Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges
Abstract
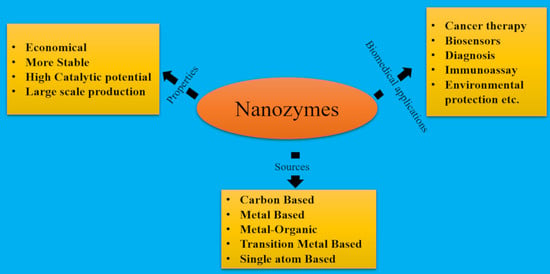
:1. Introduction
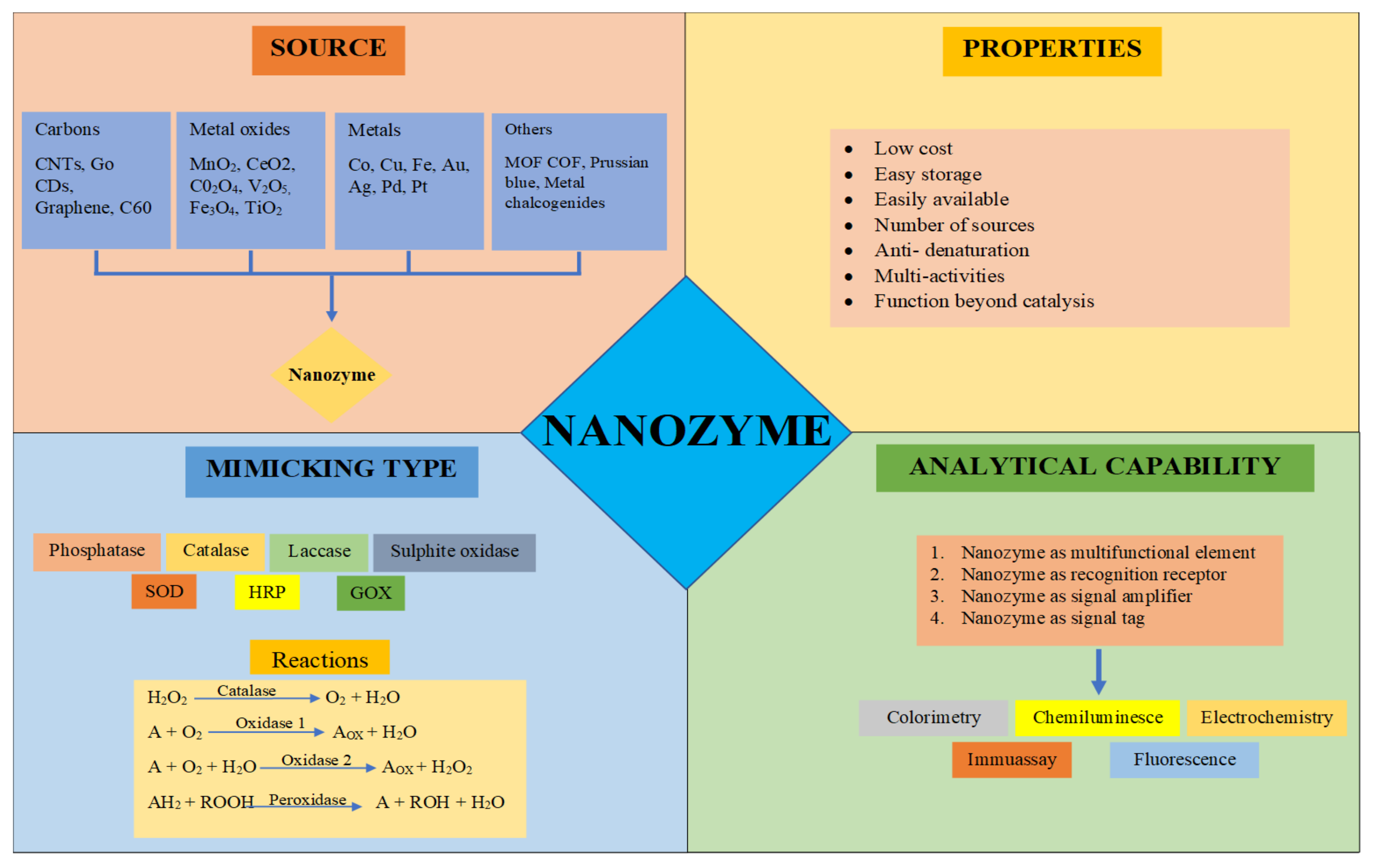
2. Types of Nanozymes
2.1. Peroxidasemimics
2.1.1. Iron-Based
2.1.2. Vanadium-Based
2.1.3. Based on Noble Metal
2.1.4. Carbon-Based
2.1.5. Based on Metal–Organic Framework
2.2. Oxidase Mimics
2.2.1. Gold-Based
2.2.2. Copper-Based
2.2.3. Molybdenum-Based
2.2.4. Based on Platinum
2.3. Catalase Mimics
2.4. Superoxide Dismustase (SOD) Mimics
2.4.1. Carbon-Based
2.4.2. Cerium-Based
2.4.3. Melanin-Based
2.5. Hydrolase Mimics
2.5.1. Carbon-Based
2.5.2. Monolayer Functionalized AuNP-Based
2.5.3. Metal–Organic Framework-Based
2.6. Other Enzyme Mimics
2.6.1. Single-Substrate Mechanism of Nanozymes
- Hydrolase;
- Peroxidase;
- Superoxide dismutase;
- Oxidase;
- Catalase [26].
2.6.2. Nanozymes with the Multi-Substrate Mechanism
- Metal-based nanozymes;
- Cu2O nanozymes;
- Nanoceria;
- Melanin nanoparticles;
- Prussian blue nanoparticles [26].
3. Synthesis of Nanozymes
3.1. Nanozyme Production
3.2. Hydrothermal and Solvothermal Methods
3.3. Chemical Reduction
3.4. Sol–Gel Method
3.5. Co-Precipitation
3.6. Electrochemical Deposition
3.7. Polymerization and Polycondensation
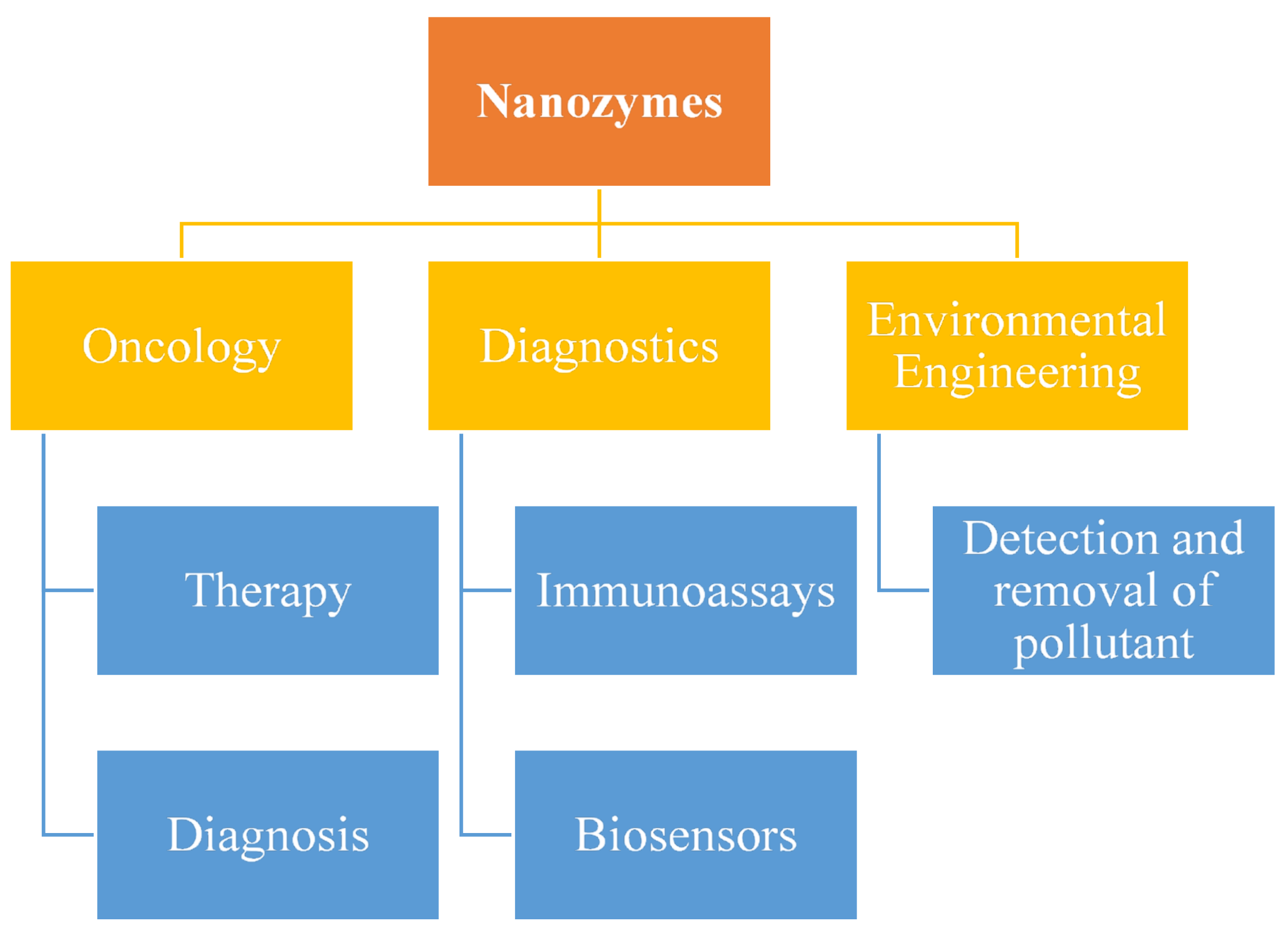
4. Nanozymes from Challenges to Opportunities
4.1. Biosensors
4.2. Detection of H2O2
4.3. Detection of Glucose
4.4. Metal Ion Sensing
4.5. Nucleic Acid Sensing
4.6. Aptasensors
4.7. Pollutant Detection
4.8. Nanozyme-Based Immunoassays
5. Nano-Enzymes Role in Diseases
5.1. Cancer Diagnostics and Therapy
5.2. Neuroprotection
5.3. Antioxidation
5.4. Anti-Inflammatory
5.5. Anti-Aging
6. Biomedical Application of Nanozymes
6.1. Nanozymes Acting by Themselves
6.2. Synergistic Nanozymes
6.3. Remote Control Nanozymes
7. Future Perspectives for Nanozymes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sindhu, R.K.; Kaur, H.; Kumar, M.; Sofat, M.; Yapar, E.A.; Esenturk, I.; Kara, B.A.; Kumar, P.; Keshavarzi, Z. The ameliorating approach of nanorobotics in the novel drug delivery systems: A mechanistic review. J. Drug Target 2021, 29, 822–833. [Google Scholar] [CrossRef]
- Cheng, M.H.; Rosentrater, K.A.; Sekhon, J.; Wang, T.; Jung, S.; Johnson, L.A. Economic feasibility of soybeanoil production by enzyme-assisted aqueous extraction processing. Food Bioprocess Technol. 2019, 12, 539–550. [Google Scholar] [CrossRef]
- Kasar, S.S.; Giri, A.P.; Pawar, P.K.; Maheshwari, V.L. A Protein α-amylase inhibitor from Withania somnifera and its role in overall quality and nutritional valuei mprovement of potato chips during processing. Food Bioprocess Technol. 2019, 12, 636–644. [Google Scholar] [CrossRef]
- Osete-Alcaraz, A.; Bautista-Ortín, A.B.; Ortega-Regules, A.E.; Gómez-Plaza, E. Combined use of pectolytic enzymes and ultra sounds for improving the extraction of phenolic compounds during vinification. Food Bioprocess Technol. 2019, 12, 1330–1339. [Google Scholar] [CrossRef]
- Wubshet, S.G.; Wold, J.P.; Afseth, N.K.; Böcker, U.; Lindberg, D.; Ihunegbo, F.N.; Måge, I. Feed-Forward Prediction of Product Qualities in Enzymatic Protein Hydrolysis of Poultry By-products: A Spectroscopic Approach. Food Bioprocess Technol. 2018, 11, 2032–2043. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.-Q.; Jiang, W.; Wu, M.; Rao, S.-Q.; Qian, J.-Y. Pulsed Electric Field as a Means to Elevate Activity and Expression of α-Amylase in Barley (Hordeum vulgare L.) Malting. Food Bioprocess Technol. 2019, 12, 1010–1020. [Google Scholar] [CrossRef]
- Sun, D.W. (Ed.) Handbook of Food Safety Engineering; Wiley Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [Green Version]
- Orive, G.; Gascon, A.R.; Hernández, R.M.; Domínguez-Gil, A.; Pedraz, J.L. Techniques: New approaches to the delivery of biopharmaceuticals. Trends Pharmacol. Sci. 2004, 25, 382–387. [Google Scholar] [CrossRef]
- Razzacki, S.Z.; Thwar, P.K.; Yang, M.; Ugaz, V.M.; Burns, M.A. Integrated microsystems for controlled drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 185–198. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, P.-L.; Wong, W.-Y.; Bian, Z.; Chui, C.-H.; Gambari, R. Recent advances in green nanoparticulate systems for drug delivery: Efficient delivery and safety concern. Nanomedicine 2017, 12, 357–385. [Google Scholar] [CrossRef]
- Antonescu (Mintas), A.-I.; Miere (Groza), F.; Fritea, L.; Ganea, M.; Zdrinca, M.; Dobjanschi, L.; Antonescu, A.; Vicas, S.I.; Bodog, F.; Sindhu, R.K.; et al. Perspectives on the Combined Effects of Ocimumbasilicum and Trifolium pratense Extracts in Terms of Phytochemical Profile and Pharmacological Effects. Plants 2021, 10, 1390. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Iram, F.; Siddiqui, S.; Sahu, K. Role of natural products in the drug discovery process. Int. J. Drug Dev. Res. 2014, 6, 172–204. [Google Scholar]
- Silva, P.; Bonifácio, B.; Ramos, M.; Negri, K.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2013, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.K.; Swamy, M.K.; Sinniah, U.R.; Anuradha, M. Leptadeniareticulata (Retz.) Wight & Arn. (Jivanti): Botanical, agronomical, phytochemical, pharmacological, and biotechnological aspects. Molecules 2017, 22, 1019. [Google Scholar]
- Beutler, J.A. Natural products as a foundation for drug discovery. Curr. Prot. Pharmacol. 2009, 46, 9–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055. [Google Scholar]
- Shin, H.Y.; Park, T.J.; Kim, M.I. Recent Research Trends and Future Prospects in Nanozymes. J. Nanomater. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, J.; Liu, Q.; Lin, A.; Li, S.; Zhang, Y.; Wang, Q.; Li, T.; An, X.; Zhou, Z.; et al. Synthesis temperature regulated multi enzyme mimicking activities of ceria nanozymes. J. Mater. Chem. B 2021, 9, 7238. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, T.; Hong, J.; Yan, X.; Liang, M. Nanozymes: A New Disease Imaging Strategy. Front. Bioeng. Biotechnol. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Chen, G.; Wu, D.; Wu, Y.; James, T.D. Enzyme Mimics for Engineered Biomimetic Cascade Nanoreactors: Mechanism, Applications, and Prospects. Adv. Funct. Mater. 2021, 2106139. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme like characteristics (nanozymes): Next-generation artificial enzymes(II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Munir, S.; Shah, A.A.; Rahman, H.; Bilal, M.; Rajoka, M.S.R.; Khan, A.A.; Khurshid, M. Nanozymes for medical biotechnology and its potential applications in biosensing and nanotherapeutics. Biotechnol. Lett. 2020, 42, 357–373. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Lin, J.; Yin, J.; Xu, J.; Chen, Y. Evaluating the bioavailability of heavy metals in natural-zeolite-amended aquatic sediments using thin-film diffusive gradients. Aquac. Fish. 2018, 3, 122–128. [Google Scholar] [CrossRef]
- Mutharaian, V.N.; Kamalakannan, R.; Mayavel, A.; Makesh, S.; Kwon, S.H.; Kang, K.-S. DNA polymorphisms and genetic relationship among populations of Acacia leucophloea using RAPD markers. J. For. Res. 2017, 29, 1013–1020. [Google Scholar] [CrossRef]
- Qiu, H.; Pu, F.; Ran, X.; Liu, C.; Ren, J.; Qu, X. Nanozyme as Artificial Receptor with Multiple Readouts for Pattern Recognition. Anal. Chem. 2018, 90, 11775–11779. [Google Scholar] [CrossRef] [Green Version]
- Yan-Yan, H.; You-Hui, L.; Fang, P.; Jin-Song, R.; Xiao-Gang, Q. The current progress of nanozymes indisease treatments. Prog. Biochem. Biophys. 2018, 45, 256–267. [Google Scholar]
