On the Size Effect of Additives in Amorphous Shape Memory Polymers
Abstract
1. Introduction
2. Model and Methods
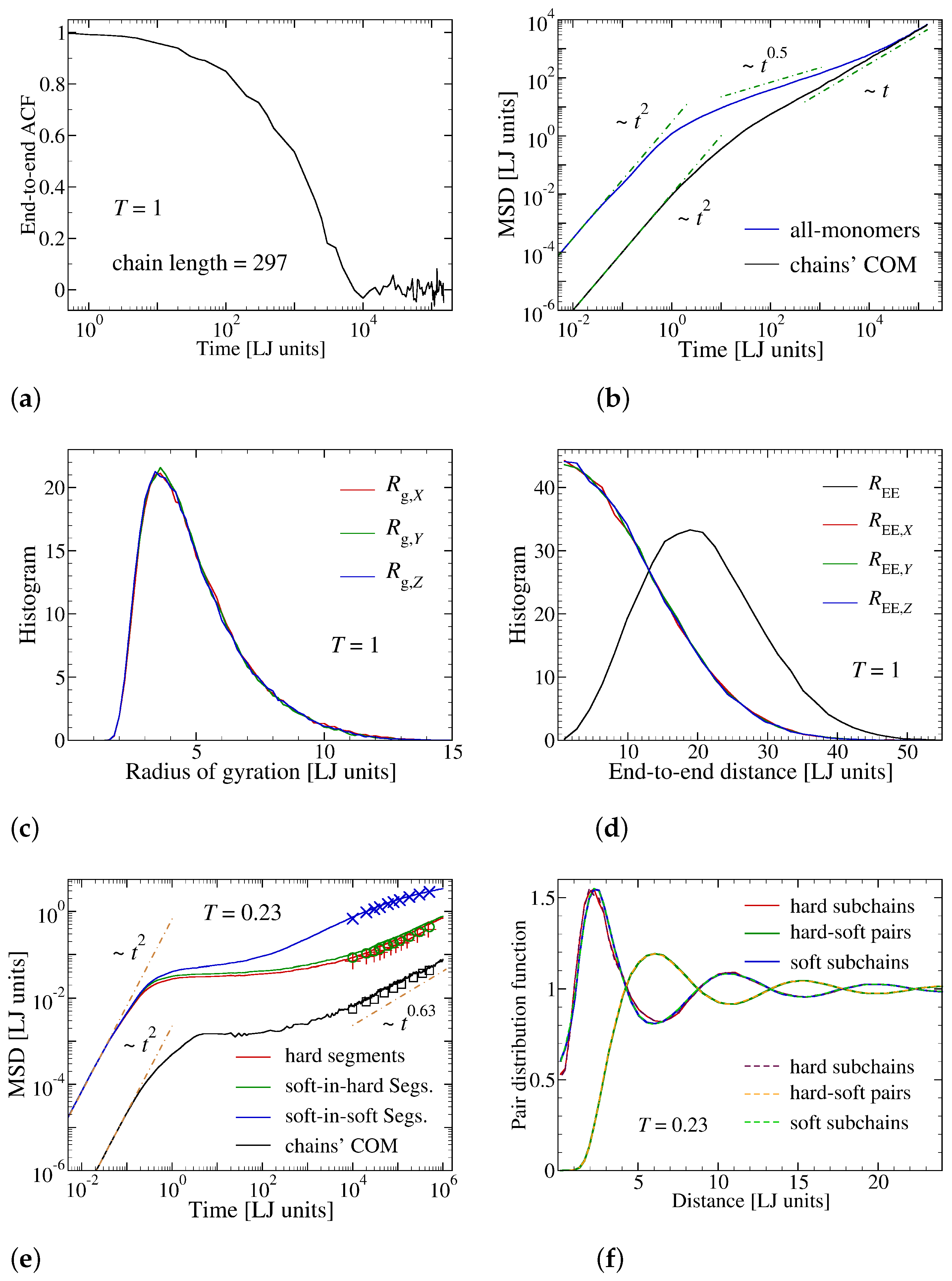
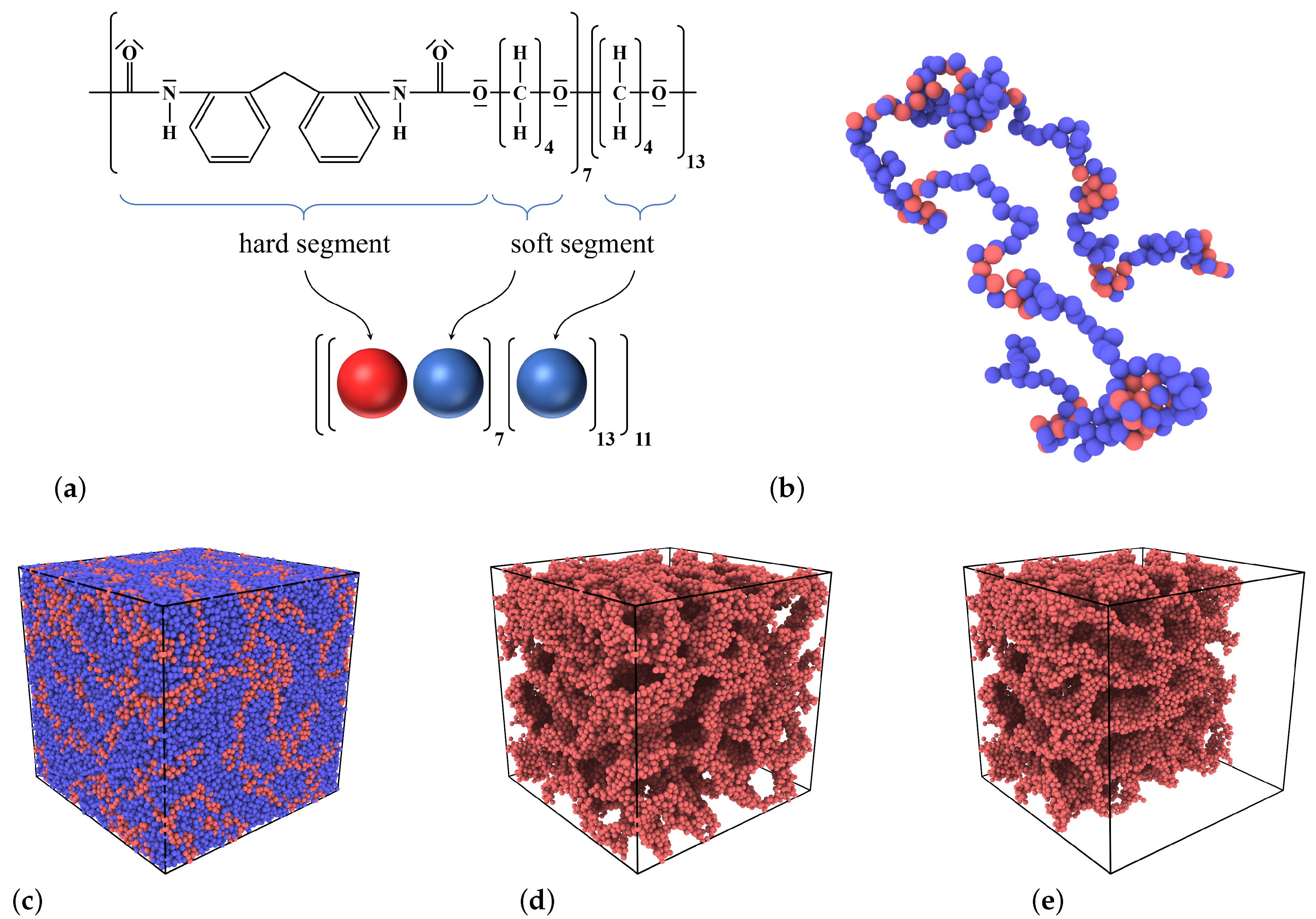
2.1. Model
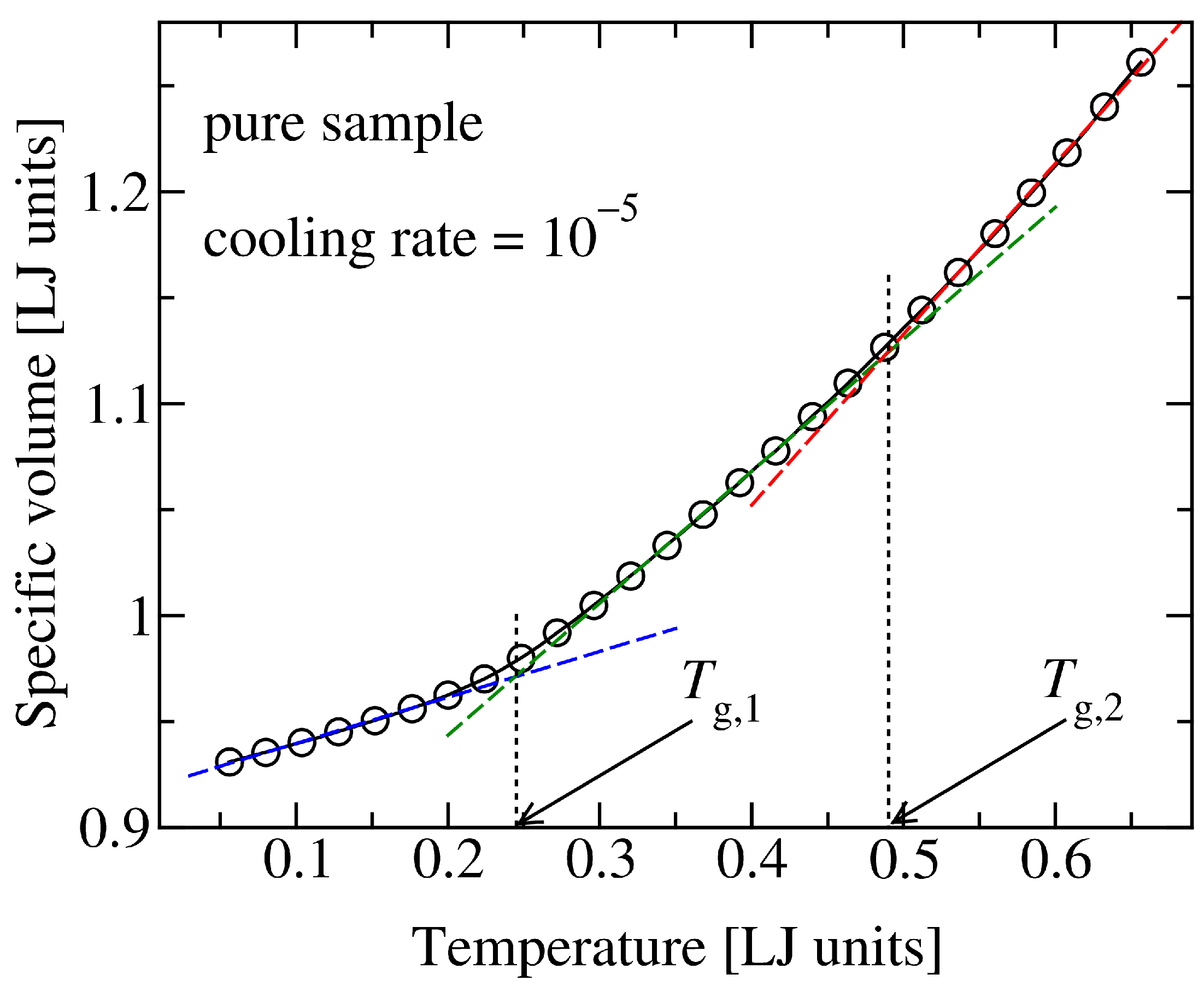
2.2. Sample Preparation
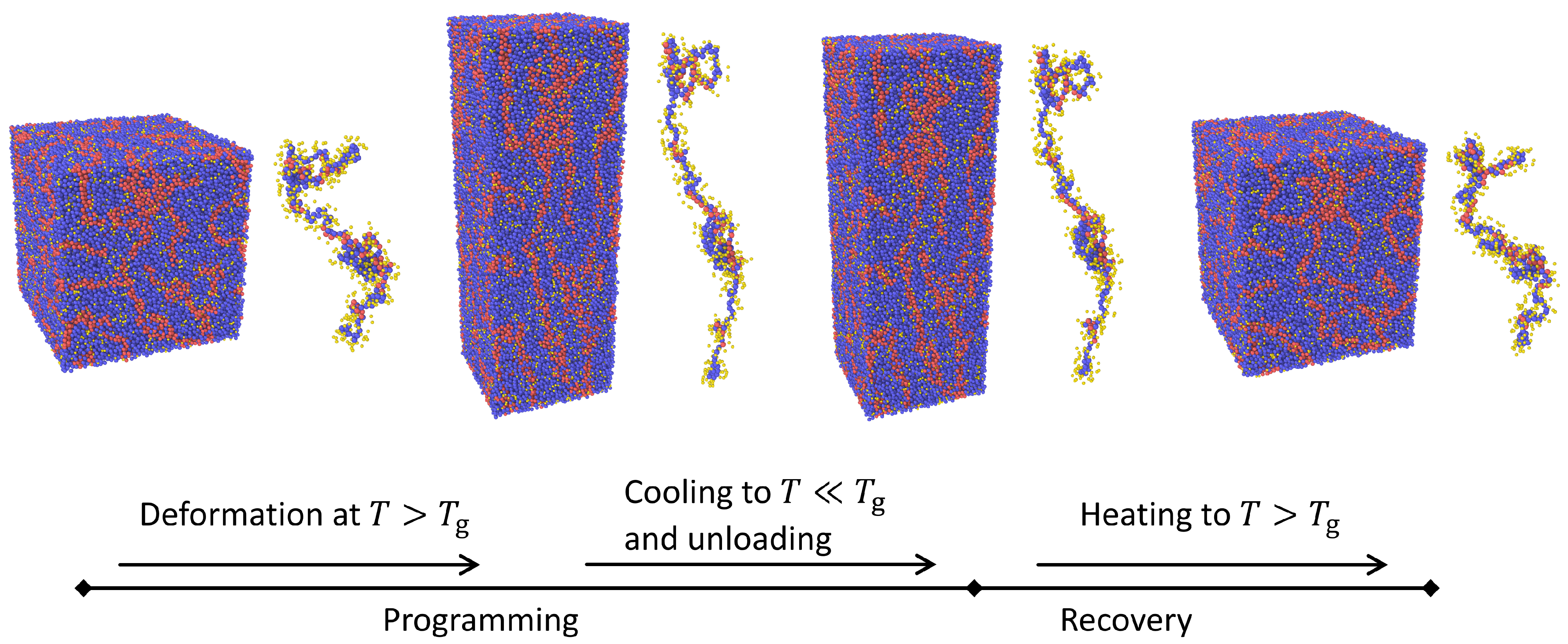
2.3. Simulating the Shape Memory Effect
3. Results and Discussion
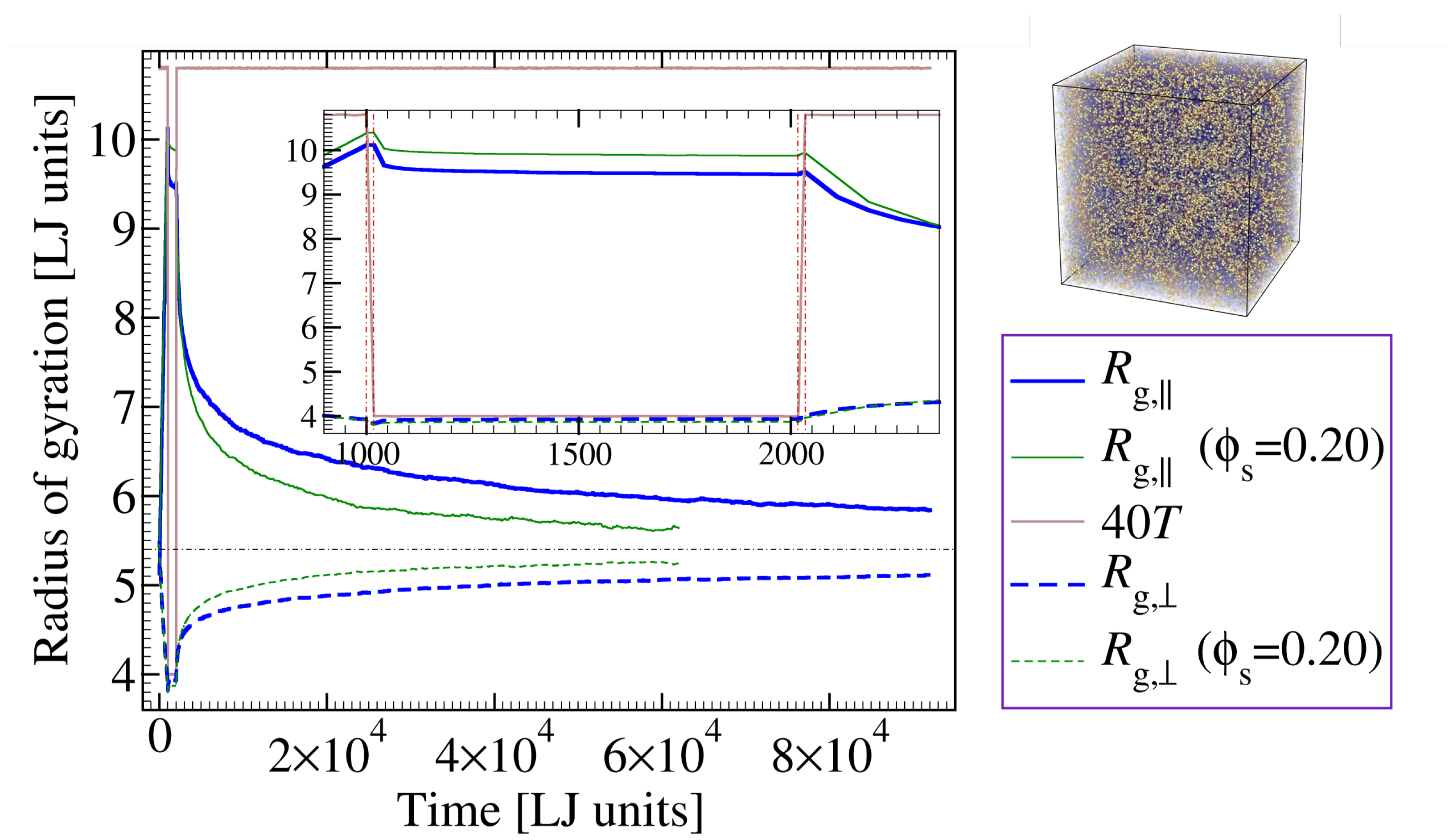
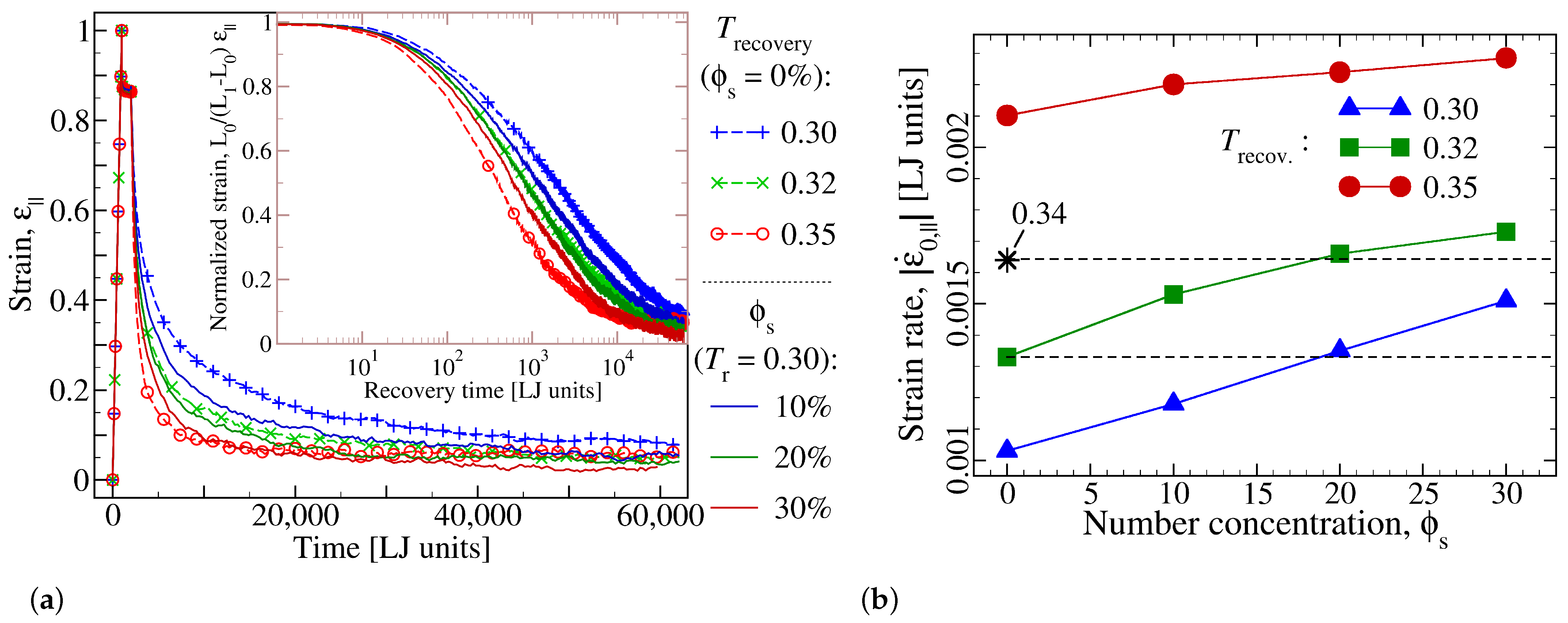
3.1. Effect of Additive Concentration on Shape Recovery
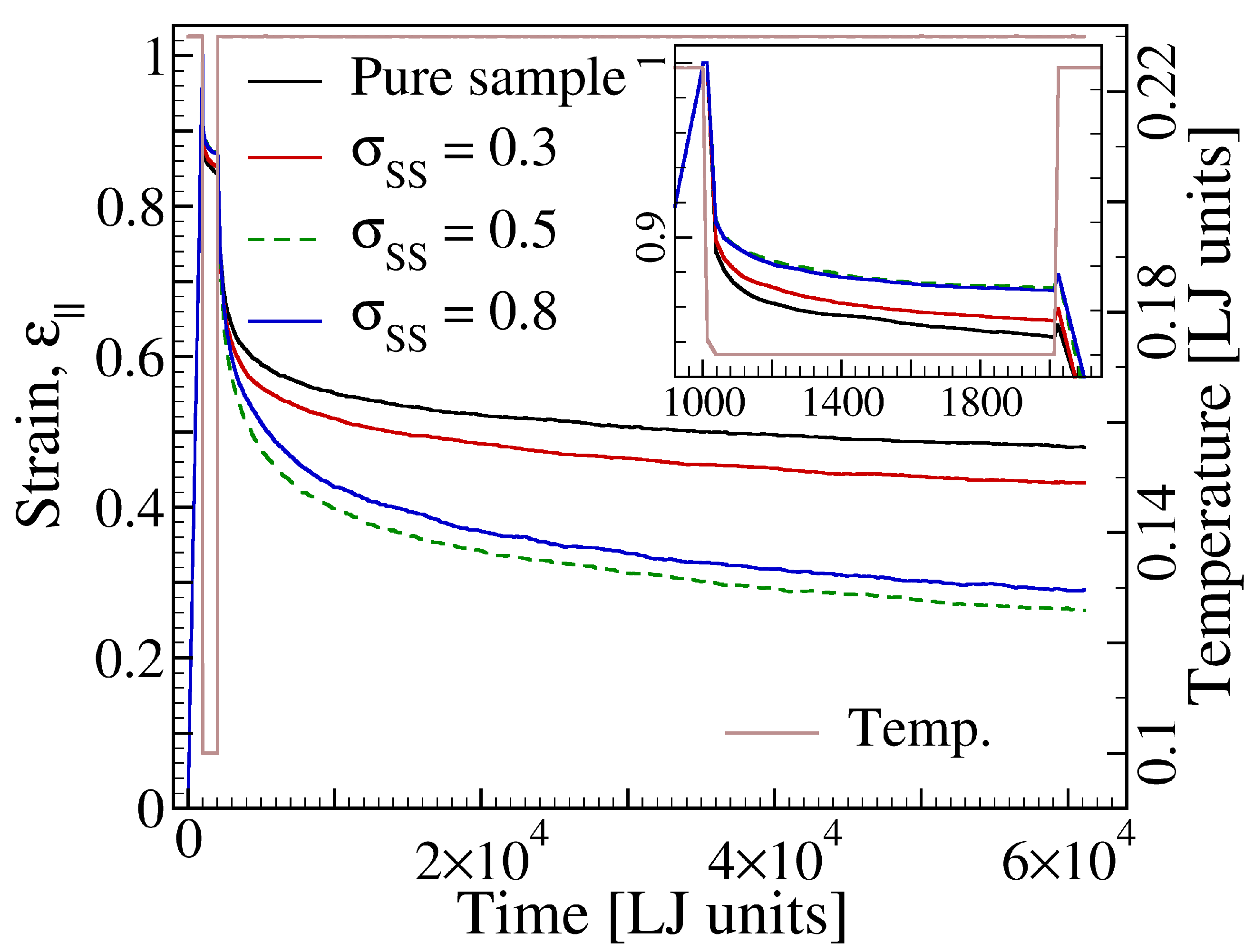
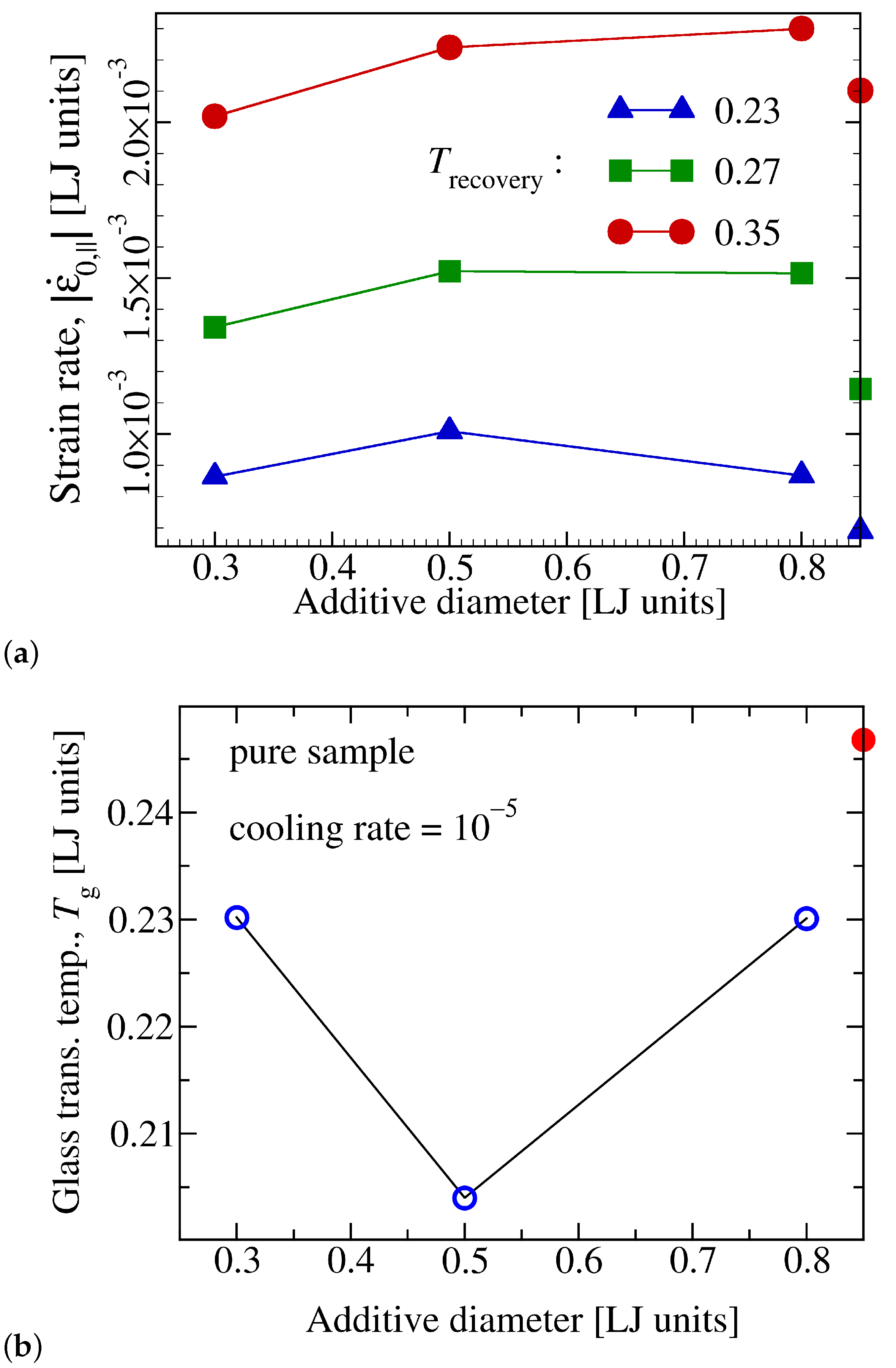
3.2. Effect of the Additive Size
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lendlein, A.; Kelch, S. Shape-Memory Polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Lendlein, A.; Behl, M.; Hiebl, B.; Wischke, C. Shape-memory polymers as a technology platform for biomedical applications. Expert Rev. Med. Devices 2010, 7, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Scence 2002, 296, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.C.; Oesterle, S.N.; Khachigian, L.M. Coronary in-stent restenosis: Current status and future strategies. J. Am. Coll. Cardiol. 2002, 39, 183–193. [Google Scholar] [CrossRef]
- Sokolowski, W.; Metcalfe, A.; Hayashi, S.; Yahia, L.; Raymond, J. Medical applications of shape memory polymers. Biomed. Mater. 2007, 2, S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Xie, T. Recent advances in polymer shape memory. Polymer 2011, 52, 4985–5000. [Google Scholar] [CrossRef]
- Behl, M.; Razzaq, M.Y.; Lendlein, A. Multifunctional shape-memory polymers. Adv. Mater. 2010, 22, 3388–3410. [Google Scholar] [CrossRef]
- Jiang, H.; Kelch, S.; Lendlein, A. Polymers Move in Response to Light. Adv. Mater. 2006, 18, 1471–1475. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007, 32, 962–990. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Ionov, L. Actively-moving materials based on stimuli-responsive polymers. J. Mater. Chem. 2010, 20, 3382. [Google Scholar] [CrossRef]
