Synthesis, Spectral, Thermal and Biological Studies of 4-Cyclohexyl-3-(4-nitrophenyl)methyl-1,2,4-triazolin-5-thione and Its Copper(II) Coordination Compound, [CuCl2(H2O)2L2]
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
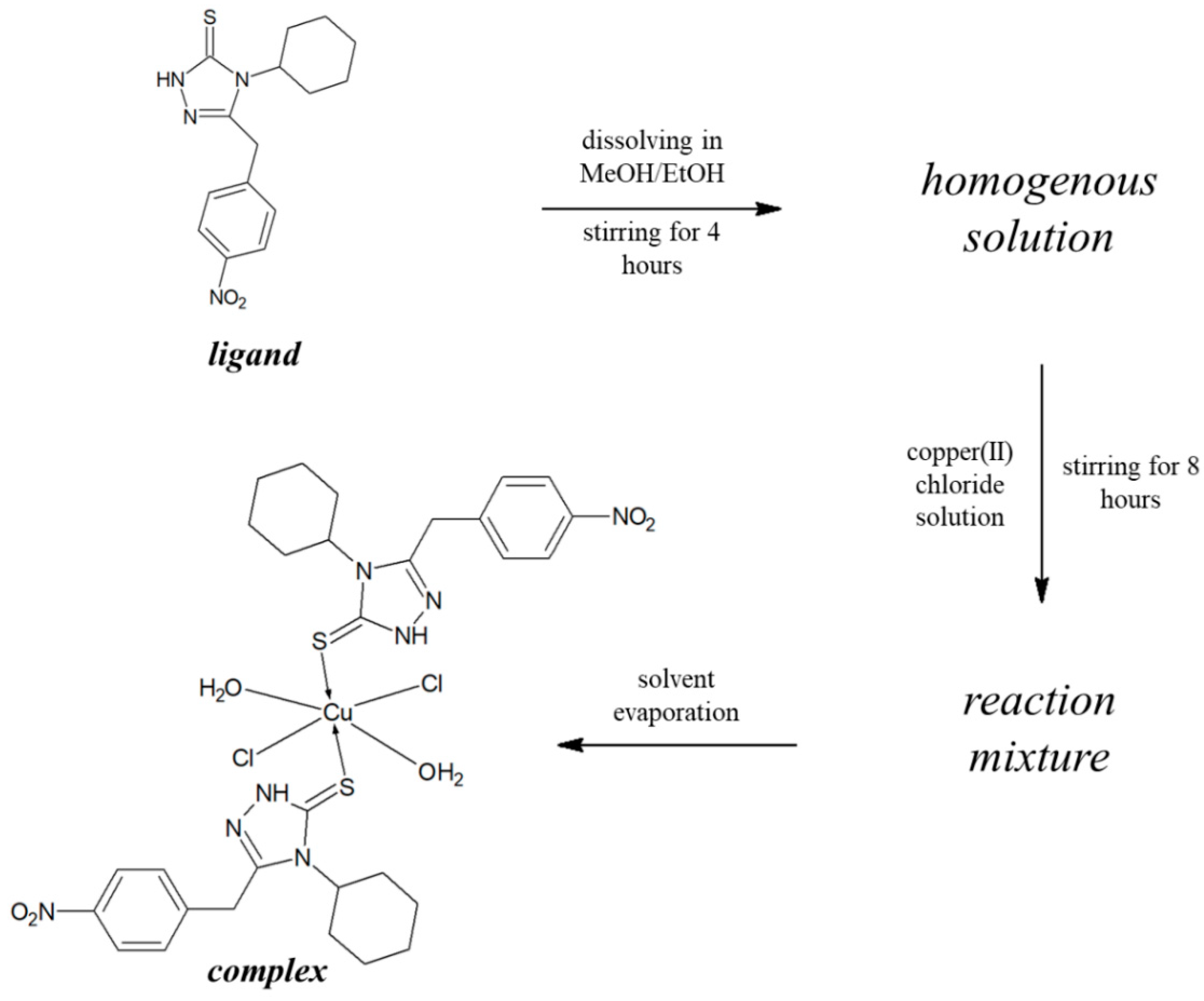
2.2. Preparations and Analysis
2.2.1. Preparation of 4-Nitrophenylacetic Acid Hydrazide (1)
2.2.2. Preparation of 4-Cyclohexyl-1-(4-nitrophenyl)acetylothiosemicarbazide (2)
2.2.3. Preparation of 4-Cyclohexyl-3-(4-nitrophenyl)methyl-1,2,4-triazolin-5-thione (3)
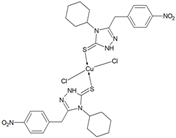
2.2.4. Preparation of Copper(II) 4-Cyclohexyl-3-(4-nitrophenyl)methyl-1,2,4-triazolin-5-thione complex (Cu-3)
3. Results and Discussion
3.1. Synthesis
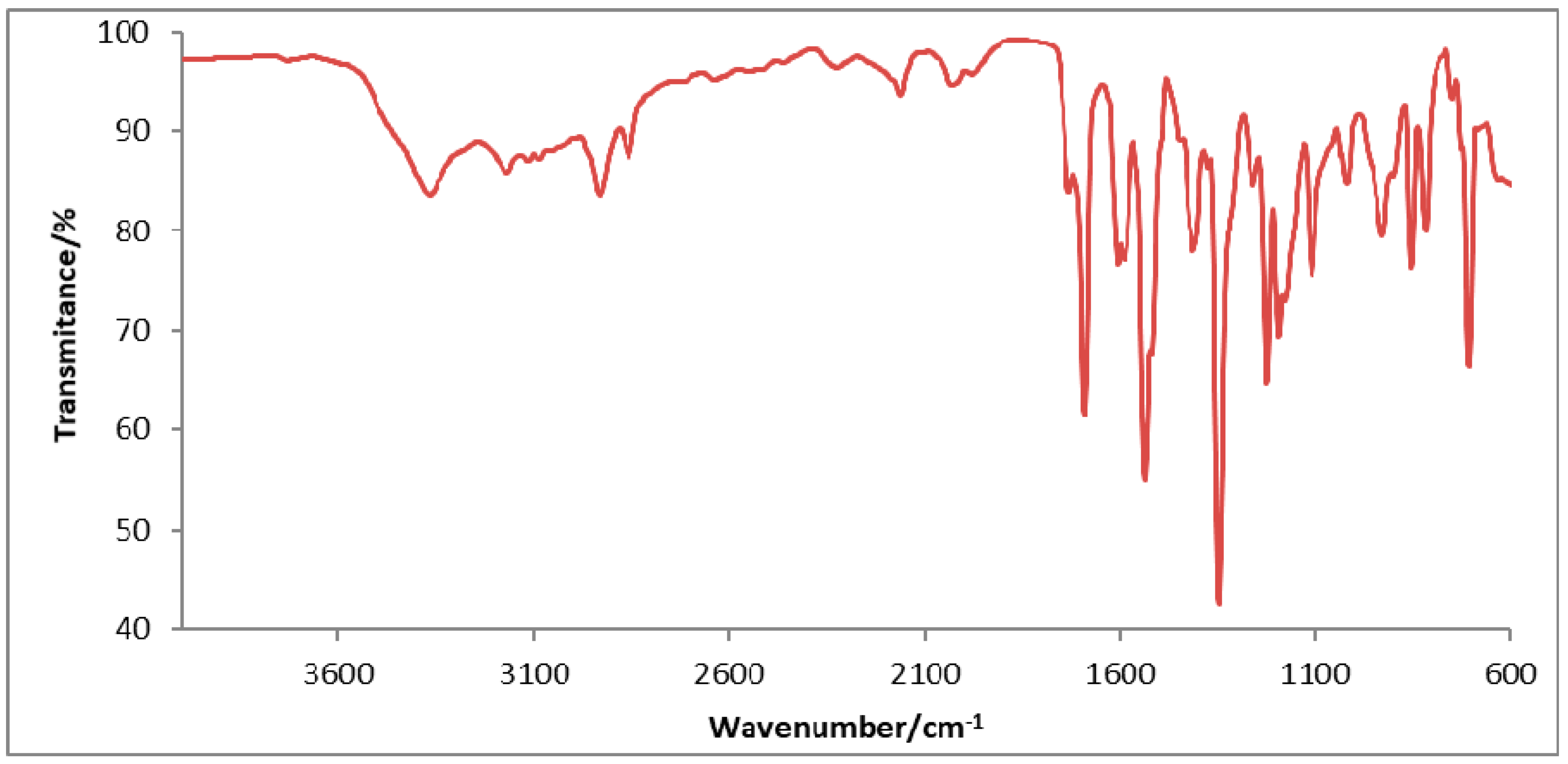
3.2. FTIR Spectra
3.3. Thermogravimetric Studies in Air
3.4. Biological Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nadeem, H.; Mohsin, M.; Afzaal, H.; Riaz, S.; Zahid, A.; Muhammad, S.A. Synthesis and in vitro biological activities of 4,5-disubstituted 1,2,4-triazole-3-thiols. Adv. Microbiol. 2013, 3, 366–375. [Google Scholar] [CrossRef][Green Version]
- Hovsepyan, T.R.; Hakobyan, M.R.; Muradyan, R.E.; Nersesyan, L.E.; Agaronyan, A.S.; Danielyan, I.S.; Minasyan, N.S. Synthesis of new substituted 1,2,4-triazoles and 1,3,4-thiadiazoles and their effects on DNA methylation level. Russ. J. Gen. Chem. 2019, 89, 673–679. [Google Scholar] [CrossRef]
- Santos, A.F.; Brotto, D.F.; Favarin, L.R.V.; Cabeza, N.A.; Andrade, G.R.; Batistote, M.; Cavalheiro, A.A.; Neves, A.; Rodrigues, D.C.M.; dos Anjos, A. Study of the antimicrobial activity of metal complexes and their ligands through bioassays applied to plant extracts. Rev. Bras. Farmacogn. 2014, 24, 309–315. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Guerreiro, S.I.; Lourenço, A.; Alves, M.M.; Montemor, M.F.; Mira, N.P.; Leitão, J.H.; Carvalho, M.F.N.N. Ag(I) camphorimine complexes with antimicrobial activity towards clinically important bacteria and species of the Candida genus. PLoS ONE 2017, 12, e0177355. [Google Scholar] [CrossRef] [PubMed]