- Huang, L.; Sun, D.W.; Pu, H.; Wei, Q. Development of Nanozymes for Food Quality and Safety Detection: Principles and Recent Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1496–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Liu, Y.; Song, A.; Zhao, Z.; Lu, H.; Hao, J. Peroxidase mimetic activity of Fe3O4 nanoparticle prepared based on magnetic hydrogels for hydrogen peroxide and glucose detection. J. Colloid Interface Sci. 2017, 506, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Fe3O4 Magnetic Nanoparticles as Peroxidase Mimetics and Their Applications in H2O2 and Glucose Detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- André, R.; Natálio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schröder, H.-C.; Müller, W.E.G.; Tremel, W. V2O5 Nanowires with an Intrinsic PeroxidaseLike Activity. Adv. Funct. Mater. 2011, 21, 501–509. [Google Scholar] [CrossRef]
- Natalio, F.; Andre, R.; Hartog, A.F.; Stoll, B.; Jochum, K.P.; Wever, R.; Tremel, W. Vanadium pentoxide nanoparticles mimic vanadium halo peroxidases and thwart biofilm formation. Nat. Nanotechnol. 2012, 7, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Zhang, L.; Lei, J.; Yang, L.; Zhang, Z.; Lu, X.; Wang, C. Monocrystalline VO2 (B) nanobelts: Largescale synthesis, intrinsic peroxidase like activity and application in biosensing. J. Mater. Chem. A 2014, 2, 2910–2914. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Jiang, H.; Wang, S.; Liu, H.; Huang, Y. V2O5 nanowires as a robust and efficient peroxidase mimic at high temperature in aqueous media. RSC Adv. 2014, 4, 26046–26049. [Google Scholar] [CrossRef]
- Niu, X.; He, Y.; Li, X.; Song, H.; Zhang, W.; Peng, Y.; Pan, J.; Qiu, F. Trace Iodide Dramatically Accelerates the Peroxidase Activity of VOx at ppb Concentration Levels. Chem. Sel. 2017, 2, 10854–10859. [Google Scholar] [CrossRef]
- Tian, R.; Sun, J.; Qi, Y.; Zhang, B.; Guo, S.; Zhao, M. Influence of VO2 Nanoparticle Morphology on the Colorimetric Assay of H2O2 and Glucose. Nanomaterials 2017, 7, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Zhu, W.; Zhang, W.; Chen, K.; Wang, J.; Wang, R.; Yang, Q.; Hu, N.; Suo, Y.; Wang, J. Layered vanadium(IV) disulfide nanosheets as a peroxidase like nanozyme for colorimetric detection of glucose. Microchim. Acta 2017, 185, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Huang, F.F.; Lin, Y.W. Fluorescent Detection of Lead in Environmental Water and Urine Samples Using Enzyme Mimics of Catechin Synthesized Au Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhou, Y.; Wang, X.-L.; Liang, L.-P.; Long, Y.-J.; Wang, Q.-L.; Zhang, H.-J.; Huang, X.-X.; Zheng, H.-Z. Detection of Hg2+ based on the selective inhibition of peroxidase mimetic activity of BSAAu clusters. Talanta 2013, 117, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.H.; Li, G.W.; Hong, L.; Liu, A.-L.; Chen, W.; Lin, X.-H.; Xia, X.-H. Colorimetric sensor based on dual-functional gold nanoparticles: Analyte-recognition and peroxidase-like activity. Food Chem. 2014, 147, 257–261. [Google Scholar] [CrossRef]
- Han, T.H.; Khan, M.M.; Lee, J.; Cho, M.H. Optimization of positively charged gold nanoparticles synthesized using a stainless-steel mesh and its application for colorimetric hydrogen peroxide detection. J. Ind. Eng. Chem. 2014, 20, 2003–2009. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Cai, N.; Long, S.; Yu, F. Negatively charged gold nanoparticles as an intrinsicper oxidase mimic and their applications in the oxidation of dopamine. J. Mater. Sci. 2014, 49, 7143–7150. [Google Scholar] [CrossRef]
- Kaur, B.; Chaterjee, J.G.; Bruno, T.K. Sharma, Defining Target Product Profiles (TPPs) for AptamerBased Diagnostics. Adv. Biochem. Eng. Biotechnol. 2020, 174, 195–209. [Google Scholar] [PubMed]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.; Mazurkiewicz-Pawlicka, M.; Malinowska, E. Peroxidase like activity of gold nanoparticles stabilized by hyperbranched poly glycidol derivatives over a wide pH range. Nanotechnology 2015, 26, 495101. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, C.; Guo, Y.; Nie, G.; Xu, L. Peroxidase like activity of apoferritin paired gold clusters for glucose detection. Biosens. Bioelectron. 2015, 64, 165–170. [Google Scholar] [CrossRef]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Zhu, J.; Li, Z.; Luo, J.; Wang, J.; Sun, Y. Chitosan–gold nanoparticles as peroxidase mimic and their application in glucose detection in serum. RSC Adv. 2017, 7, 44463–44469. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Mao, X.; Wang, Z.; Feng, C.; Chen, G.; Li, G. Fabrication of nanozymeDNA hydrogel and its application in biomedical analysis. Nano Res. 2017, 10, 959–970. [Google Scholar] [CrossRef]
- Singh, R.; Belgamwar, R.; Dhiman, M.; Polshettiwar, V. Dendritic fibrous nano-silica supported gold nanoparticles as an artificial enzyme. J. Mater. Chem. B 2018, 6, 1600–1604. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, N.; Si, Y.; Li, S.; Wen, J.; Zhu, X.; Wang, H. High-throughput colorimetric assays for mercury(ii) in blood and wastewater based on the mercury-stimulated catalytic activity of small silver nanoparticles in a temperature-switchable gelatin matrix. Chem. Commun. 2014, 50, 9196–9199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarshini, N.E. Pradhan, Gold nanoparticles as efficient sensors incolorimetric detection of toxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Hu, J.; Ni, P.; Dai, H.; Sun, Y.; Wang, Y.; Jiang, S.; Li, Z. Aptamer-based color imetric biosensing of abrinusing catalytic gold nanoparticles. Analyst 2015, 140, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Sloan-Dennison, S.; Laing, S.; Shand, N.C.; Graham, D.; Faulds, K. A novel nanozyme assay utilizing the catalytic activity of silver nanoparticles and SERRS. Analyst 2017, 142, 2484–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, M.N.; Anderson, S.R.; Singh, S.; Ramanathan, R.; Bansal, V. Nanostructured silver fabricasa free-standing Nano Zyme for colorimetric detection of glucose inurine. Biosens. Bioelectron. 2018, 110, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, H.; Dai, S.; Zhi, X.; Zhang, J.; Li, W. Glutathione-stabilized palladium nanozyme for colorimetric assay of silver(i) ions. Analyst 2015, 140, 6676–6683. [Google Scholar] [CrossRef]
- Chansuvarn, W.; Tuntulani, T.; Imyim, A. Colorimetric detection of mercury (II) based on gold nanoparticles, fluorescent gold nanoclusters and other gold-based nanomaterials. TRAC Trend Anal Chem. 2015, 65, 83–96. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Fu, Y. Synthesis and sensing application of glutathione-capped platinum nanoparticles. Anal. Methods 2015, 7, 4464–4471. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Chitkara, M.; Sandhu, I.S. Nanotechnology: Principles and Applications, 1st ed.; Jenny Stanford Publishing: Singapore, 2021; pp. 41–70. [Google Scholar]
- Lin, X.-Q.; Deng, H.-H.; Wu, G.-W.; Peng, H.-P.; Liu, A.-L.; Lin, X.-H.; Xia, X.-H.; Chen, W. Platinum nanoparticles/grapheneoxide hybrid with excellent peroxidaselike activity and its application for cysteine detection. Analyst 2015, 140, 5251–5256. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Lv, Z.; Chen, K.; Huang, L.; Wang, J.; Shao, F.; Wang, Y.; Han, H.-Y. Aqueous synthesis of porous platinum nanotubes at room temperature and their intrinsic peroxidaselike activity. Chem. Commun. 2013, 49, 6024–6026. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, M.; Hou, L.; Chen, G.; Tang, D. Irregular-shaped platinum nanoparticles as peroxidase mimics for highly efficient colorimetric immunoassay. Anal. Chim. Acta 2013, 776, 79–86. [Google Scholar] [CrossRef]
- He, S.B.; Deng, H.H.; Liu, A.L.; Li, G.W.; Lin, X.H.; Chen, W.; Xia, X.H. Synthesis and Peroxidase Like Activity of Salt Resistant Platinum Nanoparticles by Using Bovine Serum Albumin as the Scaffold. ChemCatChem 2014, 6, 1543–1548. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Li, M.; Yin, J.J.; Nie, Z. pH dependent catalytic activities of platinum nanoparticles with respect to the decomposition of hydrogen peroxide and scavenging of superoxide and singlet oxygen. Nanoscale 2014, 6, 11904–11910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, X.; Feng, J.; Tang, Y.; Jiang, Y.; He, N. Label-free detection of DNA by combining gated mesoporous silica and catalytic signal amplification of platinum nanoparticles. Analyst 2014, 139, 6088–6091. [Google Scholar] [CrossRef]
