Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study
Abstract
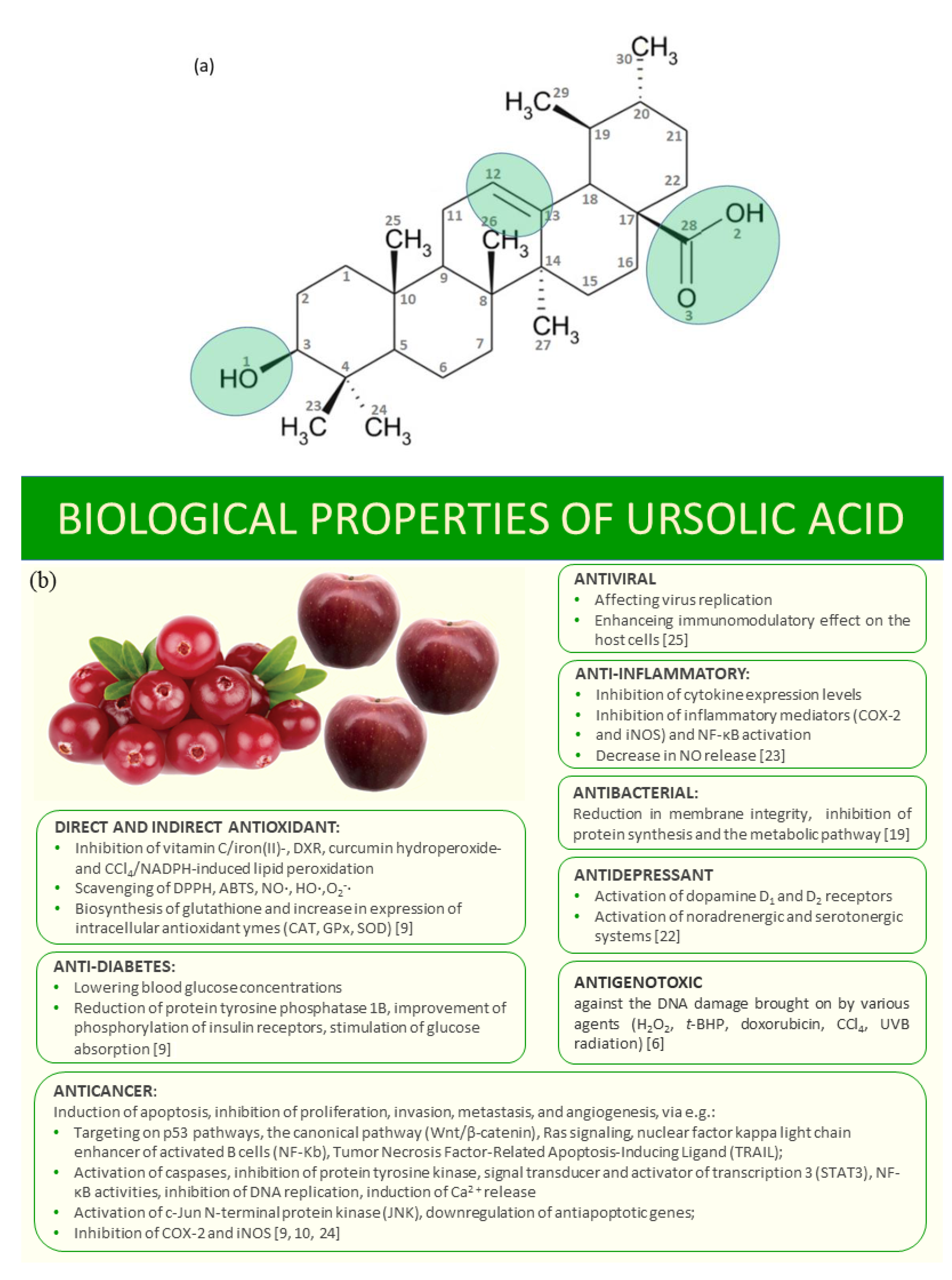
1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.1.1. Materials
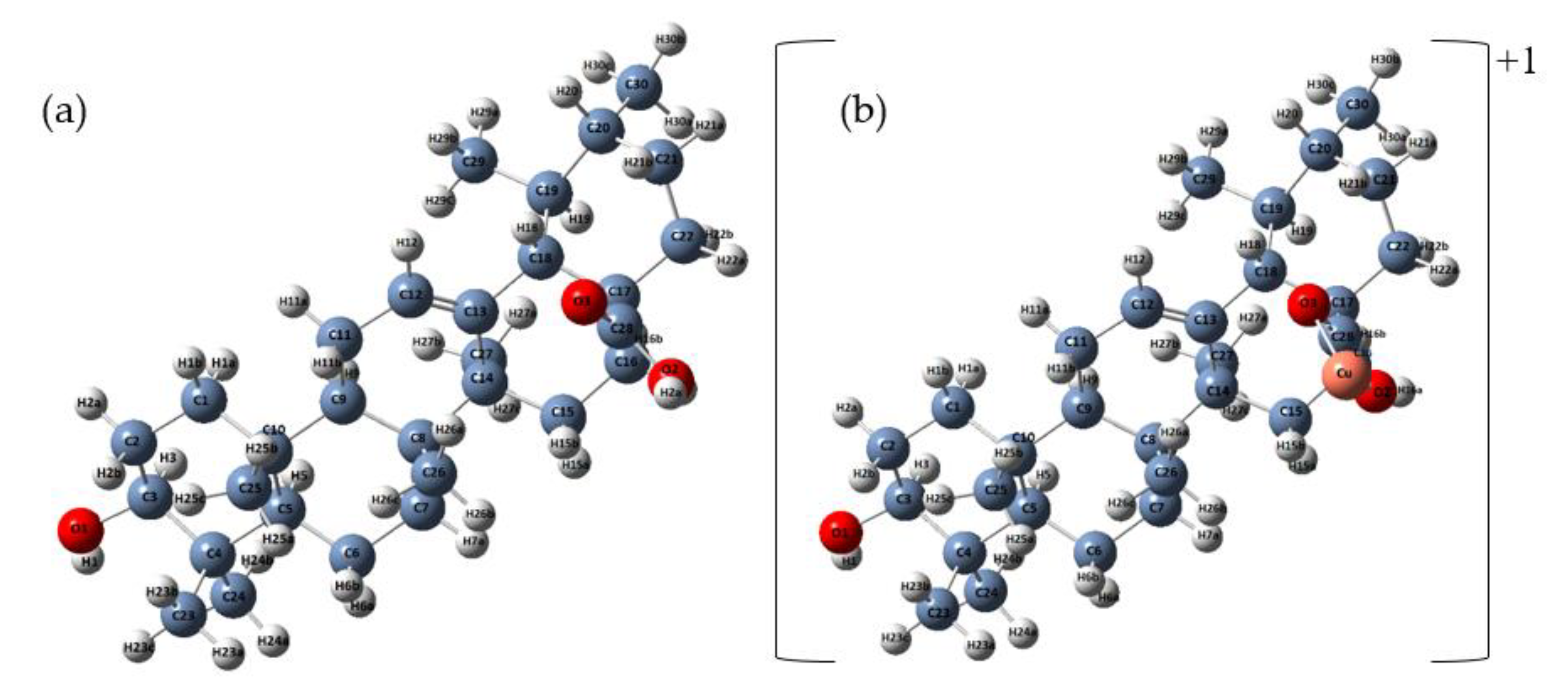
2.1.2. Preparation of Ursolic Acid–Cu (II) Complex (Cu (II) UA)
2.1.3. Instrumentation
2.1.4. Calculations
2.2. Antioxidant Activity
2.2.1. DPPH• Antiradical Assay
2.2.2. HO• Antiradical Assay
2.2.3. Ferric-Reducing Power Assay (FRAP assay)
3. Results and Discussion
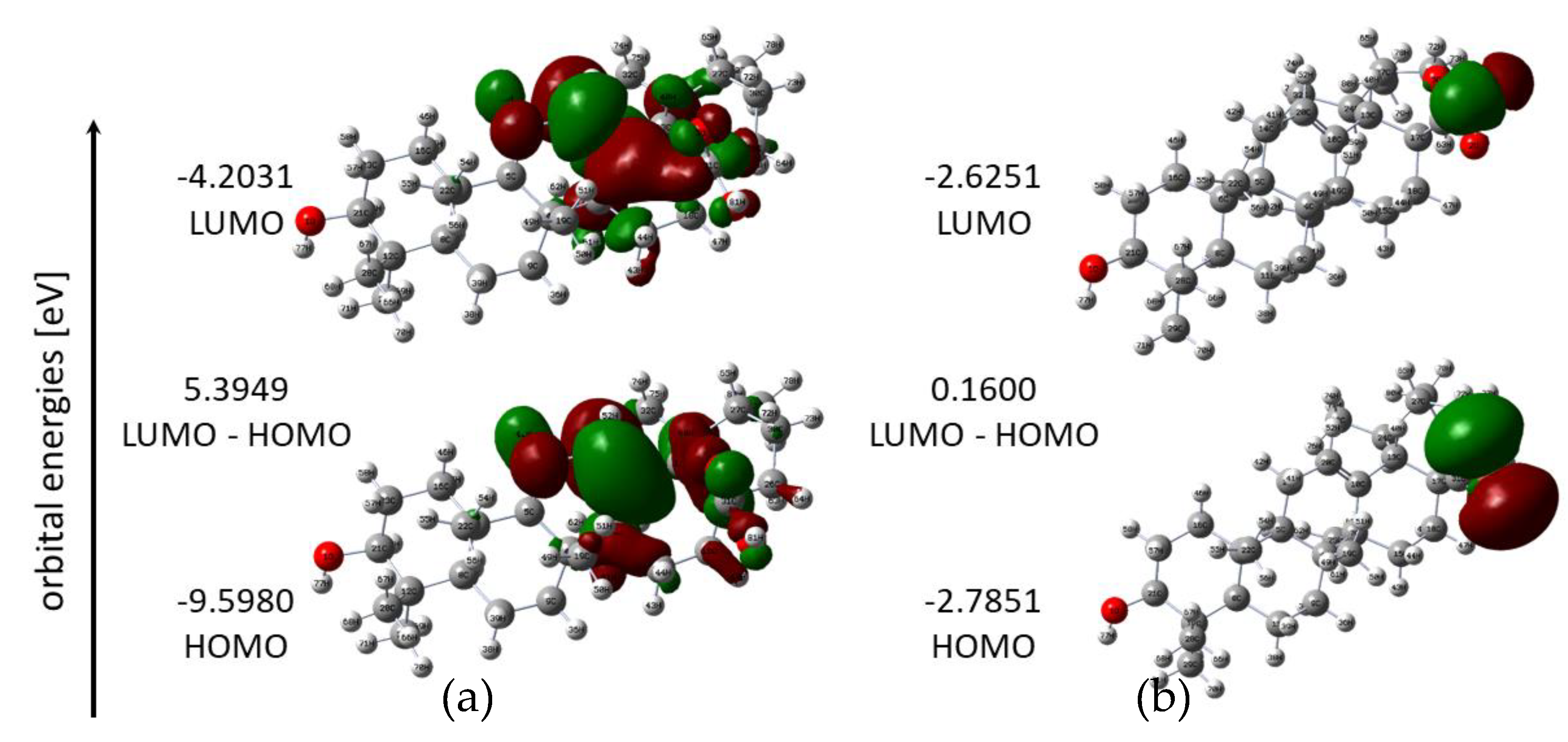
3.1. Quantum-Chemical Calculations
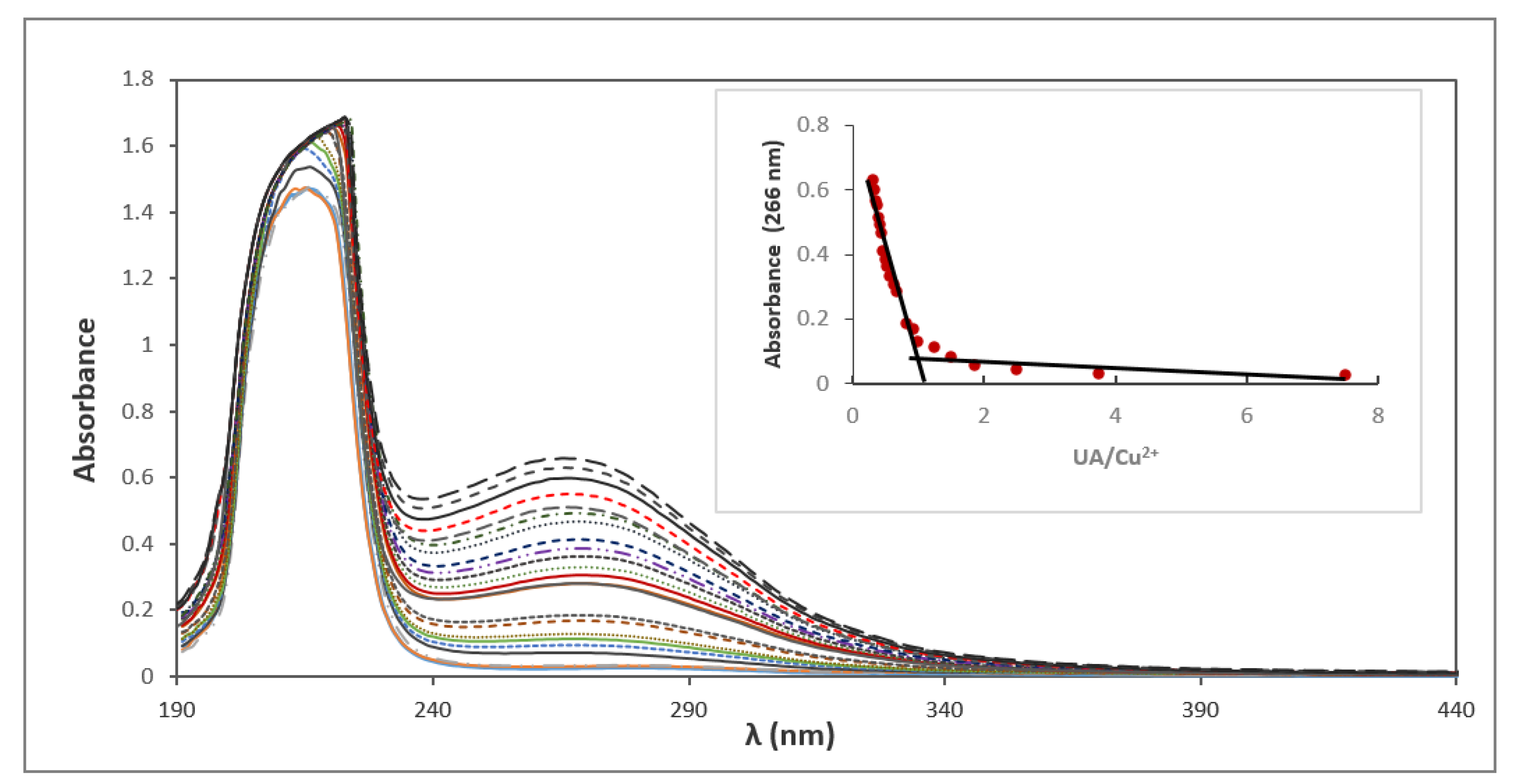
3.2. UV Spectra
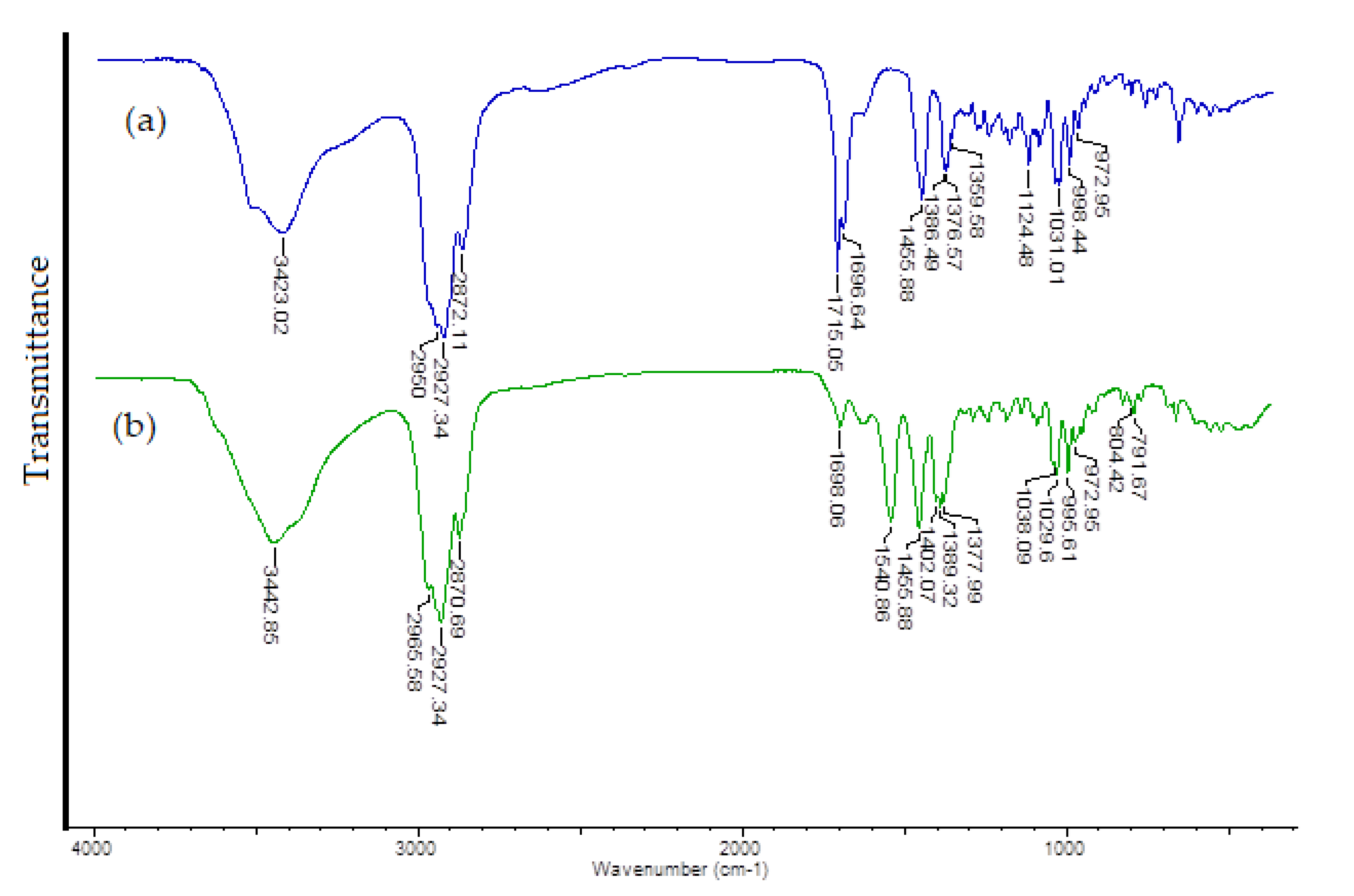
3.3. Vibrational Spectra
3.4. Antioxidant Tests
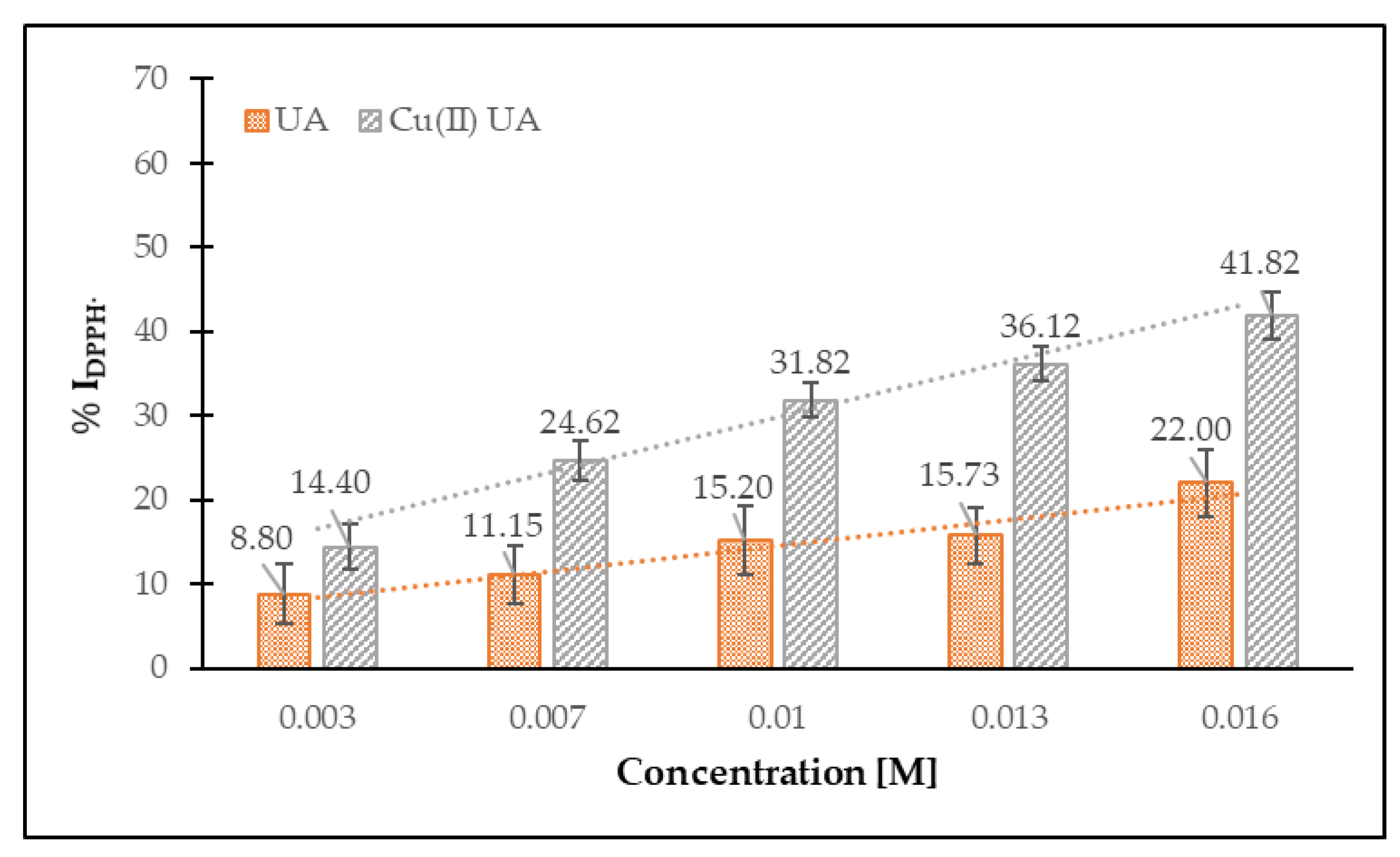
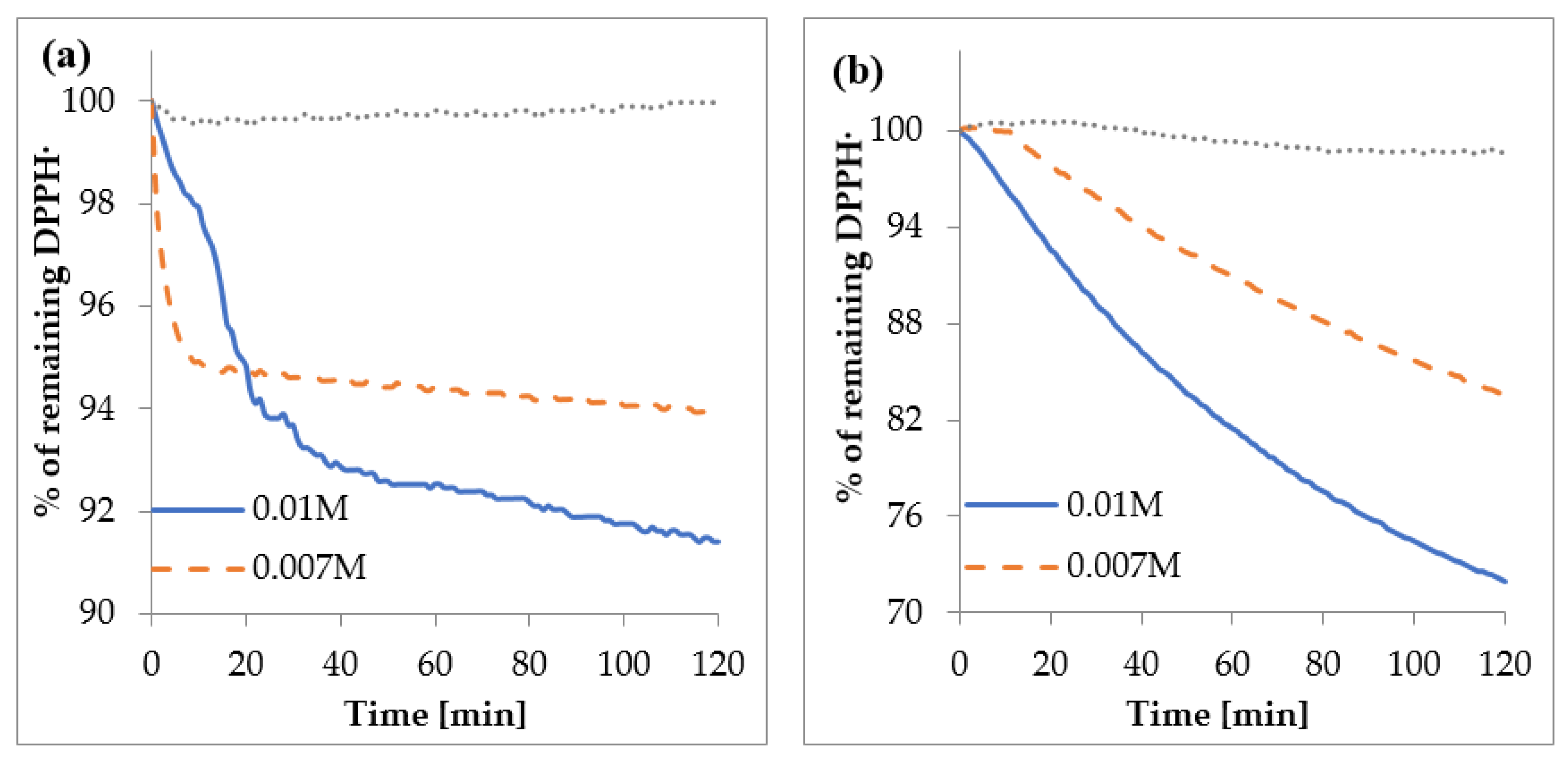
3.4.1. Antiradical DPPH• and HO• Activity
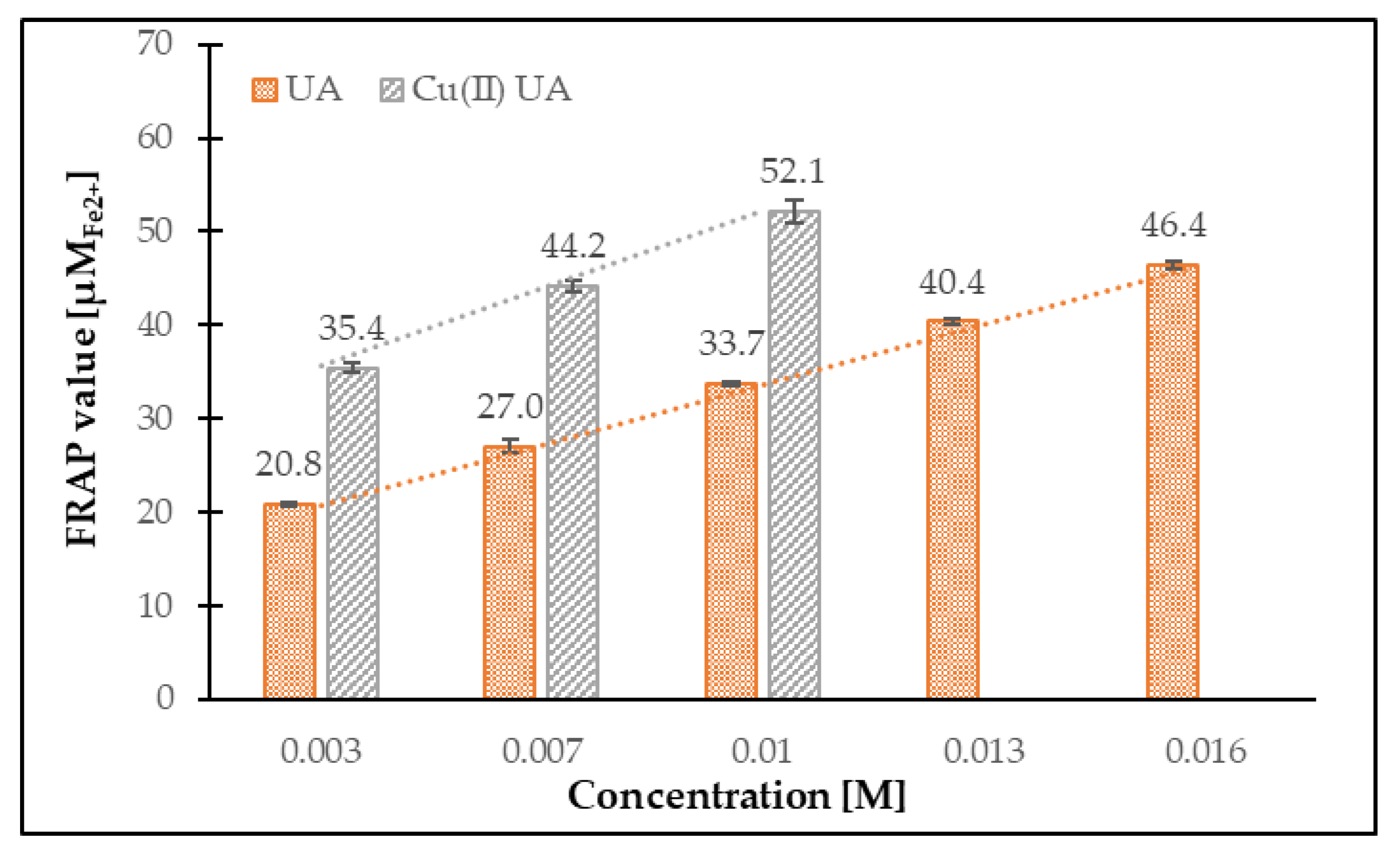
3.4.2. Ferric-Reducing Antioxidant Power (FRAP Assay)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kondo, M.; MacKinnon, S.L.; Craft, C.C.; Matchett, M.D.; Hurta, R.A.A.; Neto, C.C. Ursolic acid and its esters: Occurrence in cranberries and other Vaccinium fruit and effects on matrix metalloproteinase activity in DU145 prostate tumor cells. J. Sci. Food Agric. 2011, 91, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Waller, G.R.; Jurzysta, M.; Karns, T.K.B.; Geno, P.W. Isolation and identification of ursolic acid from Coffea arabica L. (coffee) leaves. In 14. Association Scientifique Internationale pour le Café Conferences; ASIC: San Francisco, CA, USA, 1991. [Google Scholar]
- Ellgardt, K. Triterpenes in Apple Cuticle of Organically and Commercially Cultivated Apples; Bachelor, SLU, Alnarp: St. Louis, MO, USA, 2006; ISSN 1652-1579. Available online: https://stud.epsilon.slu.se/12868/ (accessed on 30 October 2020).