- Danac, R.; Pui, A.; Coria, I.; Amarandi, R.-M.; Ciobanu, C.I.; Apostu, M.-O.; Palamarciuc, O. New M(II) (M=Mn, Co, Ni, Cu, Zn, Pb) coordinative compounds with 2-formylpyridine S-methyl-isothiosemicarbazide. J. Mol. Struct. 2020, 1207, 127747. [Google Scholar] [CrossRef]
- Jurca, T.; Marian, E.; Vicaş, L.G.; Mureşan, M.E.; Fritea, L. Metal complexes of pharmaceutical substances. Spectrosc. Anal. Dev. Appl. 2017. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; da Silva, M.F.C.G.; Florek, M.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Silver(I) 1,3,5-triaza-7-phosphaadamantane coordination polymers driven by substituted glutarate and malonate building blocks: Self-assembly synthesis, structural features, and antimicrobial properties. Inorg. Chem. 2016, 55, 5886. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Jaros, S.W.; da Silva, M.F.C.G.; Król, J.; Oliveira, M.C.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Bioactive silver–organic networks assembled from 1,3,5-triaza-7-phosphaadamantane and flexible cyclohexanecarboxylate blocks. Inorg. Chem. 2016, 55, 1486. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Morris, R.E.; Horcajada, P.; Férey, G.; Gref, R.; Couvreur, P.; Serre, C. BioMOFs: Metal-organic frameworks for biological and medical applications. Angew. Chem. Int. Ed. 2010, 49, 6260. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; da Silva, M.F.C.G.; Florek, M.; Oliveira, M.C.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Aliphatic dicarboxylate directed assembly of silver(I) 1,3,5-triaza-7-phosphaadamantane coordination networks: Topological versatility and antimicrobial activity. Cryst. Growth Des. 2014, 14, 5408. [Google Scholar] [CrossRef]
