Borosilicate Glass-Ceramics Containing Zirconolite and Powellite for RE- and Mo-Rich Nuclear Waste Immobilization
Abstract
1. Introduction
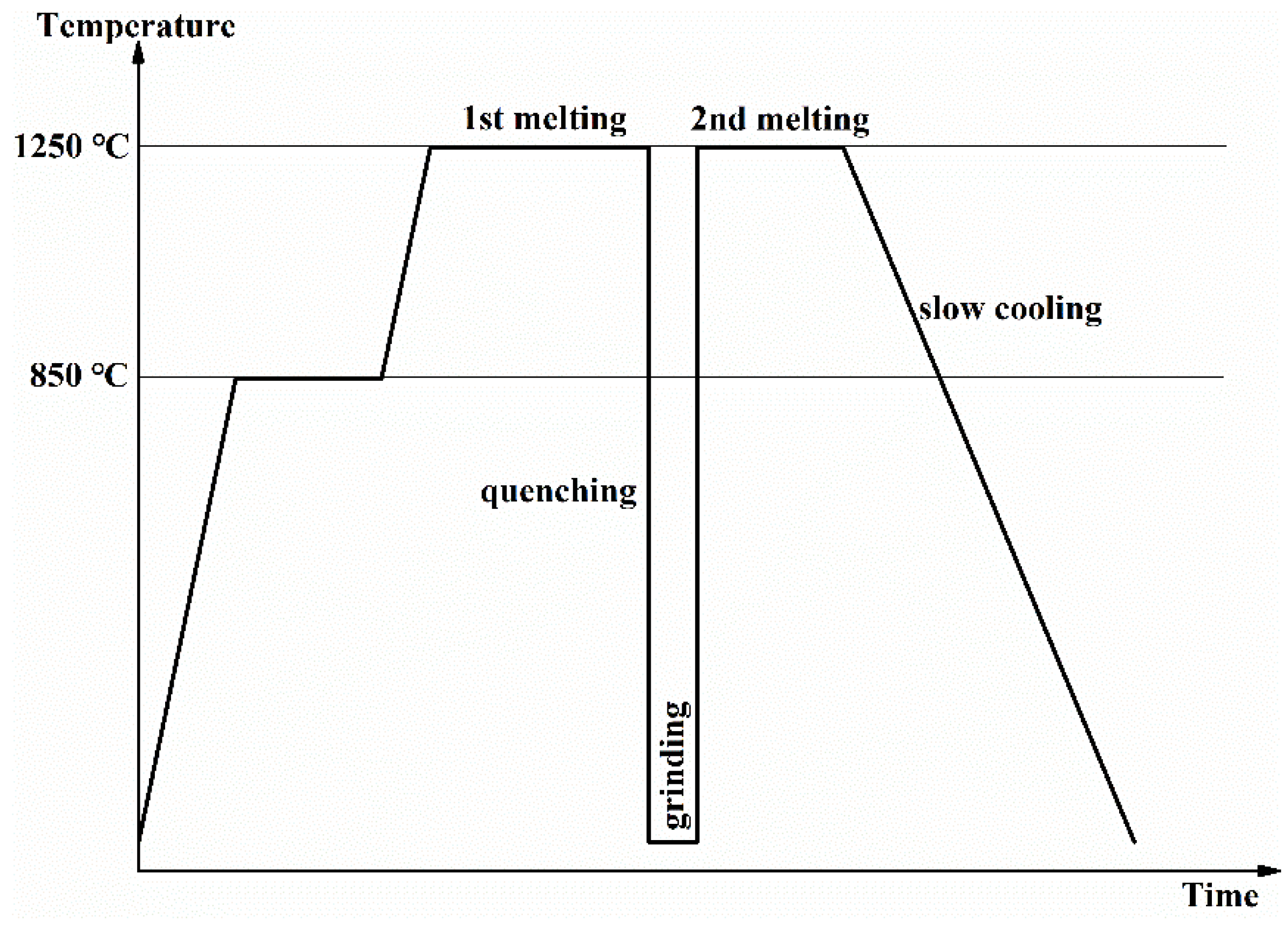
2. Experimental Procedures
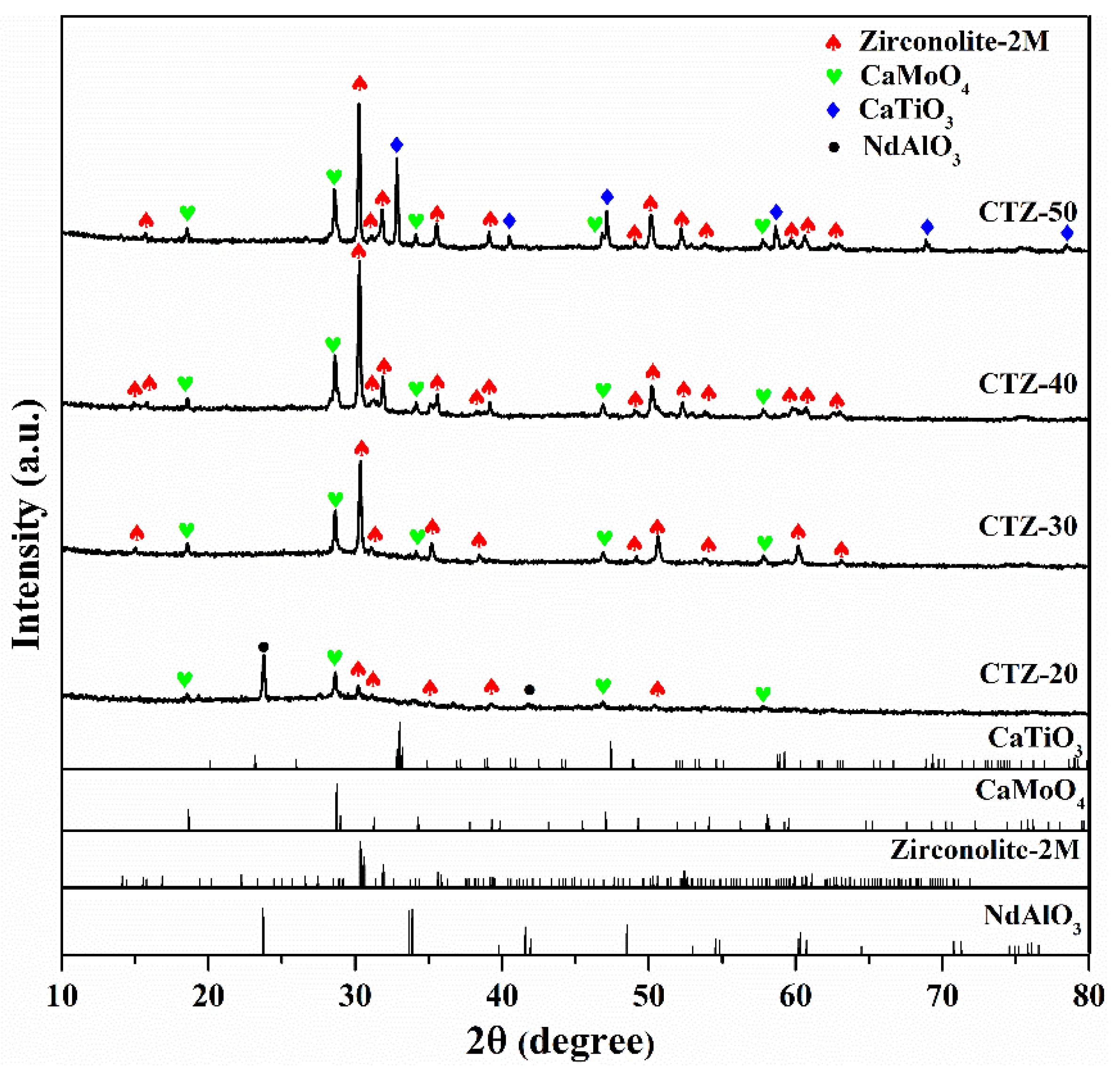
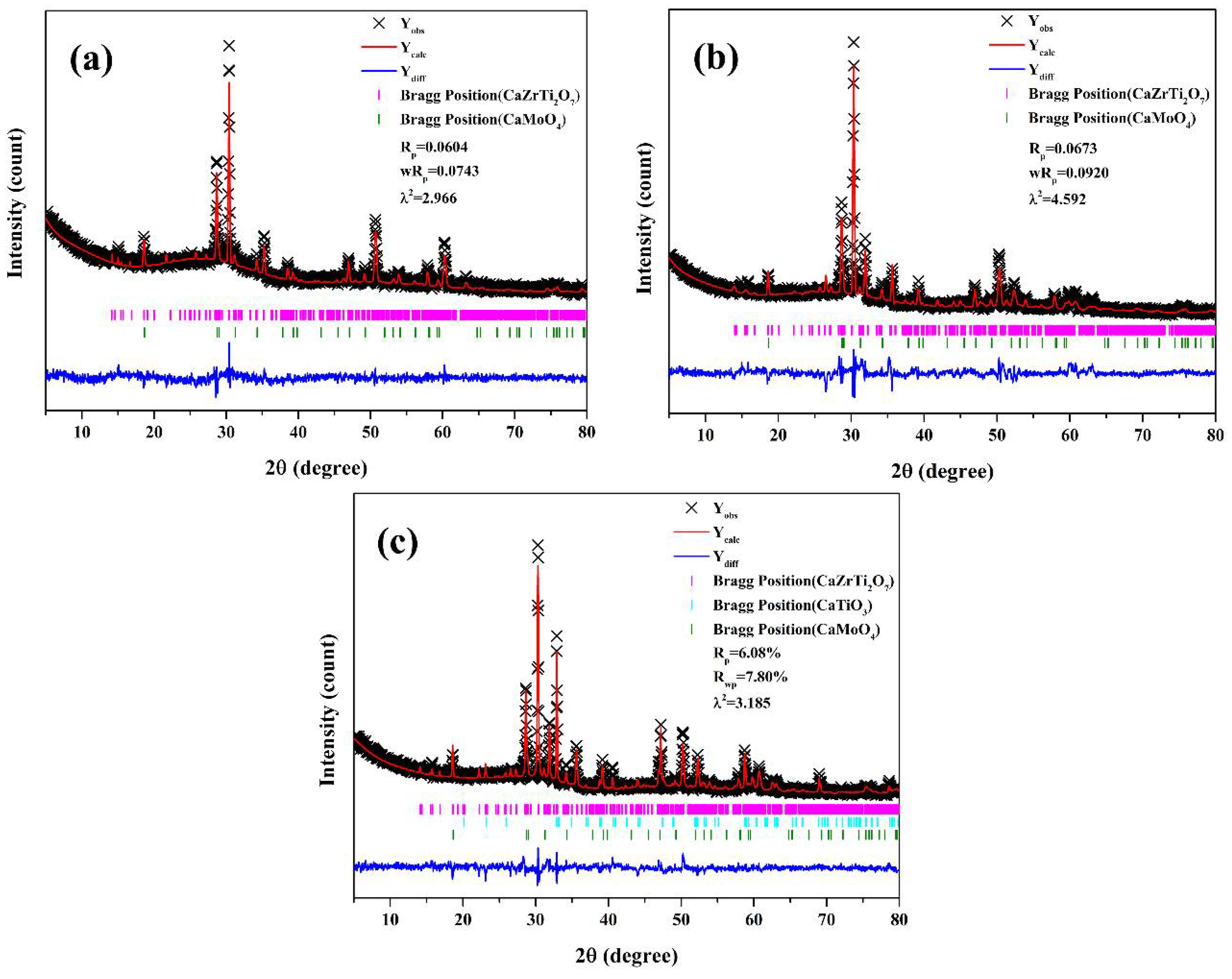
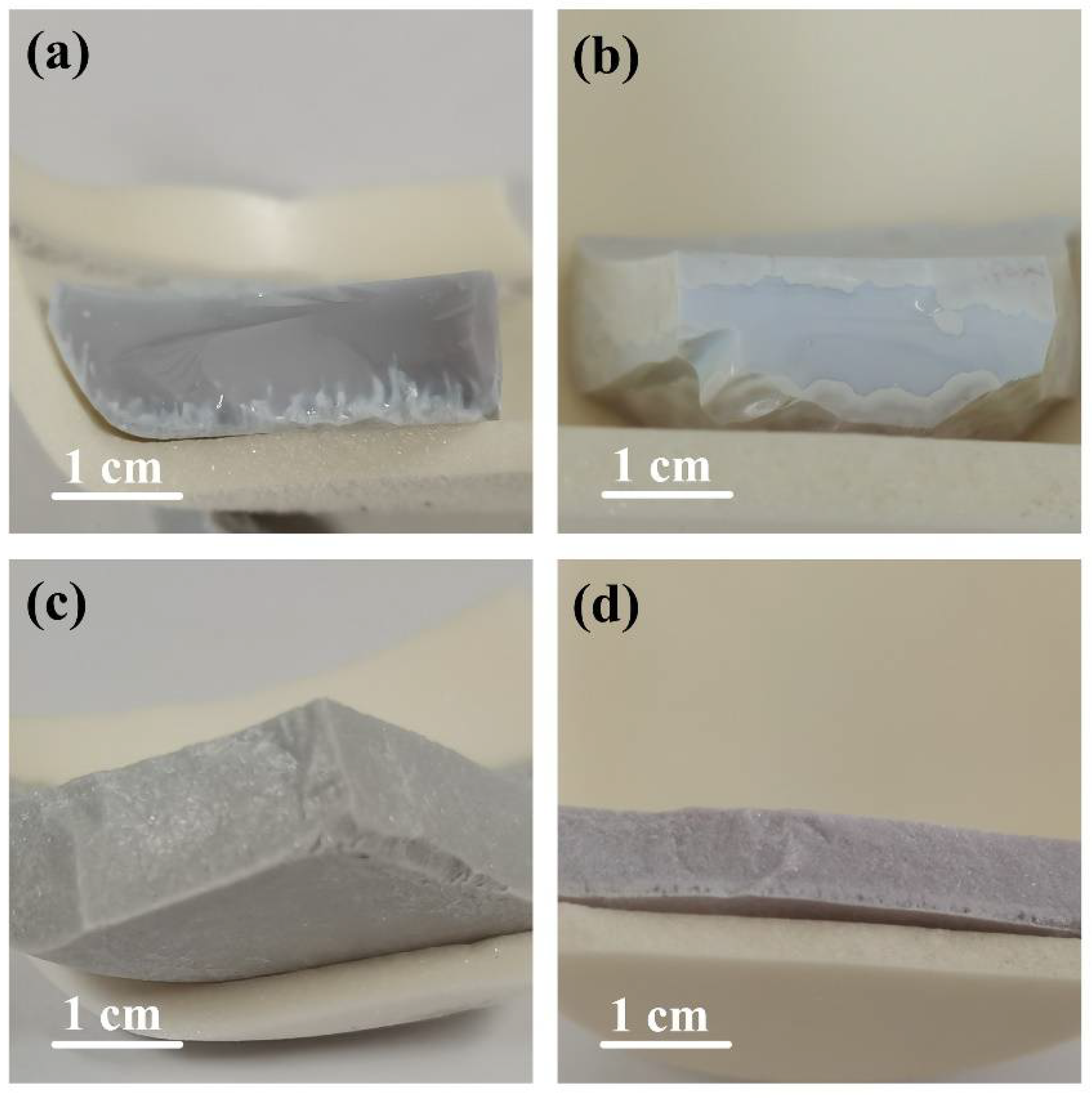
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crum, J.V.; Turo, L.; Riley, B.; Tang, M.; Kossoy, A. Multi-Phase Glass-Ceramics as a Waste Form for Combined Fission Products: Alkalis, Alkaline Earths, Lanthanides, and Transition Metals. J. Am. Ceram. Soc. 2012, 95, 1297–1303. [Google Scholar] [CrossRef]
- Caurant, D.; Majérus, O.; Loiseau, P.; Bardez, I.; Baffier, N.; Dussossoy, J. Crystallization of neodymium-rich phases in silicate glasses developed for nuclear waste immobilization. J. Nucl. Mater. 2006, 354, 143–162. [Google Scholar] [CrossRef]
- Laura, R.-P.; Soria, B.Y.M. A Review of the Nuclear Fuel Cycle Strategies and the Spent Nuclear Fuel Management Technologies. Energies 2017, 10, 1235. [Google Scholar]
- Goel, A.; McCloy, J.S.; Pokorny, R.; Kruger, A.A. Challenges with vitrification of Hanford High-Level Waste (HLW) to borosilicate glass—An overview. J. Non-Cryst. Solids X 2019, 4, 100033. [Google Scholar] [CrossRef]
- Goel, A.; McCloy, J.S.; Fox, K.M.; Leslie, C.J.; Riley, B.J.; Rodriguez, C.P.; Schweiger, M.J. Structural analysis of some sodium and alumina rich high-level nuclear waste glasses. J. Non-Cryst. Solids 2011, 358, 674–679. [Google Scholar] [CrossRef]
- McCloy, J.S.; Marcial, J.; Patil, D.; Saleh, M.; Ahmadzadeh, M.; Chen, H.; Crum, J.V.; Riley, B.J.; Kamat, H.; Bréhault, A.; et al. Glass structure and crystallization in boro-alumino-silicate glasses containing rare earth and transition metal cations: A US-UK collaborative program. MRS Adv. 2019, 4, 1029–1043. [Google Scholar] [CrossRef]
