Visible Light-Mediated Sustainable Antibacterial Activity and Osteogenic Functionality of Au and Pt Multi-Coated TiO2 Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Au and Pt Multi-Coated TiO2 Nanotubes
2.2. Surface Characteristics
2.3. Antibacterial Activity Test
2.4. Human Mesenchymal Stem Cell Cultures
2.5. Alkaline Phosphatase (ALP) Activity Assay
2.6. hMSC Morphological Changes under Visible Light Irradiation
2.7. Quantitative Real-Time PCR Assay
2.8. Alizarin Red Assay
2.9. Statistical Analysis
3. Results
3.1. Surface Characteristics
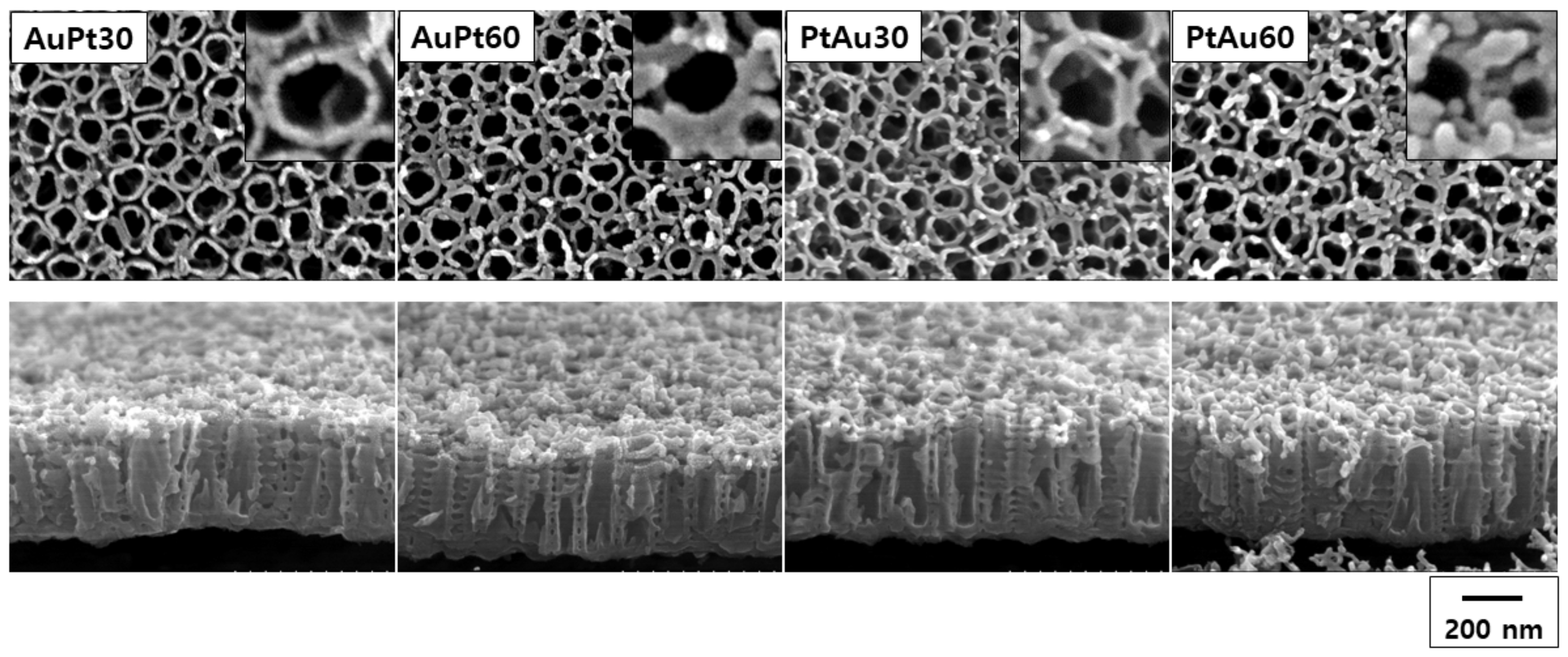
3.1.1. FE-SEM Observation
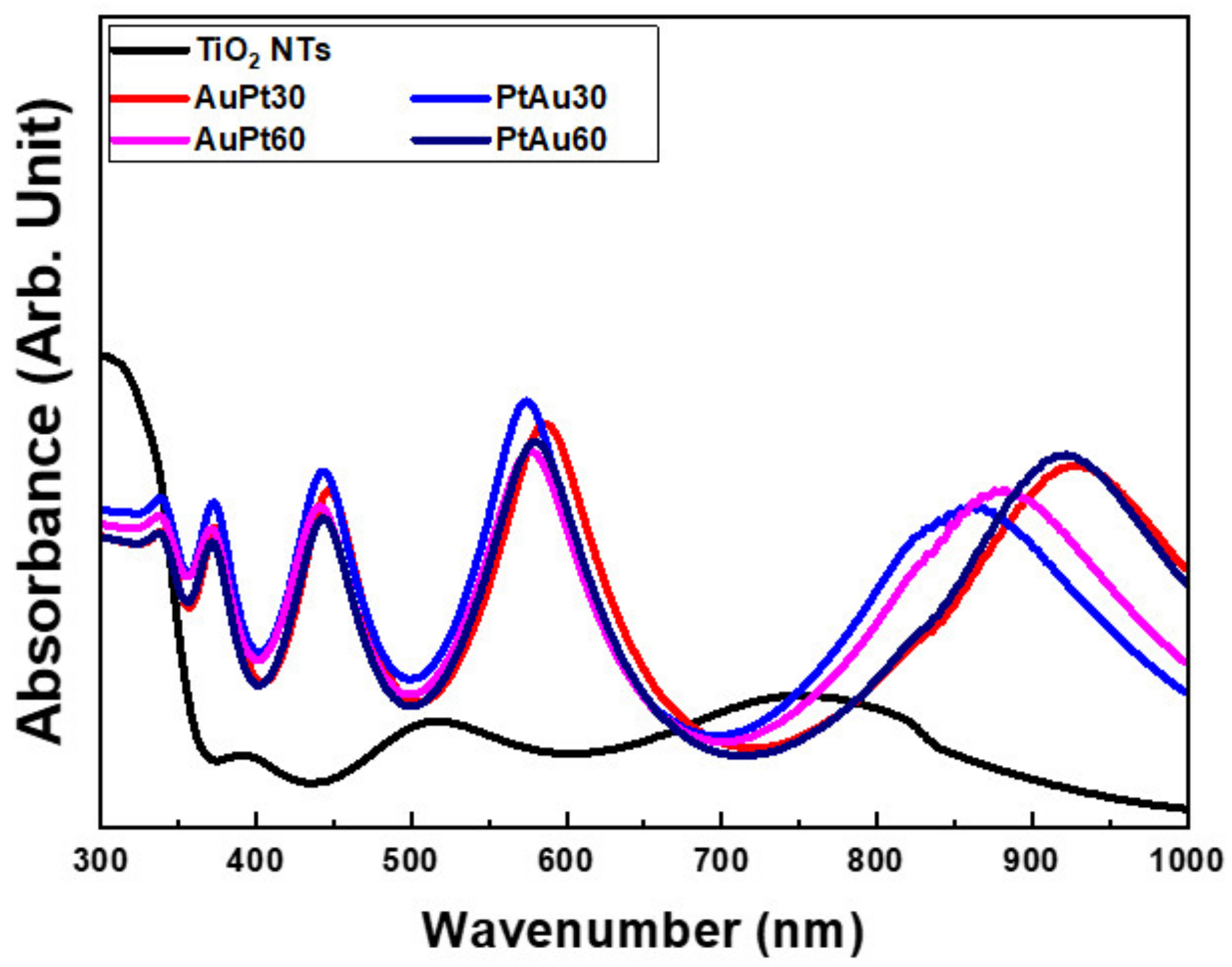
3.1.2. Diffuse Reflectance UV-Vis-NIR Spectrophotometry
3.1.3. TEM Observation and Elementary Analysis
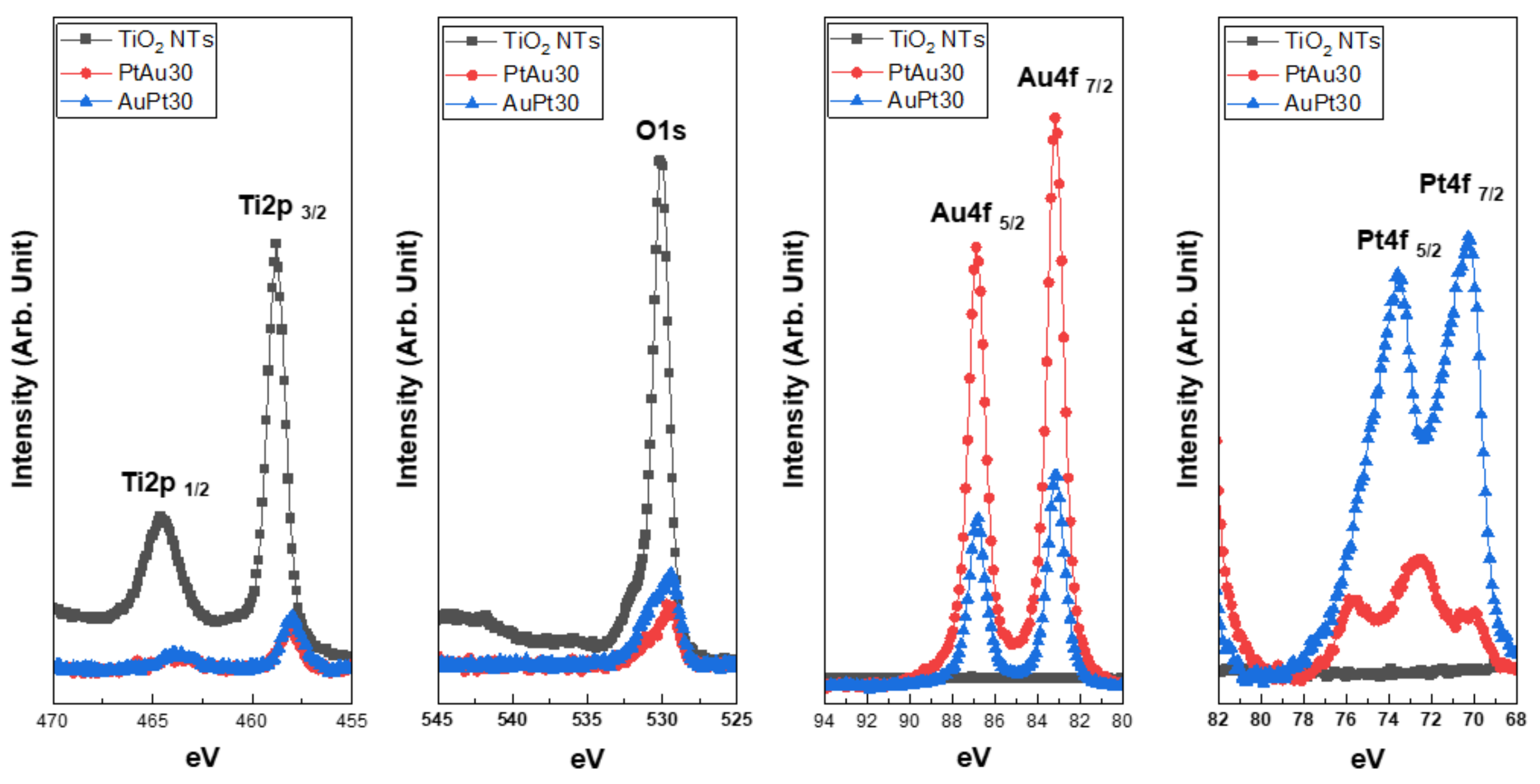
3.1.4. XPS Analysis
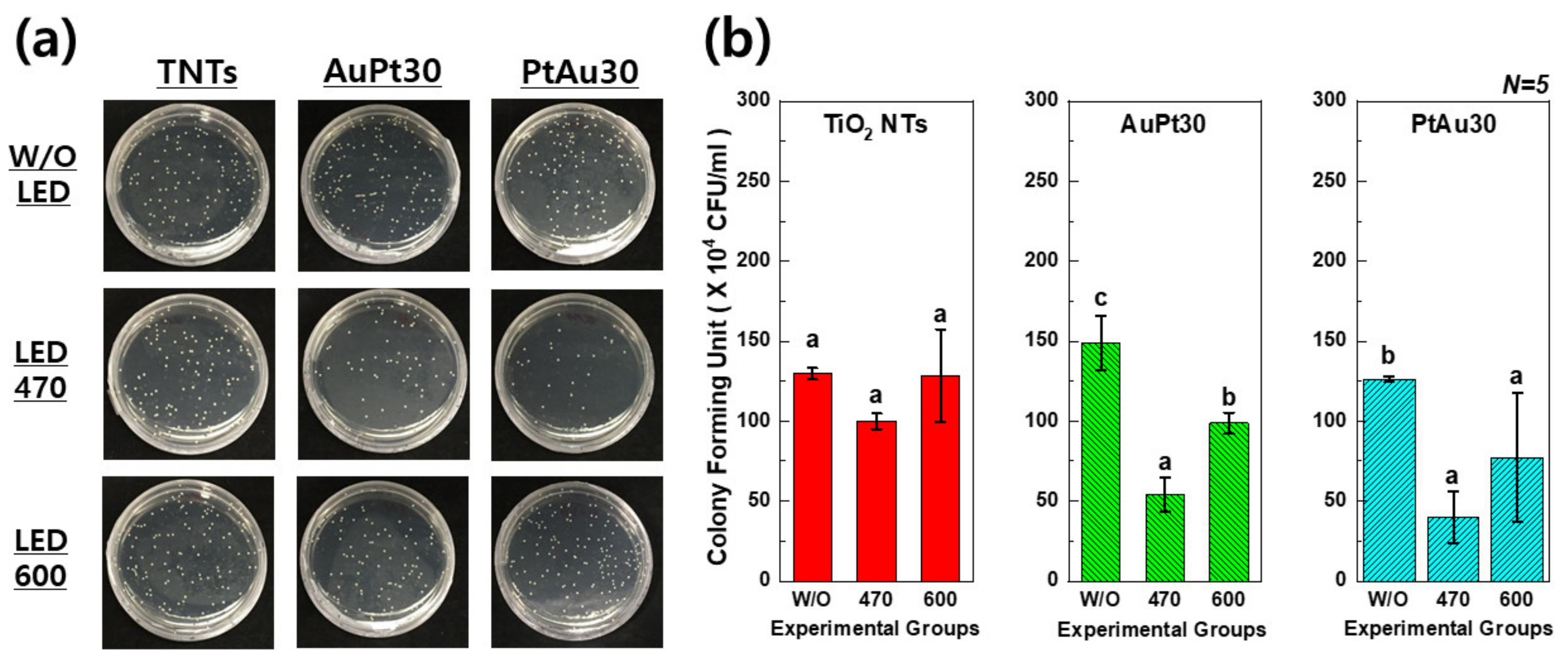
3.2. Antibacterial Activity Test
3.3. Biocompatibility and Osteogenic Functionality of hMSCs
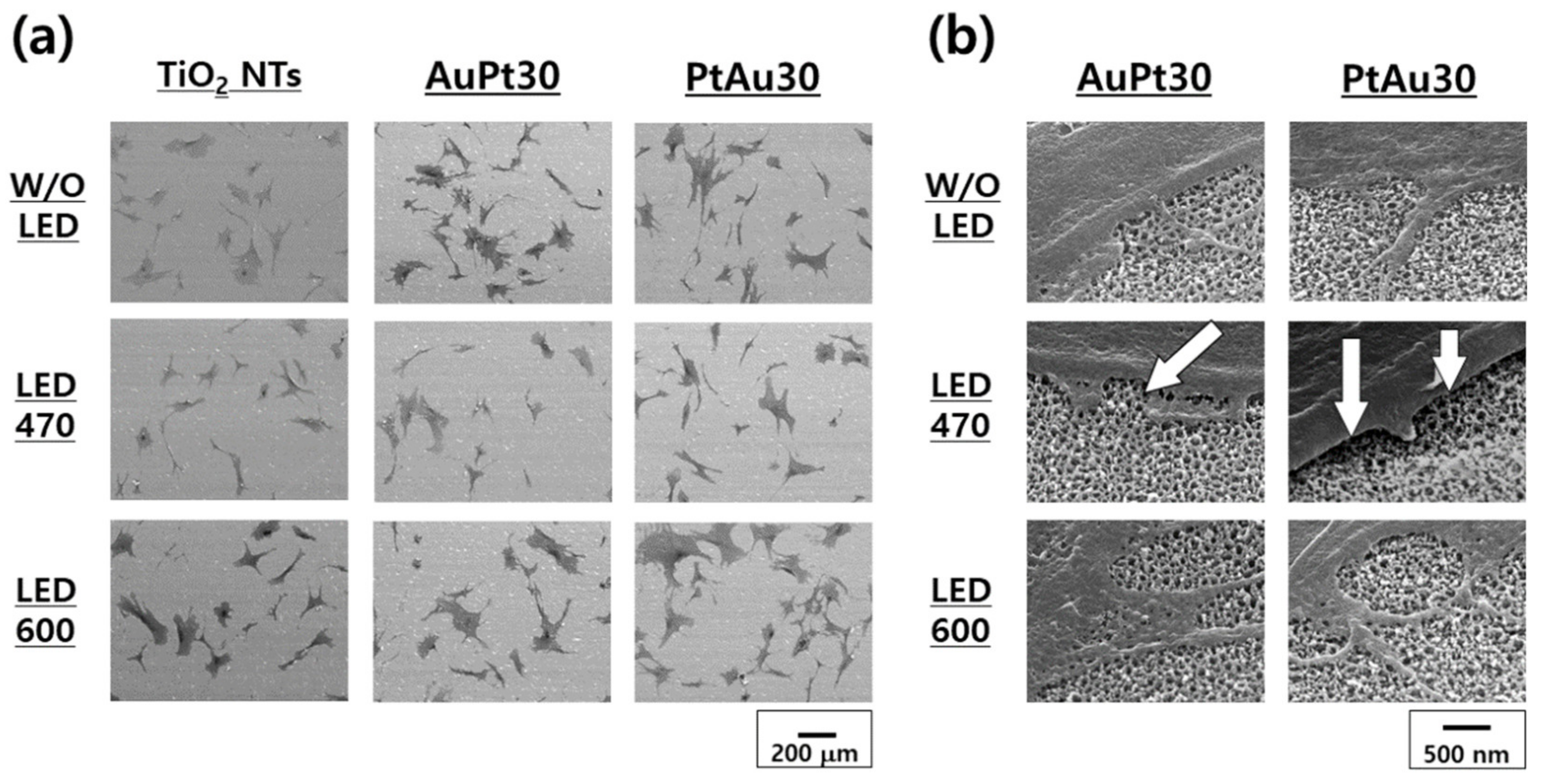
3.3.1. hMSCs Morphological Observation by FE-SEM
3.3.2. ALP Activity Assay
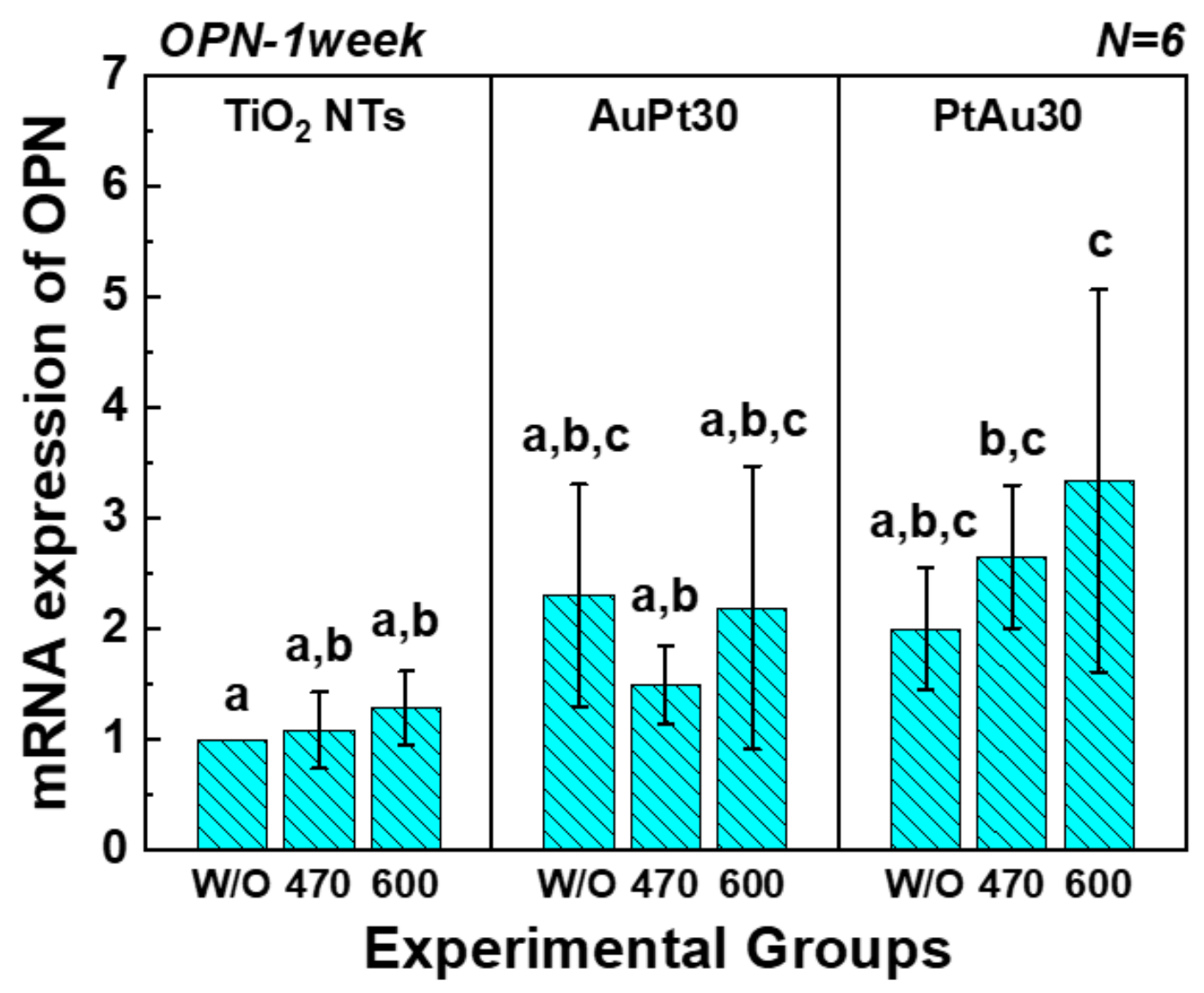
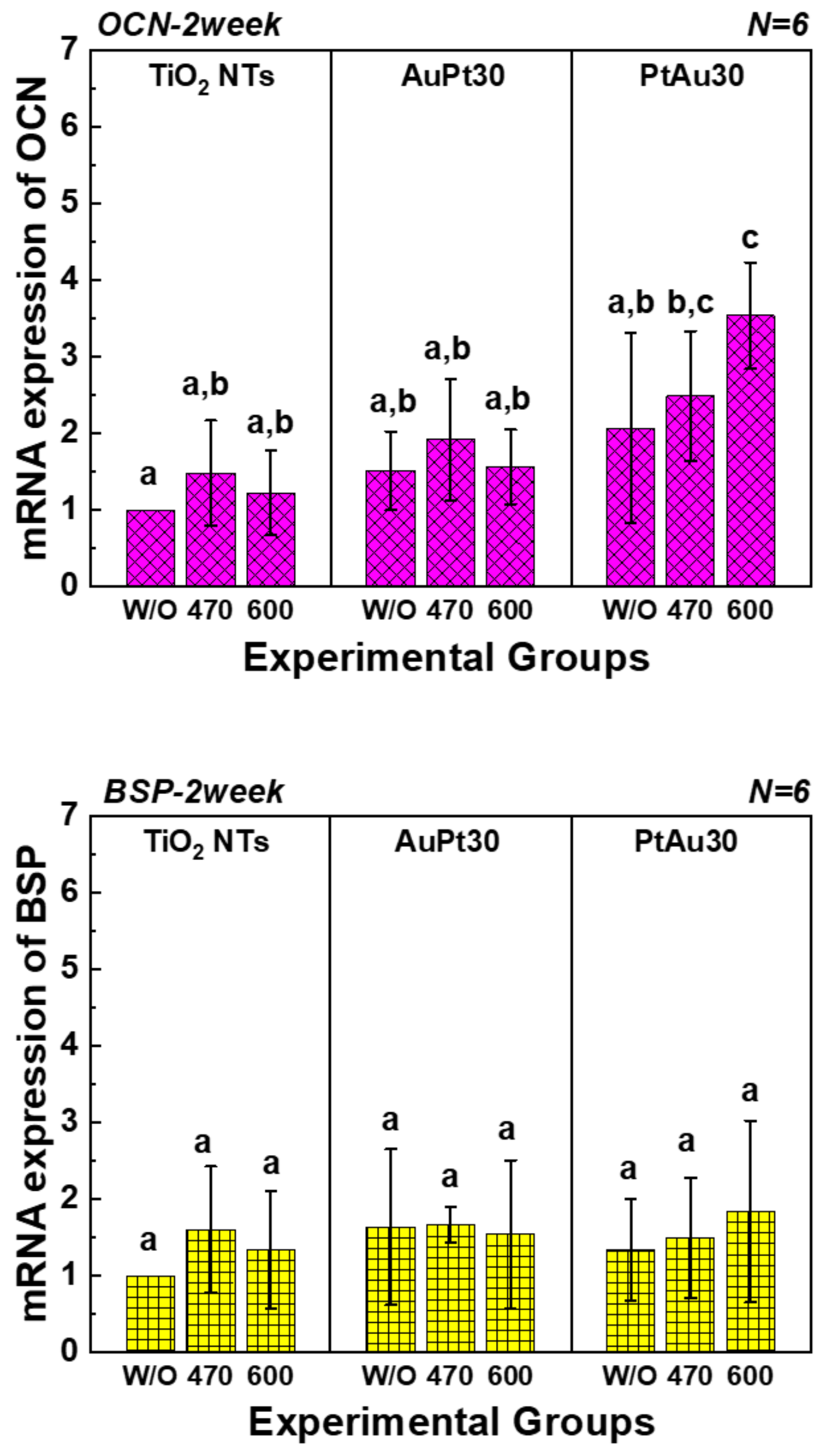
3.3.3. Quantitative Real-Time PCR Assay
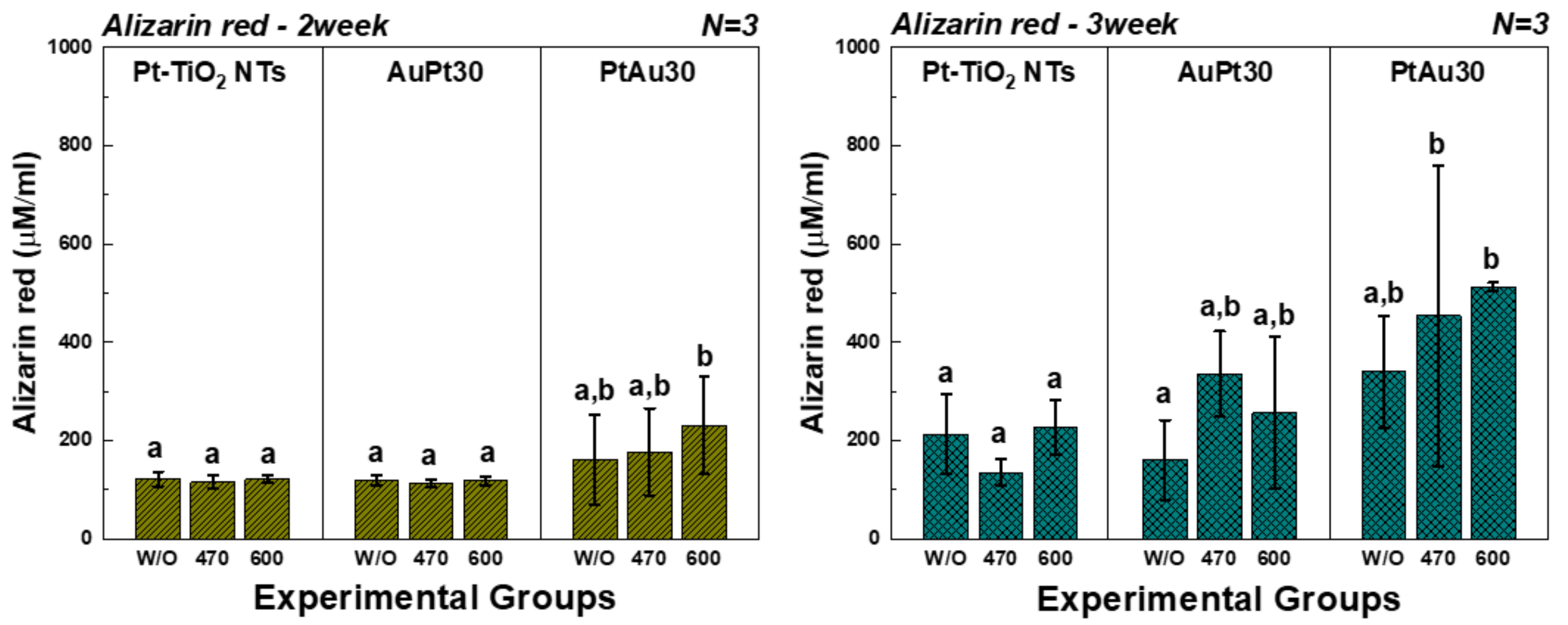
3.3.4. Alizarin Red Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karthik, K.; Sivakumar, S.; Thangaswamy, V. Evaluation of implant success: A review of past and present concepts. J. Pharm. Bioall. Sci. 2013, 5, S117–S119. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.J.; Singh, M.; Weber, H.P.; Gallucci, G.O. