Modeling of Equivalent Circuit Analysis of Degraded Electric Double-Layer Capacitors
Abstract
1. Introduction
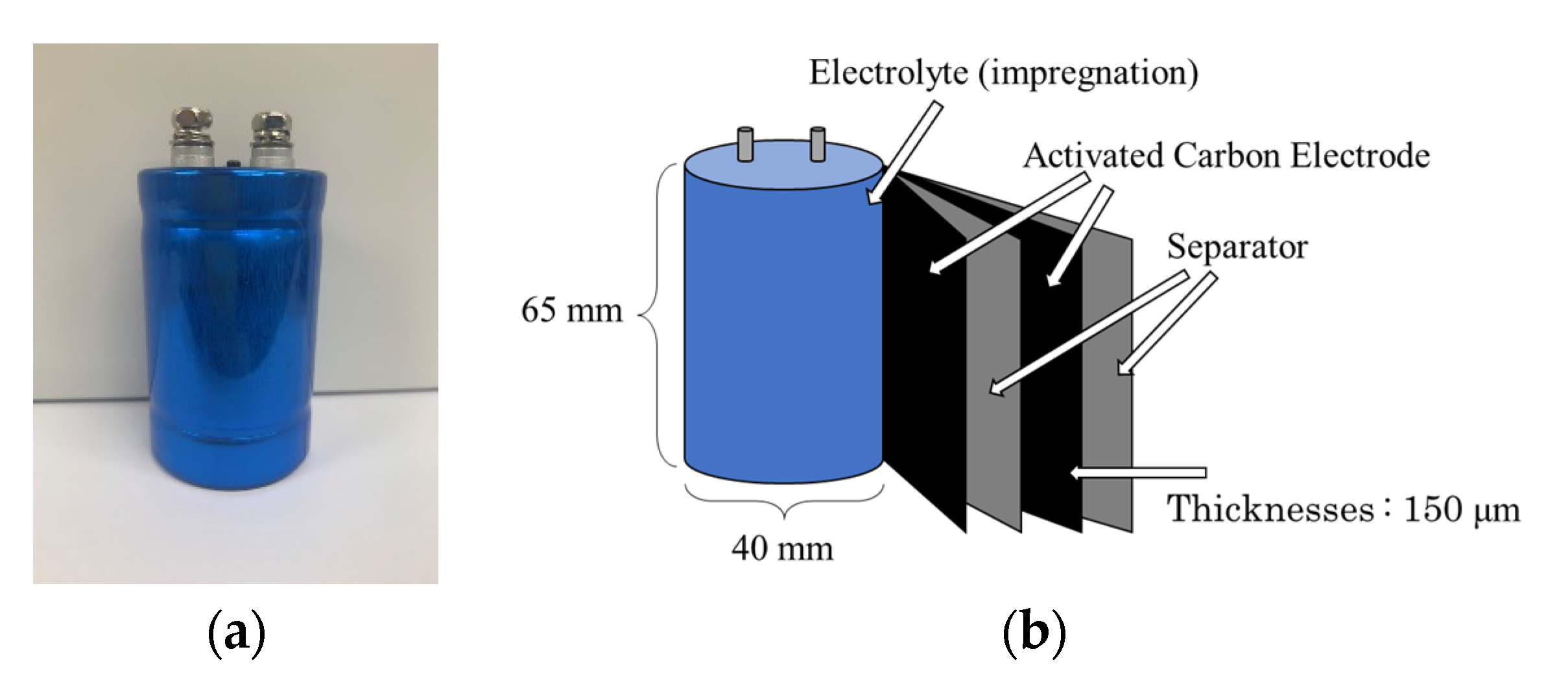
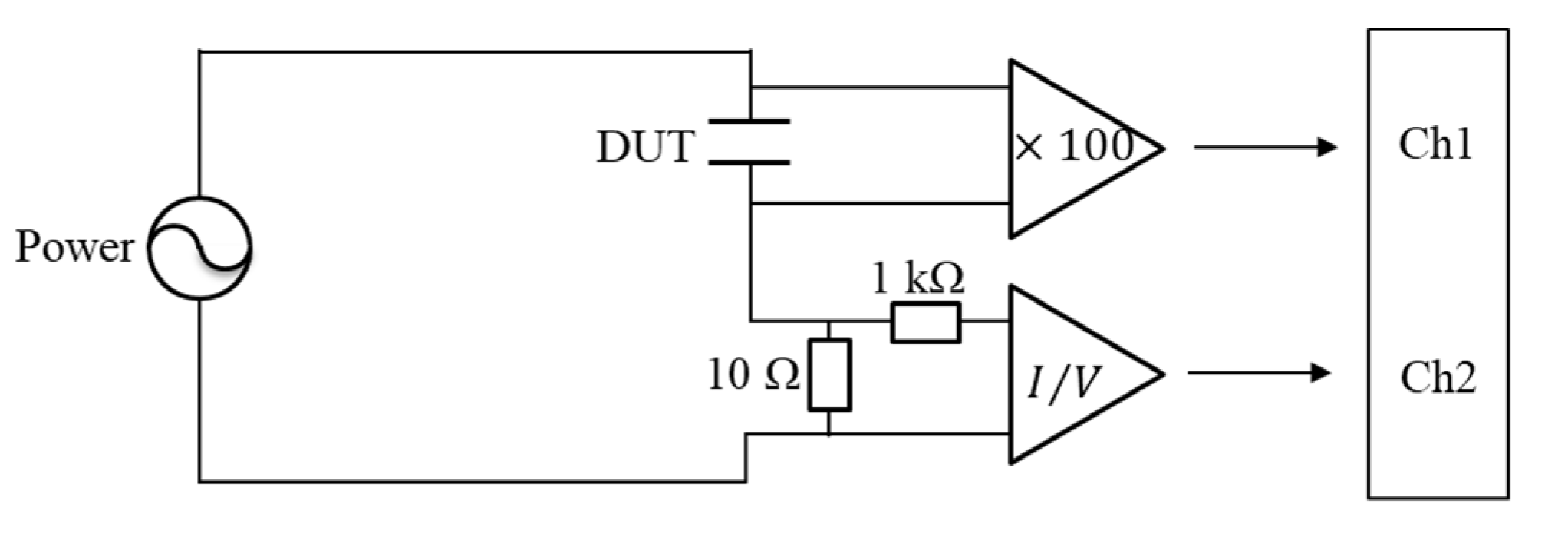
2. Materials and Methods
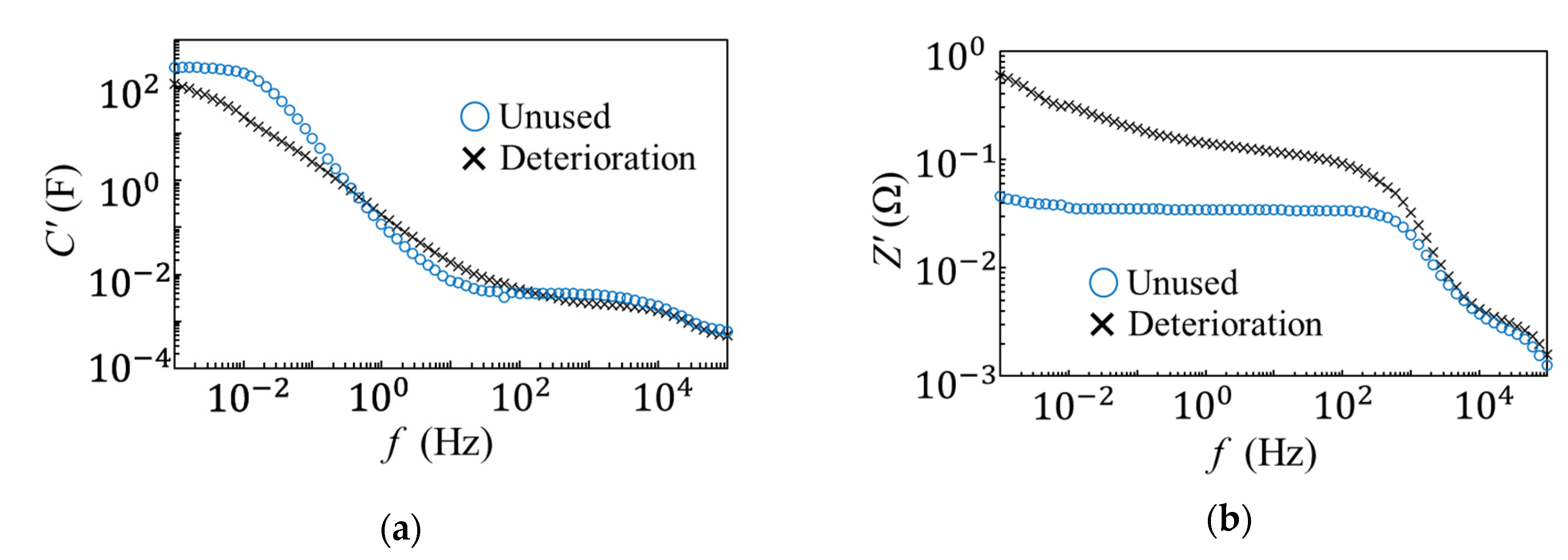
3. Results
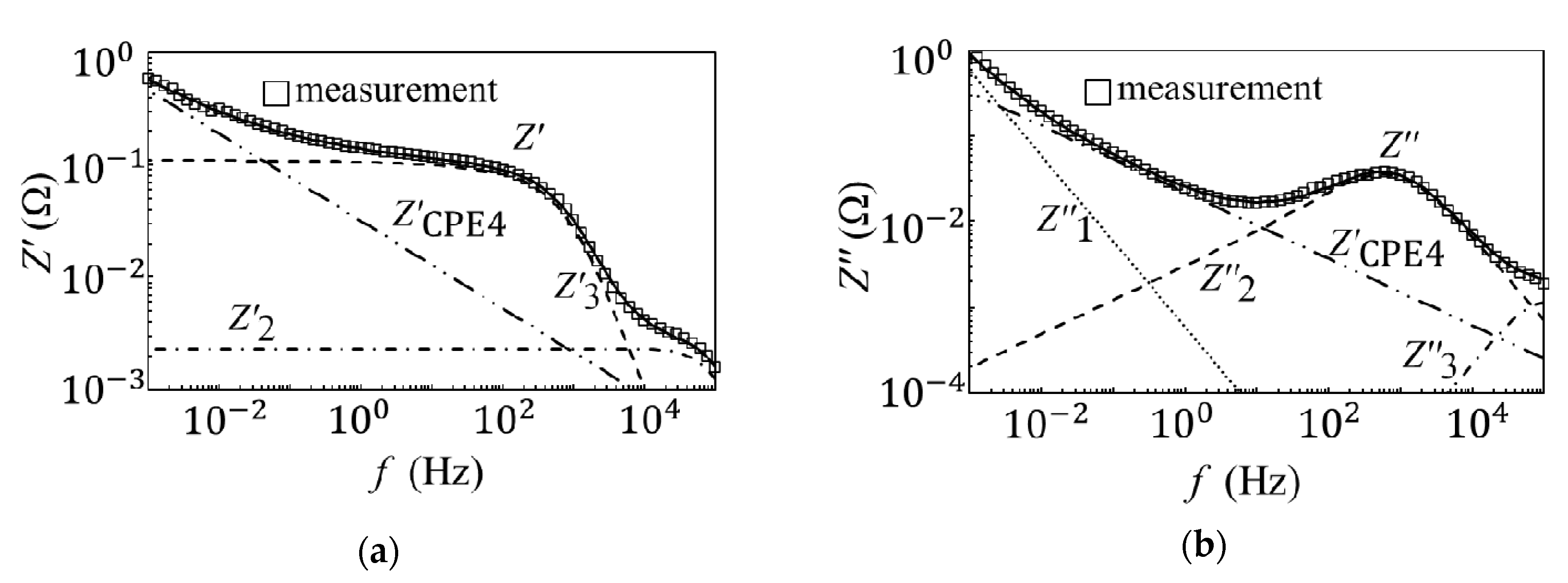
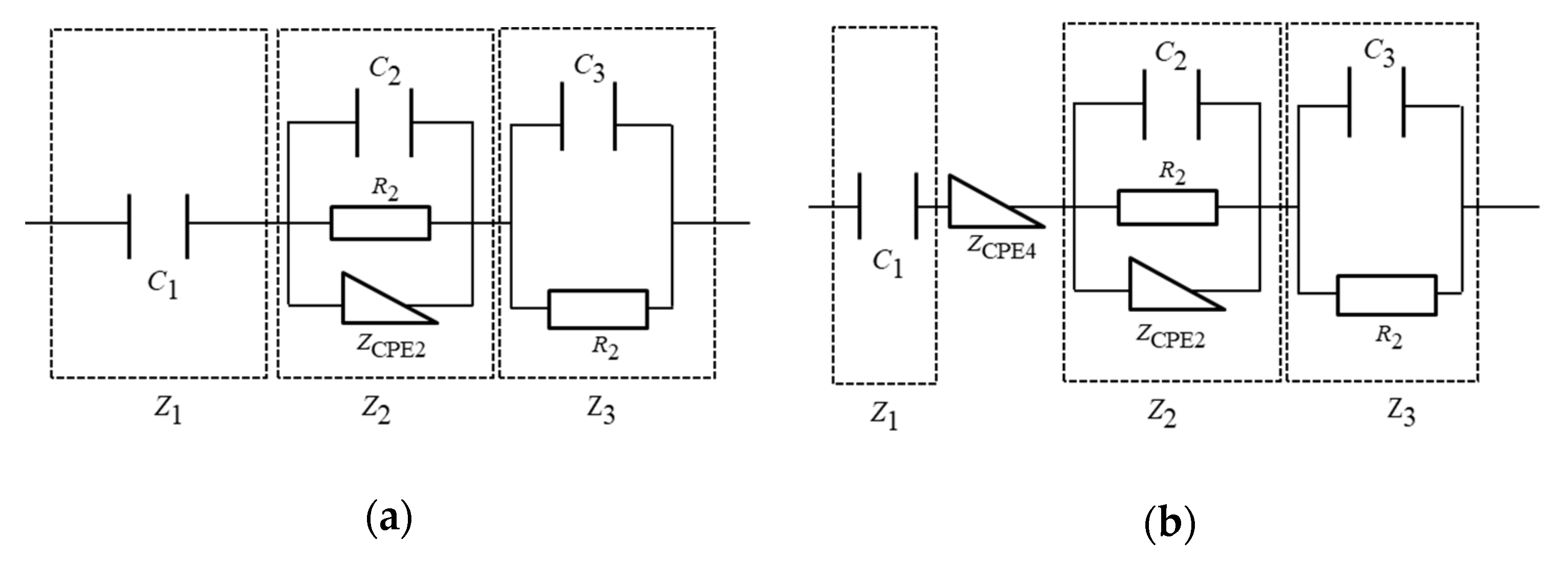
3.1. Equivalent Circuit Analysis of an EDLC
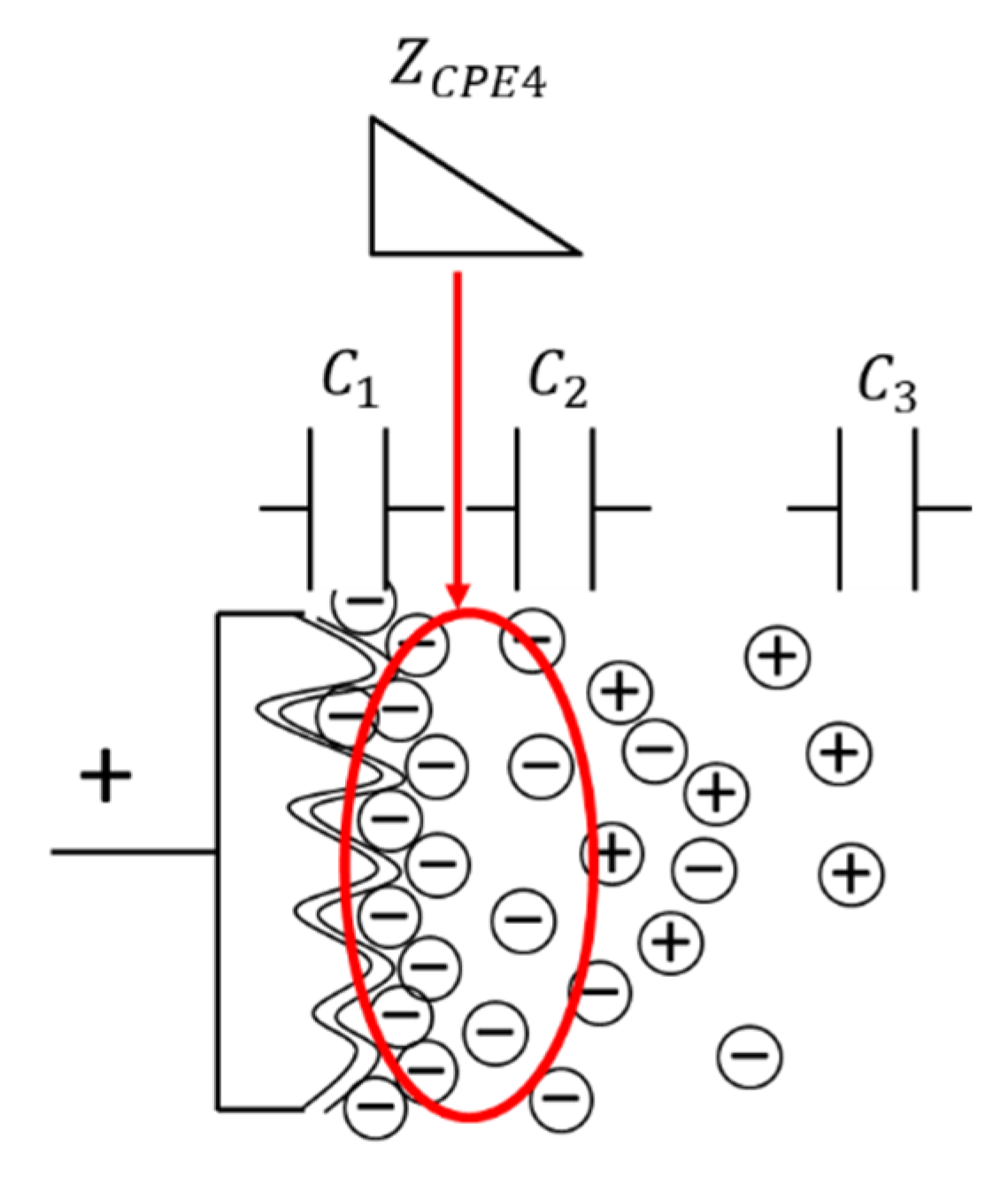
3.2. Internal Model of EDLC Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azaïs, P.; Duclaux, L.; Florian, P.; Massiot, D.; Lillo-Rodenas, M.-A.; Linares-Solano, A.; Peres, J.-P.; Jehoulet, C.; Béguin, F. Causes of supercapacitors ageing in organic electrolyte. J. Power Sources 2007, 171, 1046–1053. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Yang, J.; Wang, Y.; Chan-Park, M.B. Three-Dimensional Macroporous Graphene Foam Filled with Mesoporous Polyaniline Network for High Areal Capacitance. ACS Sustain. Chem. Eng. 2014, 2, 2291–2296. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Thounthong, P.; Raël, S.; Davat, B. Control strategy of fuel cell/supercapacitors hybrid power sources for electric vehicle. J. Power Sources 2006, 158, 806–814. [Google Scholar] [CrossRef]