- Walling, S.A.; Kauffmann, M.N.; Gardner, L.J.; Bailey, D.J.; Stennett, M.C.; Corkhill, C.L.; Hyatt, N.C. Characterisation and disposability assessment of multi-waste stream in-container vitrified products for higher activity radioactive waste. J. Hazard. Mater. 2020, 401, 123764. [Google Scholar] [CrossRef] [PubMed]
- McKeown, D.A.; Gan, H.; Pegg, I.L. X-ray absorption and Raman spectroscopy studies of molybdenum environments in borosilicate waste glasses. J. Nucl. Mater. 2017, 488, 143–149. [Google Scholar] [CrossRef]
- Taurines, T.; Boizot, B. Synthesis of powellite-rich glasses for high level waste immobilization. J. Non-Cryst. Solids 2011, 357, 2723–2725. [Google Scholar] [CrossRef]
- Crum, J.V.; Neeway, J.J.; Riley, B.; Zhu, Z.; Olszta, M.J.; Tang, M. Dilute condition corrosion behavior of glass-ceramic waste form. J. Nucl. Mater. 2016, 482, 1–11. [Google Scholar] [CrossRef]
- Kossoy, A.; Schulze, R.; Tang, M.; Safarik, D.; McCabe, R. Nd–Mo-borosilicate glass–ceramic: Synthesis, characterization and response to ionizing radiation. J. Nucl. Mater. 2013, 437, 216–221. [Google Scholar] [CrossRef]
- Caurant, D.; Majérus, O.; Fadel, E.; Quintas, A.; Gervais, C.; Charpentier, T.; Neuville, D. Structural investigations of borosilicate glasses containing MoO3 by MAS NMR and Raman spectroscopies. J. Nucl. Mater. 2010, 396, 94–101. [Google Scholar] [CrossRef]
- Patil, D.; Konale, M.; Gabel, M.; Neill, O.K.; Crum, J.V.; Goel, A.; Stennett, M.C.; Hyatt, N.C.; McCloy, J.S. Impact of rare earth ion size on the phase evolution of MoO3-containing aluminoborosilicate glass-ceramics. J. Nucl. Mater. 2018, 510, 539–550. [Google Scholar] [CrossRef]