- Liu, F. Recent advances and challenges in designing stimuli-responsive polymers. Prog. Polym. Sci. 2010, 35, 3–23. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Hu, J. A brief review of stimulus-active polymers responsive to thermal, light, magnetic, electric, and water/solvent stimuli. J. Intell. Mater. Syst. Struct. 2010, 21, 859–885. [Google Scholar] [CrossRef]
- Theodorakis, P.E.; Fytas, N.G. Microphase separation in linear multiblock copolymers under poor solvent conditions. Soft Matter 2011, 7, 1038–1044. [Google Scholar] [CrossRef]
- Bejagam, K.K.; Singh, S.K.; Ahn, R.; Deshmukh, S.A. Unraveling the Conformations of Backbone and Side Chains in Thermosensitive Bottlebrush Polymers. Macromolecules 2019, 52, 9398–9408. [Google Scholar] [CrossRef]
- Maltseva, D.; Zablotskiy, S.; Martemyanova, J.; Ivanov, V.; Shakirov, T.; Paul, W. Diagrams of States of Single Flexible-Semiflexible Multi-Block Copolymer Chains: A Flat-Histogram Monte Carlo Study. Polymers 2019, 11, 757. [Google Scholar] [CrossRef]
- Vogt, F. Long-term assessment of a novel biodegradable paclitaxel-eluting coronary polylactide stent. Eur. Heart J. 2004, 25, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Yakacki, C.M.; Shandas, R.; Lanning, C.; Rech, B.; Eckstein, A.; Gall, K. Unconstrained recovery characterization of shape-memory polymer networks for cardiovascular applications. Biomaterials 2007, 28, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Dai, S.; Li, Z. Biodegradable shape-memory block co-polymers for fast self-expandable stents. Biomaterials 2010, 31, 8132–8140. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.M.; Song, C.L.; Fu, Y.Q.; Wang, C.C.; Zhao, Y.; Purnawali, H.; Lu, H.B.; Tang, C.; Ding, Z.; Zhang, J.L. Shaping tissue with shape memory materials. Adv. Drug Deliv. Rev. 2013, 65, 515–535. [Google Scholar] [CrossRef]