- Thounthong, P.; Raël, S.; Davat, B. Energy management of fuel cell/battery/supercapacitor hybrid power source for vehicle applications. J. Power Sources 2009, 193, 376–385. [Google Scholar] [CrossRef]
- Dixon, J.; Nakashima, I.; Arcos, E.F.; Ortuzar, M. Electric Vehicle Using a Combination of Ultracapacitors and ZEBRA Battery. IEEE Trans. Ind. Electron. 2010, 57, 943–949. [Google Scholar] [CrossRef]
- Ashtiani, C.; Wright, R.; Hunt, G. Ultracapacitors for automotive applications. J. Power Sources 2006, 154, 561–566. [Google Scholar] [CrossRef]
- Al Sakka, M.; Gualous, H.; Van Mierlo, J.; Culcu, H. Thermal modeling and heat management of supercapacitor modules for vehicle applications. J. Power Sources 2009, 194, 581–587. [Google Scholar] [CrossRef]
- Bohlen, O.; Kowal, J.; Sauer, D.U. Ageing behaviour of electrochemical double layer capacitors: Part I. Experimental study and ageing model. J. Power Sources 2007, 172, 468–475. [Google Scholar] [CrossRef]
- Kötz, R.; Ruch, P.W.; Cericola, D. Aging and failure mode of electrochemical double layer capacitors during accelerated constant load tests. J. Power Sources 2010, 195, 923–928. [Google Scholar] [CrossRef]
- German, R.; Sari, A.; Venet, P.; Ayadi, M.; Briat, O.; Vinassa, J.M. Prediction of supercapacitors floating ageing with surface electrode interface based ageing law. Microelectron. Reliab. 2014, 54, 1813–1817. [Google Scholar] [CrossRef]