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Esposito, M.; Thomsen, P.; Ericson, L.E.; Lekholm, U. Histopathologic observations on early oral implant failures. Int. J. Oral. Maxillofac. Implants 1999, 14, 798–810. [Google Scholar]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Veerapandian, M.; Yun, K. Functionalization of biomolecules on nanoparticles: Specialized for antibacterial applications. Appl. Microbiol. Biotechnol. 2011, 90, 1655–1667. [Google Scholar] [CrossRef]
- Rezazadeh, N.H.; Buazar, F.; Matroodi, S. Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalized silver nanoparticles. Sci. Rep. 2020, 10, 19615. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Mu, H.B.; Tang, J.J.; Liu, Q.J.; Sun, C.L.; Wang, T.T.; Duan, J.Y. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci. Rep. 2016, 6, 18877. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Goncalves, N.L.; Borges, V.M.; de Arruda, J.A.A.; Dos Santos, E.G.; Diniz, I.M.A.; Madeira, M.F.M.; Moreno, A. Antimicrobial effects of photodynamic therapy on Staphylococcus aureus biofilm grown on a specific acrylic resin surface for ocular prostheses. Photodiagn. Photodyn. Ther. 2020, 32, 102042. [Google Scholar] [CrossRef]
- Hokari, T.; Morozumi, T.; Komatsu, Y.; Shimizu, T.; Yoshino, T.; Tanaka, M.; Tanaka, Y.; Nohno, K.; Kubota, T.; Yoshie, H. Effects of antimicrobial photodynamic therapy and local administration of minocycline on clinical, microbiological, and inflammatory markers of periodontal pockets: A pilot study. Int. J. Dent. 2018, 2018, 1748584. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Stromme, M.; Melhus, A.; Engqvist, H.; Welch, K. Photocatalytic inactivation of biofilms on bioactive dental adhesives. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 62–67. [Google Scholar] [CrossRef]