- Peterson, G.I.; Dobrynin, A.V.; Becker, M.L. α-Amino Acid-Based Poly(Ester urea)s as Multishape Memory Polymers for Biomedical Applications. ACS Macro Lett. 2016, 5, 1176–1179. [Google Scholar] [CrossRef]
- Seo, J.; Choi, J.W.; Koh, Y.H.; Seo, J.H. Enhanced Mechanical Strength, Flexibility, and Shape-Restoring Rate of a Drug-Eluting Shape-Memory Polymer by Incorporation of Supramolecular Cross-Linkers. ACS Macro Lett. 2020, 9, 389–395. [Google Scholar] [CrossRef]
- Ruderer, M.A.; Guo, S.; Meier, R.; Chiang, H.Y.; Körstgens, V.; Wiedersich, J.; Perlich, J.; Roth, S.V.; Müller-Buschbaum, P. Solvent-Induced Morphology in Polymer-Based Systems for Organic Photovoltaics. Adv. Funct. Mater. 2011, 21, 3382–3391. [Google Scholar] [CrossRef]
- Riggleman, R.A.; Yoshimoto, K.; Douglas, J.F.; de Pablo, J.J. Influence of Confinement on the Fragility of Antiplasticized and Pure Polymer Films. Phys. Rev. Lett. 2006, 97, 045502. [Google Scholar] [CrossRef]
- Riggleman, R.A.; Douglas, J.F.; de Pablo, J.J. Characterization of the potential energy landscape of an antiplasticized polymer. Phys. Rev. E 2007, 76, 011504. [Google Scholar] [CrossRef]
- Riggleman, R.A.; Douglas, J.F.; de Pablo, J.J. Tuning polymer melt fragility with antiplasticizer additives. J. Chem. Phys. 2007, 126, 234903. [Google Scholar] [CrossRef]
- Peter, S.; Meyer, H.; Baschnagel, J. MD simulation of concentrated polymer solutions: Structural relaxation near the glass transition. Eur. Phys. J. E Soft Matter Biol. Phys. 2009, 28, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Riggleman, R.A.; Douglas, J.F.; de Pablo, J.J. Antiplasticization and the elastic properties of glass-forming polymer liquids. Soft Matter 2010, 6, 292–304. [Google Scholar] [CrossRef]
- Barrat, J.L.; Baschnagel, J.; Lyulin, A. Molecular dynamics simulations of glassy polymers. Soft Matter 2010, 6, 3430–3446. [Google Scholar] [CrossRef]
