Use of Methylcellulose-Based Pellet to Enhance the Bacterial Self-Healing of Cement Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Preparation
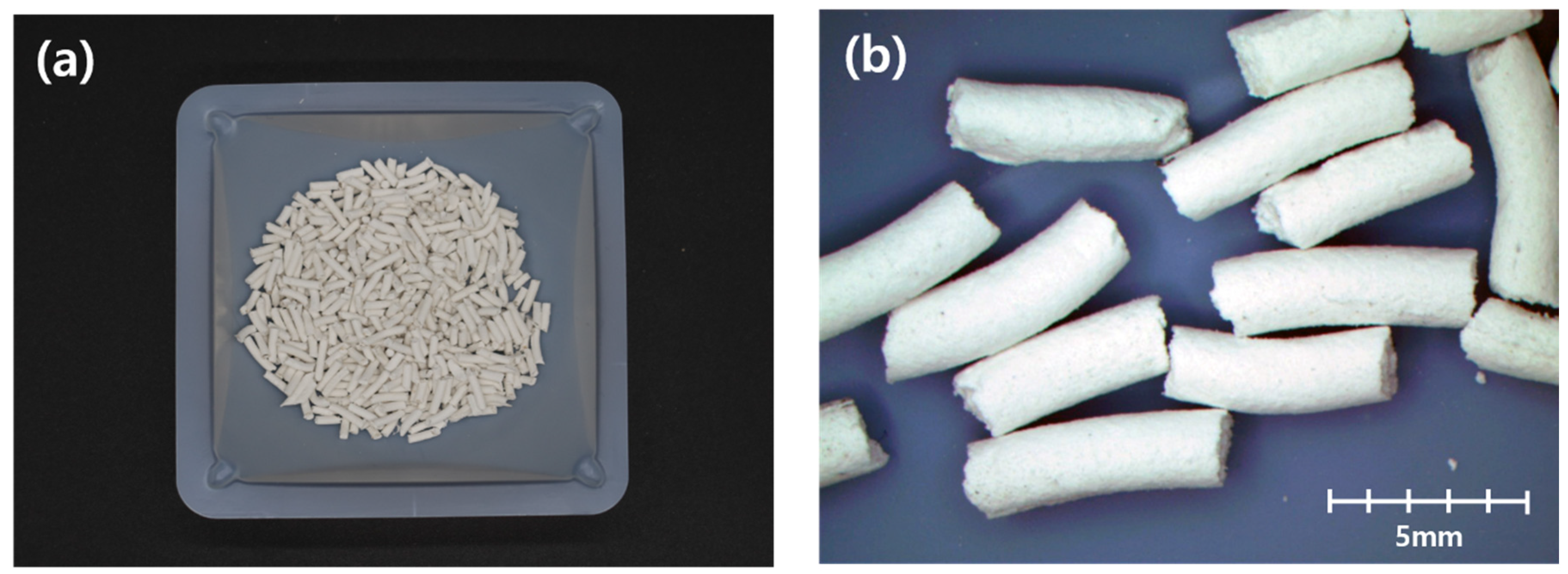
2.2. Methylcellulose-Based Pellet Materials
2.3. Methylcellulose-Based Pellet Fabrication Process
2.4. Mortar Preparation