- Torregrossa, D.; Toghill, K.E.; Amstutz, V.; Girault, H.H.; Paolone, M. Macroscopic indicators of fault diagnosis and ageing in electrochemical double layer capacitors. J. Energy Storage 2015, 2, 8–24. [Google Scholar] [CrossRef]
- Dagousset, L.; Pognon, G.; Nguyen, G.T.M.; Vidal, F.; Jus, S.; Aubert, P.-H. Electrochemical characterisations and ageing of ionic liquid/γ-butyrolactone mixtures as electrolytes for supercapacitor applications over a wide temperature range. J. Power Sources 2017, 359, 242–249. [Google Scholar] [CrossRef]
- Vališ, D.; Nováček, O.; Hasilová, K.; Leuchter, J. Modelling of degradation and a soft failure moment during the operation of a supercapacitor applying selected diffusion processes. Eng. Fail. Anal. 2017, 82, 566–582. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. A review of supercapacitor modeling, estimation, and applications: A control/management perspective. Renew. Sustain. Energy Rev. 2018, 81, 1868–1878. [Google Scholar] [CrossRef]
- Funaki, T.; Hikihara, T. Characterization and Modeling of the Voltage Dependency of Capacitance and Impedance Frequency Characteristics of Packed EDLCs. IEEE Trans. Power Electron. 2008, 23, 1518–1525. [Google Scholar] [CrossRef]