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti /pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Razborsek, M.I.; Voncina, D.B.; Dolecek, V.; Voncina, E. Determination of oleanolic, betulinic and ursolic acid in Lamiaceae and mass spectral fragmentation of their trimethylsilylated derivatives. Chromatographia 2008, 67, 433–440. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, V.S.; Trifan, A.; Miron, A. Chapter 7—Antigenotoxic Potential of Some Dietary Non-phenolic Phytochemicals. Stud. Nat. Prod. Chem. 2019, 60, 223–297. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Menichini, F.; Tundis, R. Chapter 1—Recent Insights into the Emerging Role of Triterpenoids in Cancer Therapy: Part I. Stud. Nat. Prod. Chem. 2013, 40, 1–31. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Alho, D.P.S.; Gonçalves, B.M.F.; Valdeira, A.S.; Mendes, V.I.S.; Jing, Y. Highlights of Pentacyclic Triterpenoids in the Cancer Settings. Stud. Nat. Prod. Chem. 2014, 33–73. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyed, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Zhou, X.J.; Hu, X.M.; Yi, Y.M.; Wan, J. Development and characterisation of ursolic acid nanocrystals without stabiliser having improved dissolution rate and in vitro anticancer activity. Drug Dev. Ind. Pharm. 2009, 35, 305–310. [Google Scholar] [CrossRef]
- Jabeen, M.; Ahmad, S.; Shahid, K.; Sadiq, A.; Rashid, U. Ursolic acid hydrazide based organometallic complexes: Synthesis, characterization, antibacterial, antioxidant and docking studies. Front. Chem. 2018, 6, 55. [Google Scholar] [CrossRef]