- Ju, Y.; Kim, J. Dendrimerencapsulated Pt nanoparticles with peroxidase-mimetic activity as biocatalytic labels for sensitive colorimetric analyses. Chem. Commun. 2015, 51, 13752–13755. [Google Scholar] [CrossRef]
- Raynal, M.; Ballester, P.; VidalFerran, A.; van Leeuwen, P.W.N.M. Supramolecular catalysis. Part 2: Artificial enzyme mimics. Chem Soc Rev. 2014, 43, 1734–1787. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Yang, J.; Jiang, Y.; He, N. Peroxidase-like activity of mesoporous silica encapsulated Pt nanoparticle and its application in colorimetric immunoassay. Anal. Chim. Acta 2015, 862, 53–63. [Google Scholar] [CrossRef]
- Jin, L.; Meng, Z.; Zhang, Y.; Cai, S.; Zhang, Z.; Li, C.; Shang, L.; Shen, Y. Ultrasmall Pt Nanoclusters as Robust Peroxidase Mimics for Colorimetric Detection of Glucose in Human Serum. ACS Appl. Mater. Interfaces 2017, 9, 10027–10033. [Google Scholar] [CrossRef]
- Ye, H.; Liu, Y.; Chhabra, A.; Lilla, E.; Xia, X. Poly vinylpyrrolidone (PVP) Capped Ptnanocubes with superior peroxidase-Like activity. ChemNanoMat 2017, 3, 33–38. [Google Scholar] [CrossRef]
- Lan, J.; Xu, W.; Wan, Q.; Zhang, X.; Lin, J.; Chen, J.; Chen, J. Colorimetric determination of sarcosine in urine samples of prostatic carcinoma by mimic enzyme palladium nanoparticles. Anal. Chim. Acta 2014, 825, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Purich, D.L.; Wu, C.; Wu, Y.; Chen, T.; Cui, C.; Zhang, L.; Cansiz, S.; Hou, W.; Wang, Y.; et al. Ionic function alization of hydrophobic colloidal nanoparticles to formionic nanoparticles with enzymelike properties. J. Am. Chem. Soc. 2015, 137, 14952–14958. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, X.; Shi, S.; Mo, S.; Zheng, N. An investigation of the mimetic enzyme activity of two-dimensional Pd-based nanostructures. Nanoscale 2015, 7, 19018–19026. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Z.; Liao, H.; Feng, L.Y.; Wang, M.; Fu, W.S. Accelerating the peroxidase-like activity of gold nanoclusters at neutral pH for colorimetric detection of heparin and heparinase activity. Anal Chem. 2018, 90, 6247–6252. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic per oxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Wu, D.; Deng, X.; Huang, X.; Wang, K.; Liu, Q. Low-cost preparation of photoluminescent carbon nanodots and application as peroxidase mimetics in colorimetric detection of H2O2and glucose. J. Nanosci. Nanotechnol. 2013, 13, 6611–6616. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Safavi, A.; Shamsipur, M. A new label free colorimetric chemosensor for detection of mercury ion with tunable dynamic range using carbon nanodots as enzyme mimics. Chem. Eng. J. 2014, 255, 1–7. [Google Scholar] [CrossRef]
- Shamsipur, M.; Safavi, A.; Mohammadpour, Z. Indirect colorimetric detection of glutathione based on its radical restoration ability using carbon nanodots as nanozymes. Sens. Actuators B Chem. 2014, 199, 463–469. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, J.; Jiang, Z.; Wang, W.; Liu, X. High-quality carbon dots: Synthesis, peroxidase-like activity and their application in the detection of H2O2, Ag+ and Fe3+. RSC Adv. 2014, 4, 17387–17392. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T. Carbon Nanodots as Peroxidase Nanozymes for Biosensing. Molecules 2016, 21, 1653. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Liu, J.; Yan, X.; Kang, L. Graphene oxide derived graphene quantum dots with different photoluminescence properties and peroxidase-like catalytic activity. RSC Adv. 2016, 6, 50609–50617. [Google Scholar] [CrossRef]
- Nirala, N.R.; Khandelwal, G.; Kumar, B.; Prakash, R.; Kumar, V. One step electro-oxidative preparation of graphene quantum dots from wood charcoal as a peroxidase mimetic. Talanta 2017, 173, 36–43. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Liao, W.C.; Cazelles, R.; Wang, S.; Yu, X.; Gutkin, V.; Willner, I. Mimicking Horseradish Peroxidase Functions Using Cu2+-Modified Carbon Nitride Nanoparticles or Cu2+-Modified Carbon Dots as Heterogeneous Catalysts. ACS Nano 2017, 11, 3247–3253. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Shi, L.; Guo, Z. Iron impuritiesas theactive sites for peroxidase like catalytic reaction on graphene and its derivatives. ACS Appl. Mater. Interfaces 2015, 7, 15403–15413. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Shi, L.; Xu, J.; Wang, X.; Guo, Z.; Liu, W. Grapheneoxide–iron complex: Synthesis, characterization and visible-light-driven photo catalysis. J. Mater. Chem. A 2013, 1, 644–650. [Google Scholar] [CrossRef]
- Gayathri, P.; Kumar, A.S. An Iron Impurity in Multiwalled Carbon Nanotube Complexes with Chitosan that Biomimics the Heme-Peroxidase Function. Chem.-A Eur. J. 2013, 19, 17103–17112. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
- Zhang, R.; He, S.; Zhang, C.; Chen, W. Three-dimensional Fe- and N-incorporated carbon structures as peroxidase mimics for fluorescence detection of hydrogen peroxide and glucose. J. Mater. Chem. B 2015, 3, 4146–4154. [Google Scholar] [CrossRef]
- Yang, W.; Huang, T.; Zhao, M.; Luo, F.; Weng, W.; Wei, Q.; Lin, Z.; Chen, G. High peroxidase-like activity of iron and nitrogen co-doped carbon dots and its application in immunosorbent assay. Talanta 2017, 164, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Liu, Q.; Asiri, A.M.; Qusti, A.H.; Al-Youbi, A.O.; Sun, X. Ultrathin graphitic carbon nitride nanosheets: A novel peroxidase mimetic, Fe doping-mediated catalytic performance enhancement and application to rapid, highly sensitive optical detection of glucose. Nanoscale 2013, 5, 11604–11609. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Zhong, L.; Wang, J.; Guo, L.; Wu, H.; Guo, Q.; Fu, F.; Chen, G. Graphite-like carbon nitrides as peroxidase mimetics and their applications to glucose detection. Biosens. Bioelectron. 2014, 59, 89–93. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, J.; Ai, S.; Li, L. As an ewperoxidasemimetic: The synthesis of selenium doped graphitic carbon nitride nanosheets and applications on colorimetric detection of H2O2 and xanthine. Sens. Actuators B Chem. 2015, 216, 418–427. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Y.; Hu, Y.; Ding, Y.; Lin, S.; Cao, W.; Wang, Q.; Wu, J.; Muhammad, F.; Zhao, X.; et al. Monitoring of Heparin Activity in Live Rats Using Metal–Organic Framework Nanosheets as Peroxidase Mimics. Anal. Chem. 2017, 89, 11552–11559. [Google Scholar] [CrossRef] [PubMed]
- Biella, S.; Prati, L.; Rossi, M. Selective Oxidation of D-Glucose on Gold Catalyst. J. Catal. 2002, 206, 242–247. [Google Scholar] [CrossRef]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The Catalytic Activity of Naked Gold Particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, P.; Comotti, M.; Della Pina, C.; Rossi, M. Aerobic oxidation of glucose: II. Catalysis by colloidal gold. Appl. Catal. A Gen. 2006, 297, 1–7. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Roy, P.; Wu, W.P.; Huang, Y.H.; Chang, H.T. Glucose Oxidase and Horseradish Peroxidase Like Activities of Cuprous Oxide/Polypyrrole Composites. Electrochim. Acta 2016, 215, 253–260. [Google Scholar] [CrossRef]