- Heng, M.P.; Tan, C.H.; Saad, H.M.; Sim, K.S.; Tan, K.W. Mitochondria-dependent apoptosis inducer: Testosterone-N4ethylthiosemicarbazonate and its metal complexes with selective cytotoxicity towards human colorectal carcinoma cell line (HCT 116). Inorg. Chim. Acta 2020, 507, 119581. [Google Scholar] [CrossRef]
- Grein, F.; Müller, A.; Scherer, K.M.; Liu, X.; Ludwig, K.C.; Klöckner, A.; Strach, M.; Sahl, H.-G.; Kubitscheck, U.; Schneider, T. Ca2+-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat. Commun. 2020, 11, 1455. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; Fouda, A.E.S.; Nabih, D.M. Novel Mn2+, Fe3+, Co2+, Ni2+ and Cu2+ complexes of potential thiosemicarbazide: Design, structural elucidation, anticorrosion potential study and antibacterial activity. J. Mol. Struct. 2020, 1204, 127495. [Google Scholar] [CrossRef]
- Lapasam, A.; Banothu, V.; Addepally, U.; Kollipara, M.R. Half-sandwich arene ruthenium, rhodium and iridium thiosemicarbazone complexes: Synthesis, characterization and biological evaluation. J. Chem. Sci. 2020, 32, 34–44. [Google Scholar] [CrossRef]
- Ishak, N.N.M.; Jamsari, J.; Ismail, A.Z.; Tahir, M.I.M.; Tiekink, E.R.T.; Veerakumarasivan, A.; Ravoof, T.B.S.A. Synthesis, characterisation and biological studies of mixed-ligand nickel(II) complexes containing imidazole derivatives and thiosemicarbazide Schiff bases. J. Mol. Struct. 2019, 1988, 126888. [Google Scholar] [CrossRef]
- Fiori-Duarte, A.T.; de Paiva, R.E.F.; Manzano, C.M.; Lustri, W.R.; Corb, P.P. Silver(I) and gold(I) complexes with sulfasalazine: Spectroscopic characterization, theoretical studies and antiproliferative activities over Gram-positive and Gram-negative bacterial strains. J. Mol. Struct. 2020, 1214, 128158. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, S.; Wang, Z.; Guo, H.; Yang, F. First fluorescence sensor for simultaneously detecting three kinds of IIB elements (Zn2+, Cd2+ and Hg2+) based on aggregation-induced emission. Sens. Actuators B Chem. 2020, 308, 127734. [Google Scholar] [CrossRef]