- Boué, E.; Schuller, S.; Toplis, M.; Charpentier, T.; Mesbah, A.; Pablo, H.; Monnereau, M.; Moskura, M. Kinetic and thermodynamic factors controlling the dissolution of molybdate-bearing calcines during nuclear glass synthesis. J. Nucl. Mater. 2019, 519, 74–87. [Google Scholar] [CrossRef]
- Chouard, N.; Caurant, D.; Majérus, O.; Dussossoy, J.-L.; Ledieu, A.; Peuget, S.; Baddour-Hadjean, R.; Pereira-Ramos, J.-P. Effect of neodymium oxide on the solubility of MoO3 in an aluminoborosilicate glass. J. Non-Cryst. Solids 2011, 357, 2752–2762. [Google Scholar] [CrossRef]
- Chouard, N.; Caurant, D.; Majérus, O.; Guezi-Hasni, N.; Dussossoy, J.L.; Baddour-Hadjean, R.; Pereira-Ramos, J.P. Thermal stability of SiO2-B2O3-Al2O3-Na2O-CaO glasses with high Nd2O3 and MoO3 concentrations. J. Alloy. Compd. 2016, 671, 84–99. [Google Scholar] [CrossRef]
- Caurant, D.; Majerus, O.; Fadel, E.; Lenoir, M.; Gervais, C.; Pinet, O. Effect of molybdenum on the structure and on the crystallization of SiO2-Na2O-CaO-B2O3 glasses. J. Am. Ceram. Soc. 2007, 90, 774–783. [Google Scholar] [CrossRef]
- Nicoleau, E.; Schuller, S.; Angeli, F.; Charpentier, T.; Jollivet, P.; Le Gac, A.; Fournier, M.; Mesbah, A.; Vasconcelos, F. Phase separation and crystallization effects on the structure and durability of molybdenum borosilicate glass. J. Non-Cryst. Solids 2015, 427, 120–133. [Google Scholar] [CrossRef]
- Pinet, O.; Dussossoy, J.; David, C.; Fillet, C. Glass matrices for immobilizing nuclear waste containing molybdenum and phosphorus. J. Nucl. Mater. 2008, 377, 307–312. [Google Scholar] [CrossRef]
- Brehault, A.; Patil, D.; Kamat, H.; Youngman, R.E.; Thirion, L.M.; Mauro, J.C.; Corkhill, C.L.; McCloy, J.S.; Goel, A. Compositional Dependence of Solubility/Retention of Molybdenum Oxides in Aluminoborosilicate-Based Model Nuclear Waste Glasses. J. Phys. Chem. B 2018, 122, 1714–1729. [Google Scholar] [CrossRef]