- Lewandowska, H.; Wójciuk, K.; Karczmarczyk, U. Metal Nanozymes: New Horizons in Cellular Homeostasis Regulation. Appl. Sci. 2021, 11, 9019. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Carmona, U.; Knez, M. Semi-artificial and bioactive ferroxidase with nanoparticles as the active sites. Chem. Commun. 2014, 50, 8021–8023. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, L.; Zhao, M.; Wang, Y. CO3O4 nanoparticles as an efficient catalase mimic: Properties, mechanism and its electro catalytic sensing application for hydrogen peroxide. J. Mol. Catal. A Chem. 2013, 378, 3037. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Li, T.; Tian, W.; Zhang, Q.; Cheng, Y. Generation poly amidoamine dendrimeren capsulated platinum nanoparticle mimics catalase size, shape, and catalytic activity. Langmuir 2013, 29, 5262–5270. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Gu, N. Prussian Blue Modified Ferritin Nanoparticlesas Peroxidase and Catalase Mimetics and Their Application in Glucose Detection. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2013; Volume 562, pp. 1333–1339. [Google Scholar]
- Zhu, Z.; Guan, Z.; Jia, S.; Lei, Z.; Lin, S.; Zhang, H.; Ma, Y.; Tian, Z.-Q.; Yang, C.J. Au@Pt Nanoparticle Encapsulated Target-Responsive Hydrogel with Volumetric Bar-Chart Chip Readout for Quantitative Point-of-Care Testing. Angew. Chem. Int. Ed. 2014, 53, 12503–12507. [Google Scholar]
- Nicolini, V.; Gambuzzi, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P.; D’Addato, S.; et al. Evidence of Catalase Mimetic Activity in Ce3+/Ce4+ Doped Bioactive Glasses. J. Phys. Chem. B 2015, 119, 4009–4019. [Google Scholar] [CrossRef]
- Aneesh, K.; Vusa, C.S.; Berchmans, S. Dualenzyme mimic ryexhibited by ITO nanocubes and their application inspector photometric and electro chemical sensing. Analyst 2016, 141, 4024–4028. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Korschelt, K.; Daniel, P.; Landfester, K.; Tremel, W.; Bannwarth, M.B. Fibrous Nanozyme Dressings with Catalase-Like Activity for H2O2 Reduction to Promote Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 38024–38031. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, J. Chitosan Microgels Embedded with Catalase Nanozyme-Loaded Mesocellular Silica Foam for Glucose-Responsive Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, W.; Wu, X.; Gao, X. Mechanism of pH-switchable peroxidase and catalase-like activities of gold, silver, platinum and palladium. Biomaterials 2015, 48, 37–44. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, J.; Wu, Y.; Cao, P.; Song, L.; Ma, M.; Gu, N.; Zhang, Y. Shape-dependent enzyme-like activity of CO3O4 nanoparticles and their conjugation with his-tagged EGFR single-domain antibody. Colloids Surfaces B Biointerfaces 2017, 154, 55–62. [Google Scholar] [CrossRef]
- Sobańska, K.; Pietrzyk, P.; Sojka, Z. Generation of reactive oxygen species via electro proticinter action of H2O2 with ZrO2 gel: Ionic sponge effect and pH switchable peroxidase and catalase like activity. ACS Catal. 2017, 7, 2935–2947. [Google Scholar] [CrossRef]
- Ma, X.; Hu, W.; Guo, C.; Yu, L.; Gao, L.; Xie, J.; Li, C.M. DNA-Templated Biomimetic Enzyme Sheets on Carbon Nanotubes to Sensitively In Situ Detect Superoxide Anions Released from Cells. Adv. Funct. Mater. 2014, 24, 5897–5903. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, S.; Tu, W.; Zhang, Z.; Bao, J.; Dai, Z. Biomimetic Superoxide Dismutase Stabilized by Photopolymerization for Superoxide Anions Biosensing and Cell Monitoring. Anal. Chem. 2014, 86, 4783–4790. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.N.; Pandian, K. Organically Dispersible Gold and Platinum Nanoparticles Using Aromatic Amines as Phase Transfer and Reducing Agent and Their Applications in Electro-Oxidation of Glucose. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 113–120. [Google Scholar] [CrossRef]
- Kamada, K.; Soh, N. Enzyme-Mimetic Activity of Ce-Intercalated Titanate Nanosheets. J. Phys. Chem. B 2015, 119, 5309–5314. [Google Scholar] [CrossRef]
- Liu, T.; Niu, X.; Shi, L.; Zhu, X.; Zhao, H.; Lana, M. Electro catalytic analysis of super oxideanion radical using nitrogen-doped graphene supported Prussian Blueasa biomimetic superoxide dismutase. Electrochim. Acta. 2015, 176, 1280–1287. [Google Scholar] [CrossRef]
- Shen, X.; Liu, W.; Gao, X.; Lu, Z.; Wu, X.; Gao, X. Mechanisms of Oxidase and Superoxide Dismutation-like Activities of Gold, Silver, Platinum, and Palladium, and Their Alloys: A General Way to the Activation of Molecular Oxygen. J. Am. Chem. Soc. 2015, 137, 15882–15891. [Google Scholar] [CrossRef]
- Mu, J.; Zhao, X.; Li, J.; Yang, E.-C.; Zhao, X.-J. Novel hierarchical NiO nanoflowers exhibiting intrinsic superoxide dismutase-like activity. J. Mater. Chem. B 2016, 4, 5217–5221. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, Q.; Liu, Y.; Xue, W.; Ma, L.; Feng, S.; Wan, M.; Wang, F.; Mao, C. Manganese Phosphate Self-Assembled Nanoparticle Surface and Its application for Superoxide Anion Detection. Sci. Rep. 2016, 6, 28989. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Q.; Ye, C.; Bao, S.-J.; Xu, M.-W.; Zhang, Y.; Wang, L.; Ma, X.-Q.; Guo, J.; Li, C.M. Nanostructured cobalt phosphates as excellent biomimetic enzymes to sensitively detect superoxide anions released from living cells. Biosens. Bioelectron. 2017, 87, 998–1004. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Wang, J.; Chen, W.; Wan, Y.; Wang, X.; Cai, N.; Liu, J.; Yu, F. Synergistic Peroxidase-like Activity of CeO2 -Coated Hollow Fe3O4 Nanocomposites as an Enzymatic Mimic for Low Detection Limit of Glucose. J. Taiwan Inst. Chem. Eng. 2018 83, 40–49. [CrossRef]
- Tarnuzzer, R.W.; Colon, J.; Patil, S.; Seal, S. Vacancy Engineered Ceria Nanostructures for Protection from Radiation-Induced Cellular Damage. Nano Lett. 2005, 5, 2573–2577. [Google Scholar] [CrossRef] [PubMed]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic proper ties exhibited by vacancy engineer edceria nanoparticles. Chem. Commun. 2007, 10, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.-Y.; Luo, S.-L.; Li, H.; Dong, S.-W.; He, J.; Jiang, H.; Li, R.; Yang, X.-C. Alendronate as a robust anchor for ceria nanoparticle surface coating: Facile binding and improved biological properties. RSC Adv. 2014, 4, 59965–59969. [Google Scholar] [CrossRef]
- Pulido-Reyes, G.; Rodea-Palomares, I.; Das, S.; Sakthivel, T.S.; Leganes, F.; Rosal, R.; Seal, S.; Fernández-Piñas, F. Untangling the biological effects of ceriumoxide nanoparticles: The role of surface valence states. Sci. Rep. 2015, 5, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ai, K.; Ji, X.; Askhatova, D.; Du, R.; Lu, L.; Shi, J. Comprehensive Insights into the Multi-Antioxidative Mechanisms of Melanin Nanoparticles and Their Application to Protect Brain from Injury in Ischemic Stroke. J. Am. Chem. Soc. 2017, 139, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Tokuyama, H.; Yamago, S.; Nakamura, E.; Shiraki, T.; Sugiura, Y. Photoinduced biochemical activity of fullerene carboxylic acid. J. Am. Chem. Soc. 1993, 115, 7918–7919. [Google Scholar] [CrossRef]
- Boutorine, A.S.; Takasugi, M.; Hélène, C.; Tokuyama, H.; Isobe, H.; Nakamura, E. Fullerene Oligonucleotide Conjugates: Photoinduced Sequence Specific DNA Cleavage. Angew. Chem. Int. Ed. Engl. 1995, 33, 2462–2465. [Google Scholar] [CrossRef]
- Yamakoshi, Y.N.; Yagami, T.; Sueyoshi, S.; Miyata, N. Acridine adduct of fullerene witen hanced DNA cleaving activity. J. Org. Chem. 1996, 61, 7236–7237. [Google Scholar] [CrossRef] [PubMed]