- Argibay-Otero, S.; Gano, L.; Fernandes, C.; Paulo, A.; Carballo, R.; Vázquez-López, E.M. Chemical and biological studies of Re(I)/Tc(I) thiosemicarbazonate complexes relevant for the design of radiopharmaceuticals. J. Biochem. Chem. 2020, 203, 110917. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.K.; Li, G.B.; Liu, S.G.; Zhou, X.P.; Yang, G.Z. Synthesis, crystal structure, electrochemical property, and antioxidant activity of copper(II) complex based on 4-butyloxy-2,6-bis(1-methyl-2-benzimidazolyl)pyridine. Mon. Chem. 2016, 147, 1189. [Google Scholar] [CrossRef]
- Ma, L.; Lu, L.; Zhu, M.; Wang, Q.; Li, Y.; Xing, S.; Fu, X.; Gao, Z.; Dong, Y. Mononuclear copper(II) complexes with 3,5-substituted-4-salicylidene-amino-3,5-dimethyl-1,2,4-triazole: Synthesis, structure and potent inhibition of protein tyrosine phosphatases. Dalton Trans. 2011, 40, 6532. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, F.; Haghi, F.; Bikas, R.; Heidari, A.; Gholami, M.; Kozakiewicz, A.; Zeighami, H. Synthesis, characterization and assessment of anti-quorum sensing activity of copper(II)-ciprofloxacin complex against Pseudomonas aeruginosa PAO1. AMB Expr. 2020, 10, 82. [Google Scholar] [CrossRef]
- Atheer, A.A.; Rehab, M.A.; Dhia, H.H.; Ahmed, M.R.; Hamid, S.M. Synthesis, spectroscopic, characterization, pharmacological evaluation, and cytotoxicity assays of novel nano and micro scale of copper (II) complexes against human breast cancer cells. Drug Invent. Today 2020, 14, 31–39. [Google Scholar]
- Sumrra, S.H.; Mushtaq, F.; Khalid, M.; Raza, M.A.; Nazar, M.F.; Ali, B.; Braga, A.A. Synthesis, spectral characterization and computed optical analysis of potent triazole based compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 197–207. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032. [Google Scholar] [CrossRef]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial application of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E. Dis 5.1. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Popiołek, Ł.; Biernasiuk, A. New nalidixic acid—1,3-thiazolidin-4-one hybrids: Design, synthesis and in vitro antimicrobial activity. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 23–27. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- O’Donnell, F.; Smyth, T.J.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolones. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef]
- Reddy, M.U.; Reddy, M.C. Somasekhara, Synthesis, characterization and anti-diabetic activity of vanillin based acetohydrazide-hydrazone derivatives. World J. Pharm. Res. 2017, 6, 814–825. [Google Scholar]
- Bharty, M.K.; Bharti, A.; Chaurasia, R.; Chaudhari, U.K.; Kushawaha, S.K.; Sonkar, P.K.; Ganesan, V.; Butcher, R.J. Synthesis and characterization of Mn(II) complexes of 4-phenyl(phenyl-acetyl)-3-thiosemicarbazide, 4-amino-5-phenyl-1,2,4-triazole-3-tiolate, and their application towards electrochemical oxygen reduction reaction. Polyhedron 2019, 173, 114125. [Google Scholar] [CrossRef]
- Paqhaleh, D.M.S.; Aminjanov, A.A.; Amani, V. Discrete and polymeric lead(II) complexes containing 4-methyl-1,2,4-triazole-3-thiol ligand: X-ray studies, spectroscopic characterization, and thermal analyses. J. Inorg. Organomet. Polym. 2014, 24, 340–346. [Google Scholar] [CrossRef]


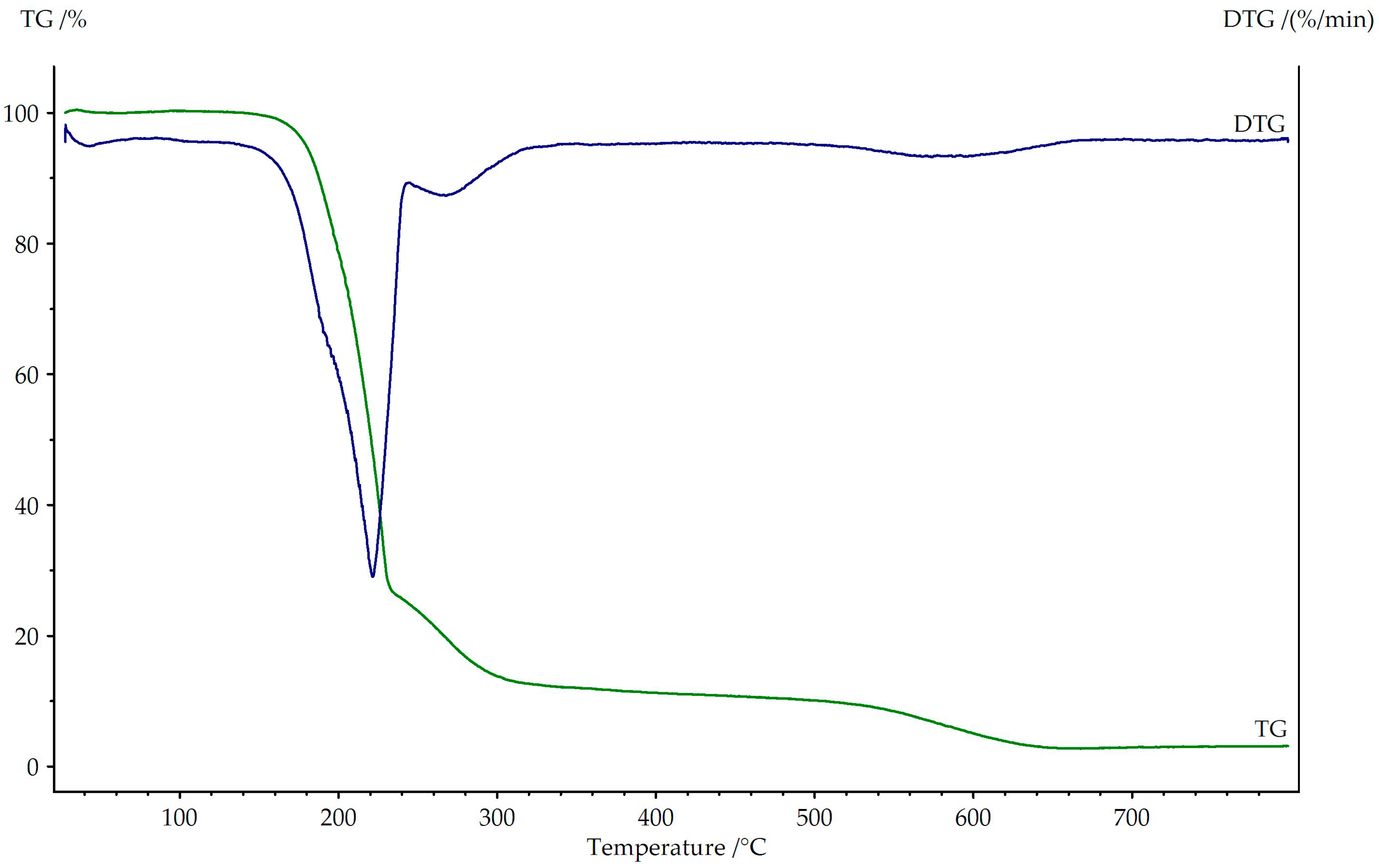

| Compound | Range of Decomposition (°C) | DTG Peak (°C) | Mass Loss (%) | Intermediate Product or Solid Residue | |
|---|---|---|---|---|---|
| Found | Calc. | ||||
 | 50–110 | 80 | 5.0 | 4.46 |  |
 | 110–310 | 210 | 54.5 | 54.33 | 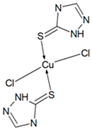 |
 | 310–640 | 580 | 31.0 | 31.36 | CuO |
| Species | MIC (MBC or MFC) [µg/mL] and {MBC/MIC or MFC/MIC} of Compounds | ||
|---|---|---|---|
| Copper(II) Complex (Cu-3) | Organic Ligand (3) | CIP/NY * | |
| Staphylococcus aureus ATCC 25923 | 250 (250) {1} | 1000 (>1000) {>1} | 0.48 (0.48) {1} |
| Staphylococcus aureus ATCC 29213 | 250 (250) {1} | >1000 (>1000) {>1} | 0.48 (0.48) {1} |