- Peterson, J.A.; Crum, J.V.; Riley, B.J.; Asmussen, R.M.; Neeway, J.J. Synthesis and characterization of oxyapatite [Ca2Nd8(SiO4)6O2] and mixed-alkaline-earth powellite [(Ca,Sr,Ba)MoO4] for a glass-ceramic waste form. J. Nucl. Mater. 2018, 510, 623–634. [Google Scholar] [CrossRef]
- Neeway, J.J.; Asmussen, R.M.; McElroy, E.M.; Peterson, J.A.; Riley, B.J.; Crum, J.V. Kinetics of oxyapatite [Ca2Nd8(SiO4)6O2] and powellite [(Ca,Sr,Ba)MoO4] dissolution in glass-ceramic nuclear waste forms in acidic, neutral, and alkaline conditions. J. Nucl. Mater. 2019, 515, 227–237. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Neeway, J.J.; Kaspar, T.C.; Crum, J.V. Corrosion Behavior and Microstructure Influence of Glass-Ceramic Nuclear Waste Forms. Corrosion 2017, 73, 1306–1319. [Google Scholar] [CrossRef]
- McCloy, J.S.; Goel, A. Glass-ceramics for nuclear-waste immobilization. MRS Bull. 2017, 42, 233–240. [Google Scholar] [CrossRef]
- Xu, A.; Wei, T.; Gregg, D.J.; Vance, E.R.; Zhang, Y.; Lumpkin, G.R. Micro-compression testing of gold ion irradiated zirconolite glass-ceramics as nuclear waste forms. J. Nucl. Mater. 2019, 527, 151813. [Google Scholar] [CrossRef]
- Gupta, M.; Kulriya, P.K.; Shukla, R.; Dhaka, R.S.; Kumar, R.; Ghumman, S.S. Reduction and structural modification of zirconolite on He+ ion irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2016, 379, 119–125. [Google Scholar] [CrossRef]
- Blackburn, L.R.; Sun, S.; Gardner, L.J.; Maddrell, E.R.; Stennett, M.C.; Hyatt, N.C. A systematic investigation of the phase assemblage and microstructure of the zirconolite CaZr1-xCexTi2O7 system. J. Nucl. Mater. 2020, 535, 152137. [Google Scholar] [CrossRef]
