Bathurst Burr (Xanthium spinosum) Powder—A New Natural Effective Adsorbent for Crystal Violet Dye Removal from Synthetic Wastewaters
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
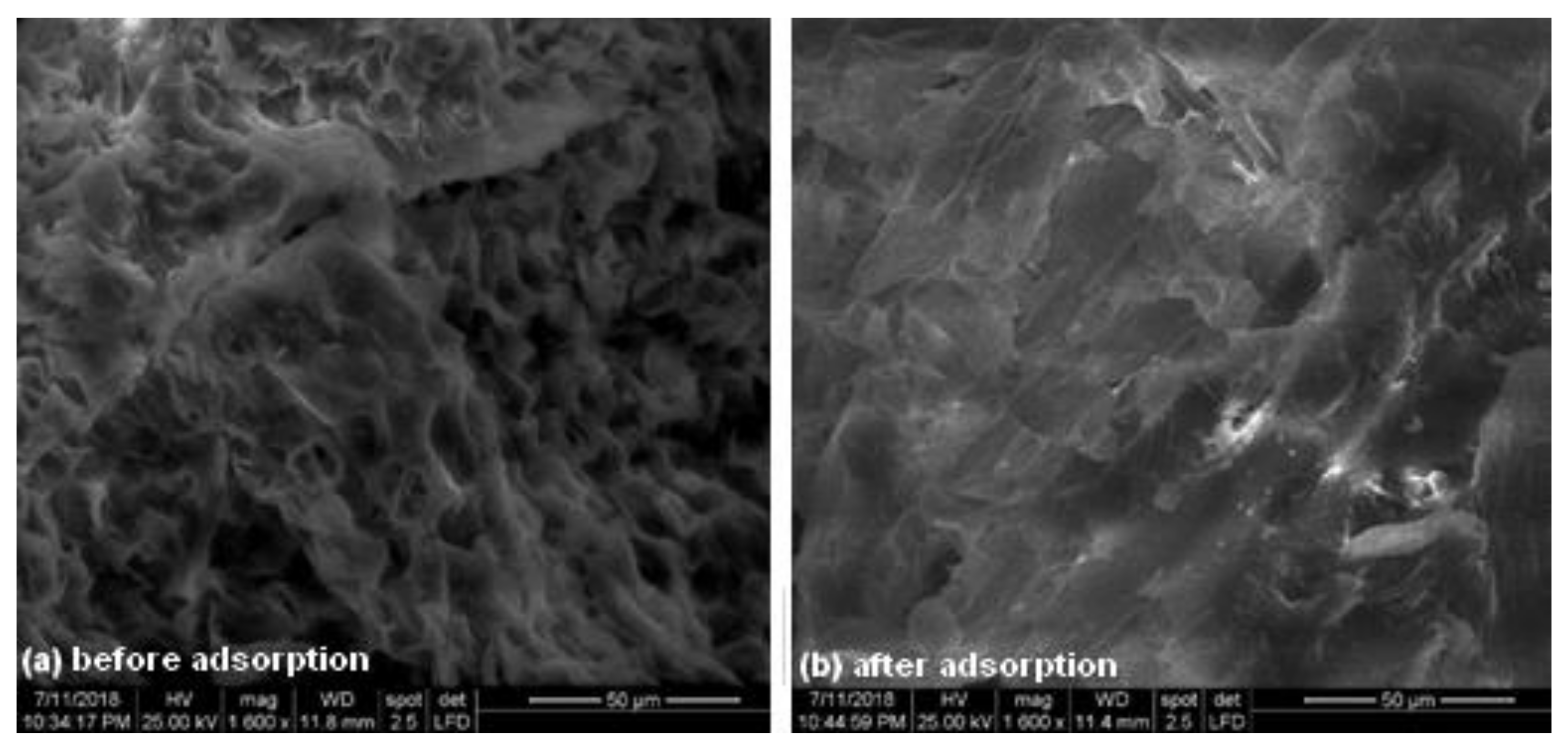
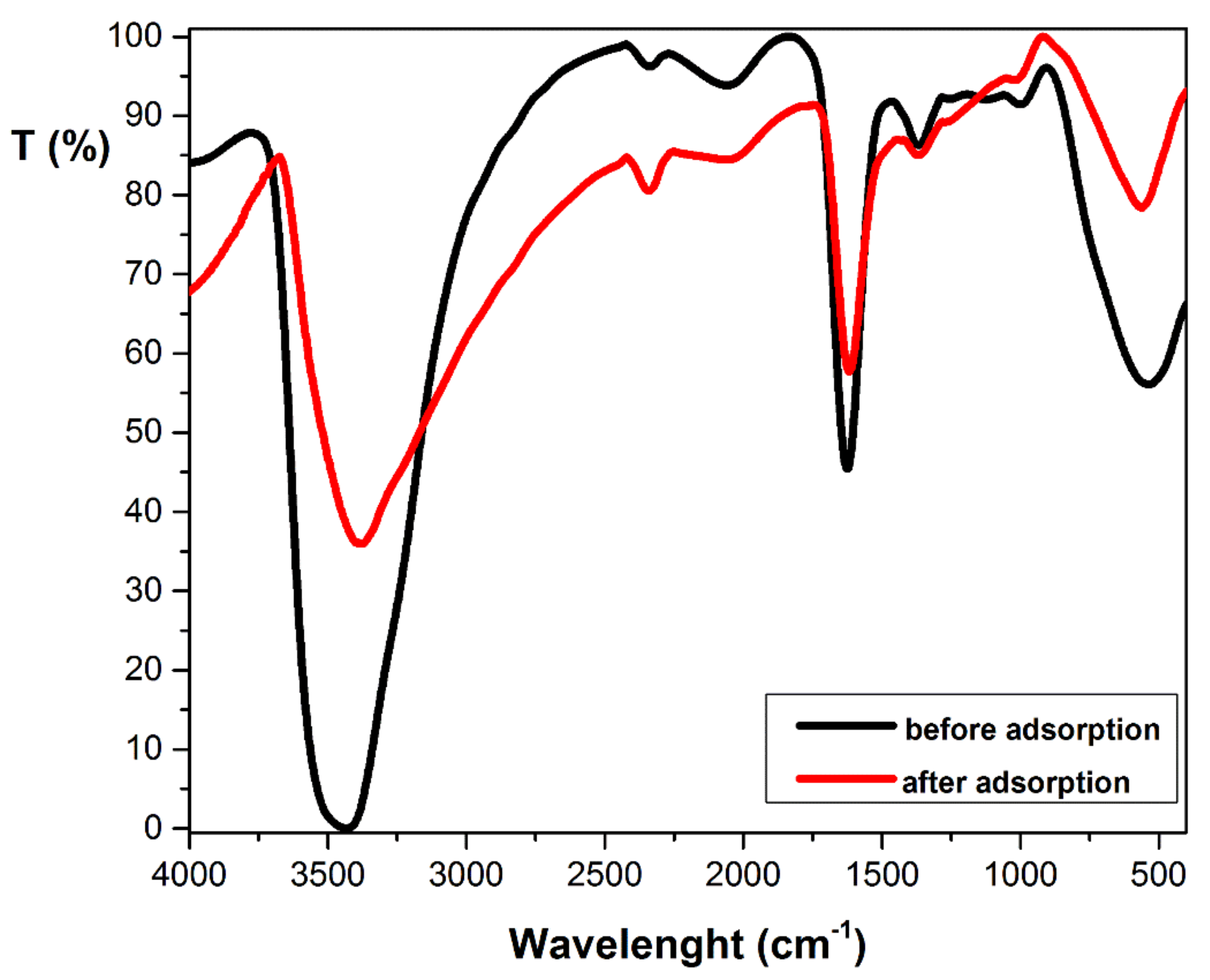
3.1. Adsorbent Characterization
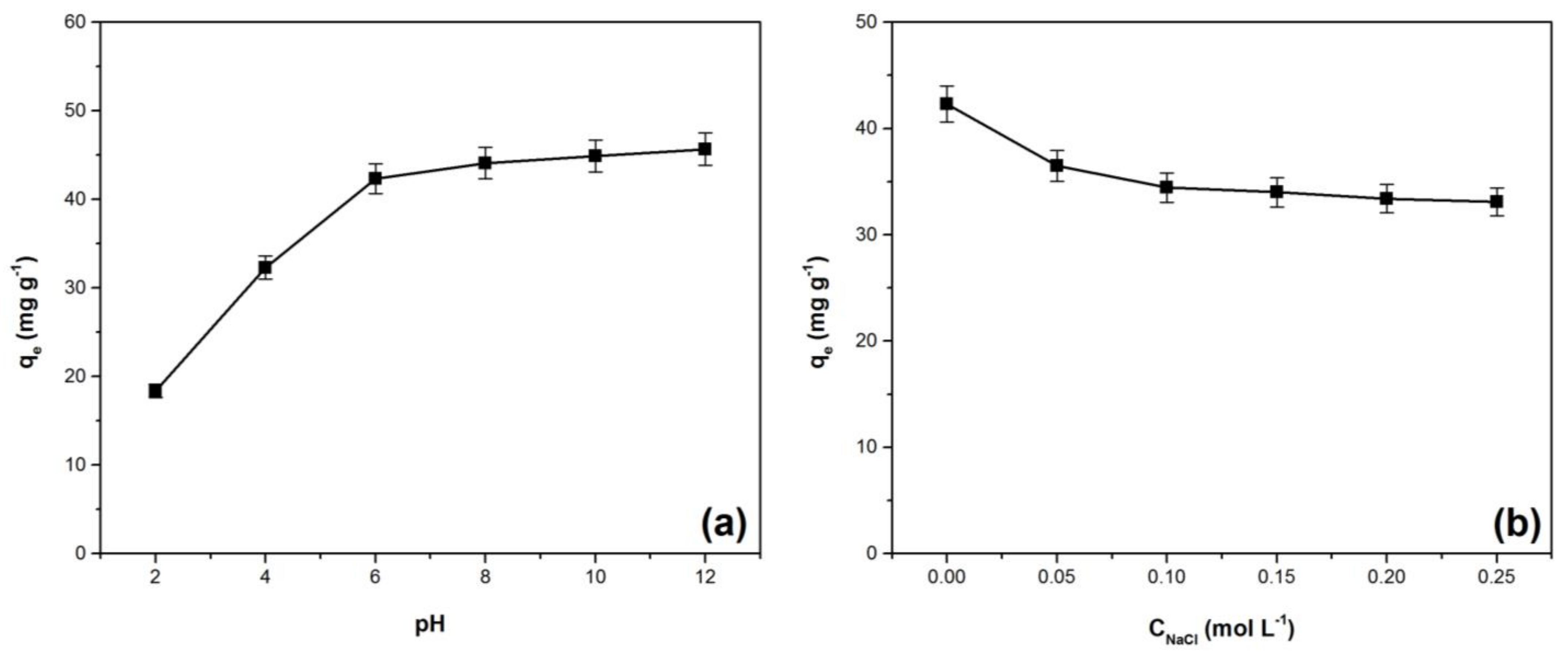
3.2. Effect of pH and Ionic Strength on Crystal Violet Adsorption
3.3. Effect of Adsorbent Dose and Initial Dye Concentration on Crystal Violet Adsorption
3.4. Equilibrum Study
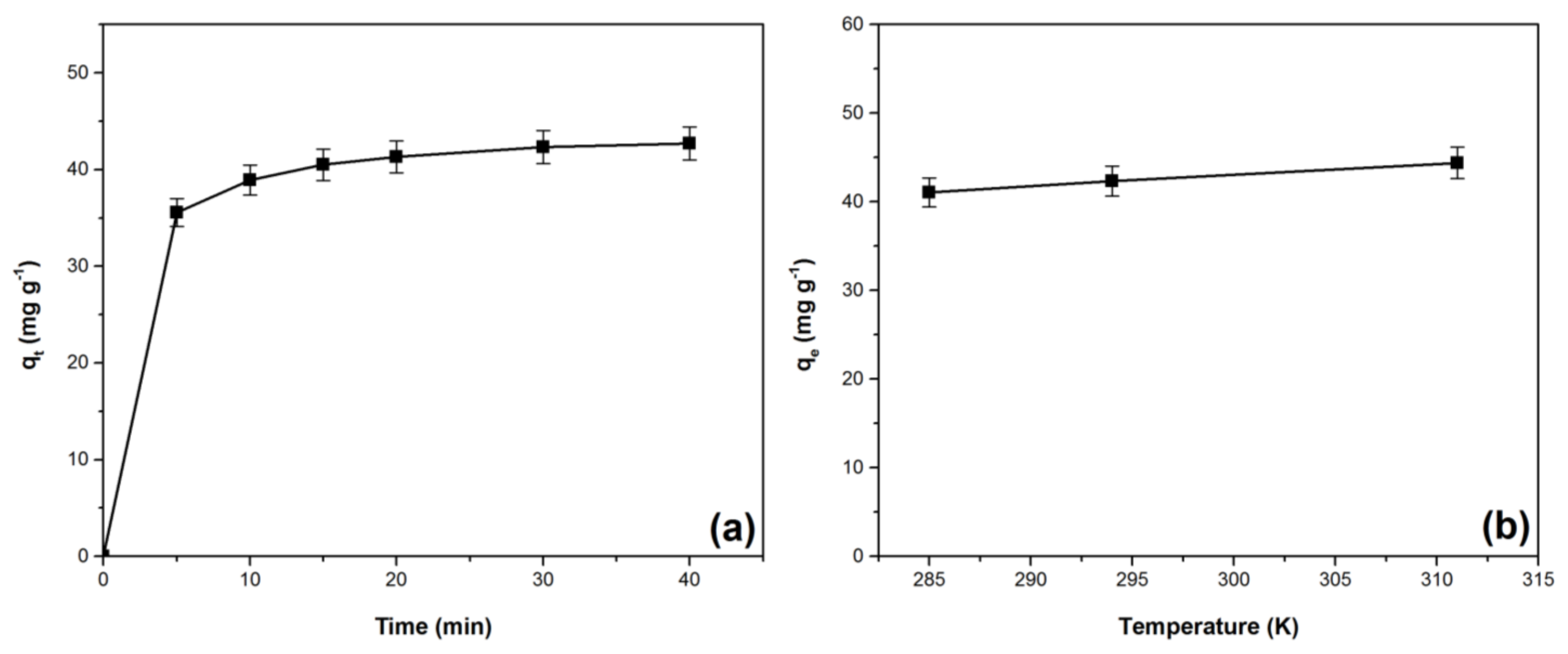
3.5. Kinetic and Thermodynamic Studies
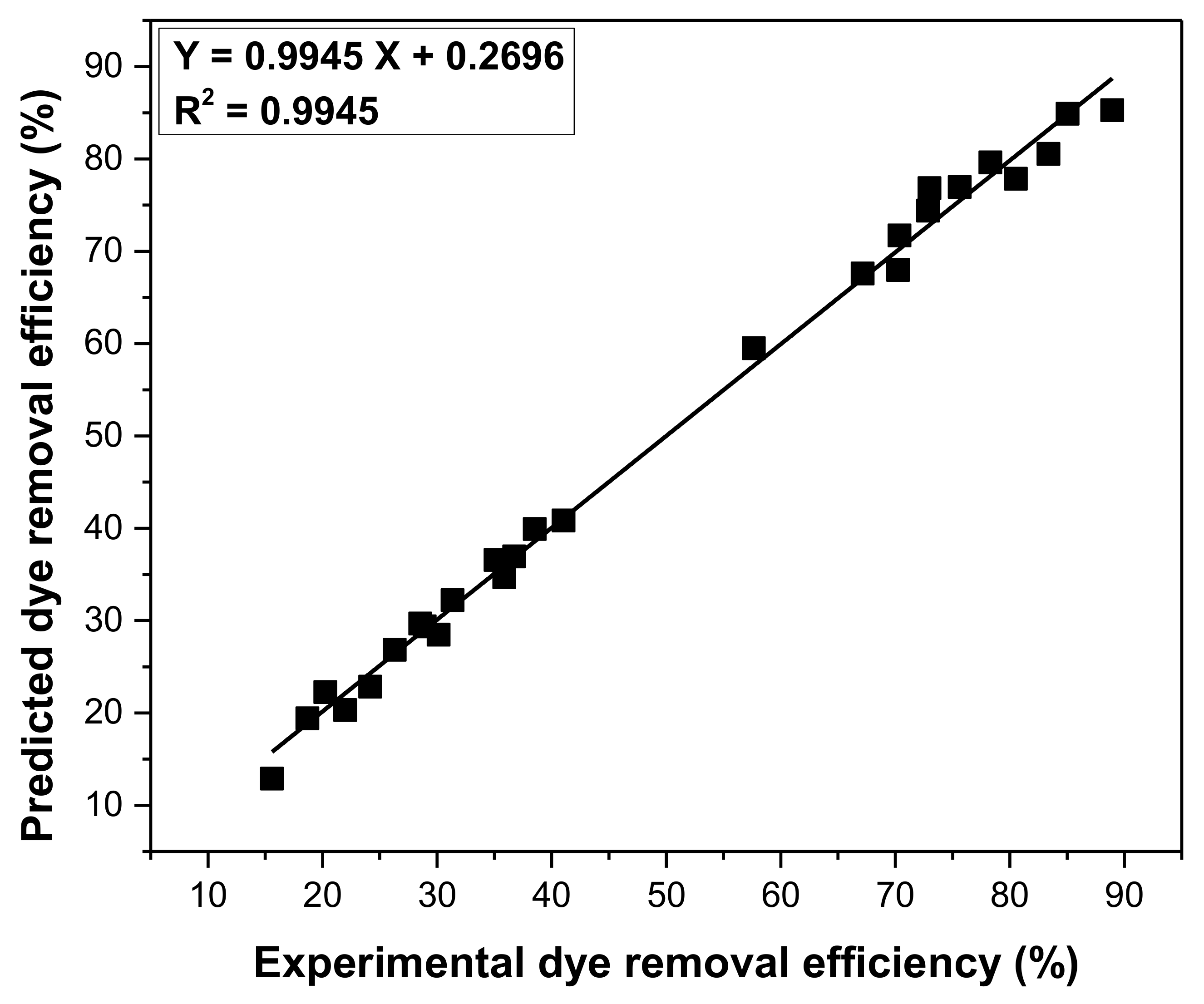
3.6. Optimization Parameters of Adsorption Process Using Taguchi Approach
3.7. Desorption Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aysu, T.; Küçük, M.M. Removal of crystal violet and methylene blue from aqueous solutions by activated carbon prepared from Ferula orientalis. Int. J. Environ. Sci. Technol. 2014, 12, 2273–2284. [Google Scholar] [CrossRef]
- Chahinez, H.-O.; Abdelkader, O.; Leila, Y.; Tran, H.N. One-stage preparation of palm petiole-derived biochar: Characterization and application for adsorption of crystal violet dye in water. Environ. Technol. Innov. 2020, 19, 100872. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Fagundes, J.L.S.; Georgin, J.; Salaua, N.P.G.; Dotto, G.L. A mass transfer study considering intraparticle diffu-sion and axial dispersion for fixed-bed adsorption of crystal violet on pecan pericarp (Carya illinoensis). Chem. Eng. J. 2020, 397, 125423. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chakraborty, S.; Das, P. Removal of Crystal Violet from Aqueous Solution by Adsorption onto Eggshells: Equilibrium, Kinetics, Thermodynamics and Artificial Neural Network Modeling. Waste Biomass- Valorization 2012, 4, 655–664. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Netto, M.S.; Allasia, D.; Oliveira, M.L.S.; Dotto, G.L. Evaluation of Ocotea puberula bark powder (OPBP) as an effective adsorbent to uptake crystal violet from colored effluents: Alternative kinetic approaches. Environ. Sci. Pollut. Res. 2020, 27, 25727–25739. [Google Scholar] [CrossRef]