- Batool, A.; Shahid, K.; Muddasir, M. Synthesis of aniline derivative of ursolic acid, its metal complexes, characterization and bioassay. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 2. [Google Scholar]
- Kalinowska, M.; Bajko, E.; Matejczyk, M.; Kaczyński, P.; Łozowicka, B. The study of anti-/pro-oxidant, lipophilic, microbial and spectroscopic properties of new alkali metal salts of 5-O-caffeoylquinic acid. Int. J. Mol. Sci. 2018, 19, 463. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Mazur, L.; Jabłońska-Trypuć, A.; Lewandowski, W. A new calcium 2,5-dihydroxybenzoate: Synthesis, characterization and antioxidant studies and stress mediated cytotoxity in MCF-7 cells. J. Saudi Chem. Soc. 2018, 22, 742–756. [Google Scholar] [CrossRef]
- Świderski, G.; Jabłońska-Trypuć, A.; Kalinowska, M.; Świsłocka, R.; Karpowicz, D.; Magnuszewska, M.; Lewandowski, W. Spectroscopic, Theoretical and Antioxidant Study of 3d-Transition Metals (Co(II), Ni(II), Cu(II), Zn(II)) Complexes with Cichoric Acid. Materials 2020, 13, 3102. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz, M.; Regulska, E.; Kalinowska, M. Hydroxyflavone metal complexes—Molecular structure, antioxidant activity and biological effects. Chem. Biol. Interact. 2017, 273, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Bisaglia, M.; Bubacco, L. Copper Ions and Parkinson’s Disease: Why Is Homeostasis So Relevant? Biomolecules 2020, 10, 195. [Google Scholar] [CrossRef]
- Wang, C.M.; Jhan, Y.L.; Tsai, S.J.; Chou, C.-H. The Pleiotropic Antibacterial Mechanisms of Ursolic Acid against Methicillin-Resistant Staphylococcus aureus. Molecules 2016, 21, 884. [Google Scholar] [CrossRef]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-Induced Oxidative Stress and Toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Cunha, M.P.; Kaster, M.P.; Lucia, A.; Rodrigues, R. Chapter 6—Natural Polyphenols and Terpenoids for Depression Treatment: Current Status. Stud. Nat. Prod. Chem. 2018, 55, 181–221. [Google Scholar] [CrossRef]
