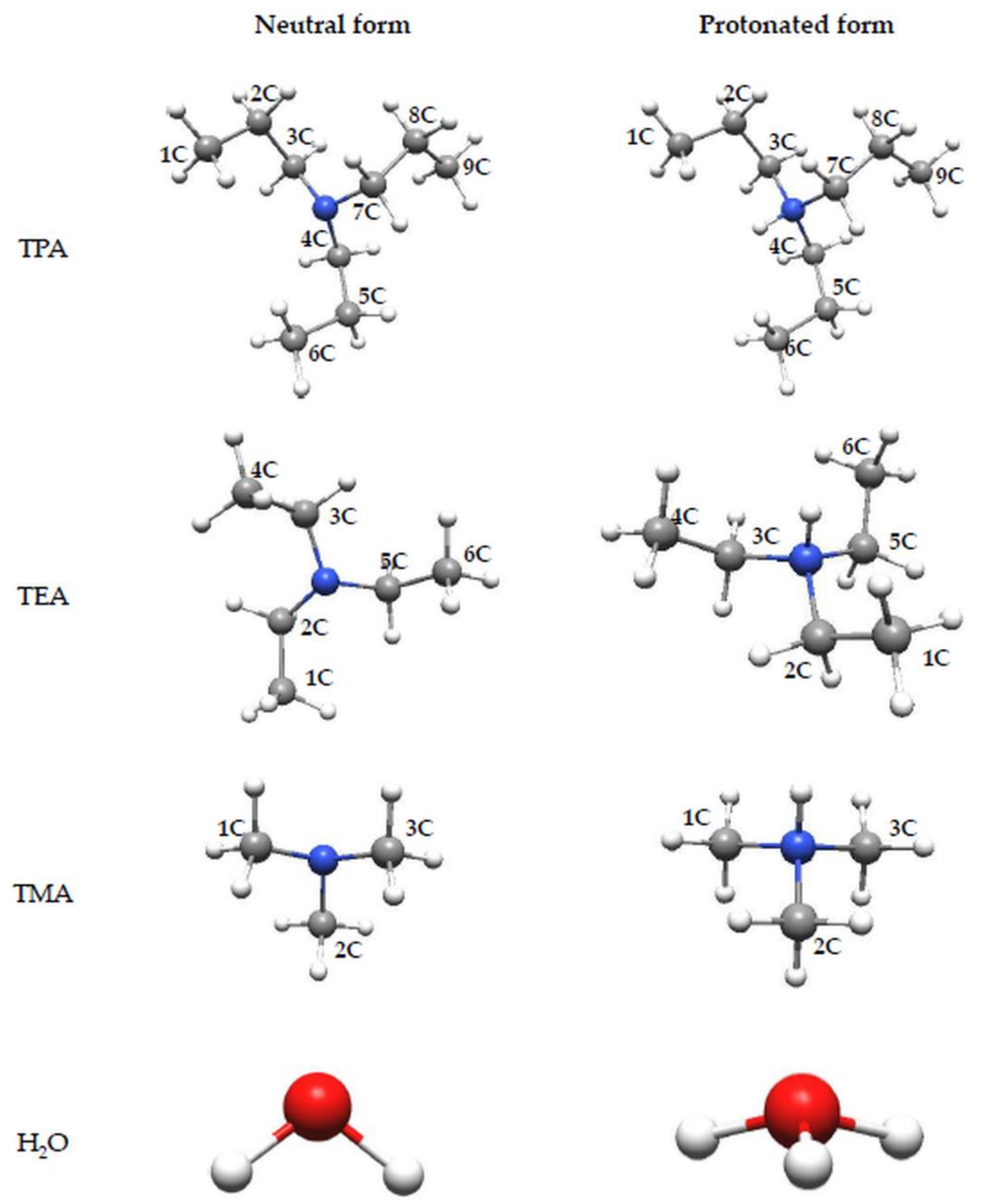

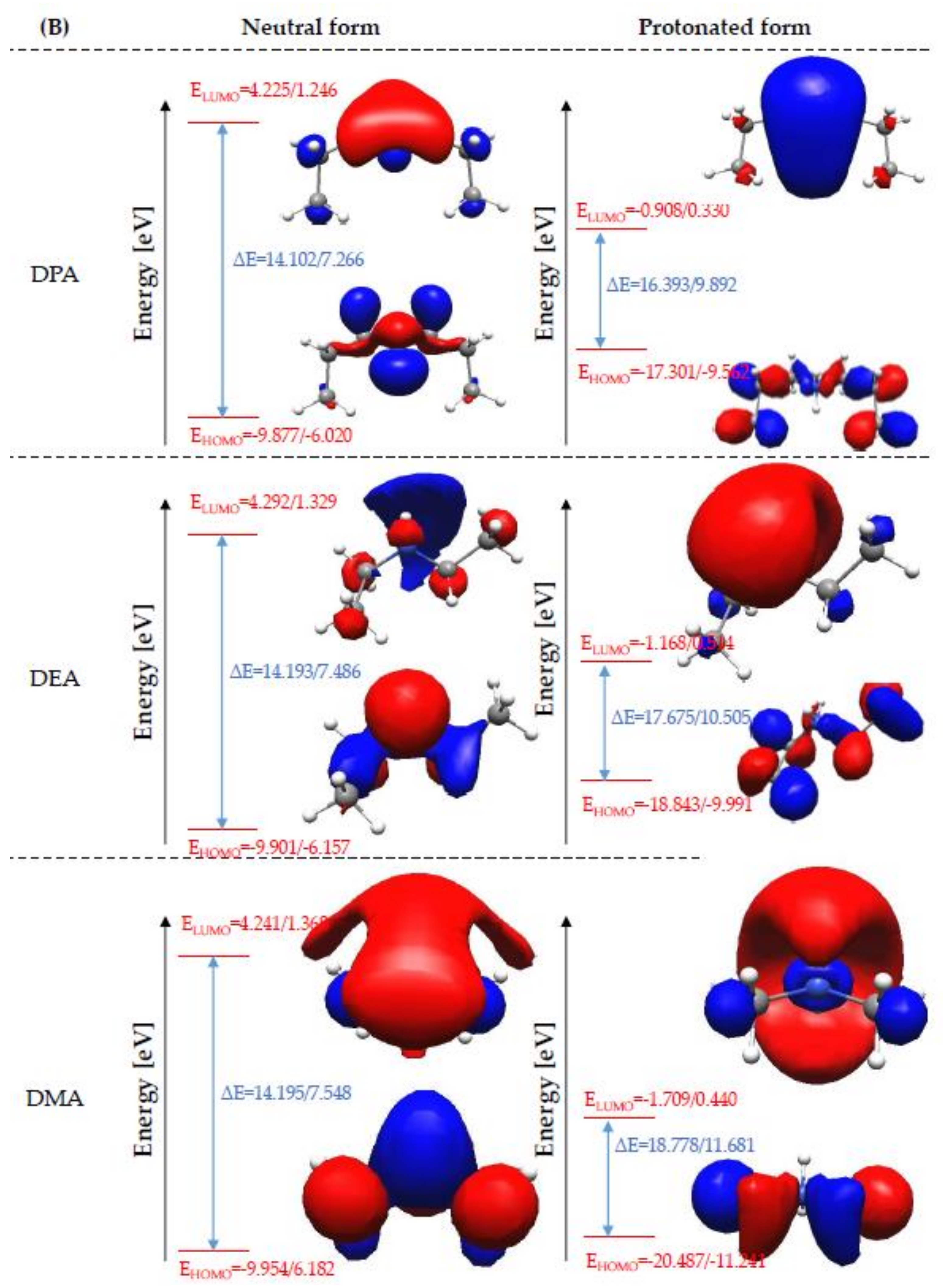
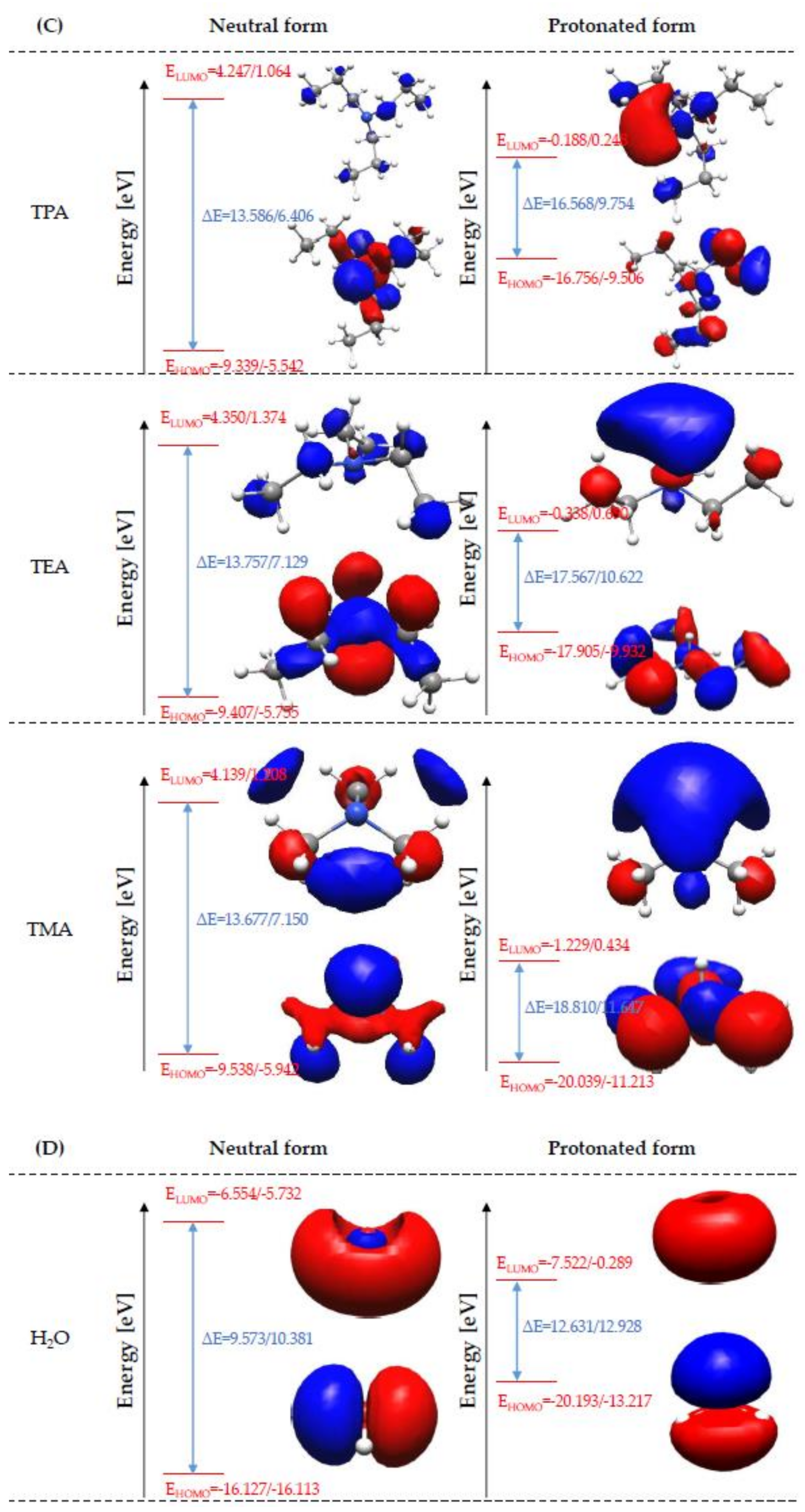
| Amine | Bond Length (Å) | Bond Angle (°) | ||||
|---|---|---|---|---|---|---|
| Gas Phase | ||||||
| Neutral Form | Protonated Form | Neutral Form | Protonated Form | |||
| MPA | 1C-N 2C-1C 3C-2C | 1.47 1.53 1.53 | 1.53 1.52 1.53 | 2C-1C-N 3C-2C-1C | 110.71 113.14 | 110.90 111.03 |
| MEA | 1C-N 2C-1C | 1.47 1.53 | 1.53 1.52 | 2C-1C-N | 110.38 | 110.74 |
| MMA | 1C-N | 1.47 | 1.52 | - | - | - |
| DPA | 1C-2C 2C-3C 3C-N N-4C 4C-5C 5C-6C | 1.53 1.53 1.47 1.46 1.53 1.53 | 1.53 1.52 1.52 1.52 1.53 1.53 | 1C-2C-3C 2C-3C-N 3C-N-4C N-4C-5C 4C-5C-6C | 113.89 111.92 113.75 111.92 113.90 | 114.51 111.79 115.47 111.79 114.51 |
| DEA | 1C-2C 2C-N N-3C 3C-4C | 1.53 1.47 1.46 1.53 | 1.52 1.52 1.52 1.52 | 1C-2C-N 2C-N-3C N-3C-4C | 112.38 114.76 110.86 | 112.51 116.44 110.99 |
| DMA | 1C-N N-2C | 1.46 1.46 | 1.51 1.51 | 1C-N-2C | 113.01 | 114.41 |
| TPA | 1C-2C 2C-3C 3C-N N-4C 4C-5C 5C-6C N-7C 7C-8C 8C-9C | 1.53 1.54 1.46 1.47 1.54 1.53 1.47 1.55 1.53 | 1.53 1.53 1.53 1.53 1.53 1.53 1.52 1.52 1.53 | 1C-2C-3C 2C-3C-N 3C-N-4C 3C-N-7C N-4C-5C 4C-5C-6C N-7C-8C 7C-8C-9C | 113.85 113.99 114.32 115.16 114.18 113.90 119.15 116.47 | 114.55 113.97 114.98 111.49 117.48 117.88 113.73 114.40 |