- Hostert, L.; Blaskievicz, S.; Fonsaca, J.; Domingues, S.; Zarbin, A.J.; Orth, E. Imidazole-derived graphene nanocatalysts for organophosphate destruction: Powder and thin film heterogeneous reactions. J. Catal. 2017, 356, 75–84. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Xia, M.; Li, S.; Zhang, X.; Zhang, Y. Mimicking the Active Sites of Organophosphorus Hydrolase on the Backbone of Graphene Oxide to Destroy Nerve Agent Simulants. ACS Appl. Mater. Interfaces 2017, 9, 21089–21093. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, J.; Yang, Y.; Su, P.; Yang, Y. CO3O4/reduced grapheneoxide nanocomposites as effective phosphotriest erase mimetics for degradation and detection of paraoxon. Ind. Eng. Chem. Res. 2017, 56, 9762–9769. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation Catalysts. Angew. Chem. 2004, 116, 6291–6295. [Google Scholar] [CrossRef]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.-F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef] [Green Version]
- Zaupa, G.; Mora, C.; Bonomi, R.; Prins, L.J.; Scrimin, P. Catalytic Self-Assembled Monolayers on Au Nanoparticles: The Source of Catalysis of a Transphosphorylation Reaction. Chem.-A Eur. J. 2011, 17, 4879–4889. [Google Scholar] [CrossRef]
- Li, P.; Klet, R.C.; Moon, S.-Y.; Wang, T.C.; Deria, P.; Peters, A.W.; Klahr, B.M.; Park, H.-J.; Al-Juaid, S.S.; Hupp, J.T.; et al. Synthesis of nanocrystals of Zr-based metal–organic frameworks with csq-net: Significant enhancement in the degradation of a nerve agent simulant. Chem. Commun. 2015, 51, 10925–10928. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.; Iii, W.C.I.; Ghosh, P.; Liao, P.; Bury, W.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Peterson, G.; et al. Destruction of chemical warfare agents using metal–organic frameworks. Nat. Mater. 2015, 14, 512–516. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Wagner, G.W.; Mondloch, J.E.; Peterson, G.W.; Decoste, J.B.; Hupp, J.T.; Farha, O.K. Effective, Facile, and Selective Hydrolysis of the Chemical Warfare Agent VX Using Zr6-Based Metal–Organic Frameworks. Inorg. Chem. 2015, 54, 10829–10833. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.; Gomes, A.C.; Pillinger, M.; Gonçalves, I.S.; Abrantes, M. Promotion of phosphor ester hydrolysis by the ZrIV based metal organic framework UiO-67. Microporous Mesoporous Mater. 2015, 208, 21–29. [Google Scholar] [CrossRef]
- López-Maya, E.; Montoro, C.; Rodríguez-Albelo, L.M.; Aznar Cervantes, S.D.; Lozano-Pérez, A.A.; Cenís, J.L.; Barea, E.; Navarro, J.A. Textile/Metal Organic Framework Composites as Self Detoxifying Filters for Chemical Warfare Agents. Angew. Int. Ed. 2015, 54, 6790–6794. [Google Scholar] [CrossRef] [PubMed]
- Nath, I.; Chakraborty, J.; Verpoort, F. Metal organic frameworks mimicking natural enzymes: A structural and functional analogy. Chem. Soc. Rev. 2016, 45, 4127–4170. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.T.; Zhao, J.; Peterson, G.W.; Parsons, G.N. A Catalytic ‘MOF-Cloth’ Formed via Directed Supramolecular Assembly of UiO-66-NH2 Crystals on ALD-coated Textiles for Rapid Degradation of Chemical Warfare Agent Simulants. Chem. Mater. 2017, 29, 4894–4903. [Google Scholar] [CrossRef]
- McCarthy, D.L.; Liu, J.; Dwyer, D.B.; Troiano, J.L.; Boyer, S.M.; De Coste, J.B.; Bernier, W.E.; Jones, W.E., Jr. Electrospunmetal organic framework polymer composites for the catalytic degradation of methylparaoxon. New J. Chem. 2017, 41, 8748–8753. [Google Scholar] [CrossRef]
- Park, H.J.; Jang, J.K.; Kim, S.-Y.; Ha, J.-W.; Moon, D.; Kang, I.-N.; Bae, Y.-S.; Kim, S.; Hwang, D.-H. Synthesis of a Zr-Based Metal–Organic Framework with Spirobifluorenetetrabenzoic Acid for the Effective Removal of Nerve Agent Simulants. Inorg. Chem. 2017, 56, 12098–12101. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Madadkhani, S.; Zand, Z.; Hołyńska, M.; Allakhverdiev, S.I. Engineered poly peptidearound nano-sized manganese calciumoxideas an artificial water oxidizing enzyme mimic king natural photosynthesis: Toward artificial enzymes with highly active site densities. Int. J. Hydrog. Energy 2016, 41, 17826–17836. [Google Scholar] [CrossRef]
- Saeed, M.; Deng, L. Carbon nanotube enhanced PVA-mimic enzyme membrane for post-combustion CO2 capture. Int. J. Greenh. Gas Control. 2016, 53, 254–262. [Google Scholar] [CrossRef]
- Nandhakumar, P.; Kim, B.; Lee, N.-S.; Yoon, Y.H.; Lee, K.; Yang, H. Nitrosoreductase-Like Nanocatalyst for Ultrasensitive and Stable Biosensing. Anal. Chem. 2017, 90, 807–813. [Google Scholar] [CrossRef]
- Xu, K.; Zhong, Z.; Xu, H.; Wang, X.; Zhao, M.; Wu, C. Highly Efficient Aerobic Oxidation of Arylalkanes with a Biomimetic Catalyst Platform. Chin. J. Appl. Chem. 2017, 34, 1079–1085. [Google Scholar]
- Xu, J.J.; Wang, M.M.; Liu, L.Z.; Li, F.; Tian, J.S. Analysis of alkaline phosphatase activity of magnetosome. J. China Agric. Univ. 2018, 23, 8–13. [Google Scholar]
- Murugan, C.; Murugan, N.; Sundramoorthy, A.K.; Sundaramurthy, A. Nanoceria Decorated Flower-like Molybdenum Sulphide Nanoflakes: An Efficient Nanozyme for Tumour Selective ROS Generation and Photo Thermal Therapy. Chem. Commun. 2019, 55, 8017–8020. [Google Scholar] [CrossRef]
- Xue, T.; Peng, B.; Xue, M.; Zhong, X.; Chiu, C.-Y.; Yang, S.; Qu, Y.; Ruan, L.; Jiang, S.; Dubin, S.; et al. Integration of molecular and enzymatic catalysts on graphene for biomimetic generation of antithrombotic species. Nat. Commun. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A Water-Stable Porphyrin-Based Metal-Organic Framework Active for Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar] [CrossRef]
- Sasan, K.; Lin, Q.; Mao, C.; Feng, P. Incorporation of iron hydrogenase active sites into a highly stable metal–organic framework for photocatalytic hydrogen generation. Chem. Commun. 2014, 50, 10390–10393. [Google Scholar] [CrossRef] [PubMed]
- Pullen, S.; Fei, H.; Orthaber, A.; Cohen, S.M.; Ott, S. Enhanced Photochemical Hydrogen Production by a Molecular Diiron Catalyst Incorporated into a Metal–Organic Framework. J. Am. Chem. Soc. 2013, 135, 16997–17003. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.C.; Jang, Y.N.; Lee, S.W. Enhanced biomimetic CO2 sequestration and CaCO3 crystallization using complex encapsulated metal organic framework. J. Cryst. Growth 2013, 373, 96–101. [Google Scholar] [CrossRef]
- Fillon, Y.; Verma, A.; Ghosh, P.; Ernenwein, D.; Rotello, V.M.; Chmielewski, J. Peptideligation catalyzed by functionalized gold nanoparticles. J. Am. Chem. Soc. 2007, 129, 6676–6677. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Hu, Y.; Wei, H. Nanozymes: Preparation and Characterization. In Nanostructure Science and Technology; Yan, X., Ed.; Springer: Singapore, 2020. [Google Scholar]
- Li, J.; Wu, Q.; Wu, J. Synthesis of Nanoparticlesvia Solvothermal and Hydrothermal Methods. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 295–328. [Google Scholar]
- Wang, H.; Wan, K.; Shi, X. Recent Advances in Nanozyme Research. Adv. Mater. 2019, 31, e1805368. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Li, F.; Si, X.; Ding, Y.; Deng, D.; Wang, T. Enzyme mimics of spinel-type CoxNi1−xFe2O4 magnetic nanomaterial for eletroctrocatalytic oxidation of hydrogen peroxide. Anal. Chim. Acta 2013, 788, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; He, L.; Jiang, Z.W.; Li, Y.; Zheng, M.C.; Cheng, Z.H.; Li, Y.F. CuO Nanoparticles Derived from Metal-Organic Gelwith Excellent Electrocatalytic and Peroxidase-Mimicking Activities for Glucose and Cholesterol Detection. Biosens. Bioelectron. 2019, 145, 111704. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, B.; Tang, Y.; Li, Q.; Xue, H.; Pang, H. Ultrathin Nanosheet Assembled [Ni3(OH)2(PTA)2(H2O)4]2H2O Hierarchical Flowers for High Performance Electrocatalysis of Glucose Oxidation Reactions. Nanoscale 2018, 10, 13270–13276. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Cai, X.; Zhao, H.; Magdassi, S.; Lan, M. Electrochemical detection of superoxide anions in HeLa cells by using two enzyme-free sensors prepared from ZIF-8-derived carbon nanomaterials. Microchim. Acta 2019, 186, 370. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, C.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Electrospun metal–organic framework derived hierarchical carbon nanofibers with high performance for supercapacitors. Chem. Commun. 2017, 53, 1751–1754. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, A.; Ruffino, F.; Sanzaro, S.; Grimaldi, M.G. Laserand Thermal Dewetting of Gold Layer onto Graphene Paperforn on Enzymatic Electrochemical Detection of Glucose and Fructose. Sens. Actuators B Chem. 2019, 301, 127113. [Google Scholar] [CrossRef]