- Zerin, T.; Lee, M.; Jang, W.S.; Nam, K.W.; Song, H.Y. Anti-inflammatory potential of ursolic acid in Mycobacterium tuberculosis-sensitized and Concanavalin A-stimulated cells. Mol. Med. Rep. 2016, 13, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Numonov, S.; Sharopov, F.; Qureshi, M.N.; Gaforzoda, L.; Gulmurodov, I.; Khalilov, Q.; Setzer, W.N.; Habasi, M.; Aisa, H.A. The Ursolic Acid-Rich Extract of Dracocephalum heterophyllum Benth. with Potent Antidiabetic and Cytotoxic Activities. Appl. Sci. 2020, 10, 6505. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial Activity of Oleanolic and Ursolic Acids: An Update. Evid. Based Complement. Alternat. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, A. 02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. Shawnee Mission, KS, GaussView, Version 6.1; Semichem, Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. II. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Zhan, C.G.; Nichols, J.A.; Dixon, D.A. Ionization Potential, Electron Affinity, Electronegativity, Hardness and Electron Excitation Energy: Molecular Properties from Density Functional Theory Orbital Energies. J. Phys. Chem. 2003, 107, 4184–4195. [Google Scholar] [CrossRef]
- Samsonowicz, M. Molecular structure of phenyl- and phenoxyacetic acids—Spectroscopic and theoretical study. Spectrochim. Acta A 2018, 118, 1386–1425. [Google Scholar] [CrossRef]