| TEA | 1C-2C 2C-N N-3C 3C-4C N-5C 5C-6C | 1.53 1.47 1.47 1.53 1.47 1.53 | 1.52 1.52 1.52 1.52 1.52 1.52 | 1C-2C-N 2C-N-3C 2C-N-5C 3C-N-5C N-3C-4C N-5C-6C | 113.09 112.11 112.12 112.11 113.09 113.10 | 112.69 111.94 112.69 111.94 112.70 111.94 |
| TMA | 1C-N 2C-N 3C-N | 1.46 1.46 1.46 | 1.51 1.51 1.51 | 1C-N-2C 2C-N-3C 1C-N-3C | 111.64 111.64 111.64 | 111.80 111.80 111.79 |
| Water Phase | ||||||
| Bond Length (Å) | Bond Angle (°) | |||||
| Neutral Form | Protonated Form | Neutral Form | Protonated Form | |||
| MPA | 1C-N 2C-1C 3C-2C | 1.48 1.53 1.53 | 1.51 1.52 1.53 | 2C-1C-N 3C-2C-1C | 110.73 112.96 | 110.82 111.26 |
| MEA | 1C-N 2C-1C | 1.48 1.53 | 1.51 1.52 | 2C-1C-N | 110.40 | 110.61 |
| MMA | 1C-N | 1.48 | 1.50 | - | ||
| DPA | 1C-2C 2C-3C 3C-N N-4C 4C-5C 5C-6C | 1.53 1.53 1.47 1.47 1.53 1.53 | 1.53 1.52 1.51 1.51 1.52 1.53 | 1C-2C-3C 2C-3C-N 3C-N-4C N-4C-5C 4C-5C-6C | 114.00 117.79 112.66 111.81 114.00 | 114.28 111.58 113.01 111.60 114.28 |
| DEA | 1C-2C 2C-N N-3C 3C-4C | 1.53 1.48 1.48 1.53 | 1.52 1.52 1.49 1.52 | 1C-2C-N 2C-N-3C N-3C-4C | 112.32 114.39 110.92 | 112.22 116.28 110.70 |
| DMA | 1C-N N-2C | 1.47 1.47 | 1.50 1.50 | 1C-N-2C | 111.71 | 113.55 |