- Blackburn, L.R.; Bailey, D.J.; Sun, S.-K.; Gardner, L.J.; Stennett, M.C.; Corkhill, C.L.; Hyatt, N.C. Review of zirconolite crystal chemistry and aqueous durability. Adv. Appl. Ceram. 2021, 120, 69–83. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Teng, Y.; Meng, G. Preparation and characterization of borosilicate glass-ceramics containing zirconolite and titanite crystalline phases. J. Non-Cryst. Solids 2013, 380, 123–127. [Google Scholar] [CrossRef]
- Loiseau, P.; Caurant, D. Glass-ceramic nuclear waste forms obtained by crystallization of SiO2-Al2O3-CaO-ZrO2-TiO2 glasses containing lanthanides (Ce, Nd, Eu, Gd, Yb) and actinides (Th): Study of the crystallization from the surface. J. Nuclear Mater. 2010, 402, 38–54. [Google Scholar] [CrossRef]
- Lv, P.; Chen, L.; Zhang, B.; Zhang, D.; Yuan, W.; Duan, B.; Guan, Y.; Pan, C.; Chen, Z.; Zhang, L.; et al. The effects of temperature and Ce-dopant concentration on the synthesis of zirconolite glass-ceramic. Ceram. Int. 2019, 45, 11819–11825. [Google Scholar] [CrossRef]
- Li, H.; Wu, L.; Xu, D.; Wang, X.; Teng, Y.; Li, Y. Structure and chemical durability of barium borosilicate glass–ceramics containing zirconolite and titanite crystalline phases. J. Nucl. Mater. 2015, 466, 484–490. [Google Scholar] [CrossRef]
- Zhang, K.; Yin, D.; Peng, L.; Wu, J. Self-propagating synthesis and CeO 2 immobilization of zirconolite-rich composites using CuO as the oxidant. Ceram. Int. 2017, 43, 1415–1423. [Google Scholar] [CrossRef]
- ASTM C1285-14, Standard Test Methods for Determining Chemical Durability of Nuclear, Hazardous, and Mixed Waste Glasses and Multiphase Glass Ceramics: The Product Consistency Test (PCT); ASTM: West Conshohocken, PA, USA, 2014.