- Rosly, N.Z.; Abdullah, A.H.; Kamarudin, M.A.; Ashari, S.E.; Ahmad, S.A.A. Adsorption of Methylene Blue Dye by Calix[6]Arene-Modified Lead Sulphide (Pbs): Optimisation Using Response Surface Methodology. Int. J. Environ. Res. Public Health 2021, 18, 397. [Google Scholar] [CrossRef]
- Filho, A.C.D.; Mazzocato, A.C.; Dotto, G.L.; Thue, P.S.; Pavan, F.A. Eragrostis plana Nees as a novel eco-friendly adsorbent for removal of crystal violet from aqueous solutions. Environ. Sci. Pollut. Res. 2017, 24, 19909–19919. [Google Scholar] [CrossRef]
- Lim, L.B.L.; Priyantha, N.; Mansor, N.H.M. Artocarpus altilis (breadfruit) skin as a potential low-cost biosorbent for the removal of crystal violet dye: Equilibrium, thermodynamics and kinetics studies. Environ. Earth Sci. 2014, 73, 3239–3247. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Adsorptive decontamination of synthetic wastewater containing crystal violet dye by employing Ter-minalia arjuna sawdust waste. Groundw. Sustain Dev. 2018, 7, 30–38. [Google Scholar] [CrossRef]
- Shoukat, S.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Mango stone biocomposite preparation and application for crystal violet ad-sorption: A mechanistic study. Micropor Mesopor Mater. 2017, 239, 180–189. [Google Scholar] [CrossRef]
- Zamouche, M.; Habib, A.; Saaidia, K.; Lehocine, M.B. Batch mode for adsorption of crystal violet by cedar cone forest waste. SN Appl. Sci. 2020, 2, 198. [Google Scholar] [CrossRef]
- Ali, H.; Muhammad, S.K. Biosorption of Crystal Violet from Water on Leaf Biomass of Calotropis procera. J. Environ. Sci. Technol. 2008, 1, 143–150. [Google Scholar] [CrossRef]
- Wang, X.S.; Liu, X.; Wen, L.; Zhou, Y.; Jiang, Y.; Li, Z. Comparison of Basic Dye Crystal Violet Removal from Aqueous Solution by Low-Cost Biosorbents. Sep. Sci. Technol. 2008, 43, 3712–3731. [Google Scholar] [CrossRef]
- Ahmad, R. Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J. Hazard. Mater. 2009, 171, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Parab, H.; Sudersanan, M.; Shenoy, N.; Pathare, T.; Vaze, B. Use of Agro-Industrial Wastes for Removal of Basic Dyes from Aqueous Solutions. CLEAN - Soil, Air, Water 2009, 37, 963–969. [Google Scholar] [CrossRef]
- Smitha, T.; Thirumalisamy, S.; Manonmani, S. Equilibrium and Kinetics Study of Adsorption of Crystal Violet onto the Peel of Cucumis sativa Fruit from Aqueous Solution. E-Journal Chem. 2012, 9, 1091–1101. [Google Scholar] [CrossRef]