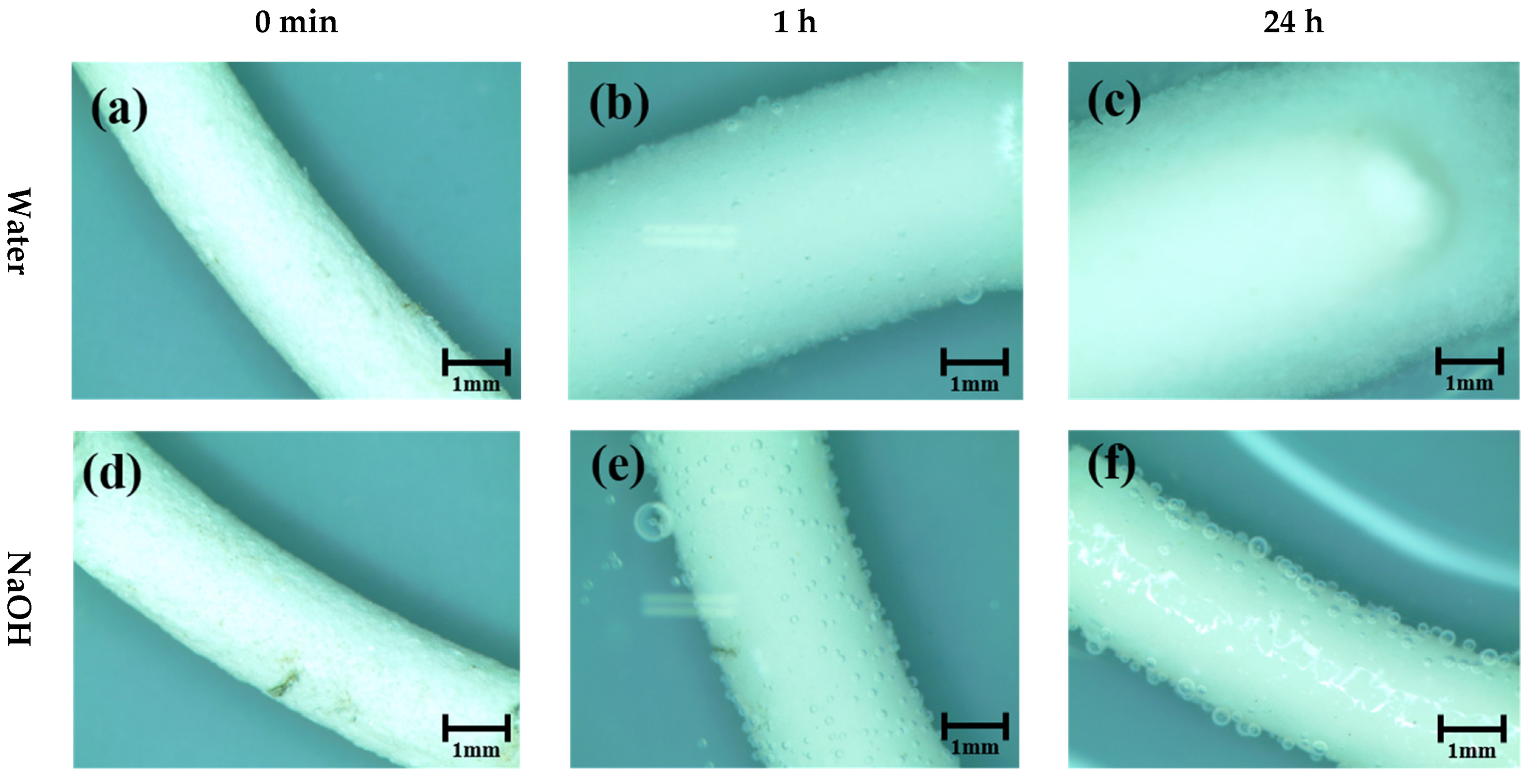
2.5. Pellet Volume Expansion in Different pH Solutions
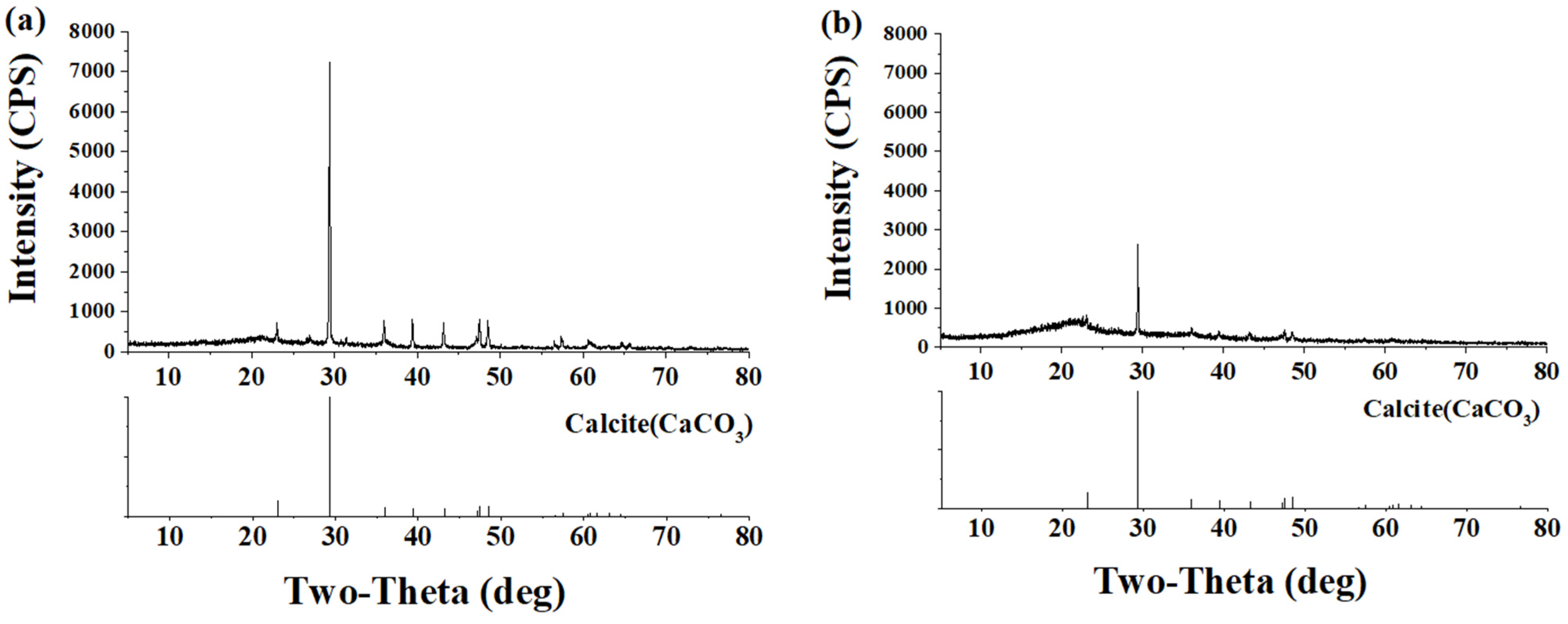
2.6. Bacterial Activity over Mortar Surface and XRD Analysis
2.7. Bacterial Viability in Cement Mortar
2.8. Mortar Flow Test and Compressive Strength
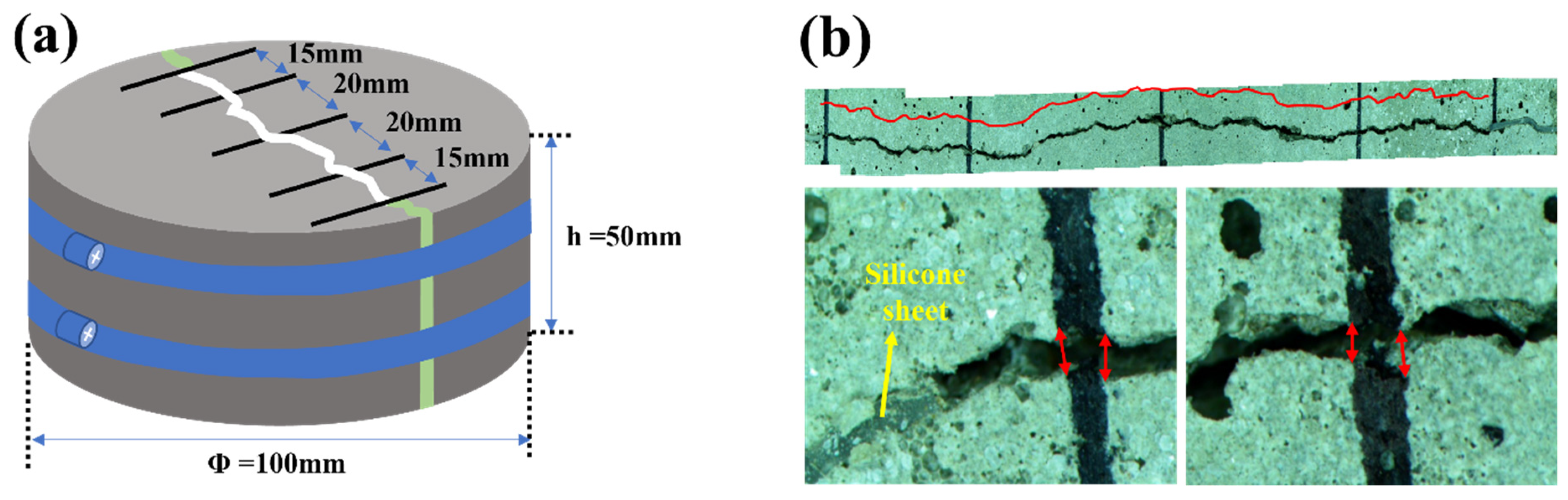
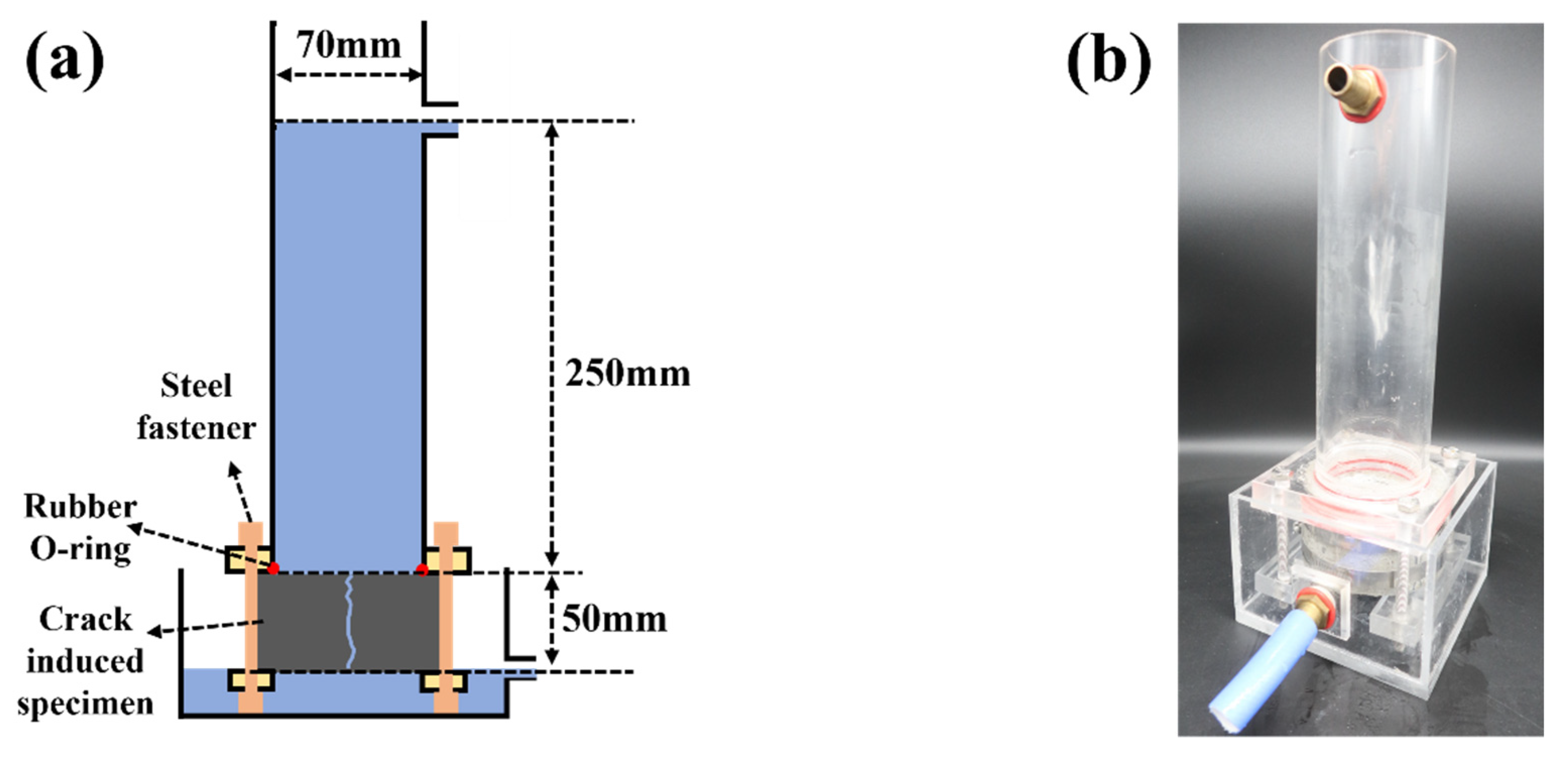