- Chou, K.-S.; Ren, C.-Y. Synthesis of nanosized silver particles by chemical reduction method. Mater. Chem. Phys. 2000, 64, 241–246. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Sabu, T.C. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials Advances and Key Technologies Micro and Nano Technologies, 1st ed.; Woodhead Publishing Company: Sawston, UK, 2018; pp. 121–139. [Google Scholar]
- Suriati, G.M.; Mariatti, M.; Azizan, A. Synthesis of silver nanoparticles buchemical reduction method: Effect of reducing agent and surfactant concentration. Int. J. Automot. Mech. Eng. 2014, 10, 1920–1927. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, X.; Nie, W.; Wang, Y.; Gao, J.; Wen, W.; Wang, S. Hollow copper sulfide nanocubesas multifunctional nanozymes for colorimetric detection of dopamine and electrochemical detection of glucose. Biosens. Bioelectron. 2019, 141, 111450. [Google Scholar] [CrossRef]
- DAS, R.; Dhiman, A.; Kapil, A.; Bansal, V.; Sharma, T.K. Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal. Bioanal. Chem. 2019, 411, 1229–1238. [Google Scholar] [CrossRef]
- Han, R.; Lu, Y.; Mingjun, L.; Yanbo, W.; Xuan, L.; Chongyang, L.; Kun, L.; Lingxing, Z.; Aihua, L. Green tide biomass templated synthesis of molybdenumoxide nanorods supported on carbon as efficient nanozyme for sensitive glucose colorimetric assay. Sens. Actuators B Chem. 2019, 296, 126517. [Google Scholar]
- Han, L.; Zhang, H.; Li, F. Bioinspired Nanozymes with pH Independent and MetalIons-Controllable Activity: Field Programmable Logic Conversion of Sole Logic Gate System. Part. Part. Syst. Char. 2018, 35, 1800207. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly Sensitive Glucose Sensor Based on Pt Nanoparticle/Polyaniline Hydrogel Heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef]
- Chen, H.-I.; Chang, H.-Y. Synthesis of nanocrystalline cerium oxide particles by the precipitation method. Ceram. Int. 2005, 31, 795–802. [Google Scholar] [CrossRef]
- Dashtestani, F.; Ghourchian, H.; Eskandari, K.; RafieePour, H.A. A superoxide dismutase mimic nanocomposite for ampere metric sensing of superoxide anions. Microchim. Acta 2015, 82, 1045–1053. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bat’hi, S.A.M. Electrode position of nanostructure materials. In Electroplating of Nanostructures; Aliofkhazraei, M., Ed.; InTech: Rijeka, Croatia, 2015; pp. 3–25. [Google Scholar]
- Wurm, F.R.; Weiss, C.K. Nanoparticles from renewable polymers. Front. Chem. 2014, 2, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhosh, P.; Manesh, K.M.; Lee, S.-H.; Uthayakumar, S.; Gopalan, A.I.; Lee, K.-P. Sensitive electrochemical detection of superoxide anion using gold nanoparticles distributed poly(methyl methacrylate)–polyaniline core–shell electrospun composite electrode. Analyst 2011, 136, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2016, 3, 41–60. [Google Scholar] [CrossRef]
- Gao, L.; Fan, K.; Yan, X. Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications. Theranostics 2017, 7, 3207–3227. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, X.; Liu, J. Iron oxide nanozyme catalyzed synthesis of fluorescent polydopamine for light-up Zn2+ detection. Nanoscale 2016, 8, 13620–13626. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef]
- Feng, L.; Musto, C.J.; Suslick, K.S. A Simple and Highly Sensitive Colorimetric Detection Method for Gaseous Formaldehyde. J. Am. Chem. Soc. 2010, 132, 4046–4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, J.E. Enzyme-linked immune sorbent assay. J. Immunoass. 2000, 21, 165–209. [Google Scholar] [CrossRef]
- Deng, J.; Wang, K.; Wang, M.; Yu, P.; Mao, L. Mitochondria Targeted Nanoscale Zeolitic Imidazole Framework-90 for ATP Imaging in Live Cells. J. Am. Chem. Soc. 2017, 139, 5877–5882. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Liu, W.; Liu, H.; Liu, F.; Yu, J.; Yan, M.; Huang, J. Colorimetric detection of the flux of hydrogen peroxide released from living cells based on the high peroxidase-like catalytic performance of porous PtPd nanorods. Biosens. Bioelectron. 2015, 71, 456–462. [Google Scholar] [CrossRef]
- Chi, M.; Nie, G.; Jiang, Y.; Yang, Z.; Zhang, Z.; Wang, C.; Lu, X. Self-Assembly Fabrication of Coaxial Tepoly(3,4-ethylenedioxythiophene) Nanocables and Their Conversion to Pdpoly(3,4-ethylenedioxythiophene) Nanocables with a High Peroxidase-like Activity. ACS Appl. Mater. Interfaces 2016, 8, 1041–1049. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J. Enzyme nanoarchitectonics: Organization and device application. Chem. Soc. Rev. 2013, 42, 6322–6345. [Google Scholar] [CrossRef]
- Chen, H.C.; Tu, Y.M.; Hou, C.C.; Lin, Y.C.; Chen, C.H.; Yang, K.H. Direct electron transfer of glucose oxidase and dual hydrogen peroxide and glucose detection based on water-dispersible carbon nanotubes derivative. Anal. Chim. Acta 2015, 867, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shi, J.; Liu, A. Novel biotemplated MnO2 1D nanozyme with controllable peroxidase-like activity and unique catalytic mechanism and its application for glucose sensing. Sens. Actuators B Chem. 2017, 252, 919–926. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Huang, L.; Zhang, Z.; Dong, S. Onepotsynthesis of Fe3O4 nanoparticle loaded 3D porous graphene nanocomposites with enhanced nanozyme activity for glucose detection. ACS Appl. Mater. Interfaces 2017, 9, 7465–7471. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.-W.; Chen, Y.-C.; Chang, H.-T.; Huang, C.-C. Logical regulation of the enzyme-like activity of gold nanoparticles by using heavy metal ions. Nanoscale 2013, 5, 8227–8234. [Google Scholar] [CrossRef]
- Wang, G.L.; Jin, L.-Y.; Wu, X.M.; Dong, Y.M.; Li, Z.J. Label-free colorimetric sensor for mercury(II) and DNA on the basis of mercury(II) switched-on the oxidase-mimicking activity of silver nanoclusters. Anal. Chim. Acta 2015, 871, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Xu, C.; Ren, J.; Qu, X. Visual and quantitative detection of copper ions using magnetic silica nanoparticles clicked on multiwalled carbon nanotubes. Chem. Commun. 2010, 46, 6572–6574. [Google Scholar] [CrossRef]
- Li, W.; Chen, B.; Zhang, H.; Sun, Y.; Wang, J.; Zhang, J.; Fu, Y. BSA-stabilized Pt nanozyme for peroxidase mimetics and its application on colorimetric detection of mercury(II) ions. Biosens. Bioelectron. 2015, 66, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Deng, L.; Li, J.; Guo, S.; Wang, E.; Dong, S. Hemin−Graphene Hybrid Nanosheets with Intrinsic Peroxidase-like Activity for Label-free Colorimetric Detection of Single-Nucleotide Polymorphism. ACS Nano 2011, 5, 1282–1290. [Google Scholar] [CrossRef]
- Qu, K.; Shi, P.; Ren, J.; Qu, X. Nanocomposite Incorporating V2O5Nanowires and Gold Nanoparticles for Mimicking an Enzyme Cascade Reaction and Its Application in the Detection of Biomolecules. Chem. A Eur. J. 2014, 20, 7501–7506. [Google Scholar] [CrossRef]
- Thiramanas, R.; Jangpatarapongsa, K.; Tangboriboonrat, P.; Polpanich, D. Detection of Vibrio cholerae Using the Intrinsic Catalytic Activity of a Magnetic Polymeric Nanoparticle. Anal. Chem. 2013, 85, 5996–6002. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Stimuli-responsive peroxidase mimicking at a smart graphene interface. Chem. Commun. 2012, 48, 7055. [Google Scholar] [CrossRef]