- Dishari, S.K.; Hickner, M.A. Antiplasticization and water uptake of nafion thin films. ACS Macro Lett. 2012, 1, 291–295. [Google Scholar] [CrossRef]
- Mangalara, J.H.; Simmons, D.S. Tuning Polymer Glass Formation Behavior and Mechanical Properties with Oligomeric Diluents of Varying Stiffness. ACS Macro Lett. 2015, 4, 1134–1138. [Google Scholar] [CrossRef]
- Mahmoudinezhad, E.; Marquardt, A.; Eggeler, G.; Varnik, F. Molecular dynamics simulations of entangled polymers: The effect of small molecules on the glass transition temperature. Procedia Comput. Sci. 2017, 108, 265–271. [Google Scholar] [CrossRef]
- Meng, H.; Li, G. A review of stimuli-responsive shape memory polymer composites. Polymer 2013, 54, 2199–2221. [Google Scholar] [CrossRef]
- Yang, B.; Huang, W.M.; Li, C.; Lee, C.M.; Li, L. On the effects of moisture in a polyurethane shape memory polymer. Smart Mater. Struct. 2004, 13, 191–195. [Google Scholar] [CrossRef]
- Huang, W.M.; Yang, B.; An, L.; Li, C.; Chan, Y.S. Water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism. Appl. Phys. Lett. 2005, 86, 114105. [Google Scholar] [CrossRef]
- Lv, H.B.; Leng, J.S.; Liu, Y.J.; Du, S.Y. Shape-Memory Polymer in Response to Solution. Adv. Eng. Mater. 2008, 10, 592–595. [Google Scholar] [CrossRef]
- Lv, H.B.; Liu, Y.J.; Zhang, D.X.; Leng, J.S.; Du, S.Y. Solution-Responsive Shape-Memory Polymer Driven by Forming Hydrogen Bonding. Adv. Mater. Res. 2008, 47, 258–261. [Google Scholar] [CrossRef]
- Du, H.; Zhang, J. Solvent induced shape recovery of shape memory polymer based on chemically cross-linked poly(vinyl alcohol). Soft Matter 2010, 6, 3370. [Google Scholar] [CrossRef]
- Xiao, R.; Guo, J.; Safranski, D.L.; Nguyen, T.D. Solvent-driven temperature memory and multiple shape memory effects. Soft Matter 2015, 11, 3977–3985. [Google Scholar] [CrossRef]