2.9. Water Permeability of Crack-Induced Mortar
3. Results
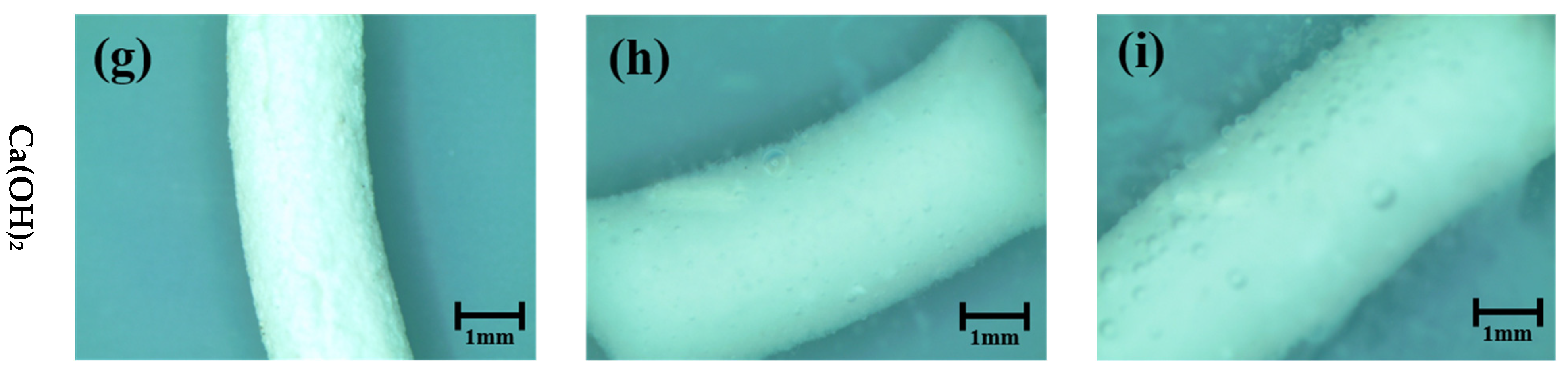
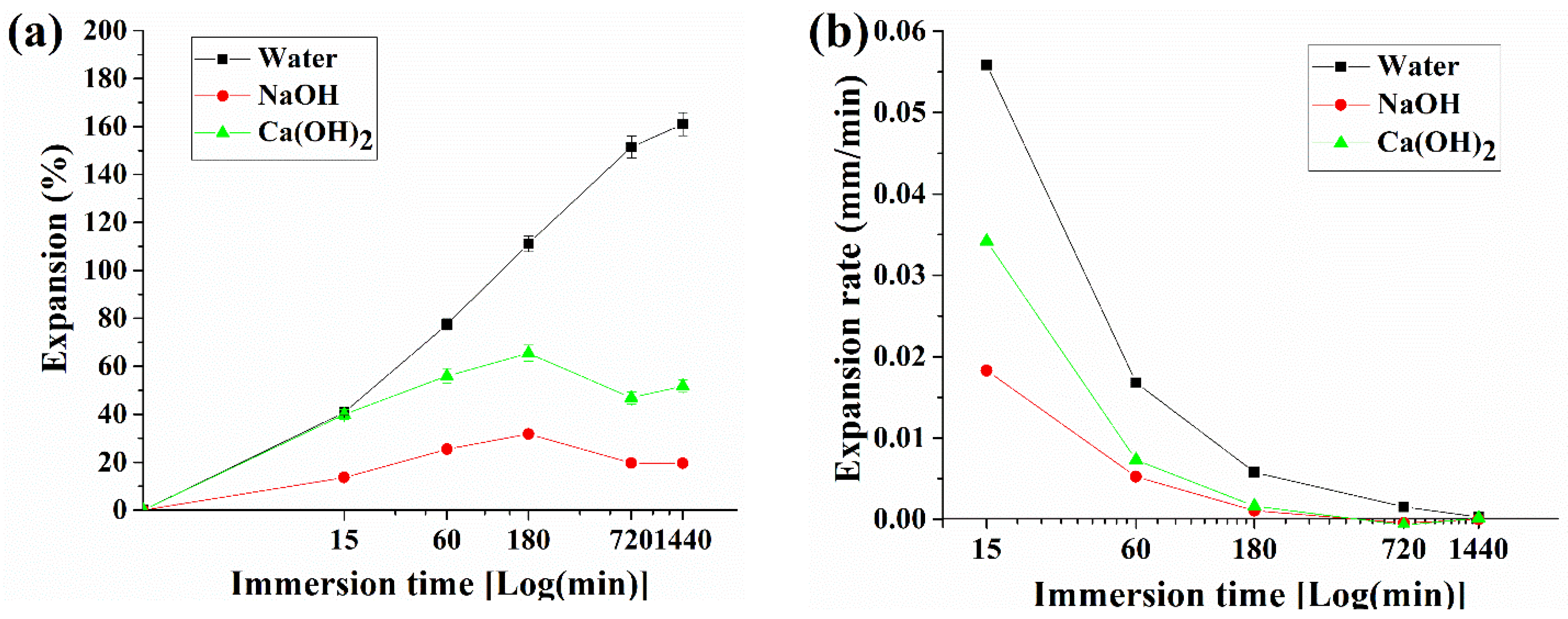
3.1. Pellet Behavior in Different pH Solutions
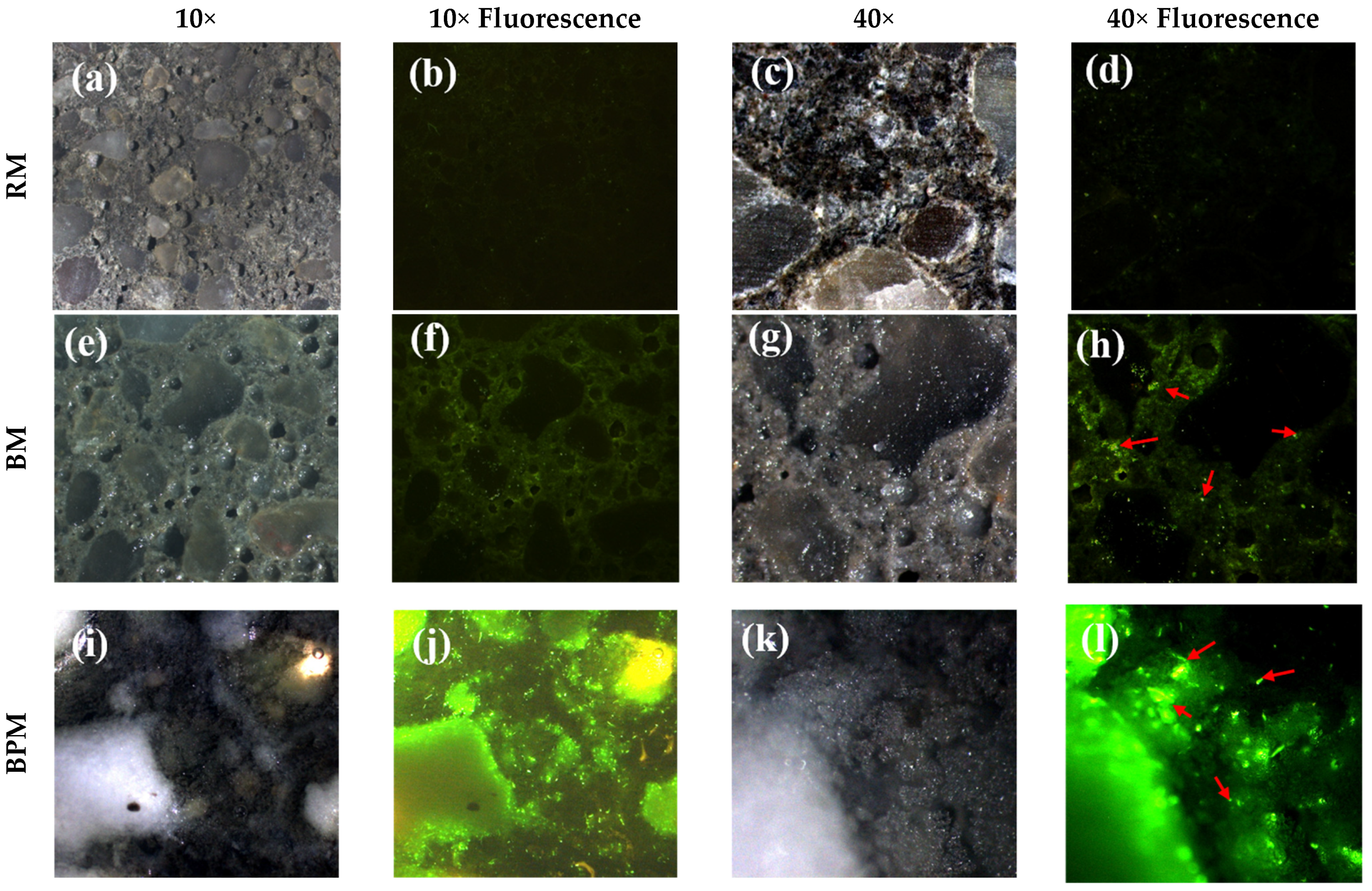
3.2. Bacterial Activity over Mortar Surface
3.3. Bacterial Viability in Cement Mortar