| TPA | 1C-2C 2C-3C 3C-N N-4C 4C-5C 5C-6C N-7C 7C-8C 8C-9C | 1.53 1.53 1.47 1.47 1.54 1.53 1.46 1.55 1.53 | 1.53 1.53 1.53 1.53 1.54 1.53 1.51 1.53 1.53 | 1C-2C-3C 2C-3C-N 3C-N-4C 3C-N-7C N-4C-5C 4C-5C-6C N-7C-8C 7C-8C-9C | 113.75 113.82 112.99 112.99 114.20 113.89 119.46 116.48 | 114.22 114.09 114.36 115.61 113.99 114.03 116.91 116.71 |
| TEA | 1C-2C 2C-N N-3C 3C-4C N-5C 5C-6C | 1.53 1.47 1.48 1.53 1.46 1.53 | 1.52 1.52 1.51 1.52 1.52 1.52 | 1C-2C-N 2C-N-3C 2C-N-5C 3C-N-5C N-3C-4C N-5C-6C | 112.92 111.24 112.18 112.06 113.01 113.06 | 112.93 111.85 111.11 112.00 112.60 112.45 |
| TMA | 1C-N 2C-N 3C-N | 1.47 1.47 1.47 | 1.50 1.50 1.50 | 1C-N-2C 2C-N-3C 1C-N-3C | 110.49 111.35 111.36 | 111.63 111.63 111.63 |
| Amine | µ (Debye) | |||
|---|---|---|---|---|
| Gas Phase | Water Phase | |||
| Neutral Form | Protonated Form | Neutral Form | Protonated Form | |
| MPA | 1.34 | 6.74 | 1.77 | 8.15 |
| MEA | 1.41 | 4.14 | 1.83 | 5.12 |
| MMA | 1.46 | 2.34 | 1.82 | 2.96 |
| DPA | 1.04 | 1.90 | 1.43 | 2.40 |
| DEA | 1.02 | 1.97 | 1.41 | 2.69 |
| DMA | 1.10 | 1.70 | 1.39 | 2.26 |
| TPA | 0.69 | 0.99 | 0.78 | 1.44 |
| TEA | 0.69 | 0.41 | 0.89 | 1.49 |
| TMA | 0.69 | 1.03 | 0.81 | 1.43 |
| Atom | Neutral Amine | Ionized Amine | Atom | Neutral Amine | Ionized Amine | ||
|---|---|---|---|---|---|---|---|
| Primary amine | MMA | N | −0.472 | −0.324 | 1C | −0.205 | −0.210 |