- Kötz, R.; Hahn, M.; Gallay, R. Temperature behavior and impedance fundamentals of supercapacitors. J. Power Sources 2006, 154, 550–555. [Google Scholar] [CrossRef]
- German, R.; Venet, P.; Sari, A.; Briat, O.; Vinassa, J.M. Comparison of EDLC impedance models used for ageing monitoring. In Proceedings of the 2012 First International Conference on Renewable Energies and Vehicular Technology, Nabeul, Tunisia, 26–28 March 2012; pp. 224–229. [Google Scholar]
- Yoshida, A.; Nonaka, S.; Aoki, I.; Nishino, A. Electric double-layer capacitors with sheet-type polarizable electrodes and application of the capacitors. J. Power Sources 1996, 60, 213–218. [Google Scholar] [CrossRef]
- Martin, R.; Quintana, J.J.; Ramos, A.; Nuez, I.d.l. Modeling electrochemical double layer capacitor, from classical to fractional impedance. In Proceedings of the MELECON 2008—The 14th IEEE Mediterranean Electrotechnical Conference, Ajaccio, France, 5–7 May 2008; pp. 61–66. [Google Scholar]
- Schiffer, J.; Linzen, D.; Sauer, D.U. Heat generation in double layer capacitors. J. Power Sources 2006, 160, 765–772. [Google Scholar] [CrossRef]
- Bohlen, O.; Kowal, J.; Dirk Uwe, S. Ageing behaviour of electrochemical double layer capacitors: Part II. Lifetime simulation model for dynamic applications. J. Power Sources 2007, 173, 626–632. [Google Scholar] [CrossRef]