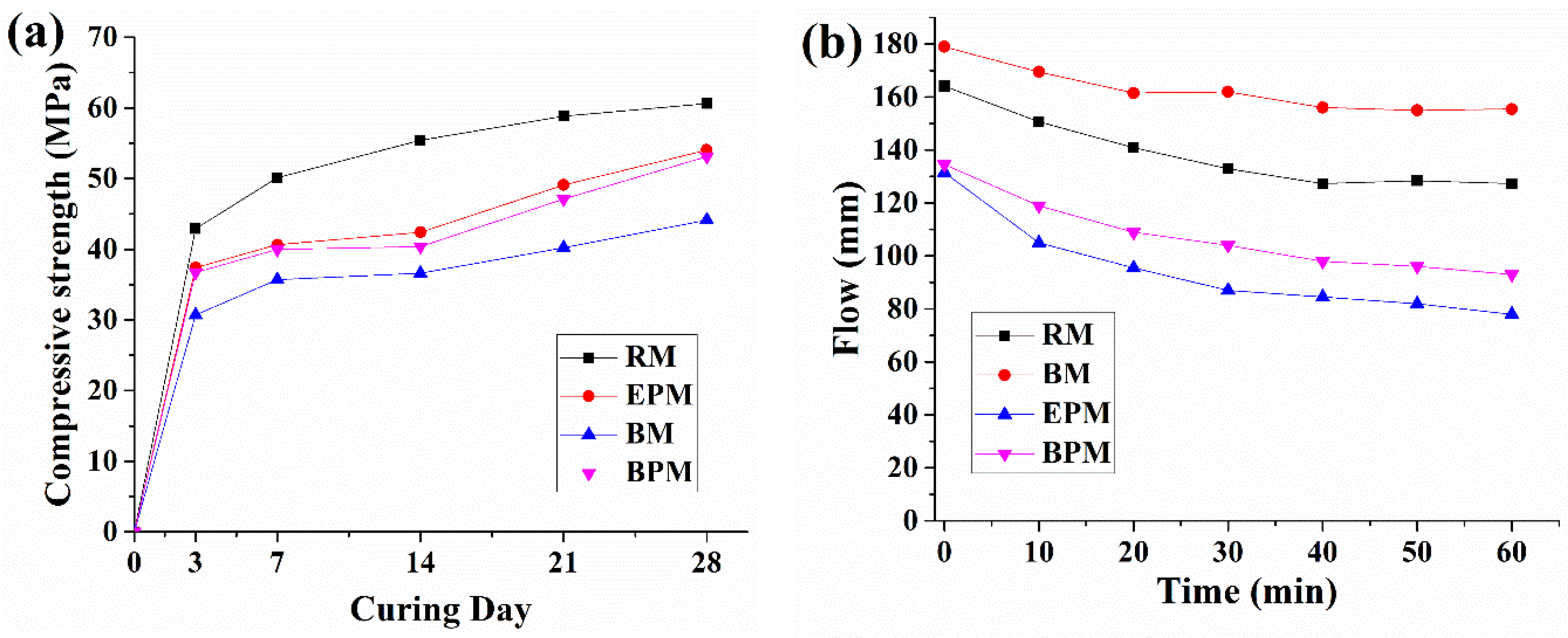
3.4. Compressive Strength and Flow
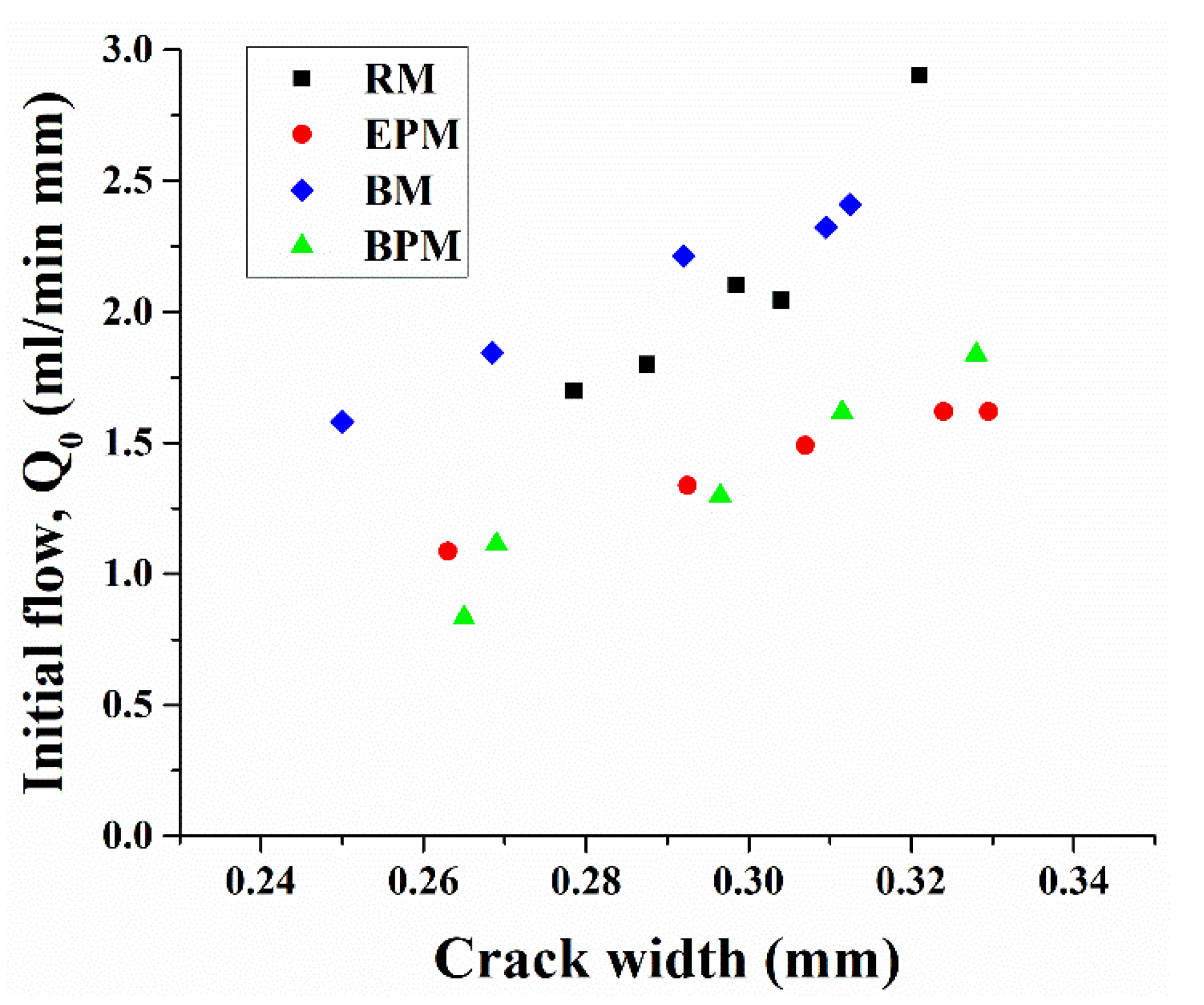
3.5. Water Permeability of Crack-Induced Mortar
4. Discussion
4.1. Effects of MC-Based Pellet on Bacterial Activity
4.2. Effects of MC-Based Pellet on Self-Healing of the Cement Mortar
4.3. Effects of MC-Based Pellet on Mechanical Properties of the Cement Mortar
5. Conclusions
- Pellets increased the survival rate of bacterial spores in the mortar by about 3.5 times in 28 days.
- Fluorescence imaging showed that the use of pellets enhanced bacterial activity after the cracks’ cross-section contact with the water
- crack permeability decreased by 90% or more within 14 days when the bacterial pellet was incorporated into the mortar, compared with the specimen containing only the bacteria for 28 days or more.
- However, the workability of the fresh mortar was adversely affected, and the 28-day compressive strength was degraded by 10% compared to the reference mortar.
