Effect of Chloride Ions Concentrations to Breakdown the Passive Film on Rebar Surface Exposed to L-Arginine Containing Pore Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Studies
2.3. Analysis of the Passive Film and Corrosion Products
3. Results and Discussion
3.1. Electrochemical Studies
3.1.1. Measurement of Open Circuit Potential (OCP)
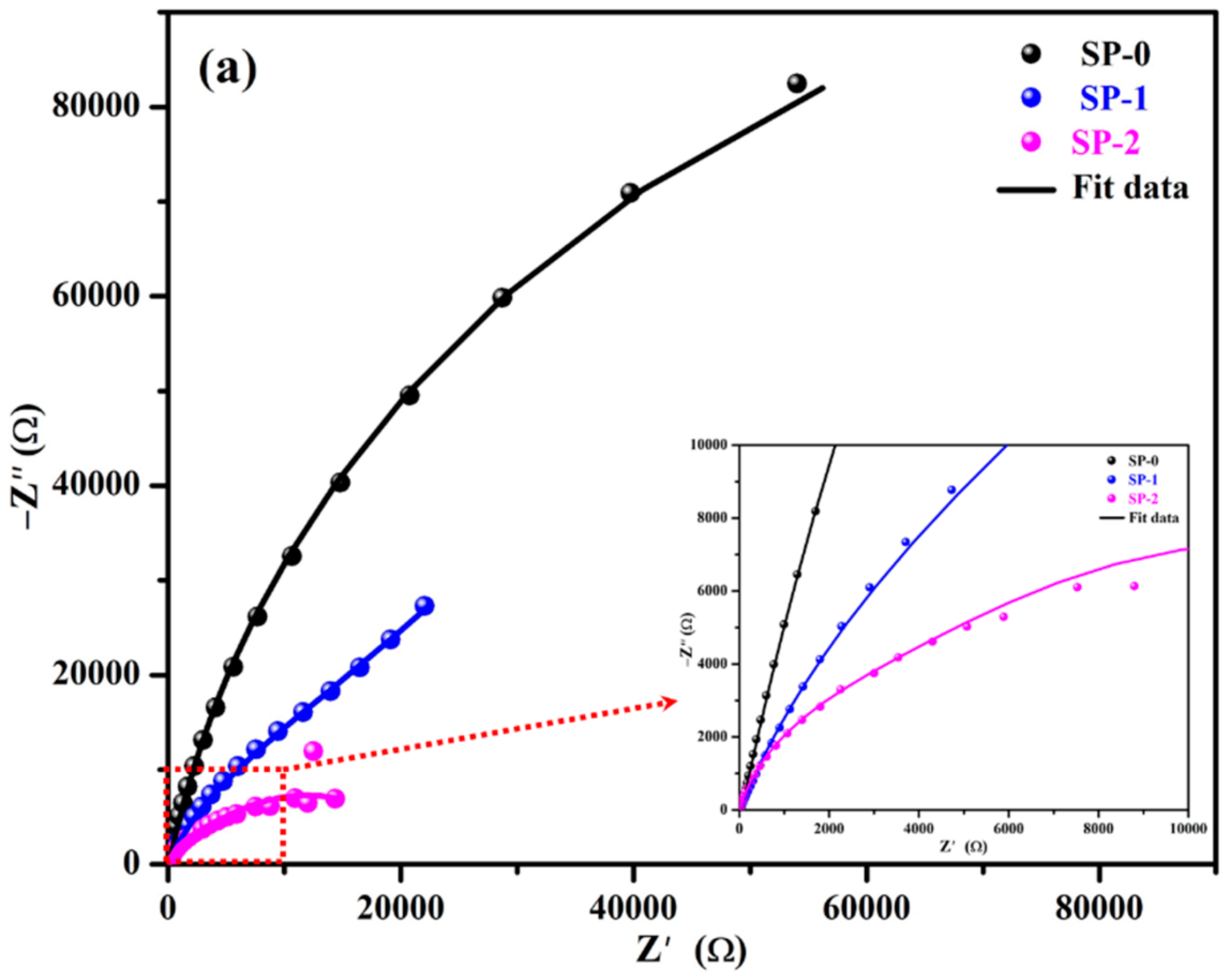