- Das, P.; Chakraborty, S.; Chowdhury, S. Batch and continuous (fixed-bed column) biosorption of crystal violet by Artocarpus heterophyllus (jackfruit) leaf powder. Colloids Surf. B Colloid Surface B 2012, 92, 262–270. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Das, P. Insight into biosorption equilibrium, kinetics and thermodynamics of crystal violet onto Ananas comosus (pineapple) leaf powder. Appl. Water Sci. 2012, 2, 135–141. [Google Scholar] [CrossRef][Green Version]
- Pavan, F.A.; Camacho, E.S.; Lima, E.C.; Dotto, G.L.; Branco, V.T.; Dias, S. Formosa papaya seed powder (FPSP): Preparation, characterization and application as an alternative adsorbent for the removal of crystal violet from aqueous phase. J. Environ. Chem. Eng. 2014, 2, 230–238. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Georgin, J.; Marques, B.S.; Peres, E.C.; Allasia, D.; Dotto, G.L. Biosorption of cationic dyes by Pará chestnut husk (Bertholletia excelsa). Water Sci. Technol. 2018, 77, 1612–1621. [Google Scholar] [CrossRef]
- Ghazali, A.; Shirani, M.; Semnania, A.; Zare-Shahabadic, V.; Nekoeiniad, M. Optimization of crystal violet adsorption onto Date palm leaves as a potent biosorbent from aqueous solutions using response surface methodology and ant colony. J. Environ. Chem. Eng. 2018, 6, 3942–3950. [Google Scholar] [CrossRef]
- Muhammad, U.L.; Zango, Z.U.; Kadir, H.A.; Usman, A. Crystal violet removal from aqueous solution using corn stalk bio-sorbent. Sci. World J. 2019, 14, 133–138. [Google Scholar]
- Keereerak, A.; Chinpa, W. A potential biosorbent from Moringa oleifera pod husk for crystal violet adsorption: Kinetics, iso-therms, thermodynamic and desorption studies. Sci. Asia. 2020, 46, 186–194. [Google Scholar] [CrossRef]
- Loulidi, I.; Boukhlifi, F.; Ouchabi, M.; Amar, A.; Jabri, M.; Kali, A.; Chraibi, S.; Hadey, C.; Aziz, F. Adsorption of Crystal Violet onto an Agricultural Waste Residue: Kinetics, Isotherm, Thermodynamics, and Mechanism of Adsorption. Sci. World, J. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.R.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds. Distribution and Biology; Krieger Pub. Co.: Malabar, FL, USA, 1977; p. 610. [Google Scholar]