- Zhao, Z.; Chen, H.; Xiang, H.; Dai, F.-Z.; Wang, X.; Xu, W.; Sun, K.; Peng, Z.; Zhou, Y. High-entropy (Y0.2Nd0.2Sm0.2Eu0.2Er0.2)AlO3: A promising thermal/environmental barrier material for oxide/oxide composites. J. Mater. Sci. Technol. 2020, 47, 45–51. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Li, H.; Xiao, J.; Cai, X.; Teng, Y. Preparation and characterization of SO3-doped barium borosilicate glass-ceramics containing zirconolite and barite phases. Ceram. Int. 2017, 43, 534–539. [Google Scholar] [CrossRef]
- Rossell, H.J. Zirconolite—a fluorite-related superstructure. Nature 1980, 283, 282–283. [Google Scholar] [CrossRef]
- Gürmen, E.; Daniels, E.; King, J.S. Crystal Structure Refinement of SrMoO4, SrWO4, CaMoO4, and BaWO4 by Neutron Diffraction. J. Chem. Phys. 1971, 55, 1093–1097. [Google Scholar] [CrossRef]
- Beran, A.; Libowitzky, E.; Armbruster, T. A single-crystal infrared spectroscopic and X-ray diffraction study of untwinned San Benito perovskite containing O-H groups. Can. Mineral. 1996, 34, 803–809. [Google Scholar]
- Loiseau, P.; Caurant, D.; Baffier, N.; Mazerolles, L.; Fillet, C. Glass–ceramic nuclear waste forms obtained from SiO2–Al2O3–CaO–ZrO2–TiO2 glasses containing lanthanides (Ce, Nd, Eu, Gd, Yb) and actinides (Th): Study of internal crystallization. J. Nucl. Mater. 2004, 335, 14. [Google Scholar] [CrossRef]
- Begg, B.D.; Vance, E.R. The incorporation of cerium in zirconolite. Mater. Res. Soc. 1997, 465, 333–340. [Google Scholar] [CrossRef]
- Vance, E.; Ball, C.; Day, R.; Smith, K.; Blackford, M.; Begg, B.; Angel, P. Actinide and rare earth incorporation into zirconolite. J. Alloy. Compd. 1994, 213-214, 406–409. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.; Wang, X.; Xiao, J.; Teng, Y.; Li, Y. Effects of Nd Content on Structure and Chemical Durability of Zirconolite-Barium Borosilicate Glass-Ceramics. J. Am. Ceram. Soc. 2016, 99, 4093–4099. [Google Scholar] [CrossRef]
- Liao, C.-Z.; Shih, K.; Lee, W.E. Crystal Structures of Al–Nd Codoped Zirconolite Derived from Glass Matrix and Powder Sintering. Inorg. Chem. 2015, 54, 7353–7361. [Google Scholar] [CrossRef]