- Chen, L.; Sha, L.; Qiu, Y.; Wang, G.; Jiang, H.; Zhang, X. An amplified electrochemical aptasensor based on hybridization chain reactions and catalysis of silver nanoclusters. Nanoscale 2015, 7, 3300–3308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghdisi, S.M.; Danesh, N.M.; Lavaee, P.; Emrani, A.S.; Ramezani, M.; Abnous, K. A novel colorimetric triple-helix molecular switch aptasensor based on peroxidase-like activity of gold nanoparticles for ultrasensitive detection of lead(ii). RSC Adv. 2015, 5, 43508–43514. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Shukla, R.; Sharma, T.K.; Bansal, V. Aptamer-Controlled Reversible Inhibition of Gold Nanozyme Activity for Pesticide Sensing. Anal. Chem. 2014, 86, 11937–11941. [Google Scholar] [CrossRef]
- Ding, N.; Yan, N.; Ren, C.; Chen, X. Colorimetric Determination of Melamine in Dairy Products by Fe3O4 Magnetic Nanoparticles−H2O2−ABTS Detection System. Anal. Chem. 2010, 82, 5897–5899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhuang, J.; Gao, L.; Zhang, Y.; Gu, N.; Feng, J.; Yang, D.; Zhu, J.; Yan, X. Decomposing phenol by the hidden talent of ferromagnetic nanoparticles. Chemosphere 2008, 73, 1524–1528. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, J.; Zhu, L.; Huang, L.; Jiang, H.; Zhang, Y. Degradation of Methylene Blue with H2O2 Activated by Peroxidase-Like Fe3O4 Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 4793–4799. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, M.; Lu, M.; Chen, G.; Tang, D. Urchin-like (goldcore) & (platinumshell) nanohybrids: A highly efficient peroxidase mimetic system for insitu amplified colorimetric immune assay. Biosens. Bioelectron. 2015, 70, 194–201. [Google Scholar]
- Kim, M.; Kim, M.S.; Kweon, S.H.; Jeong, S.; Kang, M.H.; Kim, M.I.; Lee, J.; Doh, J. Simple and Sensitive Point-of-Care Bioassay System Based on Hierarchically Structured Enzyme-Mimetic Nanoparticles. Adv. Heal. Mater. 2015, 4, 1311–1316. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Kim, M.I.; Ye, Y.; Woo, M.-A.; Lee, J.; Park, H.G. A Highly Efficient Colorimetric Immunoassay Using a Nanocomposite Entrapping Magnetic and Platinum Nanoparticles in Ordered Mesoporous Carbon. Adv. Heal. Mater. 2014, 3, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, J.; Zhang, Z.; Gao, W.; Zhou, X.; Qu, Y. Highly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detection. Biomaterials 2015, 59, 116–124. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-Like Activity of Polymer-Coated Cerium Oxide Nanoparticles. Angew. Chem. 2009, 121, 2344–2348. [Google Scholar] [CrossRef]
- Tao, Y.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Incorporating graphen eoxide and gold nanoclusters: A synergistic catalyst with surprisingly high peroxidase like activity over abroad pH range and its application for cancer cell detection. Adv. Mater. 2013, 25, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.K.; Mandal, A.K.; Nguyen, K.T.; Borah, P.; Zhao, Y. Cancer Cell Detection and Therapeutics Using Peroxidase-Active Nanohybrid of Gold Nanoparticle-Loaded Mesoporous Silica-Coated Graphene. ACS Appl. Mater. Interfaces 2015, 7, 9807–9816. [Google Scholar] [CrossRef]
- Pratt, A.; MacRae, I.J. The RNA-induced Silencing Complex: A Versatile Gene-silencing Machine. J. Biol. Chem. 2009, 284, 17897–17901. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, H.; Yang, S.H.; Wang, T.; Liu, C.; Cao, Y.C. Nanoparticle-based artificial RNA silencing machinery for antiviral therapy. Proc. Natl. Acad. Sci. USA 2012, 109, 12387–12392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme Decorated Metal–Organic Frameworks for Enhanced Photodynamic Therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef]
- Atilgan, A.; Islamoglu, T.; Howarth, A.J.; Hupp, J.T.; Farha, O.K. Detoxification of aS ulfur Mustard Simulant Using a BODIPY-Functionalized Zirconium Based Metal Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 24555–24560. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Liu, M.-D.; Zhang, M.-K.; Wang, S.-B.; Xu, L.; Li, C.-X.; Gao, F.; Xie, B.-R.; Zhong, Z.-L.; Zhang, X.-Z. Interfering with Lactate-Fueled Respiration for Enhanced Photodynamic Tumor Therapy by a Porphyrinic MOF Nanoplatform. Adv. Funct. Mater. 2018, 28, 1803498. [Google Scholar] [CrossRef]
- Vaupel, P.; Harrison, L. Tumor Hypoxia: Causative Factors, Compensatory Mechanisms, and Cellular Response. Oncologist 2004, 9, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritinnanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef] [Green Version]
- Ziech, D.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Reactive Oxygen Species (ROS)Induced genetic and epigenetic alterations in human carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 2011, 711, 167–173. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Ju, E.; Liu, Z.; Cao, F.; Chen, Z.; Ren, J.; Qu, X. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Cai, S.; Qi, C.; Li, Y.; Han, Q.; Yang, R.; Wang, C. PtCo bimetallic nanoparticles with high oxidase-like catalytic activity and their applications for magnetic-enhanced colorimetric biosensing. J. Mater. Chem. B 2016, 4, 1869–1877. [Google Scholar] [CrossRef]
- Dugan, L.L.; Gabrielsen, J.K.; Yu, S.P.; Lin, T.-S.; Choi, D.W. Buckminsterfuller enol Free Radical Scavengers Reduce Excitotoxic and Apoptotic Death of Cultured Cortical Neurons. Neurobiol. Dis. 1996, 3, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Dugan, L.L.; Turetsky, D.M.; Du, C.; Lobner, D.; Wheeler, M.; Almli, C.R.; Shen, C.K.-F.; Luh, T.-Y.; Choi, D.W.; Lin, T.-S. Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. USA 1997, 94, 9434–9439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.S.; Hardt, J.I.; Quick, K.L.; Kim-Han, J.S.; Erlanger, B.F.; Huang, T.-T.; Epstein, C.J.; Dugan, L.L. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free. Radic. Biol. Med. 2004, 37, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, T.; Choi, I.-Y.; Soh, M.; Kim, D.; Kim, Y.-J.; Jang, H.; Yang, H.-S.; Kim, J.Y.; Park, H.-K.; et al. Ceria Nanoparticles that can Protect against Ischemic Stroke. Angew. Chem. Int. Ed. 2012, 51, 11039–11043. [Google Scholar] [CrossRef]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Dowding, J.M.; Song, W.; Bossy, K.; Karakoti, A.; Kumar, A.; Kim, A.; Bossy, B.; Seal, S.; Ellisman, M.H.; Perkins, G.; et al. Ceriumoxide nanoparticles protect against Aβ-induced mitochondrial fragmentation and neuronal cell death. Cell Death Differ. 2014, 21, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Robinson, K.A.; Gabbita, S.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free. Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Redox signaling: Hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 1999, 31, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlett, B.S.; Stadtman, E.R. Protein Oxidation in Aging, Disease, and Oxidative Stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Cordy, A.; Yeh, K.N. Blue dye identification on cellulosic fibers: Indigo, logwood and Prussian blue. J. Am. Inst. Conserv. 1984, 24, 33–39. [Google Scholar] [CrossRef]
- Thompson, D. Management of Thallium Poisoning. Clin. Toxicol. 1981, 18, 979–990. [Google Scholar] [CrossRef]
- Cheng, L.; Gong, H.; Zhu, W.; Liu, J.; Wang, X.; Liu, G.; Liu, Z. PEGylated Prussian blue nanocubes as a theragnostic agent for simultaneous cancer imaging and photothermal therapy. Biomaterials 2014, 35, 9844–9852. [Google Scholar] [CrossRef]
- Cai, X.; Gao, W.; Ma, M.; Wu, M.; Zhang, L.; Zheng, Y.; Chen, H.; Shi, J.A. Prussian Blue Based Core Shell Hollow Structured Mesoporous Nanoparticleasa Smart Theragnostic Agent with Ultrahigh pH Responsive Longitudinal Relativity. Adv. Mater. 2015, 27, 6382–6389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, S.; Yin, J.-J.; He, W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Lenz, A.; Franklin, G.A.; Cheadle, W.G. Systemic inflammation after trauma. Injury 2007, 38, 1336–1345. [Google Scholar] [CrossRef]