- Raman, G.; Park, K.T.; Kim, J.-H.; Park, S. Characteristics of the completed chloroplast genome sequence of Xanthium spinosum: Comparative analyses, identification of mutational hotspots and phylogenetic implications. BMC Genom. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Global Invasive Species Database (GISD) 2021. Species Profile Xanthium Spinosum. Available online: http://www.iucngisd.org/gisd/species.php?sc=1347 (accessed on 23 September 2021).
- Amin, S.; Barkatullah; Khan, H. Pharmacology of Xanthium species. A review. J. Phytopharm. 2016, 5, 126–127. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Angosto, J.M.; Roca, M.J.; Miñarro, M.D. Taguchi design-based enhancement of heavy metals bioremoval by agroindustrial waste biomass from artichoke. Sci. Total. Environ. 2018, 653, 55–63. [Google Scholar] [CrossRef]
- Santra, D.; Joarder, R.; Sarkar, M. Taguchi design and equilibrium modeling for fluoride adsorption on cerium loaded cellulose nanocomposite bead. Carbohydr. Polym. 2014, 111, 813–821. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Rastgordani, M. Optimization of simultaneous removal of binary mixture of indigo carmine and methyl orange dyes by cobalt hydroxide nano-particles through Taguchi method. J. Mol. Liq. 2018, 262, 405–414. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Sanjay, M.R.; Bhat, K.S.; Padmaraj, N.H.; Jawaid, M. Characterization of natural cellulosic fiber frombark of Albizia amara. J. Nat. Fibers 2018, 1–8. [Google Scholar] [CrossRef]
- Tsuboi, M. Infrared spectrum and crystal structure of cellulose. J. Polym. Sci. 1957, 25, 159–171. [Google Scholar] [CrossRef]
- Karimi, S.; Tahir, P.M.; Karimi, A.; Dufresne, A.; Abdulkhani, A. Kenaf bast cellulosic fibers hierarchy: A comprehensive approach from micro to nano. Carbohydr. Polym. 2013, 101, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Labbe, N.; Rials, T.G.; Kelley, S.S.; Cheng, Z.M.; Kim, J.Y.; Li, Y. FT-IR imaging and pyrolysis-molecular beam mass spec-trometry: New tools to investigate wood tissues. Wood Sci. Technol. 2005, 39, 61–76. [Google Scholar] [CrossRef]