- Fang, Z.; Kuang, Y.; Zhou, P.; Ming, S.; Zhu, P.; Liu, Y.; Ning, H.; Chen, G. Programmable Shape Recovery Process of Water-Responsive Shape-Memory Poly(vinyl alcohol) by Wettability Contrast Strategy. ACS Appl. Mater. Interfaces 2017, 9, 5495–5502. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhang, C.; Gou, X.; Huang, W.M. Tunable shape-memory behaviors in amorphous polymers through bound solvent. Mater. Lett. 2017, 209, 131–133. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Mori, T.; Fujimori, A.; Jikei, M.; Sawada, H.; Oishi, Y. Poly(amide–ether) Thermoplastic Elastomers Based on Monodisperse Aromatic Amide Hard Segments as Shape-Memory and Moisture-Responsive Materials. Macromolecules 2018, 51, 9430–9441. [Google Scholar] [CrossRef]
- Ghobadi, E.; Marquardt, A.; Zirdehi, E.M.; Neuking, K.; Varnik, F.; Eggeler, G.; Steeb, H. The Influence of Water and Solvent Uptake on Functional Properties of Shape-Memory Polymers. Int. J. Polym. Sci. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Garces, I.; Aslanzadeh, S.; Boluk, Y.; Ayranci, C. Effect of Moisture on Shape Memory Polyurethane Polymers for Extrusion-Based Additive Manufacturing. Materials 2019, 12, 244. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, J.; Liu, K.; Li, X.; Xu, W.; Song, H.; Shan, G.; Bao, Y.; Zhao, Q.; Pan, P. Sequence-Rearranged Cocrystalline Polymer Network with Shape Reconfigurability and Tunable Switching Temperature. ACS Macro Lett. 2020, 9, 588–594. [Google Scholar] [CrossRef]
- Diani, J.; Gall, K. Molecular dynamics simulations of the shape-memory behaviour of polyisoprene. Smart Mater. Struct. 2007, 16, 1575–1583. [Google Scholar] [CrossRef]
- Abberton, B.C.; Liu, W.K.; Keten, S. Coarse-grained simulation of molecular mechanisms of recovery in thermally activated shape-memory polymers. J. Mech. Phys. Solids 2013, 61, 2625–2637. [Google Scholar] [CrossRef]
- Davidson, J.D.; Li, Y.; Goulbourne, N.C. The shape memory effect in crosslinked polymers: Effects of polymer chemistry and network architecture. Behav. Mech. Multifunct. Mater. Compos. 2013 2013, 8689, 86890K. [Google Scholar] [CrossRef]