- Sharma, R.; Tepas, J.J.; Hudak, M.L.; Mollitt, D.L.; Wludyka, P.S.; Teng, R.J.; Premachandra, B.R. Neonatal gut barrier and multiple organ failure: Role of endotoxin and proinflammatory cytokines insepsis and necrotizing enterocolitis. J. Pediatric Surg. 2007, 42, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Quick, K.L.; Ali, S.S.; Arch, R.; Xiong, C.; Wozniak, D.; Dugan, L.L. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol. Aging 2008, 29, 117–128. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-Free Colorimetric Detection of Single Nucleotide Polymorphism by Using Single-Walled Carbon Nanotube Intrinsic Peroxidase-Like Activity. Chem. A Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef]
- Ragg, R.; Natalio, F.; Tahir, M.N.; Janssen, H.; Kashyap, A.; Strand, D.; Strand, S.; Tremel, W. Molybdenum Trioxide Nanoparticles with Intrinsic Sulfite Oxidase Activity. ACS Nano. 2014, 8, 5182–5189. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, M.; Han, S.; Lai, Z.; Yang, J.; Tan, C.; Ma, Q.; Lu, Q.; Chen, J.; Zhang, X.; et al. Growth of Au Nanoparticles on 2D Metalloporphyrinic Metal-Organic Framework Nanosheets Used as Biomimetic Catalysts for Cascade Reactions. Adv. Mater. 2017, 29, 1700102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, Y.; Zhu, H.; Zhu, Q.; Xia, Y. Three-in-One: Sensing, Self-Assembly, and Cascade Catalysis of Cyclodextrin Modified Gold Nanoparticles. J. Am. Chem. Soc. 2016, 138, 16645–16654. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, L.; Chen, Y.; Shi, J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Neri, S.; Martin, S.G.; Pezzato, C.; Prins, L.J. Photoswitchable Catalysis by a Nanozyme Mediated by a LightSensitive Cofactor. J. Am. Chem. Soc. 2017, 139, 1794–1797. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.-L.; Lin, Y.-J.; Chen, H.-L.; Huang, C.-C.; Shih, P.-C.; Bow, Y.-R.; Chia, W.-T.; Sung, H.-W. In Situ Nanoreactor for Photosynthesizing H2 Gas to Mitigate Oxidative Stress in Tissue Inflammation. J. Am. Chem. Soc. 2017, 139, 12923–12926. [Google Scholar] [CrossRef]
- Ye, H.; Yang, K.; Tao, J.; Liu, Y.; Zhang, Q.; Habibi, S.; Nie, Z.; Xia, X. An Enzyme-Free Signal Amplification Technique for Ultrasensitive Colorimetric Assay of Disease Biomarkers. ACS Nano 2017, 11, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, C.; Ren, J.; Qu, X. Using Thermally Regenerable Cerium Oxide Nanoparticles in Biocomputing to Perform Label-free, Resettable, and Colorimetric Logic Operations. Angew. Chem. Int. Ed. 2012, 51, 12579–12583. [Google Scholar] [CrossRef]
- Li, W.-P.; Su, C.-H.; Chang, Y.-C.; Lin, Y.-J.; Yeh, C.-S. Ultrasound-Induced Reactive Oxygen Species Mediated Therapy and Imaging Using a Fenton Reaction Activable Polymersome. ACS Nano 2016, 10, 2017–2027. [Google Scholar] [CrossRef]

| Sl. No | Nanozymes | Subtypes | Features | Reference |
|---|---|---|---|---|
| 1. | Peroxidase mimics | Iron-based | The Fe3O4 NPs, which are magnetic, have functions such as imitating the intrinsic peroxidase. The MNP nanozymes have the occurring mechanism which is known as ping pong catalysis and is suggested in the kinetics studies. | [35] |
| Vanadium-based | V2O5 nanowire-based peroxidase mimics were the first demonstration done. Other research included the peculiar vanadium haloperoxidase imitating the functions of V2O5 wires which are nano, along with their anti-biofouling and marine applications. | [37,38] | ||
| Based on noble metal | Multi metallic NPs of the noble metals (Pd,Ag,Pt) which are known as peroxidase imitates and are utilized for antibodies, therapy, and biosensing. | [44,45,46,47,48] | ||
| Carbon-based | They possess pH, temp and hydrogen peroxide concentration dependent functions. These have been possessed by nanotubes which have a single wall and oxides of graphene. | [79,80] | ||
| Based on metal–organic framework | 2D MOFs are believed to exhibit high functions of catalysis as compared to the analogs of 3D, hence giving effective sensibility for the detection of biomolecules. | [98] | ||
| 2. | Oxidase mimics | Gold-based | According to the kinetics measurements, mechanism of Eley–Rideal was suggested for AuNP-based imitates of oxidase. | [101] |
| Copper-based | Nanoparticles that contained copper were also used as imitates of oxidase. For example, Goximitating composites of Cu2O or polypyrrole were accounted for the oxidative catalysis of glucose for creating hydrogen peroxidase in fundamental terms. | [102] | ||
| Molybdenum-based | It has been reported that the molybdenum trioxidenanoparticles can mimic sulfite oxidase for converting it to sulfate beneath the terms of physiology. | [103] | ||
| Based on platinum | Some research linked with PtNPs as the imitates of ferroxidase for oxidizing them were noted. Examples such as, Zhang, Knez, and collaborators used apoferritin which is a light chain as the platform for PtNPs to get ready. | [104] | ||
| 3. | Catalase mimics | -------------- | There are many nanomaterials such as metal oxides, metals, and PB which exhibit the type of activities linked with catalase. Pt and Pd were demonstrated for possessing the good imitating functions of catalase compared to those of gold and silver. | [105,106,107,108,109,110,111,112,113] |
| 4. | Superoxide dismutase (SOD) mimics | Carbon-based | C60[C(COOH)2]3 comprised of symmetry linked with C3 has been approved for possessing more properties such as acting against oxidation. | [125] |
| Cerium-based | Nanoceria was classified as one of the first nanomaterials possessing SOD mimicking activity. These have been allocated to the shuttle of electrons between the mixed states of oxidation. | [127,128] | ||
| Melanin-based | The mixture of the hydrochloride of dopamine along with NH3 in the ethanol and water led to the synthesis of melanin nanoparticles. | [132] | ||
| 5. | Hydrolase mimics | Carbon-based | Aqueous-solvent fullerene worked with the corrosive carboxylic, known as C 60-1, and it was exhibited for catalyzing the phosphodiester cleavage obligation occurring in DNA and illuminated through the light. | [134,135] |
| Monolayer functionalized AuNP based | AuNPs worked along with monolayers, which are catalytic throughout the bonds of gold and silver, are amongst the very first nanomaterials imitating as hydrolases that deserve acknowledgments. | [139,140,141] | ||
| MOF-based | MOFs based on Zr are used as imitates of phosphor triesterase for the occurrence of cleavage of the bond of phosphate ester of chemical warfare agent. | [142,143,144,145,146,147,148,149,150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindhu, R.K.; Najda, A.; Kaur, P.; Shah, M.; Singh, H.; Kaur, P.; Cavalu, S.; Jaroszuk-Sierocińska, M.; Rahman, M.H. Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges. Materials 2021, 14, 5965. https://doi.org/10.3390/ma14205965
Sindhu RK, Najda A, Kaur P, Shah M, Singh H, Kaur P, Cavalu S, Jaroszuk-Sierocińska M, Rahman MH. Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges. Materials. 2021; 14(20):5965. https://doi.org/10.3390/ma14205965
Chicago/Turabian StyleSindhu, Rakesh K., Agnieszka Najda, Prabhjot Kaur, Muddaser Shah, Harmanpreet Singh, Parneet Kaur, Simona Cavalu, Monika Jaroszuk-Sierocińska, and Md. Habibur Rahman. 2021. "Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges" Materials 14, no. 20: 5965. https://doi.org/10.3390/ma14205965
APA StyleSindhu, R. K., Najda, A., Kaur, P., Shah, M., Singh, H., Kaur, P., Cavalu, S., Jaroszuk-Sierocińska, M., & Rahman, M. H. (2021). Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges. Materials, 14(20), 5965. https://doi.org/10.3390/ma14205965