| MEA | N | −0.457 | −0.323 | 1C 2C | −0.119 −0.297 | −0.162 −0.292 | |
| MPA | N | −0.457 | −0.329 | 1C 2C 3C | −0.095 −0.227 −0.299 | −0.131 −0.253 −0.292 | |
| Secondary amine | DMA | N | −0.385 | −0.327 | 1C 2C | −0.193 −0.193 | −0.197 −0.197 |
| DEA | N | −0.379 | −0.332 | 1C 2C 3C 4C | −0.279 −0.193 −0.193 −0.302 | −0.303 −0.142 −0.141 −0.302 | |
| DPA | N | −0.383 | −0.339 | 1C 2C 3C 4C 5C 6C | −0.283 −0.241 −0.080 −0.080 −0.241 −0.283 | −0.316 −0.252 −0.112 −0.112 −0.252 −0.317 | |
| Tertiary amine | TMA | N | −0.331 | −0.333 | 1C 2C 3C | −0.182 −0.182 −0.182 | −0.190 −0.190 −0.190 |
| TEA | N | −0.353 | −0.342 | 1C 2C 3C 4C 5C 6C | −0.266 −0.113 −0.113 −0.226 −0.113 −0.226 | −0.298 −0.138 −0.138 −0.298 −0.138 −0.298 | |
| TPA | N | −0.368 | −0.368 | 1C 2C 3C 4C 5C 6C 7C 8C 9C | −0.280 −0.238 −0.081 −0.074 −0.224 −0.289 −0.100 −0.224 −0.295 | −0.289 −0.282 −0.101 −0.100 −0.282 −0.289 −0.101 −0.282 −0.289 | |
| H2O | O | −0.474 | −0.208 | - | - | - | |
| Atom | Neutral Amine | Ionized Amine | Atom | Neutral Amine | Ionized Amine | ||
|---|---|---|---|---|---|---|---|
| Primary amine | MMA | N | −0.545 | −0.288 | 1C | −0.218 | −0.204 |
| MEA | N | −0.528 | −0.292 | 1C 2C | −0.119 −0.316 | −0.134 −0.313 | |
| MPA | N | −0.530 | −0.298 | 1C 2C 3C | −0.010 −0.232 −0.320 | −0.106 −0.242 −0.316 | |