- Ghobadi, E.; Heuchel, M.; Kratz, K.; Lendlein, A. Atomistic Simulation of the Shape-Memory Effect in Dry and Water Swollen Poly[(rac-lactide)-co-glycolide] and Copolyester Urethanes Thereof. Macromol. Chem. Phys. 2014, 215, 65–75. [Google Scholar] [CrossRef]
- Davidson, J.D.; Goulbourne, N.C. Microscopic mechanisms of the shape memory effect in crosslinked polymers. Smart Mater. Struct. 2015, 24, 055014. [Google Scholar] [CrossRef]
- Moon, J.; Choi, J.; Cho, M. Programmed shape-dependence of shape memory effect of oriented polystyrene: A molecular dynamics study. Polymer 2016, 102, 1–9. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Ji, F.; Li, X.; Han, J.; Wu, Y. Revealing the morphological architecture of a shape memory polyurethane by simulation. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Ghobadi, E.; Heuchel, M.; Kratz, K.; Lendlein, A. Influence of the addition of water to amorphous switching domains on the simulated shape-memory properties of poly(l-lactide). Polymer 2013, 54, 4204–4211. [Google Scholar] [CrossRef]
- Marquardt, A.; Mogharebi, S.; Neuking, K.; Varnik, F.; Eggeler, G. Diffusion of small molecules in a shape memory polymer. J. Mater. Sci. 2016, 51, 9792–9804. [Google Scholar] [CrossRef]
- Kremer, K.; Grest, G.S.; Carmesin, I. Crossover from Rouse to Reptation Dynamics: A Molecular-Dynamics Simulation. Phys. Rev. Lett. 1988, 61, 566–569. [Google Scholar] [CrossRef]
- Baschnagel, J.; Varnik, F. Computer simulations of supercooled polymer melts in the bulk and in confined geometry. J. Phys. Condens. Matter 2005, 17, R851–R953. [Google Scholar] [CrossRef]
- Cooper, S.L.; Tobolsky, A.V. Properties of linear elastomeric polyurethanes. J. Appl. Polym. Sci. 1966, 10, 1837–1844. [Google Scholar] [CrossRef]
- Gibson, P.; Vallance, M.; Cooper, S. Developments in Block Copolymers—I; Appl. Science Series; Elsevier: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Oertel, G.; Abele, L. Polyurethane Handbook: Chemistry, Raw Materials, Processing, Application, Properties; Hanser: Cincinnati, OH, USA, 1994. [Google Scholar]
