Ion Capture and Release Ability of Glass Ionomer Cement Containing Nanoporous Silica Particles with Different Pore and Particle Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GIC-NPS Test Pieces and Controls
2.3. Evaluation of Dye Capture and Release Abilities
2.4. Evaluation of Mechanical Property
3. Results
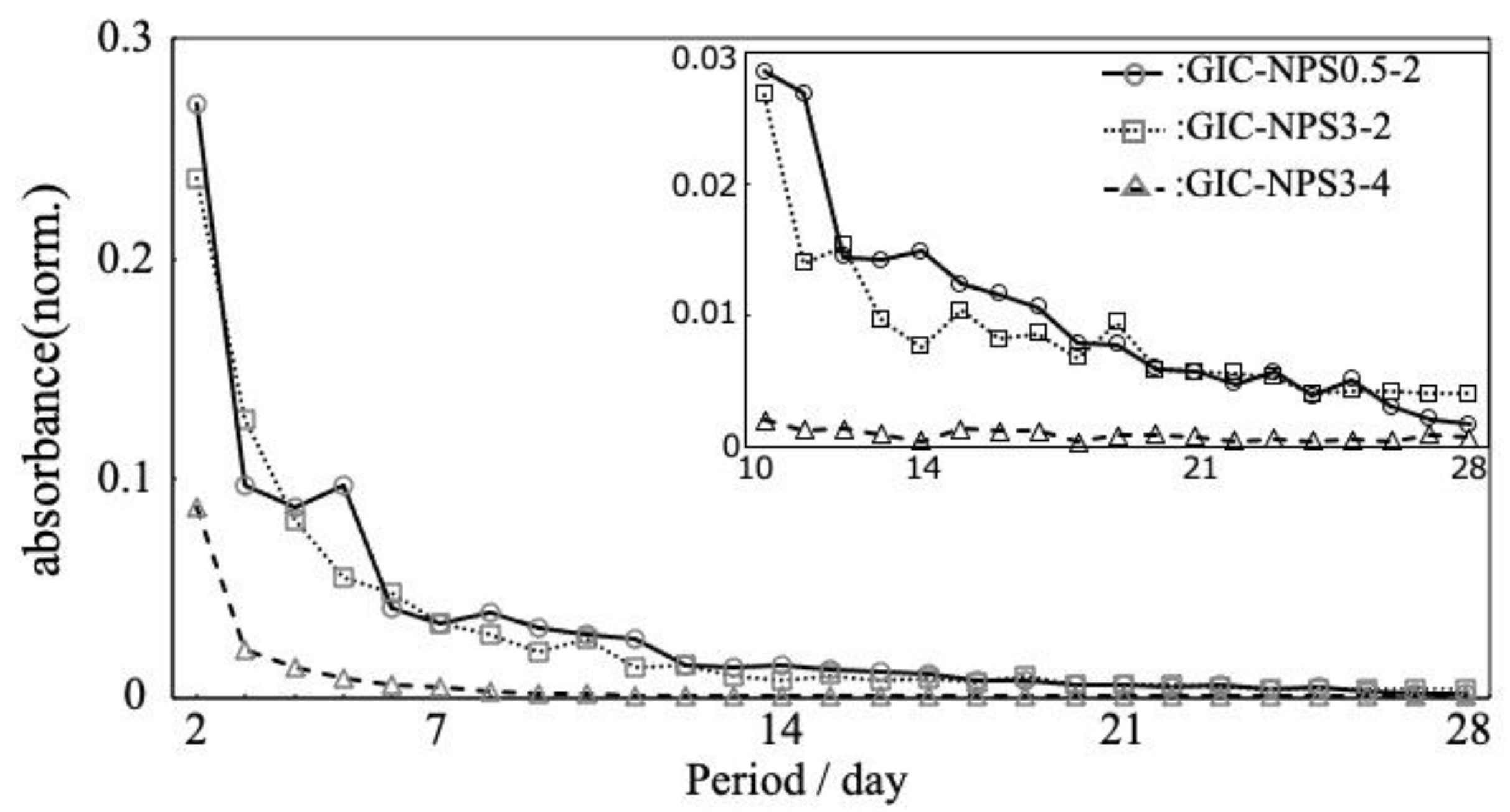
3.1. Dye Capture and Release Properties
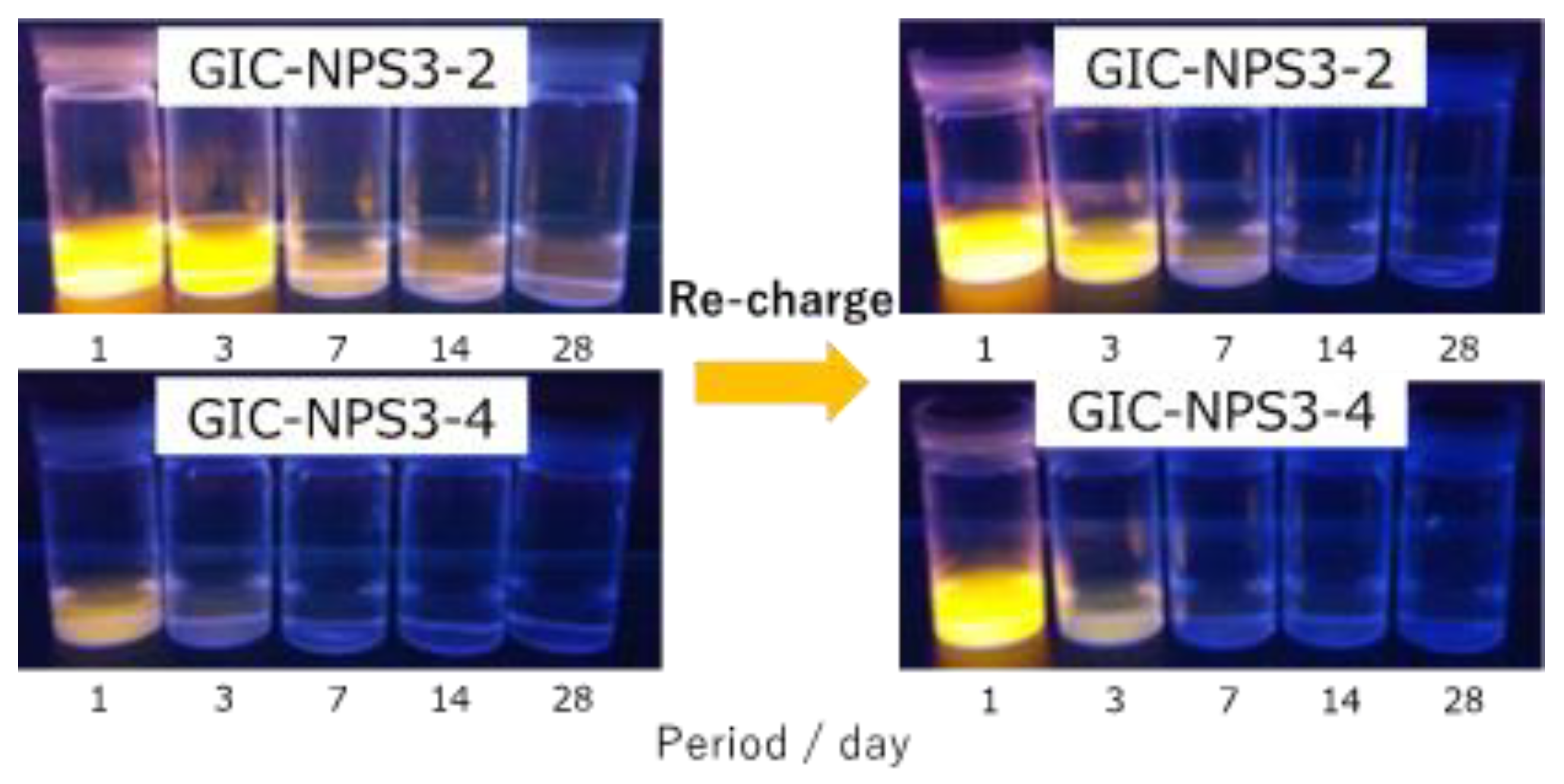
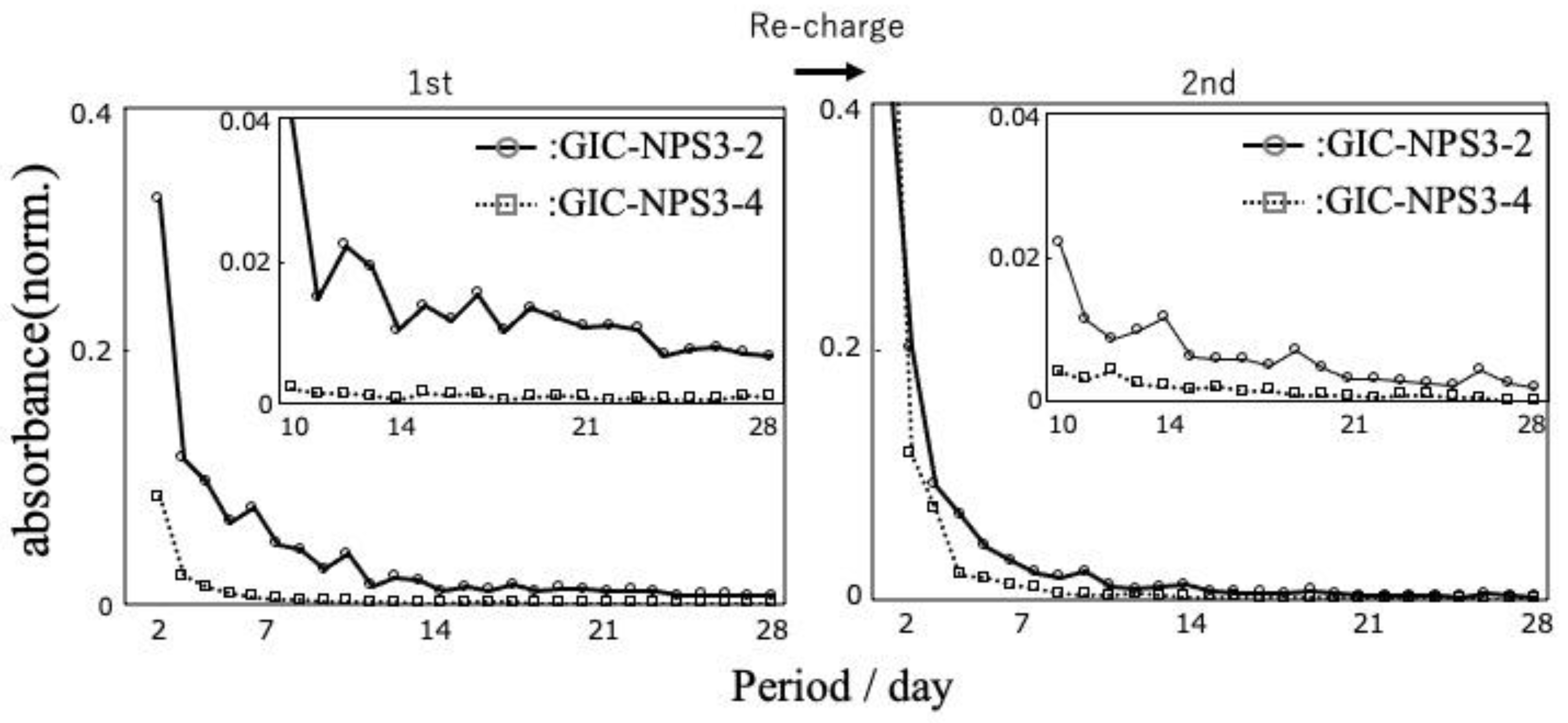
3.2. Recharge Ability of GIC–NPS
3.3. Mechanical Property of GIC–NPS
4. Discussion
4.1. Materials
4.2. Dye Capture and Release Properties
4.3. Mechanical Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Richter, A.E.; Arruda, A.O.; Peters, M.C.; Sohn, W. Incidence of caries lesions among patients treated with comprehensive orthodontics. Am. J. Orthod. Dentofac. Orthop. 2011, 135, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, L.; Geiger, A.M.; Gwinnett, A.J. Incidence of white spot formation after bonding and banding. Am. J. Orthod. 1982, 81, 93–98. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral. Biol. Craniofac. Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khetawat, S.; Lodha, S. Nanotechnology (nanohydroxyapatite crystals): Recent advancement in treatment of dentinal hypersensitivity. J. Interdiscipl. Med. Dent. Sci. 2015, 3, 1000181. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Tao, J.; Xu, X.; Mao, C.; Gu, X.; Tang, R. Repair of enamel by using hydroxyapatite nanoparticles as the builing blocks. J. Mater. Chem. 2008, 18, 4079–4084. [Google Scholar] [CrossRef]
- Chen, M.H. Update on dental nanonanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef]
- Mitra, S.B.; Wu, D.; Holmes, B.N. An application of nanotechnology in advanced dental materials. J. Am. Dent. Assoc. 2003, 134, 1382–1390. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, R.; Sokolov, I. Silica nanoparticles to polish tooth surfaces for caries prevention. J. Dent. Res. 2008, 87, 980–983. [Google Scholar] [CrossRef]
- Fullriede, H.; Abendroth, P.; Ehlert, N.; Doll, K.; Schäske, J.; Winkel, A.; Stumpp, S.N.; Stiesch, M.; Behrens, P. pH-responsive release of chlorhexidine from modified nanoporous silica nanoparticles for dental applications. Bio Nano Mat. 2016, 17, 59–72. [Google Scholar] [CrossRef]
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- He, Q.J.; Shi, J. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Tagaya, M.; Ikoma, T.; Yoshioka, T.; Motozuka, S.; Xu, Z.; Minami, F.; Tanaka, J. Synthesis and luminescence properties of Eu(III)-doped nanoporous silica spheres. J. Colloid Interface Sci. 2011, 363, 456–464. [Google Scholar] [CrossRef]
- Carriazo, D.; Arco, M.D.; Fernández, A.; Martín, C.; Rives, V. Inclusion and release of fenbufen in mesoporous silica. J. Pharm. Sci. 2010, 99, 3372–3380. [Google Scholar] [CrossRef]
- Liong, M.; France, B.; Bradley, K.A.; Zink, J.I. Antimicrobial activity of silver nanocrystals encapsulated in mesoporous silica nanoparticles. Adv. Mater. 2009, 21, 1684–1689. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Vallet-Regí, M.; Kupferschmidt, N.; Terasaki, O.; Schmidtchen, A.; Malmsten, M. Incorporation of antimicrobial compounds in mesoporous silica film monolith. Biomaterials 2009, 30, 5729–5736. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial Dental Composites with Chlorhexidine and Mesoporous Silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef]
- Nakamura, K.; Abe, S.; Minamikawa, H.; Yawaka, Y. Calcium Charge and Release of Conventional Glass-Ionomer Cement Containing Nanoporous Silica. Materials 2018, 11, 1295. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Yang, H.; Li, K.; Yu, J.; Huang, C. Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement. Molecules 2017, 22, 1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bando, Y.; Nakanishi, K.; Abe, S.; Yamagata, S.; Yoshida, Y.; Iida, J. Electric Charge Dependence of Controlled Dye-Release Behavior in Glass Ionomer Cement Containing Nano-Porous Silica Particles. J. Nanosci. Nanotechnol. 2018, 18, 75–79. [Google Scholar] [PubMed]
- GC Europe Fuji1. Available online: https://europe.gc.dental/en-BH/products/fuji1?language_content_entity=en-BH (accessed on 16 September 2021).