| Secondary amine | DMA | N | −0.443 | −0.297 | 1C 2C | −0.209 −0.209 | −0.194 −0.194 |
| DEA | N | −0.430 | −0.319 | 1C 2C 3C 4C | −0.318 −0.103 −0.121 −0.300 | −0.317 −0.115 −0.120 −0.316 | |
| DPA | N | −0.409 | −0.331 | 1C 2C 3C 4C 5C 6C | −0.307 −0.246 −0.087 −0.087 −0.246 −0.307 | −0.323 −0.251 −0.094 −0.094 −0.251 −0.323 | |
| Tertiary amine | TMA | N | −0.368 | −0.318 | 1C 2C 3C | −0.203 −0.203 −0.203 | −0.189 −0.189 −0.189 |
| TEA | N | −0.370 | −0.350 | 1C 2C 3C 4C 5C 6C | −0.288 −0.117 −0.117 −0.288 −0.117 −0.288 | −0.307 −0.124 −0.124 −0.307 −0.124 −0.307 | |
| TPA | N | −0.380 | −0.362 | 1C 2C 3C 4C 5C 6C 7C 8C 9C | −0.314 −0.239 −0.111 −0.077 −0.243 −0.300 −0.088 −0.228 −0.302 | −0.330 −0.250 −0.108 −0.134 −0.255 −0.324 −0.108 −0.228 −0.302 | |
| H2O | O | −0.570 | −0.215 | - | - | - | |
| Amine | ΔN | Eb-d (eV) | ||||||
|---|---|---|---|---|---|---|---|---|
| Gas Phase | Water Phase | Gas Phase | Water Phase | |||||
| Neutral Form | Protonated Form | Neutral Form | Protonated Form | Neutral Form | Protonated Form | Neutral Form | Protonated Form | |
| MPA | 0.32 | −0.12 | 0.67 | 0.34 | 1.85 | 1.96 | 0.99 | 1.25 |
| MEA | 0.32 | −0.12 | 0.67 | 0.29 | 1.84 | 2.13 | 0.98 | 1.32 |
| MMA | 0.32 | −0.20 | 0.67 | 0.20 | 1.85 | 2.38 | 0.99 | 1.48 |