- Funaki, T. Evaluating Energy Storage Efficiency by Modeling the Voltage and Temperature Dependency in EDLC Electrical Characteristics. IEEE Trans. Power Electron. 2010, 25, 1231–1239. [Google Scholar] [CrossRef]
- He, H.; Xiong, R.; Fan, J. Evaluation of Lithium-Ion Battery Equivalent Circuit Models for State of Charge Estimation by an Experimental Approach. Energies 2011, 4, 582–598. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, L.; Hu, X.; Wang, Z.; Wik, T.; Pecht, M. A review of fractional-order techniques applied to lithium-ion batteries, lead-acid batteries, and supercapacitors. J. Power Sources 2018, 390, 286–296. [Google Scholar] [CrossRef]
- Zhu, M.; Weber, C.J.; Yang, Y.; Konuma, M.; Starke, U.; Kern, K.; Bittner, A.M. Chemical and electrochemical ageing of carbon materials used in supercapacitor electrodes. Carbon 2008, 46, 1829–1840. [Google Scholar] [CrossRef]
- Ruch, P.W.; Cericola, D.; Foelske-Schmitz, A.; Kötz, R.; Wokaun, A. Aging of electrochemical double layer capacitors with acetonitrile-based electrolyte at elevated voltages. Electrochim. Acta 2010, 55, 4412–4420. [Google Scholar] [CrossRef]
- Cericola, D.; Kötz, R.; Wokaun, A. Effect of electrode mass ratio on aging of activated carbon based supercapacitors utilizing organic electrolytes. J. Power Sources 2011, 196, 3114–3118. [Google Scholar] [CrossRef]