- Liang, C.Y.; Marchessault, R.H. Infrared spectra of crystalline polysaccharides. II.Native celluloses in the region from 640 to 1700 cm−1. J. Polym. Sci. 1959, 39, 269–278. [Google Scholar] [CrossRef]
- Pardo, L.M.F.; Córdoba, A.G.; Galán, J.E.L. Characterization of hemicelluloses from leaves and tops of the CC 8475, CC 8592, and V 7151 varieties of sugarcane (Saccharum officinarum L.). DYNA 2019, 86, 98–107. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural Changes of Oak Wood Main Components Caused by Thermal Modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-Ray diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef]
- Blázquez, G.; Martín-Lara, M.; Tenorio, G.; Calero, M. Batch biosorption of lead(II) from aqueous solutions by olive tree pruning waste: Equilibrium, kinetics and thermodynamic study. Chem. Eng. J. 2011, 168, 170–177. [Google Scholar] [CrossRef]
- Putri, K.N.A.; Keereerak, A.; Chinpa, W. Novel cellulose-based biosorbent from lemongrass leaf combined with cellulose acetate for adsorption of crystal violet. Int. J. Biol. Macromol. 2020, 156, 762–772. [Google Scholar] [CrossRef]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A. Artocarpus odoratissimus leaf-based cellulose as adsorbent for removal of methyl violet and crystal violet dyes from aqueous solution. Cellulose 2018, 25, 3037–3049. [Google Scholar] [CrossRef]
- Zehra, T.; Priyantha, N.; Lim, L.B.L. Removal of crystal violet dye from aqueous solution using yeast-treated peat as adsorbent: Thermodynamics, kinetics, and equilibrium studies. Environ. Earth Sci. 2016, 75, 1–15. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Saha, P.D. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; Ma, X. Adsorption characteristics of methylene blue onto low cost biomass material lotus leaf. Chem. Eng. J. 2011, 171, 1–8. [Google Scholar] [CrossRef]
- Pang, J.; Fu, F.; Ding, Z.; Lu, J.; Li, N.; Tang, B. Adsorption behaviors of methylene blue from aqueous solution on mesoporous birnessite. J. Taiwan Inst. Chem. Eng. 2017, 77, 168–176. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Sabaa, M.W.; ElHafeez, E.A.; Mohamed, R.R. Crystal violet dye removal using crosslinked grafted xanthan gum. Int. J. Biol. Macromol. 2019, 137, 1086–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.