- Parker, A.J.; Rottler, J. Using soft potentials for the simulation of block copolymer morphologies. Macromol. Theory Simulations 2014, 23, 401–409. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18. [Google Scholar] [CrossRef]
- Rottler, J.; Barsky, S.; Robbins, M.O. Cracks and Crazes: On Calculating the Macroscopic Fracture Energy of Glassy Polymers from Molecular Simulations. Phys. Rev. Lett. 2002, 89, 148304. [Google Scholar] [CrossRef]
- Ge, T.; Tzoumanekas, C.; Anogiannakis, S.D.; Hoy, R.S.; Robbins, M.O. Entanglements in Glassy Polymer Crazing: Cross-Links or Tubes? Macromolecules 2017, 50, 459–471. [Google Scholar] [CrossRef]
- Sharafi, S.; Li, G. Multiscale modeling of vibration damping response of shape memory polymer fibers. Compos. Part B Eng. 2016, 91, 306–314. [Google Scholar] [CrossRef]
- Varnik, F.; Franosch, T. Non-monotonic effect of confinement on the glass transition. J. Phys. Condens. Matter 2016, 28, 133001. [Google Scholar] [CrossRef]
- Mittal, J.; Truskett, T.M.; Errington, J.R.; Hummer, G. Layering and Position-Dependent Diffusive Dynamics of Confined Fluids. Phys. Rev. Lett. 2008, 100, 145901. [Google Scholar] [CrossRef]
- Bollinger, J.A.; Jain, A.; Carmer, J.; Truskett, T.M. Communication: Local structure-mobility relationships of confined fluids reverse upon supercooling. J. Chem. Phys. 2015, 142, 161102. [Google Scholar] [CrossRef]
- Zirdehi, E.M.; Varnik, F. Non-monotonic effect of additive particle size on the glass transition in polymers. J. Chem. Phys. 2019, 150, 024903. [Google Scholar] [CrossRef] [PubMed]
- Zirdehi, E.M.; Voigtmann, T.; Varnik, F. Multiple character of non-monotonic size-dependence for relaxation dynamics in polymer-particle and binary mixtures. J. Phys. Condens. Matter 2020, 32, 275104. [Google Scholar] [CrossRef] [PubMed]