- Bittner, A.M.; Zhu, M.; Yang, Y.; Waibel, H.F.; Konuma, M.; Starke, U.; Weber, C.J. Ageing of electrochemical double layer capacitors. J. Power Sources 2012, 203, 262–273. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Gong, Q.; Weng, M.; Bai, J.; Liu, X.; Jiang, Y.; Wang, J.; Wang, D.; Shao, Y.; et al. Experimental and Correlative Analyses of the Ageing Mechanism of Activated Carbon Based Supercapacitor. Electrochim. Acta 2017, 228, 214–225. [Google Scholar] [CrossRef]
- Zheng, F.; Li, Y.; Wang, X. Study on effects of applied current and voltage on the ageing of supercapacitors. Electrochim. Acta 2018, 276, 343–351. [Google Scholar] [CrossRef]
- Kurzweil, P.; Chwistek, M. Electrochemical stability of organic electrolytes in supercapacitors: Spectroscopy and gas analysis of decomposition products. J. Power Sources 2008, 176, 555–567. [Google Scholar] [CrossRef]
- Ishimoto, S.; Asakawa, Y.; Shinya, M.; Naoi, K. Degradation Responses of Activated-Carbon-Based EDLCs for Higher Voltage Operation and Their Factors. J. Electrochem. Soc. 2009, 156, A563. [Google Scholar] [CrossRef]
- Marracci, M.; Tellini, B.; Catelani, M.; Ciani, L. Ultracapacitor Degradation State Diagnosis via Electrochemical Impedance Spectroscopy. IEEE Trans. Instrum. Meas. 2015, 64, 1916–1921. [Google Scholar] [CrossRef]