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Mora, E.P. Life cycle, sustainability and the transcendent quality of building materials. Build. Environ. 2007, 42, 1329–1334. [Google Scholar] [CrossRef]
- Edvardsen, C. Water permeability and autogenous healing of cracks in concrete. In Innovation in Concrete Structures: Design and Construction; Thomas Telford Publishing: London, UK, 1999; pp. 473–487. [Google Scholar]
- Wu, M.; Johannesson, B.; Geiker, M. A review: Self-healing in cementitious materials and engineered cementitious composite as a self-healing material. Constr. Build. Mater. 2012, 28, 571–583. [Google Scholar] [CrossRef]
- Nishiwaki, T.; Kwon, S.; Homma, D.; Yamada, M.; Mihashi, H. Self-Healing Capability of Fiber-Reinforced Cementitious Composites for Recovery of Watertightness and Mechanical Properties. Materials 2014, 7, 2141–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Inoue, M.; Kwon, S.; Choi, H.; Lim, M. Effective Crack Control of Concrete by Self-Healing of Cementitious Composites Using Synthetic Fiber. Materials 2016, 9, 248. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ding, S.; Han, B.; Ni, Y.-Q.; Ou, J. Self-healing properties of reactive powder concrete with nanofillers. Smart Mater. Struct. 2018, 27, 115033. [Google Scholar] [CrossRef]
- Bentz, D.P. Internal curing of high-performance blended cement mortars. ACI Mater. J. 2007, 104, 408. [Google Scholar]
- Justs, J.; Wyrzykowski, M.; Bajare, D.; Lura, P. Internal curing by superabsorbent polymers in ultra-high performance concrete. Cem. Concr. Res. 2015, 76, 82–90. [Google Scholar] [CrossRef]
- Ryu, J.-S.; Otsuki, N. Crack closure of reinforced concrete by electrodeposition technique. Cem. Concr. Res. 2002, 32, 159–164. [Google Scholar] [CrossRef]
- Chu, H.; Jiang, L.; Song, Z.; Xu, Y.; Zhao, S.; Xiong, C. Repair of concrete crack by pulse electro-deposition technique. Constr. Build. Mater. 2017, 148, 241–248. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Z.; Yang, Z.; Zhao, N.; Yuan, W. Self-Healing Efficiency of Cementitious Materials Containing Microcapsules Filled with Healing Adhesive: Mechanical Restoration and Healing Process Monitored by Water Absorption. PLoS ONE 2013, 8, e81616. [Google Scholar] [CrossRef]
- Sangadji, S.; Schlangen, E. Self Healing of Concrete Structures—Novel Approach Using Porous Network Concrete. J. Adv. Concr. Technol. 2012, 10, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Joseph, C.; Jefferson, A.; Isaacs, B.; Lark, R.; Gardner, D. Experimental investigation of adhesive-based self-healing of cementitious materials. Mag. Concr. Res. 2010, 62, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Dry, C. Matrix cracking repair and filling using active and passive modes for smart timed release of chemicals from fibers into cement matrices. Smart Mater. Struct. 1994, 3, 118–123. [Google Scholar] [CrossRef]
- Minnebo, P.; Van Tittelboom, K.; De Belie, N.; Van Hemelrijck, D. Vascular self-healing of a reinforced concrete beams under 4-point bending. In Proceedings of the 14th International Conference on Durability of Building Materials and Components (XIV DBMC), Ghent, Belgium, 29–31 May 2017. [Google Scholar]
- Li, L.; Li, Q.; Zhang, F. Behavior of Smart Concrete Beams with Embedded Shape Memory Alloy Bundles. J. Intell. Mater. Syst. Struct. 2016, 18, 1003–1014. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Wang, J.; Mignon, A.; Snoeck, D.; Wiktor, V.; Van Vliergerghe, S.; Boon, N.; De Belie, N. Application of modified-alginate encapsulated carbonate producing bacteria in concrete: A promising strategy for crack self-healing. Front. Microbiol. 2015, 6, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Jonkers, H.; Boon, N.; De Belie, N. Bacillus sphaericus LMG 22257 is physiologically suitable for self-healing concrete. Appl. Microbiol. Biotechnol. 2017, 101, 5101–5114. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Ghorbel, E.; Fares, H.; Cousture, A. Bacterial self-healing of concrete and durability assessment. Cem. Concr. Compos. 2019, 104, 103340. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next generation of self-healing concrete. Appl. Microbiol. Biotechnol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifan, M.; Sarmah, A.K.; Ebrahiminezhad, A.; Ghasemi, Y.; Samani, A.K.; Berenjian, A. Bio-reinforced self-healing concrete using magnetic iron oxide nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 2167–2178. [Google Scholar] [CrossRef]
- Jonkers, H.; Schlangen, E. Development of a bacteria-based self healing concrete. In Tailor Made Concrete Structures; Taylor & Francis Group: London, UK, 2008; p. 109. [Google Scholar]
- Wang, J.Y.; De Belie, N.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. Biotechnol. 2012, 39, 567–577. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Feng, T.; Zhou, M.; Zhao, L.; Zhou, A.; Li, Z. Immobilizing bacteria in expanded perlite for the crack self-healing in concrete. Constr. Build. Mater. 2017, 148, 610–617. [Google Scholar] [CrossRef]