- Voigtmann, T.H. Multiple glasses in asymmetric binary hard spheres. EPL 2011, 96. [Google Scholar] [CrossRef]
- Zhang, R.; Schweizer, K.S. Microscopic Theory of Coupled Slow Activated Dynamics in Glass-Forming Binary Mixtures. J. Phys. Chem. B 2018, 122, 3465–3479. [Google Scholar] [CrossRef]
- Hendricks, J.; Capellmann, R.; Schofield, A.B.; Egelhaaf, S.U.; Laurati, M. Different mechanisms for dynamical arrest in largely asymmetric binary mixtures. Phys. Rev. E 2015, 91, 032308. [Google Scholar] [CrossRef]
- Lázaro-Lázaro, E.; Perera-Burgos, J.A.; Laermann, P.; Sentjabrskaja, T.; Pérez-Ángel, G.; Laurati, M.; Egelhaaf, S.U.; Medina-Noyola, M.; Voigtmann, T.; Castañeda-Priego, R.; et al. Glassy dynamics in asymmetric binary mixtures of hard spheres. Phys. Rev. E 2019, 99, 42603. [Google Scholar] [CrossRef]
- Felder, R.; Huvard, G. 17. Permeation, Diffusion, and Sorption of Gases and Vapors. In Polymers; Fava, R., Ed.; Academic Press: Cambridge, MA, USA, 1980; Volume 16, pp. 315–377. [Google Scholar] [CrossRef]
| 1 | 1 | {0.3, 0.5, 0.8} | 1 | arithmetic mean | arithmetic mean |
| 1 | 0.5 | 0.5 | 0.3 | 0.5 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zirdehi, E.M.; Dumlu, H.; Eggeler, G.; Varnik, F. On the Size Effect of Additives in Amorphous Shape Memory Polymers. Materials 2021, 14, 327. https://doi.org/10.3390/ma14020327
Zirdehi EM, Dumlu H, Eggeler G, Varnik F. On the Size Effect of Additives in Amorphous Shape Memory Polymers. Materials. 2021; 14(2):327. https://doi.org/10.3390/ma14020327
Chicago/Turabian StyleZirdehi, Elias M., Hakan Dumlu, Gunther Eggeler, and Fathollah Varnik. 2021. "On the Size Effect of Additives in Amorphous Shape Memory Polymers" Materials 14, no. 2: 327. https://doi.org/10.3390/ma14020327
APA StyleZirdehi, E. M., Dumlu, H., Eggeler, G., & Varnik, F. (2021). On the Size Effect of Additives in Amorphous Shape Memory Polymers. Materials, 14(2), 327. https://doi.org/10.3390/ma14020327