-Z. Studies of adsorption of crystal violet from aqueous solution by nano mesocellular foam silica: Process equilibrium, kinetic, isotherm, and thermodynamic studies. Water Sci. Technol. 2020, 81. [Google Scholar] [CrossRef]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S. Adsorption of crystal violet onto functionalized multi-walled carbon nanotubes: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2016, 134, 390–397. [Google Scholar] [CrossRef]
- Grassi, P.; Reis, C.; Drumm, F.C.; Georgin, J.; Tonato, D.; Escudero, L.B.; Kuhn, R.; Jahn, S.L.; Dotto, G.L. Biosorption of crystal violet dye using inactive biomass of the fungus Diaporthe schini. Water Sci. Technol. 2019, 79, 709–717. [Google Scholar] [CrossRef]
- Patil, S.R.; Sutar, S.S.; Jadhav, J.P. Sorption of crystal violet from aqueous solution using live roots of Eichhornia crassipes: Kinetic, isotherm, phyto and cyto-genotoxicity studies. Environ. Technol. Innov. 2020, 18, 100648. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Markou, G.; Çelekli, A.; Bozkurt, H. Biosorption of methylene blue onto Arthrospira platensis biomass: Kinetic, equilibrium and thermodynamic studies. J. Environ. Chem. Eng. 2015, 3, 670–680. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Abu Elella, M.H.; Sabaa, M.W.; Saad, G.R. Synthesis of an efficient adsorbent hydrogel based on biodegradable polymers for removing crystal violet dye from aqueous solution. Cellulose 2018, 25, 6513–6529. [Google Scholar] [CrossRef]
- Dotto, G.L.; Salau, N.P.G.; Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A. Adsorption Kinetics in Liquid Phase: Modeling for Discontinuous and Continuous Systems; Springer: Berlin, Germany, 2017; pp. 52–76. [Google Scholar]
- Fabryanty, R.; Valencia, C.; Soetaredjo, F.E.; Putro, J.; Santoso, S.P.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Removal of crystal violet dye by adsorption using bentonite – alginate composite. J. Environ. Chem. Eng. 2017, 5, 5677–5687. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling and Interpretations; Springer: Berlin, Germany, 2017; pp. 19–51. [Google Scholar]