- Rupp, F.; Haupt, M.; Eichler, M.; Doering, C.; Klostermann, H.; Scheideler, L.; Lachmann, S.; Oehr, C.; Wendel, H.P.; Decker, E.; et al. Formation and photocatalytic decomposition of a pellicle on anatase surfaces. J. Dent. Res. 2012, 91, 104–109. [Google Scholar] [CrossRef]
- Wu, H.; Xie, L.; He, M.; Zhang, R.T.; Tian, Y.; Liu, S.R.; Gong, T.; Huo, F.J.; Yang, T.; Zhang, Q.Y.; et al. A wear-resistant TiO2 nanoceramic coating on titanium implants for visible-light photocatalytic removal of organic residues. Acta Biomater. 2019, 97, 597–607. [Google Scholar] [CrossRef]
- Nagay, B.E.; Dini, C.; Cordeiro, J.M.; Ricomini-Filho, A.P.; de Avila, E.D.; Rangel, E.C.; da Cruz, N.C.; Barao, V.A.R. Visible-light-induced photocatalytic and antibacterial activity of TiO2 codoped with nitrogen and bismuth: New perspectives to control implant-biofilm-related diseases. ACS Appl. Mater. Interfaces 2019, 11, 18186–18202. [Google Scholar] [CrossRef] [PubMed]
- Gyorgyey, A.; Janovak, L.; Adam, A.; Kopniczky, J.; Toth, K.L.; Deak, A.; Panayotov, I.; Cuisinier, F.; Dekany, I.; Turzo, K. Investigation of the in vitro photocatalytic antibacterial activity of nanocrystalline TiO2 and coupled TiO2/Ag containing copolymer on the surface of medical grade titanium. J. Biomater. Appl. 2016, 31, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.M.; Zhang, Y.Q.; Zheng, Y.F.; Xu, C.S.; Liu, G.M. Enhanced visible light photocatalytic activity of N, F-codoped TiO2 powders with high thermal stability. Environ. Technol. 