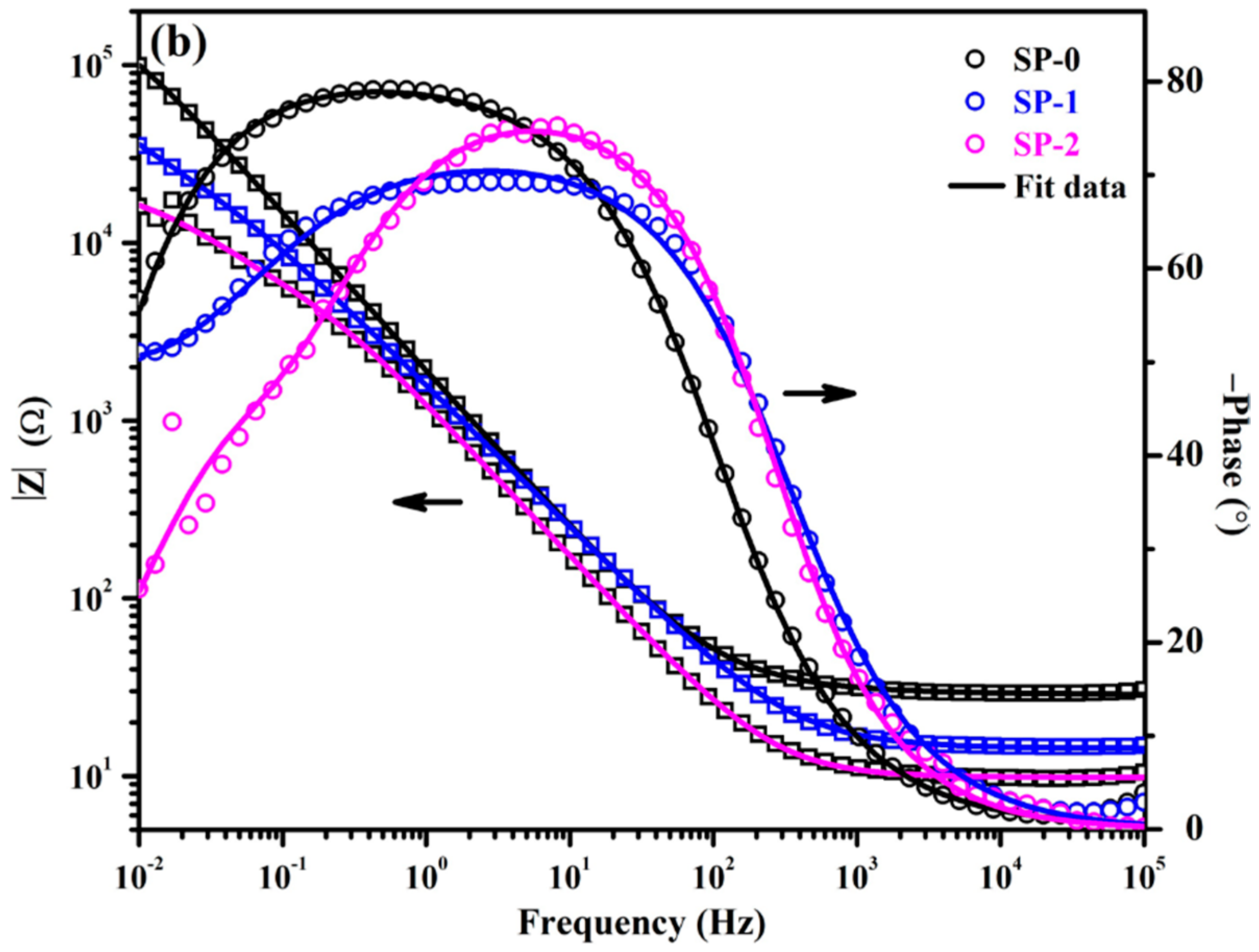
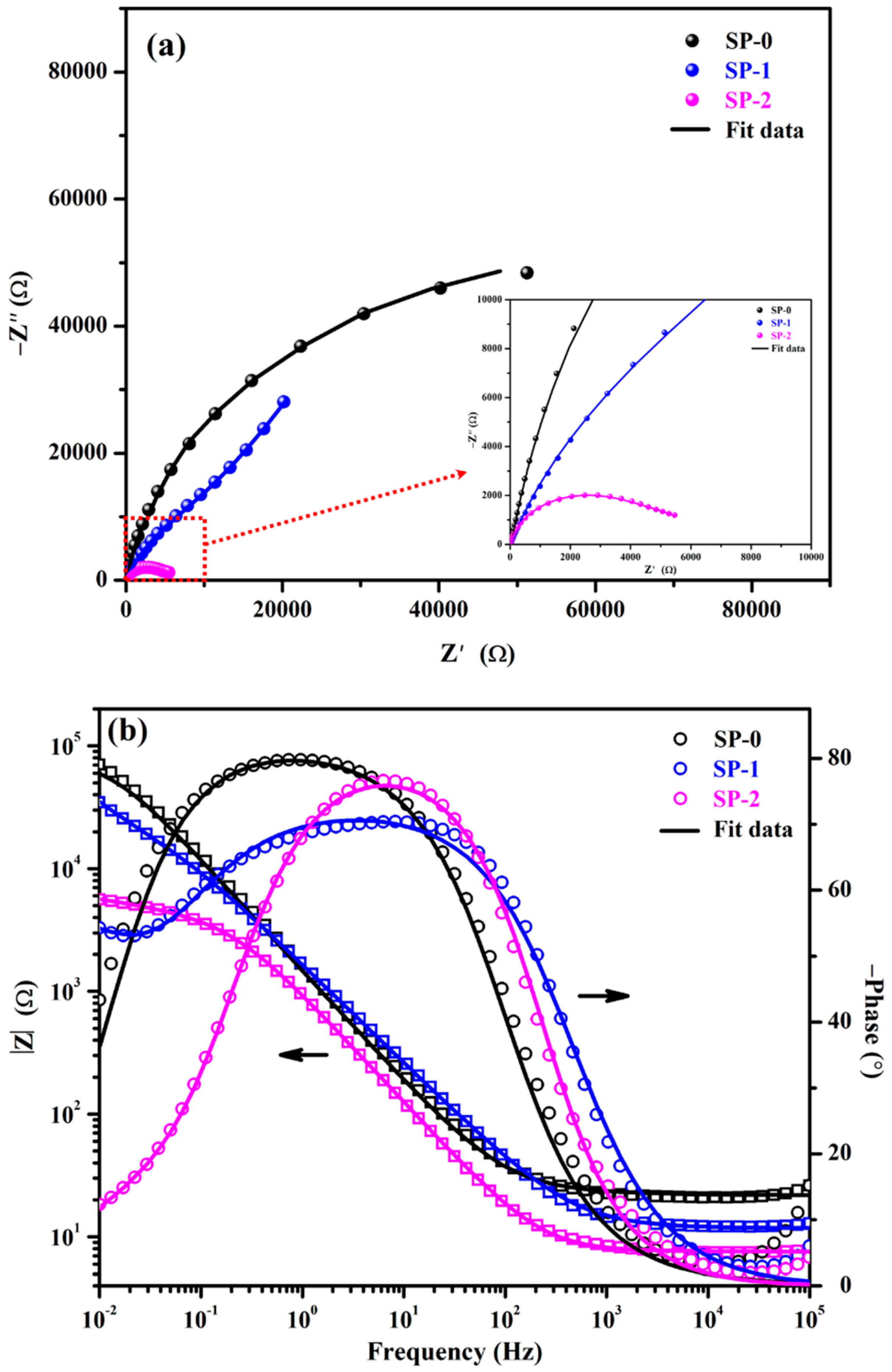
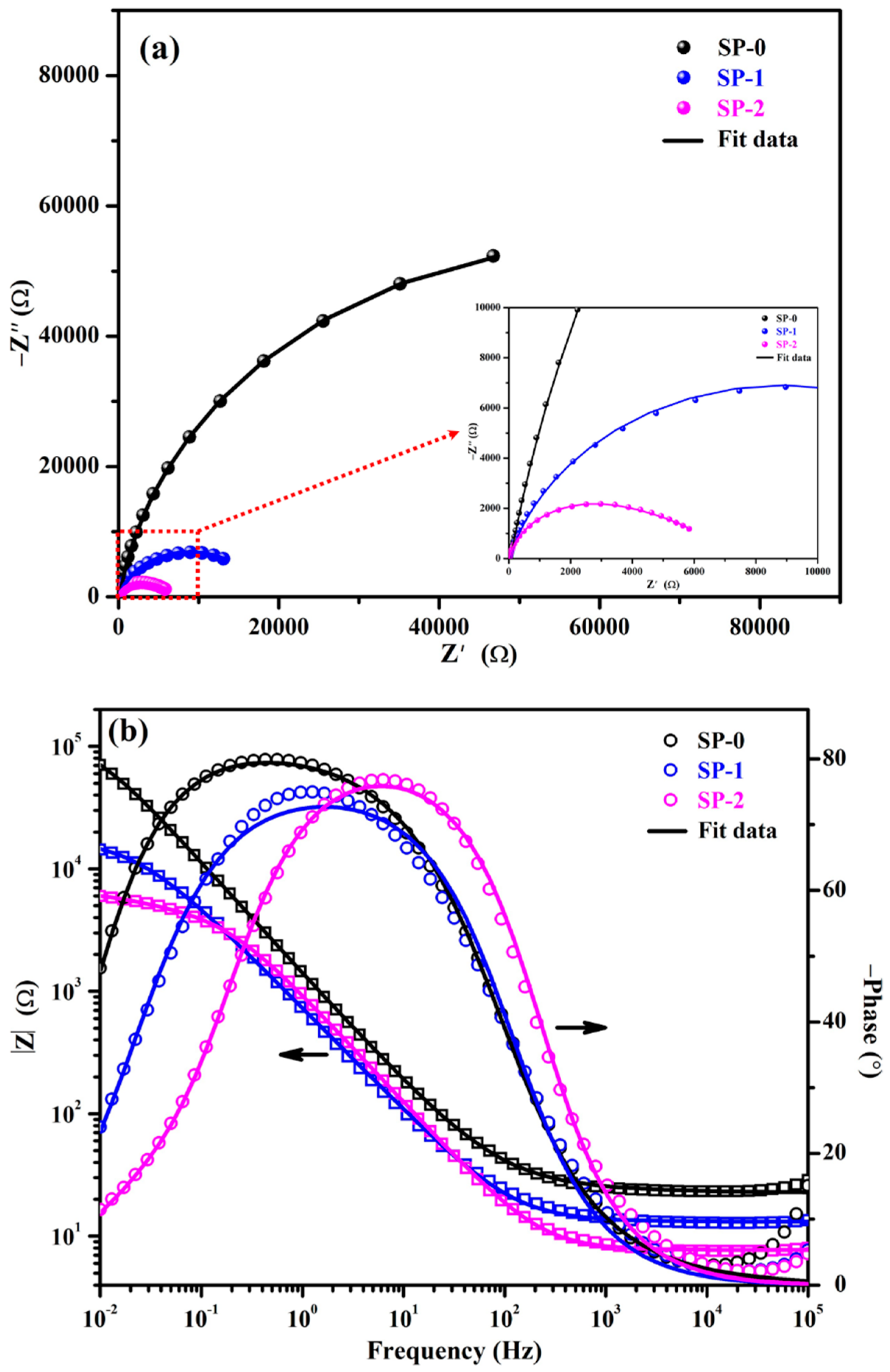