- Hossain, M.A.; al-Hassan, M.T. Kinetic and thermodynamic studies of the adsorption of crystal violet onto used black tea leaves. Orbital: Electron. J. Chem. 2013, 5, 148–156. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Adsorption of Crystal Violet from Wastewater by Modified Bambusa Tulda. KSCE J. Civ. Eng. 2017, 22, 2755–2763. [Google Scholar] [CrossRef]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide inter-calated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surfaces B: Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2012, 181–182, 449–457. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2018, 276, 105–114. [Google Scholar] [CrossRef]

| Factor | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| pH | 2 | 6 | 12 |
| Time (min) | 5 | 20 | 40 |
| Adsorbent dose (mg·L−1) | 0.5 | 2.0 | 3.0 |
| Initial dye concentration (mg·L−1) | 50 | 150 | 250 |
| Temperature (K) | 285 | 294 | 311 |
| Ionic strength (mol L−1) | 0 | 0.10 | 0.25 |
| FTIR Bands | Assignment | Reference |
|---|---|---|
| 3448 cm−1 | O–H stretching | [31,36,38] |
| 2340 cm−1 | O–H bending of adsorbed water | [32] |
| 1630 cm−1 | O–H bending of adsorbed water | [33] |
| 1368 cm−1 | C–H bending | [34,36,37] |
| 1000 cm−1 | C–O stretching | [35] |
| Isotherm Model | Parameters | Value |
|---|---|---|
| Langmuir non-linear | KL (L mg−1) | 0.024 ± 0.001 |
| qmax (mg·g−1) | 164.1 ± 4.23 | |
| R2 | 0.9967 | |
| χ2 | 0.222 | |
| SSE | 11.47 | |
| ARE (%) | 2.57 | |
| Freundlich non-linear | Kf (mg·g−1) | 8.62 ± 1.47 |
| 1/n | 0.60 ± 0.03 | |
| R2 | 0.9828 | |
| χ2 | 1.249 | |
| SSE | 62.12 | |
| ARE (%) | 6.94 |
| Adsorbent | Maximum Adsorption Capacity (mg·g−1) | Reference |
|---|---|---|
| Water hyacinth root powder | 322.58 | [20] |
| Used black tea leaves | 200.00 | [58] |
| Bathurst burr powder | 164.10 | This study |
| Moringa oleifera pod husk | 156.25 | [24] |
| Breadfruit skin | 145.80 | [8] |
| Papaya seeds | 85.99 | [19] |
| Pará chestnut husk | 83.60 | [21] |
| Pineapple leaf powder | 78.22 | [18] |
| Wheat bran | 69.15 | [13] |
| Laminaria japonica | 66.64 | [13] |
| Coir pith | 65.53 | [15] |
| Eragrostis plana nees | 60.10 | [7] |
| Jackfruit leaf powder | 43.39 | [17] |
| Rice bran | 41.68 | [13] |
| Sawdust | 37.83 | [15] |
| Date palm leaves powder | 37.73 | [22] |
| Peel of Cucumis sativa fruit | 34.24 | [16] |
| Coniferous pinus bark powder | 32.78 | [14] |
| Cedar cones | 13.64 | [11] |