- Patel, K.B.; Peuget, S.; Schuller, S.; Lampronti, G.I.; Facq, S.P.; Grygiel, C.; Monnet, I.; Farnan, I. Discovery of a maximum damage structure for Xe-irradiated borosilicate glass ceramics containing powellite. J. Nucl. Mater. 2018, 510, 229–242. [Google Scholar] [CrossRef]
- Brinkman, K.; Fox, K.; Marra, J.; Reppert, J.; Crum, J.; Tang, M. Single phase melt processed powellite (Ba,Ca)MoO4 for the immobilization of Mo-rich nuclear waste. J. Alloy. Compd. 2013, 551, 136–142. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, F.; Liao, Q.; Wang, Y.; Zhu, Y. Effect of CeO2 and Nd2O3 on phases, microstructure and aqueous chemical durability of borosilicate glass-ceramics for nuclear waste immobilization. Mater. Chem. Phys. 2020, 249, 122936. [Google Scholar] [CrossRef]
- Bunker, B.C.; Arnold, G.W.; Day, D.E.; Bray, P.J. The effect of molecular structure on borosilicate glass leaching. J. Non-Cryst. Solids 1986, 87, 226–253. [Google Scholar] [CrossRef]
- Crawford, C.; Marra, J.; Bibler, N. Glass fabrication and product consistency testing of lanthanide borosilicate glass for plutonium disposition. J. Alloy. Compd. 2007, 444-445, 569–579. [Google Scholar] [CrossRef][Green Version]
- Martin, C.; Ribet, I.; Frugier, P.; Gin, S. Alteration kinetics of the glass-ceramic zirconolite and role of the alteration film—Comparison with the SON68 glass. J. Nucl. Mater. 2007, 366, 277–287. [Google Scholar] [CrossRef]
- Rebiscoul, D.; Van der Lee, A.; Rieutord, F.; Né, F.; Spalla, O.; El-Mansouri, A.; Frugier, P.; Ayral, A.; Gin, S. Morphological evolution of alteration layers formed during nuclear glass alteration: New evidence of a gel as a diffusive barrier. J. Nucl. Mater. 2004, 326, 9–18. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.; Li, H.; Teng, Y.; Peng, L. The effects of sulfate content on crystalline phase, microstructure, and chemical durability of zirconolite-barium borosilicate glass-ceramics. J. Nucl. Mater. 2016, 478, 303–309. [Google Scholar] [CrossRef]


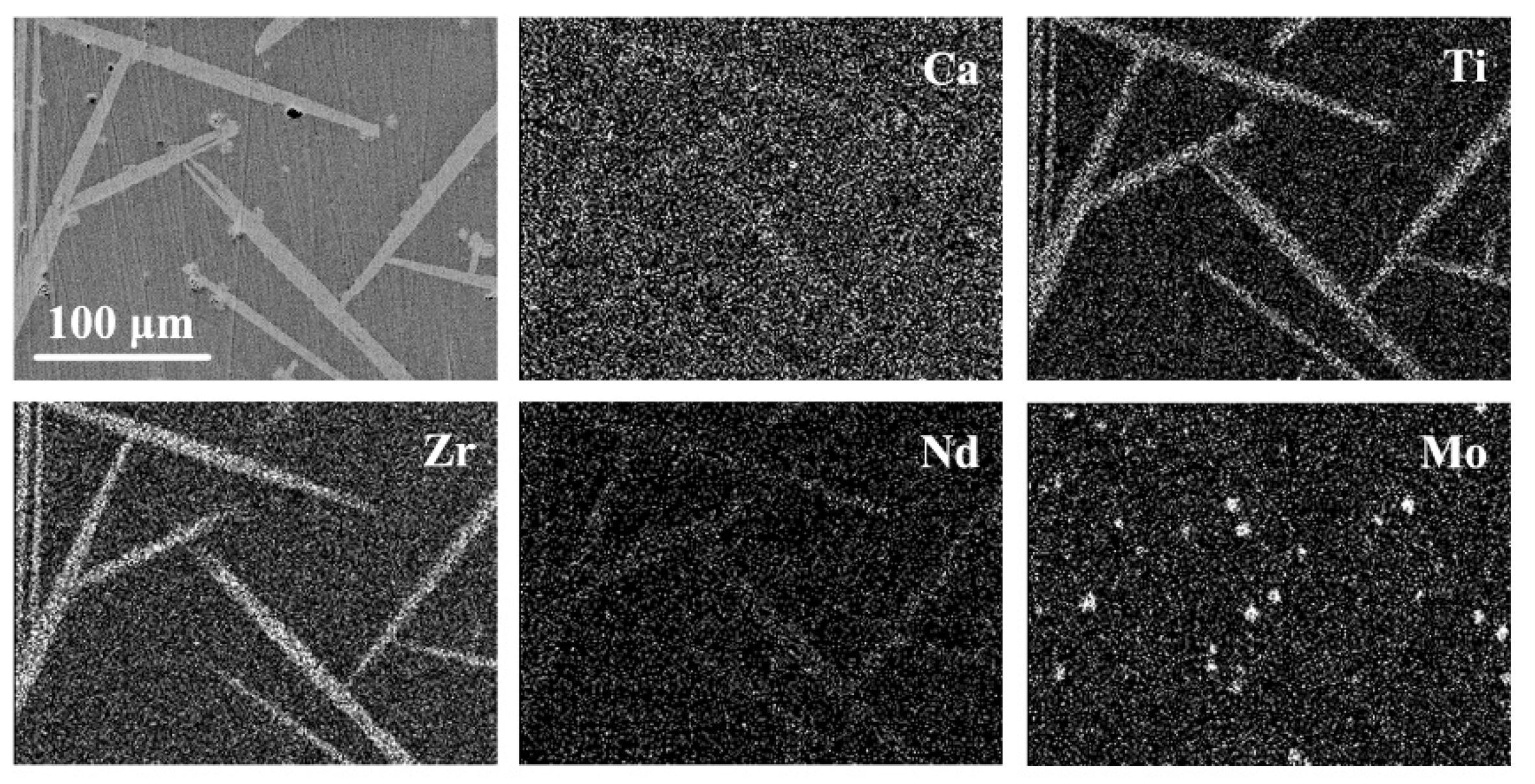
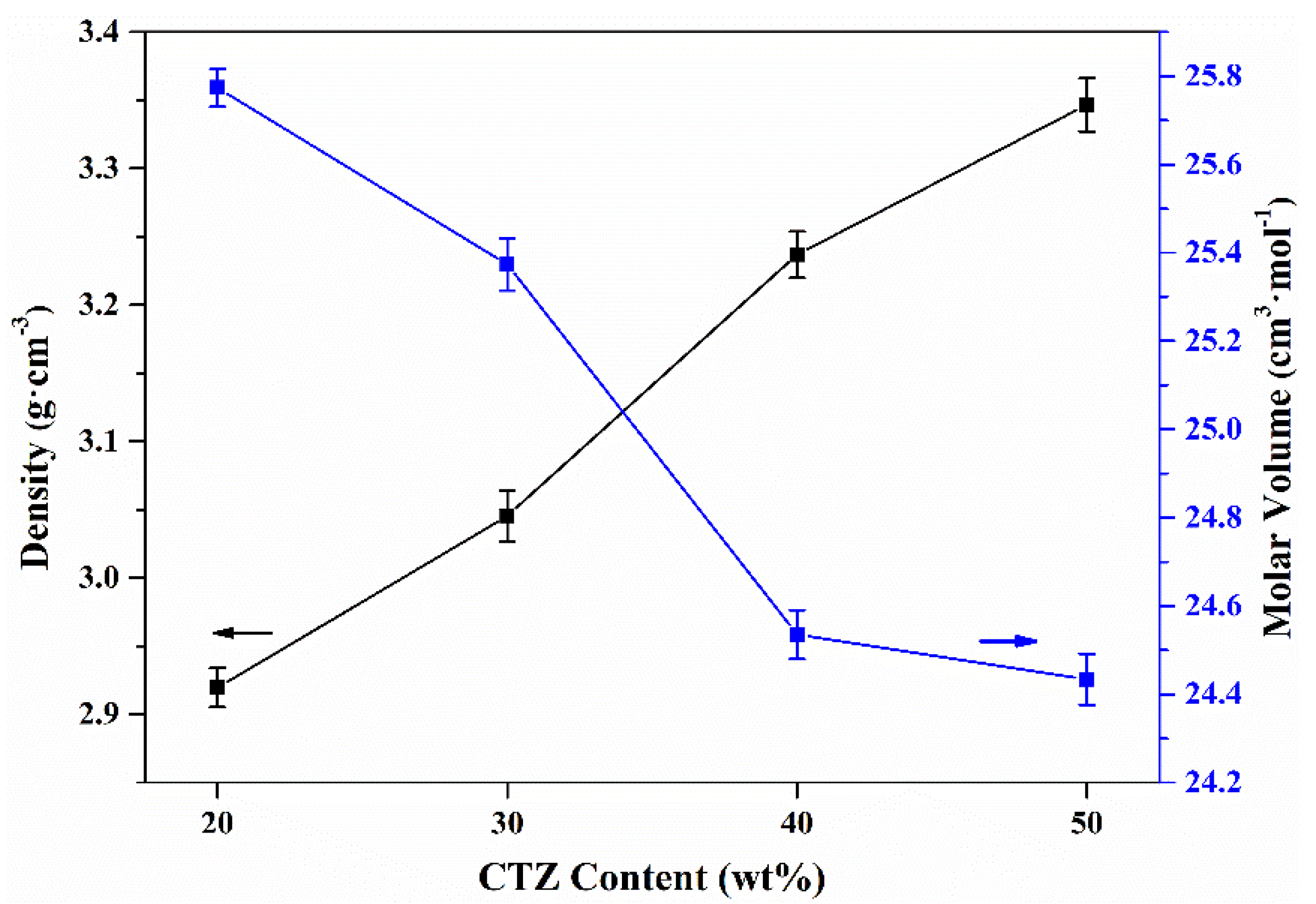

| SiO2 | B2O3 | Na2O | Al2O3 | CaO | TiO2 | ZrO2 | Nd2O3 | MoO3 | |
|---|---|---|---|---|---|---|---|---|---|
| CTZ-20 | 48.80 | 10.73 | 12.07 | 1.21 | 7.63 | 7.83 | 3.41 | 2.67 | 3.07 |
| CTZ-30 | 43.85 | 9.64 | 13.16 | 1.08 | 9.04 | 12.07 | 5.25 | 2.75 | 3.15 |