- Wilhelm, P.; Stephan, D. Photodegradation of rhodamine B in aqueous solution via SiO2@TiO2 nano-spheres. J. Photochem. Photobiol. A 2007, 185, 19–25. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. Prevention and Reversal of Dental Caries: Role of Low Level Fluoride. Community Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef]
- Ten Cate, J.M.; Featherstone, J.D. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit. Rev. Oral Biol. 1991, 2, 283–296. [Google Scholar] [CrossRef]
- Featherstone, J.D.B.; Glena, R.; Shariati, M.; Shields, C.P. Dependence of in vitro demineralization of apatite and remineralization of dental enamel on fluoride concentration. J. Dent. Res. 1990, 69, 620–625. [Google Scholar] [CrossRef]
- Whitford, G.M.; Schuster, G.S.; Pashley, D.H.; Ven Kateswarlu, P. Fluoride Uptake by Streptococcus mutans 6715. Infect. Immun. 1977, 18, 660–687. [Google Scholar] [CrossRef] [Green Version]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry, 1st ed.; Wiley: New York, NY, USA, 1979; pp. 375–378. [Google Scholar]
- Xu, T.; He, Y.; Qin, Y.; Zhao, C.; Peng, C.; Hu, J.; Liu, H. Facile preparation of porous organic copolymer based on triptycene and crown ether for efficient organic dye adsorption. RSC Adv. 2018, 8, 4963–4968. [Google Scholar] [CrossRef] [Green Version]
- Alataxi, R.A.S.; Elsayed, N.H.; Mohamed, W.S. Influence of hydroxyapatite nanoparticles on the properties of glass ionomer cement. J. Mater. Res. Technol. 2019, 8, 344–349. [Google Scholar] [CrossRef]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Alobiedy, A.N.; Alhille, A.H.; Al-Hamaoy, A.R. Mechanical Properties Enhancement of Conventional Glass Ionomer Cement by Adding Zirconium Oxide Micro and Nanoparticles. J. Eng. 2019, 25, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Okabe, S.; Iida, T. Effects of particle shape, size and interfacial adhesion on the fracture strength of silica-filled epoxy resin. Polym. Polym. Compos. 1999, 7, 177–186. [Google Scholar]
- Mackay, M.E.; Tuteja, A.; Duxbury, P.M.; Hawker, C.J.; Van Horn, B.; Guan, Z.; Chen, G.; Krishnan, R.S. General Strategies for Nanoparticle Dispersion. Science 2006, 311, 1740–1743. [Google Scholar] [CrossRef]
- Johnsen, B.B.; Kinloch, A.J.; Mohammed, R.D.; Taylor, A.C.; Sprenger, S. Toughening mechanisms of nanoparticle-modified epoxy polymers. Polymer 2007, 48, 530–541. [Google Scholar] [CrossRef]
- Witten, T.A.; Rubinstein, M.; Colby, R.H. Reinforcement of rubber by fractal aggregates. J. Phys. II Fr. 1993, 3, 367–383. [Google Scholar] [CrossRef]
- Jancar, J.; Douglas, J.F.; Starr, F.W.; Kumar, S.K.; Cassagnau, P.; Lesser, A.J.; Sternstein, S.S.; Buehler, M.J. Current issues in research on structureeproperty relationships in polymer nanocomposites. Polymer 2010, 51, 3321–3343. [Google Scholar] [CrossRef]



| Diameter /mm | Pore Size /mm | Surface Area /m2·g−2 | |
|---|---|---|---|
| NPS3-4 | 3 | 4 | 300–400 |
| NPS3-2 | 3 | 2 | 900–1000 |
| NPS0.5-2 | 0.5 | 2 | 900–1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, R.; Nakanishi, K.; Bando, Y.; Abe, S.; Maruoka, H.; Nakamura, M.; Akasaka, T.; Yoshida, Y.; Sato, Y. Ion Capture and Release Ability of Glass Ionomer Cement Containing Nanoporous Silica Particles with Different Pore and Particle Size. Materials 2021, 14, 5742. https://doi.org/10.3390/ma14195742
Endo R, Nakanishi K, Bando Y, Abe S, Maruoka H, Nakamura M, Akasaka T, Yoshida Y, Sato Y. Ion Capture and Release Ability of Glass Ionomer Cement Containing Nanoporous Silica Particles with Different Pore and Particle Size. Materials. 2021; 14(19):5742. https://doi.org/10.3390/ma14195742
Chicago/Turabian StyleEndo, Ryoshun, Ko Nakanishi, Yosuke Bando, Shigeaki Abe, Haruhi Maruoka, Mariko Nakamura, Tsukasa Akasaka, Yasuhiro Yoshida, and Yoshiaki Sato. 2021. "Ion Capture and Release Ability of Glass Ionomer Cement Containing Nanoporous Silica Particles with Different Pore and Particle Size" Materials 14, no. 19: 5742. https://doi.org/10.3390/ma14195742
APA StyleEndo, R., Nakanishi, K., Bando, Y., Abe, S., Maruoka, H., Nakamura, M., Akasaka, T., Yoshida, Y., & Sato, Y. (2021). Ion Capture and Release Ability of Glass Ionomer Cement Containing Nanoporous Silica Particles with Different Pore and Particle Size. Materials, 14(19), 5742. https://doi.org/10.3390/ma14195742