- Krishnapriya, S.; Babu, D.V. Isolation and identification of bacteria to improve the strength of concrete. Microbiol. Res. 2015, 174, 48–55. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Lactose mother liquor as an alternative nutrient source for microbial concrete production by Sporosarcina pasteurii. J. Ind. Microbiol. Biotechnol. 2009, 36, 433–438. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Erşan, Y.Ç.; Da Silva, F.B.; Boon, N.; Verstraete, W.; De Belie, N. Screening of bacteria and concrete compatible protection materials. Constr. Build. Mater. 2015, 88, 196–203. [Google Scholar] [CrossRef]
- Wang, J.; Soens, H.; Verstraete, W.; De Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Pei, R.; Liu, J.; Wang, S.; Yang, M. Use of bacterial cell walls to improve the mechanical performance of concrete. Cem. Concr. Compos. 2013, 39, 122–130. [Google Scholar] [CrossRef]
- Wang, J.; Van Tittelboom, K.; De Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Sharma, T.K.; Alazhari, M.; Heath, A.; Paine, K.; Cooper, R.M. Alkaliphilic Bacillus species show potential application in concrete crack repair by virtue of rapid spore production and germination then extracellular calcite formation. J. Appl. Microbiol. 2017, 122, 1233–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. BioTechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Räsänen, V.; Penttala, V. The pH measurement of concrete and smoothing mortar using a concrete powder suspension. Cem. Concr. Res. 2004, 34, 813–820. [Google Scholar] [CrossRef]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology 2007, 5, 401–411. [Google Scholar] [CrossRef]
- Jang, I.; Son, D.; Kim, W.; Park, W.; Yi, C. Effects of spray-dried co-cultured bacteria on cement mortar. Constr. Build. Mater. 2020, 243, 118206. [Google Scholar] [CrossRef]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzym. Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Dürig, T.; Karan, K. Binders in Wet Granulation. In Handbook of Pharmaceutical Wet Granulation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 317–349. [Google Scholar]
- Brady, J.; Dürig, T.; Lee, P.; Li, J.-X. Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms; Academic Press: Cambridge, MA, USA, 2017; pp. 181–223. [Google Scholar]
- Lee, Y.S.; Kim, H.J.; Park, W. Non-ureolytic calcium carbonate precipitation by Lysinibacillus sp. YS11 isolated from the rhizosphere of Miscanthus sacchariflorus. J. Microbiol. 2017, 55, 440–447. [Google Scholar] [CrossRef]
- Shin, B.; Park, C.; Lee, B.-H.; Lee, K.-E.; Park, W. Bacillus miscanthi sp. nov., a alkaliphilic bacterium from the rhizosphere of Miscanthus sacchariflorus. Int. J. Syst. Evol. Microbiol. 2020, 70, 1843–1849. [Google Scholar] [CrossRef]
- Jett, B.D.; Hatter, K.L.; Huycke, M.M.; Gilmore, M.S. Simplified Agar Plate Method for Quantifying Viable Bacteria. Biotechniques 1997, 23, 648–650. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. The role of bacterial extracellular polymeric substances in geomicrobiology. Chem. Geol. 2014, 386, 115–132. [Google Scholar] [CrossRef]
- Petersson, P. Fracture energy of concrete: Practical performance and experimental results. Cem. Concr. Res. 1980, 10, 91–101. [Google Scholar] [CrossRef]
- Rastiello, G.; Boulay, C.; Pont, S.D.; Tailhan, J.; Rossi, P. Real-time water permeability evolution of a localized crack in concrete under loading. Cem. Concr. Res. 2014, 56, 20–28. [Google Scholar] [CrossRef]



| Components | Constituents | CAS No. (Manufacturer) | Mixing Ratio by Weight | |
|---|---|---|---|---|
| Bacterial Pellet (BP) | Empty Pellet (EP) | |||
| Binder | Methylcellulose (MC) | 9004-67-5 (Junsei) | 1.0 | 1.0 |
| Microcrystalline cellulose (MCC) | 9004-34-6 (Daejung) | 0.1 | 0.1 | |
| Filler | Silicon dioxide (SiO2) | 7631-86-9 (Samchun) | 3.0 | 3.0 |
| Diatomaceous earth (DE) | 68855-54-9 (Celite) | 0.485 | 0.4 | |
| Active substances | Cocultured bacteria | L. boronitolerans YS11 Bacillus sp. AK13 | 0.025 | - |
| Calcium lactate pentahydrate | 5743-47-5 (Duksan) | 0.05 | 0.05 | |
| Yeast extract | 8013-01-2 (Samchun) | 0.05 | 0.05 | |
| Mixture Title * | Cement | Water | Sand | Bacterial Mix ** | Empty Pellet (EP) | Bacterial Pellet (BP) |
|---|---|---|---|---|---|---|
| RM | 1.0 | 0.4 | 2.0 | - | - | - |
| BM | 0.03 | - | - | |||
| EPM | - | 0.05 | - | |||
| BPM | - | - | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, I.; Son, D.; Son, Y.; Min, J.; Yi, C. Use of Methylcellulose-Based Pellet to Enhance the Bacterial Self-Healing of Cement Composite. Materials 2021, 14, 6113. https://doi.org/10.3390/ma14206113
Jang I, Son D, Son Y, Min J, Yi C. Use of Methylcellulose-Based Pellet to Enhance the Bacterial Self-Healing of Cement Composite. Materials. 2021; 14(20):6113. https://doi.org/10.3390/ma14206113
Chicago/Turabian StyleJang, Indong, Dasom Son, Yongjun Son, Jihyeon Min, and Chongku Yi. 2021. "Use of Methylcellulose-Based Pellet to Enhance the Bacterial Self-Healing of Cement Composite" Materials 14, no. 20: 6113. https://doi.org/10.3390/ma14206113
APA StyleJang, I., Son, D., Son, Y., Min, J., & Yi, C. (2021). Use of Methylcellulose-Based Pellet to Enhance the Bacterial Self-Healing of Cement Composite. Materials, 14(20), 6113. https://doi.org/10.3390/ma14206113