2019, 40, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Chen, X.; Tong, L.; Wang, D.; Liu, L.; Ye, J. Remarkable visible-light photocatalytic activity enhancement over Au/p-type TiO2 promoted by efficient interfacial charge transfer. ACS Appl. Mater. Interfaces 2019, 11, 24154–24163. [Google Scholar] [CrossRef]
- Qin, L.; Wang, G.; Tan, Y. Plasmonic Pt nanoparticles-TiO2 hierarchical nano-architecture as a visible light photocatalyst for water splitting. Sci. Rep. 2018, 8, 16198. [Google Scholar] [CrossRef] [Green Version]
- Ueda, T.; Ueda, K.; Ito, K.; Ogasawara, K.; Kanetaka, H.; Mokudai, T.; Niwano, Y.; Narushima, T. Visible-light-responsive antibacterial activity of Au-incorporated TiO2 layers formed on Ti–(0–10)at% Au alloys by air oxidation. J. Biomed. Mater. Res. A 2019, 107, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.S.; Bae, J.M.; Jin, S.; Oh, S. Infrared-mediated drug elution activity of gold nanorod-grafted TiO2 Nanotubes. J. Nanomater. 2014, 2014, 750813. [Google Scholar] [CrossRef]
- Moon, K.S.; Park, Y.B.; Bae, J.M.; Oh, S. Near-infrared laser-mediated drug release and antibacterial activity of gold nanorod–sputtered titania nanotubes. J. Tissue Eng. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Moon, K.S.; Choi, E.J.; Bae, J.M.; Park, Y.B.; Oh, S. Visible light-enhanced antibacterial and osteogenic functionality of Au and Pt nanoparticles deposited on TiO2 nanotubes. Materials 2020, 13, 3721. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhu, B.L.; Wang, G.C.; Liu, Z.F.; Huang, W.P.; Zhang, S.M. Enhanced CO catalytic oxidation over an Au-Pt alloy supported on TiO2 nanotubes: Investigation of the hydroxyl and Au/Pt ratio influences. Catal. Sci. Technol. 2018, 8, 6109–6122. [Google Scholar] [CrossRef]