- Simon, A.; Delage, C.; Saux, M.; Chulia, A.J.; Najid, A.; Rigaud, M. Structure of ursolic acid ethanol solvate. Acta Cryst. 1992, 48, 726–728. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E.; Kowczyk-Sadowy, M.; Butarewicz, A.; Lewandowski, W. The study on molecular structure and microbiological activity of alkali metal 3-hydroxyphenylycetates. J. Mol. Struct. 2017, 1146, 755–765. [Google Scholar] [CrossRef]
- Mikulski, D.; Eder, K.; Molski, M. Quantum-chemical study on relationship between structure and antioxidant properties of hepatoprotective compounds occurring in Cynara scolymus and Silybum marianum. JTCC 2014, 13, 1450004. [Google Scholar] [CrossRef]
- Teles Fujishima, M.A.; Silva, N.; Ramos, R.; Batista Ferreira, E.F.; Santos, K.; Silva, C.; Silva, J.; Campos Rosa, J.M.; Santos, C. An Antioxidant Potential, Quantum-Chemical and Molecular Docking Study of the Major Chemical Constituents Present in the Leaves of Curatella americana Linn. Pharmaceuticals 2018, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz, M.; Kowczyk-Sadowy, M.; Regulska, E.; Lewandowski, W. Molecular structure and spectroscopic analysis of homovanillic acid and its sodium salt—NMR, FT-IR and DFT studies. Spectrochim. Acta Part A 2014, 118, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tong, H.H.Y.; Li, L.; Shek, F.L.Y.; Lv, Y.; Zheng, Y. Synthesis, characterization and thermal analysis of ursolic acid solid forms. Cryst. Res. Technol. 2015, 50, 538–548. [Google Scholar] [CrossRef]
- Pai, S.R.; Upadhya, V.; Hegde, H.V.; Joshi, R.K.; Kholkute, S.D. Determination of betulinic acid, oleanolic acid and ursolic acid from Achyranthes aspera using RP-UFLC-DAD analysis and evaluation of various parameters for their optimum yield. Indian J. Exp. Biol. 2016, 54, 196–202. [Google Scholar]