| Almond shells | 12.20 | [25] |
| Sugarcane fiber | 10.44 | [15] |
| Corn stalk | 9.64 | [23] |
| Calotropis procera leaf | 4.14 | [12] |
| Kinetic Model | Parameters | Value |
|---|---|---|
| Pseudo-first order non-linear | k1 (min−1) | 0.371 ± 0.021 |
| qe,calc (mg·g−1) | 41.40 ± 0.62 | |
| R2 | 0.9960 | |
| χ2 | 0.138 | |
| SSE | 5.56 | |
| ARE (%) | 16.09 | |
| Pseudo-second order non-linear | k2 (min−1) | 0.019 ± 0.008 |
| qe,calc (mg·g−1) | 43.85 ± 0.21 | |
| R2 | 0.9999 | |
| χ2 | 0.007 | |
| SSE | 0.14 | |
| ARE (%) | 14.59 |
| ΔG (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (J mol−1·K−1) | ||
|---|---|---|---|---|
| 285 K | 294 K | 311 K | ||
| −21.62 | −22.51 | −24.35 | 1.01 | 12.66 |
| Level | pH | Ionic Strength | Adsorbent Dose | Initial Dye Concentration | Time | Temperature |
|---|---|---|---|---|---|---|
| 1 | 27.40 | 33.76 * | 29.14 | 32.97 * | 31.75 | 32.40 |
| 2 | 35.04 | 32.36 | 34.34 | 32.96 | 33.05 | 32.66 |
| 3 | 35.70 * | 32.01 | 34.65 * | 32.19 | 33.33 * | 33.07 * |
| Delta | 8.30 | 1.74 | 5.50 | 0.78 | 1.59 | 0.68 |
| Rank | 1 | 3 | 2 | 5 | 4 | 6 |
| Contribution (%) | 65.00 | 2.60 | 29.23 | 0.62 | 2.19 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosoarca, G.; Vancea, C.; Popa, S.; Boran, S. Bathurst Burr (Xanthium spinosum) Powder—A New Natural Effective Adsorbent for Crystal Violet Dye Removal from Synthetic Wastewaters. Materials 2021, 14, 5861. https://doi.org/10.3390/ma14195861
Mosoarca G, Vancea C, Popa S, Boran S. Bathurst Burr (Xanthium spinosum) Powder—A New Natural Effective Adsorbent for Crystal Violet Dye Removal from Synthetic Wastewaters. Materials. 2021; 14(19):5861. https://doi.org/10.3390/ma14195861
Chicago/Turabian StyleMosoarca, Giannin, Cosmin Vancea, Simona Popa, and Sorina Boran. 2021. "Bathurst Burr (Xanthium spinosum) Powder—A New Natural Effective Adsorbent for Crystal Violet Dye Removal from Synthetic Wastewaters" Materials 14, no. 19: 5861. https://doi.org/10.3390/ma14195861
APA StyleMosoarca, G., Vancea, C., Popa, S., & Boran, S. (2021). Bathurst Burr (Xanthium spinosum) Powder—A New Natural Effective Adsorbent for Crystal Violet Dye Removal from Synthetic Wastewaters. Materials, 14(19), 5861. https://doi.org/10.3390/ma14195861