- Ataee-Esfahani, H.; Wang, L.; Nemoto, Y.; Yamauchi, Y. Synthesis of bimetallic Au@ Pt nanoparticles with Au core and nanostructured Pt shell toward highly active electrocatalysts. Chem. Mater. 2010, 22, 6310–6318. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Qian, S.; Liu, Z.; Feng, J.; Jin, P.; Liu, X. Plasmonic gold nanoparticles modified titania nanotubes for antibacterial application. Appl. Phys. Lett. 2014, 104, 261110. [Google Scholar] [CrossRef]
- Kibis, L.S.; Svintsitskiy, D.A.; Stadnichenko, A.I.; Slavinskaya, E.M.; Romanenko, A.V.; Fedorova, E.A.; Stonkus, O.A.; Svetlichnyi, V.A.; Fakhrutdinova, E.D.; Vorokhta, M.; et al. In situ probing of Pt/TiO2 activity in low-temperature ammonia oxidation. Catal. Sci. Technol. 2021, 11, 250–263. [Google Scholar] [CrossRef]
- Parkinson, C.R.; Walker, M.; McConville, C.F. Reaction of atomic oxygen with a Pt(111) surface: Chemical and structural determination using XPS, CAICISS and LEED. Surf. Sci. 2003, 545, 19–33. [Google Scholar] [CrossRef]
- Rod, H.; Sina, S.; John, Y.; Partrick, C. Target material selection for sputter coating of SEM samples. Micros. Today 2019, 27, 32–36. [Google Scholar]
- Gu, D.; Wang, Y.; Li, Z.D.; Liu, Y.; Wang, B.H.; Wu, H.J. UV-light aided photoelectrochemical synthesis of Au/TiO2 NTs for photoelectrocatalytic degradation of HPAM. RSC Adv. 2016, 6, 63711–63716. [Google Scholar] [CrossRef]