- Pereira, J.R.; Queiroz, R.F.; De Siqueira, E.A.; Brasileiro-Vidal, A.C.; Sant’ana, A.E.G.; Silva, D.M.; De Mello Affonso, P.R.A. Evaluation of cytogenotoxicity, antioxidant and hypoglycemiant activities of isolate compounds from Mansoa hirsuta D.C. (Bignoniaceae). An. Acad. Bras. Ciênc. 2017, 89, 317–331. [Google Scholar] [CrossRef]
- Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.M.C.; Arriaga, Â.M.C.; Ferreira, D.A.; Santiago, G.M.P.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Chang, M.Y.; Liu, C.M.; Shieh, D.E.; Chen, C.Y. Evaluation and analysis of phytochemical antioxidant capacity. Biomed. Res. 2017, 28, 6431–6434. [Google Scholar]
- Santiago, L.A.; Dayrit, K.C.; Correa, P.C.B. Mayor, Comparison of antioxidant and free radical scavenging activity of triterpenesα-amyrin, oleanolic acid and ursolic acid. J. Nat. Prod. 2014, 7, 29–36. [Google Scholar]
- Psomas, G. Copper(II) and zinc(II) coordination compounds of non-steroidal anti-inflammatory drugs: Structural features and antioxidant activity. Coord. Chem. Rev. 2020, 412, 213259. [Google Scholar] [CrossRef]
- Guerreiro, J.F.; Gomes, M.A.G.B.; Pagliari, F.; Jansen, J.; Marafioti, M.G.; Nistico, C.; Hanley, R.; Costa, R.O.; Ferreira, S.S.; Mendes, V.; et al. Iron and copper complexes with antioxidant activity as inhibitors of the metastatic potential of glioma cells. RSC Adv. 2020, 10, 12699. [Google Scholar] [CrossRef]
- Călinescu, M.; Fiastru, M.; Bala, D.; Mihailciuc, C.; Negreanu-Pîrjol, T.; Jurcă, B. Synthesis, characterization, electrochemicalbehavior and antioxidant activity of new copper(II) coordination compounds with curcumin derivatives. J. Saudi Chem. Soc. 2019, 23, 817–827. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstractions. 