| CTZ-40 | 38.63 | 8.50 | 11.60 | 0.95 | 10.53 | 16.53 | 7.19 | 2.82 | 3.24 |
| CTZ-50 | 33.11 | 7.28 | 9.94 | 0.82 | 12.11 | 21.26 | 9.25 | 2.90 | 3.33 |
| Parameters | Raw Lattice | CTZ-30 | CTZ-40 | CTZ-50 |
|---|---|---|---|---|
| a(Å) | 12.4458 | 12.5298(4) | 12.7909(1) | 12.6149(4) |
| b(Å) | 7.2734 | 7.2264(2) | 7.3946(4) | 7.2780(9) |
| c(Å) | 11.3942 | 11.9817(4) | 11.6172(1) | 11.4489(3) |
| α(°) | 90.000 | 90.000(0) | 90.000(0) | 90.000(0) |
| β(°) | 100.533 | 100.095(2) | 100.900(6) | 100.697(2) |
| γ(°) | 90.000 | 90.000(0) | 90.000(0) | 90.000(0) |
| V(Å3) | 1014.06 | 1068.09(2) | 1078.97(8) | 1032.86(6) |
| Parameters | Raw Lattice | CTZ-30 | CTZ-40 | CTZ-50 |
|---|---|---|---|---|
| a(Å) | 5.226 | 5.241(8) | 5.249(10) | 5.237(1) |
| b(Å) | 5.226 | 5.241(8) | 5.249(10) | 5.237(1) |
| c(Å) | 11.43 | 11.49(2) | 11.49(2) | 11.47(1) |
| α(°) | 90.0 | 90.0(0) | 90.0(0) | 90.0(0) |
| β(°) | 90.0 | 90.0(0) | 90.0(0) | 90.0(0) |
| γ(°) | 90.0 | 90.0(0) | 90.0(0) | 90.0(0) |
| V(Å3) | 312.17 | 315.48(6) | 316.59(9) | 314.62(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, W.; Zhu, Y.; Zhang, X.; Yang, D.; Huo, Y.; Xu, C.; Yu, H.; Zhao, J.; Huo, J.; Meng, B. Borosilicate Glass-Ceramics Containing Zirconolite and Powellite for RE- and Mo-Rich Nuclear Waste Immobilization. Materials 2021, 14, 5747. https://doi.org/10.3390/ma14195747
Wan W, Zhu Y, Zhang X, Yang D, Huo Y, Xu C, Yu H, Zhao J, Huo J, Meng B. Borosilicate Glass-Ceramics Containing Zirconolite and Powellite for RE- and Mo-Rich Nuclear Waste Immobilization. Materials. 2021; 14(19):5747. https://doi.org/10.3390/ma14195747
Chicago/Turabian StyleWan, Wei, Yongchang Zhu, Xingquan Zhang, Debo Yang, Yonglin Huo, Chong Xu, Hongfu Yu, Jian Zhao, Jichuan Huo, and Baojian Meng. 2021. "Borosilicate Glass-Ceramics Containing Zirconolite and Powellite for RE- and Mo-Rich Nuclear Waste Immobilization" Materials 14, no. 19: 5747. https://doi.org/10.3390/ma14195747
APA StyleWan, W., Zhu, Y., Zhang, X., Yang, D., Huo, Y., Xu, C., Yu, H., Zhao, J., Huo, J., & Meng, B. (2021). Borosilicate Glass-Ceramics Containing Zirconolite and Powellite for RE- and Mo-Rich Nuclear Waste Immobilization. Materials, 14(19), 5747. https://doi.org/10.3390/ma14195747