- Yoo, S.M.; Rawal, S.B.; Lee, J.E.; Kim, J.; Ryu, H.Y.; Park, D.W.; Lee, W.I. Size-dependence of plasmonic Au nanoparticles in photocatalytic behavior of Au/TiO2 and Au@SiO2/TiO2. Appl. Catal. A-Gen. 2015, 499, 47–54. [Google Scholar] [CrossRef]
- Lin, Z.J.; Wang, X.H.; Liu, J.; Tian, Z.Y.; Dai, L.C.; He, B.B.; Han, C.; Wu, Y.G.; Zeng, Z.G.; Hu, Z.Y. On the role of localized surface plasmon resonance in UV-Vis light irradiated Au/TiO2 photocatalysis systems: Pros and cons. Nanoscale 2015, 7, 4114–4123. [Google Scholar] [CrossRef]
- Chen, Y.S.; Frey, W.; Kim, S.; Homan, K.; Kruizinga, P.; Sokolov, K.; Emelianov, S. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt. Express 2010, 18, 8867–8878. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Khlebtsov, N.G. Multipole plasmons in metal nanorods: Scaling properties and dependence on particle size, shape, orientation, and dielectric environment. J. Phys. Chem. C 2007, 111, 11516–11527. [Google Scholar] [CrossRef]
- Liao, Q.; Mu, C.; Xu, D.S.; Ai, X.C.; Yao, J.N.; Zhang, J.P. Gold nanorod arrays with good reproducibility for high-performance surface-enhanced raman scattering. Langmuir 2009, 25, 4708–4714. [Google Scholar] [CrossRef]
- Zhang, K.; Xiang, Y.; Wu, X.; Feng, L.; He, W.; Liu, J.; Zhou, W.; Xie, S. Enhanced optical responses of Au@Pd core/shell nanobars. Langmuir 2009, 25, 1162–1168. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Choso, T.; Karasuda, T.; Shimomura, S.; Ouyang, F.; Tabata, K.; Yamaguchi, Y. Photoemission study of the interaction of a reduced thin film SnO2 with oxygen. Surf. Sci. 1999, 433–435, 226–229. [Google Scholar] [CrossRef]