1. The Reactions of Phenols with 2,2-Diphenyl-1-picrylhydrazyl (DPPH•) in Alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Sendra, J.M.; Sentandreu, E.; Navarro, J.L. Reduction kinetics of the free stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH•) for determination of the antiradical activity of citrus juices. Eur. Food Res. Technol. 2006, 223, 615–624. [Google Scholar] [CrossRef]




| Atom Numbers * | UA | Cu (II) UA |
|---|---|---|
| Distances Between Atoms (Å) | ||
| O2–H2a/Cu | 0.976 | 1.898 |
| O2–C28 | 1.361 | 1.285 |
| C28–O3 | 1.213 | 1.293 |
| O3–H2a/Cu | 2.247 | 1.886 |
| C28–C17 | 1.533 | 1.508 |
| C17–C22 | 1.566 | 1.574 |
| C19–C18 | 1.565 | 1.578 |
| C18–C17 | 1.561 | 1.568 |
| C14–C13 | 1.540 | 1.532 |
| C13–C18 | 1.537 | 1.522 |
| C11–C12 | 1.501 | 1.488 |
| C12–C13 | 1.340 | 1.363 |
| Angles | UA | Cu (II) UA |
|---|---|---|
| O3–C28–O2 | 120.95 | 115.48 |
| C28–O2–H2a/Cu | 105.46 | 86.92 |
| C28–O3–H2a/Cu | 56.50 | 87.21 |
| O2–H2a–O3 | 77.09 | 70.38 |
| C28–C17–C22 | 105.86 | 104.83 |
| O3–C28–C17 | 126.15 | 121.59 |
| O2–C28–C17 | 112.79 | 122.83 |
| C17–C22–H22a | 108.30 | 107.01 |
| C17–C18–H18 | 106.47 | 107.60 |
| H19–C19–C18 | 108.82 | 107.66 |
| C13–C14–C27 | 108.38 | 107.17 |
| C18–C13–C14 | 121.15 | 122.28 |
| C12–C11–C9 | 113.11 | 114.12 |
| C8–C14–C27 | 112.11 | 110.46 |
| Parameter | UA | Cu (II) UA |
|---|---|---|
| Energy (Hartree *) | −1397.7661 | −3037.1805 |
| Dipole moment (Debye) | 2.5264 | 13.8294 |
| EHOMO (eV) | −9.5980 | −2.7851 |
| ELUMO (eV) | −4.2031 | −2.6251 |
| Energy gap (eV) | 5.3949 | 0.1600 |
| Ionization potential, I = −EHOMO (eV) | 9.5980 | 2.7851 |
| Electronaffinity, A = −ELUMO (eV) | 4.2031 | 2.6251 |