- Tokita, M.; Yoshimoto, N.; Fujii, K.; Morita, M. Degradation Characteristics of Electric Double-Layer Capacitors Consisting of High Surface Area Carbon Electrodes with Organic Electrolyte Solutions. Electrochim. Acta 2016, 209, 210–218. [Google Scholar] [CrossRef]
- Oz, A.; Gelman, D.; Goren, E.; Shomrat, N.; Baltianski, S.; Tsur, Y. A novel approach for supercapacitors degradation characterization. J. Power Sources 2017, 355, 74–82. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omori, T.; Nakanishi, M.; Tashima, D. Modeling of Equivalent Circuit Analysis of Degraded Electric Double-Layer Capacitors. Materials 2021, 14, 435. https://doi.org/10.3390/ma14020435
Omori T, Nakanishi M, Tashima D. Modeling of Equivalent Circuit Analysis of Degraded Electric Double-Layer Capacitors. Materials. 2021; 14(2):435. https://doi.org/10.3390/ma14020435
Chicago/Turabian StyleOmori, Tomoki, Masahiro Nakanishi, and Daisuke Tashima. 2021. "Modeling of Equivalent Circuit Analysis of Degraded Electric Double-Layer Capacitors" Materials 14, no. 2: 435. https://doi.org/10.3390/ma14020435
APA StyleOmori, T., Nakanishi, M., & Tashima, D. (2021). Modeling of Equivalent Circuit Analysis of Degraded Electric Double-Layer Capacitors. Materials, 14(2), 435. https://doi.org/10.3390/ma14020435