| DPA | 0.36 | −0.07 | 0.76 | 0.33 | 1.76 | 2.04 | 0.90 | 1.23 |
| DEA | 0.36 | −0.12 | 0.73 | 0.30 | 1.77 | 2.20 | 0.94 | 1.31 |
| DMA | 0.36 | −0.17 | 0.73 | 0.21 | 1.77 | 2.34 | 0.94 | 1.46 |
| TPA | 0.39 | −0.03 | 0.90 | 0.34 | 1.69 | 2.07 | 0.80 | 1.21 |
| TEA | 0.39 | −0.07 | 0.80 | 0.31 | 1.71 | 2.20 | 0.89 | 1.32 |
| TMA | 0.38 | −0.15 | 0.77 | 0.22 | 1.70 | 2.35 | 0.89 | 1.46 |
| H2O | −0.36 | −0.47 | −0.29 | 0.09 | 1.20 | 1.58 | 1.30 | 1.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowski, S.; Wróbel, M.; Woszuk, A. Quantum Chemical Analysis of the Corrosion Inhibition Potential by Aliphatic Amines. Materials 2021, 14, 6197. https://doi.org/10.3390/ma14206197
Malinowski S, Wróbel M, Woszuk A. Quantum Chemical Analysis of the Corrosion Inhibition Potential by Aliphatic Amines. Materials. 2021; 14(20):6197. https://doi.org/10.3390/ma14206197
Chicago/Turabian StyleMalinowski, Szymon, Michał Wróbel, and Agnieszka Woszuk. 2021. "Quantum Chemical Analysis of the Corrosion Inhibition Potential by Aliphatic Amines" Materials 14, no. 20: 6197. https://doi.org/10.3390/ma14206197
APA StyleMalinowski, S., Wróbel, M., & Woszuk, A. (2021). Quantum Chemical Analysis of the Corrosion Inhibition Potential by Aliphatic Amines. Materials, 14(20), 6197. https://doi.org/10.3390/ma14206197