| Electronegativity, (eV) | 6.9005 | 2.7051 |
| Electronic chemical potential, (eV) | −6.9005 | −2.7051 |
| Chemical hardness, (eV) | 2.6975 | 0.0800 |
| Chemical softness, S (eV) | 0.1854 | 6.2499 |
| Electrophilicity index, (eV) | 8.8263 | 45.7334 |
| UA | Cu (II) UA Complex | Assignments | ||
|---|---|---|---|---|
| IR KBr | IR–ATR | IR KBr | IR–ATR | |
| 3423 s | 3402w | 3443 s | ν (OH) | |
| - | 2966 s | ν(OH) | ||
| 2950 vs | 2948 vs | - | ν (CH) | |
| 2927vs | 2926 vs | 2927 vs | ν(CH) | |
| 2872 s | 2835 s | 2871 s | 2844vw | ν(CH) |
| 1715 s | - | ν(C=O) | ||
| 1697 s | 1659 w | 1698 w | 1636w | ν(C=O) |
| - | 1541 m | νas(COO-) | ||
| 1456 m | 1452 m | 1456 s | 1454 w | βas (OH) |
| - | 1402 m | νs(COO−) | ||
| 1386 m | 1389 m | 1387w | δs(CH3) | |
| 1377 m | 1378 m | βs(OH) | ||
| 1360 w | - | ν(C-OH) | ||
| 1031 m | 1029 m | 1030 m | (C-OH) | |
| 998 m | 996 m | γ(CH) – C = C − | ||
| 973 w | 973 w | ν (C-C), C-H, C-O | ||
| - | 804 w | δs(CH3) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsonowicz, M.; Kalinowska, M.; Gryko, K. Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study. Materials 2021, 14, 264. https://doi.org/10.3390/ma14020264
Samsonowicz M, Kalinowska M, Gryko K. Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study. Materials. 2021; 14(2):264. https://doi.org/10.3390/ma14020264
Chicago/Turabian StyleSamsonowicz, Mariola, Monika Kalinowska, and Kamila Gryko. 2021. "Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study" Materials 14, no. 2: 264. https://doi.org/10.3390/ma14020264
APA StyleSamsonowicz, M., Kalinowska, M., & Gryko, K. (2021). Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study. Materials, 14(2), 264. https://doi.org/10.3390/ma14020264