| Stphylococcus epidermidis ATCC 12228 | 250 (250) {1} | 1000 (1000) {1} | 0.12 (0.12) {1} |
| Micrococcus luteus ATCC 10240 | 250 (250) {1} | 1000 (1000) {1} | 0.98 (1.95) {2} |
| Bacillus cereus ATCC 10876 | 500 (>1000) {>2} | 1000 (>1000) {>1} | 0.06 (0.12) {2} |
| Bacillus subtilis ATCC 6633 | 250 (250) {1} | 1000 (>1000) {>1} | 0.03 (0.03) {1} |
| Bordetella bronchiseptica ATCC 4617 | 500 (1000) {2} | 500 (1000) {2} | 0.98 (0.98) {1} |
| Candida albicans ATCC 10231 | 500 (1000) {2} | 500 (1000) {2} | 0.48 * (0.48) {1} |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czylkowska, A.; Drozd, M.; Biernasiuk, A.; Rogalewicz, B.; Malm, A.; Pitucha, M. Synthesis, Spectral, Thermal and Biological Studies of 4-Cyclohexyl-3-(4-nitrophenyl)methyl-1,2,4-triazolin-5-thione and Its Copper(II) Coordination Compound, [CuCl2(H2O)2L2]. Materials 2020, 13, 4135. https://doi.org/10.3390/ma13184135
Czylkowska A, Drozd M, Biernasiuk A, Rogalewicz B, Malm A, Pitucha M. Synthesis, Spectral, Thermal and Biological Studies of 4-Cyclohexyl-3-(4-nitrophenyl)methyl-1,2,4-triazolin-5-thione and Its Copper(II) Coordination Compound, [CuCl2(H2O)2L2]. Materials. 2020; 13(18):4135. https://doi.org/10.3390/ma13184135
Chicago/Turabian StyleCzylkowska, Agnieszka, Monika Drozd, Anna Biernasiuk, Bartłomiej Rogalewicz, Anna Malm, and Monika Pitucha. 2020. "Synthesis, Spectral, Thermal and Biological Studies of 4-Cyclohexyl-3-(4-nitrophenyl)methyl-1,2,4-triazolin-5-thione and Its Copper(II) Coordination Compound, [CuCl2(H2O)2L2]" Materials 13, no. 18: 4135. https://doi.org/10.3390/ma13184135
APA StyleCzylkowska, A., Drozd, M., Biernasiuk, A., Rogalewicz, B., Malm, A., & Pitucha, M. (2020). Synthesis, Spectral, Thermal and Biological Studies of 4-Cyclohexyl-3-(4-nitrophenyl)methyl-1,2,4-triazolin-5-thione and Its Copper(II) Coordination Compound, [CuCl2(H2O)2L2]. Materials, 13(18), 4135. https://doi.org/10.3390/ma13184135