- Vuong, N.M.; Kim, D.; Kim, H. Porous Au-embedded WO3 nanowire structure for efficient detection of CH4 and H2S. Sci. Rep. 2015, 5, 11040. [Google Scholar] [CrossRef] [Green Version]
- Nishijima, Y.; Ueno, K.; Kotake, Y.; Murakoshi, K.; Inoue, H.; Misawa, H. Near-infrared plasmon-assisted water oxidation. J. Phys. Chem. Lett. 2012, 3, 1248–1252. [Google Scholar] [CrossRef]
- Yuzawa, H.; Yoshida, T.; Yoshida, H. Gold nanoparticles on titanium oxide effective for photocatalytic hydrogen formation under visible light. Appl. Catal. B-Environ. 2012, 115, 294–302. [Google Scholar] [CrossRef]
- Tanaka, A.; Hashimoto, K.; Kominami, H. A very simple method for the preparation of Au/TiO2 plasmonic photocatalysts working under irradiation of visible light in the range of 600–700 nm. Chem. Commun. 2017, 53, 4759–4762. [Google Scholar] [CrossRef]
- Sutherland, J.C. Biological effects of polychromatic light. Photochem. Photobiol. 2002, 76, 164–170. [Google Scholar] [CrossRef]
- Kim, W.S.; Calderhead, R.G. Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Thera. 2011, 20, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, J.T.; McLoda, T.A.; Seegmiller, J.G.; Baxter, G.D. Low-level laser therapy facilitates superficial wound healing in humans: A triple-blind, sham-controlled study. J. Athl. Train. 2004, 39, 223–229. [Google Scholar]
- Aimbire, F.; Albertini, R.; Pacheco, M.T.; Castro-Faria-Neto, H.C.; Leonardo, P.S.; Iversen, V.V.; Lopes-Martins, R.A.; Bjordal, J.M. Low-level laser therapy induces dose-dependent reduction of TNF alpha levels in acute inflammation. Photomed. Laser. Surg. 2006, 24, 33–37. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Kong, N.; Zhang, Y.C.; Yang, W.R.; Yan, F.H. Size-dependent effects of gold nanoparticles on osteogenic differentiation of human periodontal ligament progenitor cells. Theranostics 2017, 7, 1214–1224. [Google Scholar] [CrossRef]
- Ko, W.K.; Kim, S.J.; Heo, D.N.; Han, I.B.; Kim, S.; Kwon, I.K.; Sohn, S. Double layers of gold nanoparticles immobilized titanium implants improve the osseointegration in rabbit models. Nanomed. Nanotechnol. 2020, 24, 102129. [Google Scholar] [CrossRef]
- Li, J.C.; Li, J.J.; Zhang, J.; Wang, X.L.; Kawazoe, N.; Chen, G.P. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, K.-S.; Park, Y.-B.; Bae, J.-M.; Choi, E.-J.; Oh, S.-H. Visible Light-Mediated Sustainable Antibacterial Activity and Osteogenic Functionality of Au and Pt Multi-Coated TiO2 Nanotubes. Materials 2021, 14, 5976. https://doi.org/10.3390/ma14205976
Moon K-S, Park Y-B, Bae J-M, Choi E-J, Oh S-H. Visible Light-Mediated Sustainable Antibacterial Activity and Osteogenic Functionality of Au and Pt Multi-Coated TiO2 Nanotubes. Materials. 2021; 14(20):5976. https://doi.org/10.3390/ma14205976
Chicago/Turabian StyleMoon, Kyoung-Suk, Young-Bum Park, Ji-Myung Bae, Eun-Joo Choi, and Seung-Han Oh. 2021. "Visible Light-Mediated Sustainable Antibacterial Activity and Osteogenic Functionality of Au and Pt Multi-Coated TiO2 Nanotubes" Materials 14, no. 20: 5976. https://doi.org/10.3390/ma14205976
APA StyleMoon, K.-S., Park, Y.-B., Bae, J.-M., Choi, E.-J., & Oh, S.-H. (2021). Visible Light-Mediated Sustainable Antibacterial Activity and Osteogenic Functionality of Au and Pt Multi-Coated TiO2 Nanotubes. Materials, 14(20), 5976. https://doi.org/10.3390/ma14205976