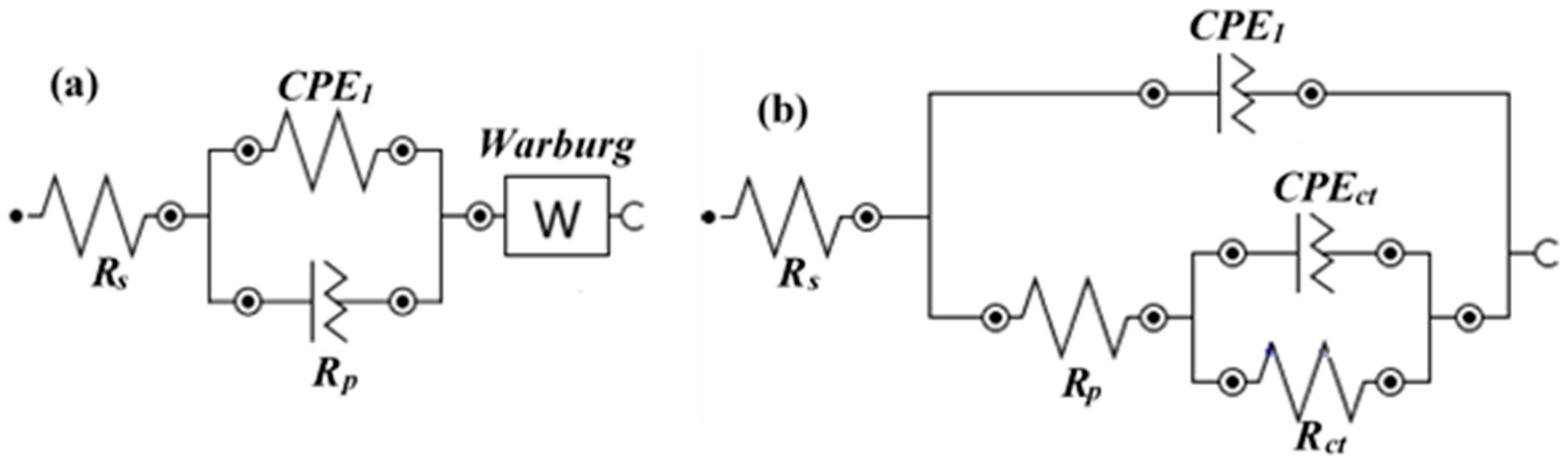
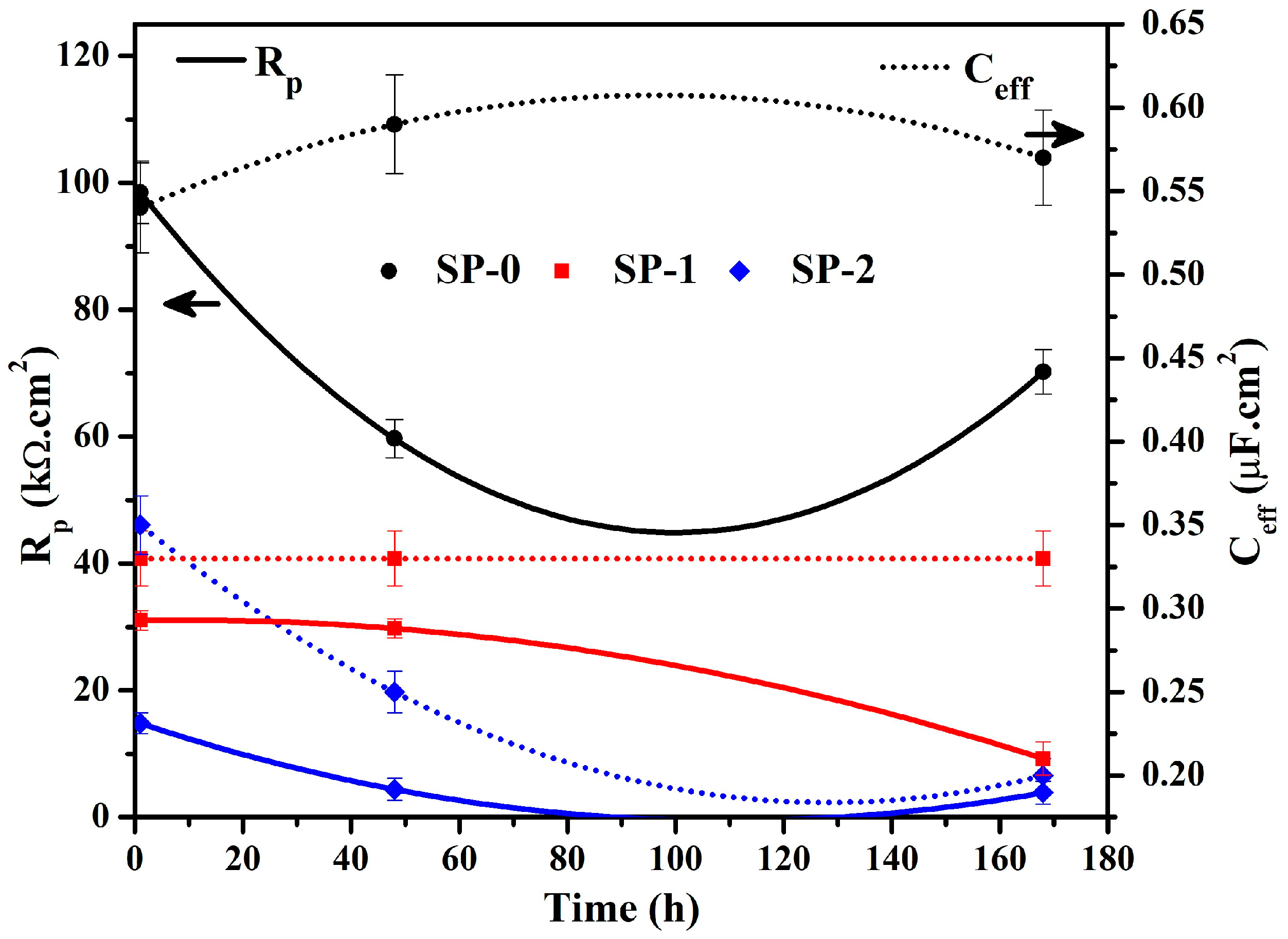
3.1.2. EIS with Time
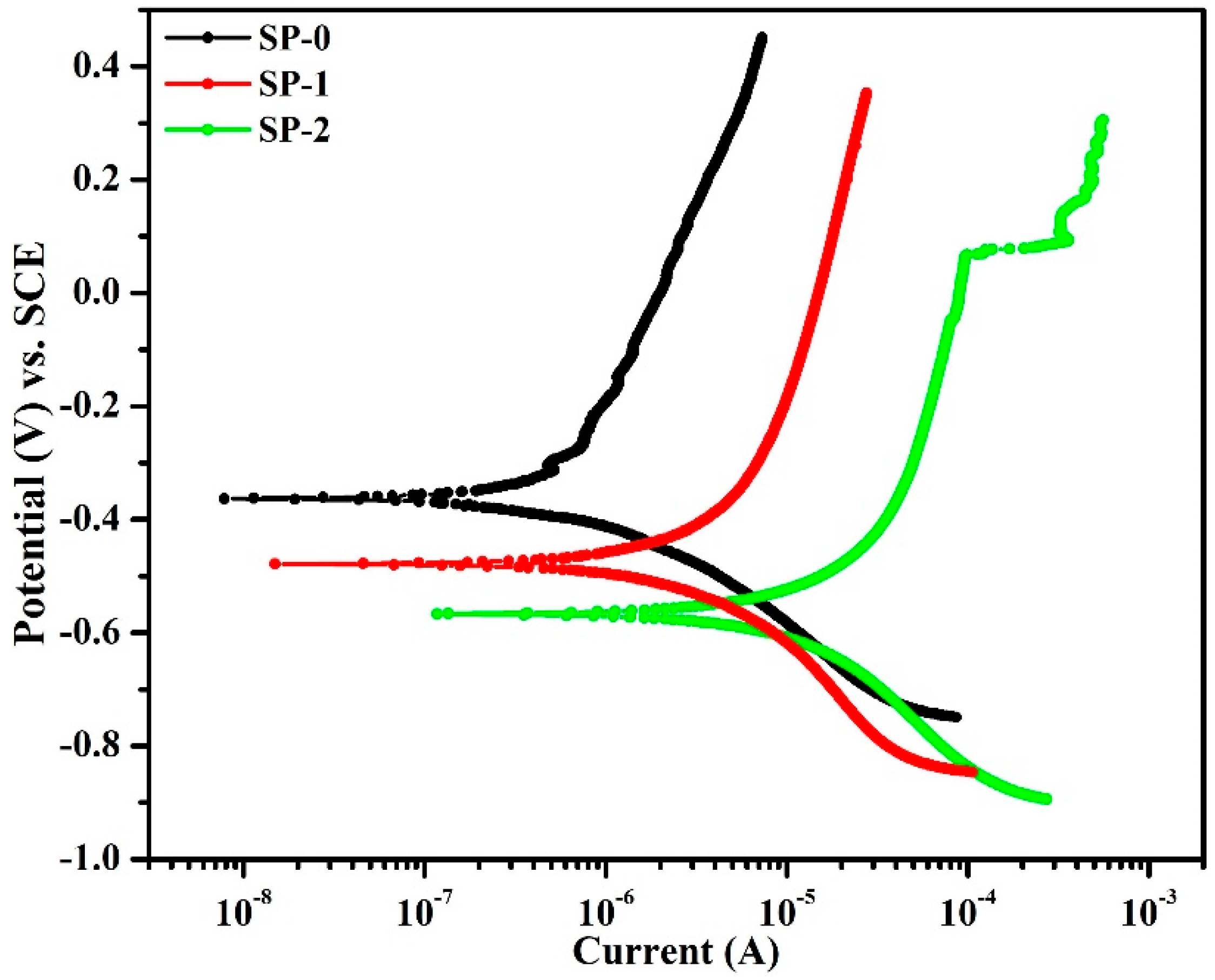
3.1.3. Potentiodynamic Polarization Studies after 168 h of Exposure in Different Solutions
3.2. Characterization
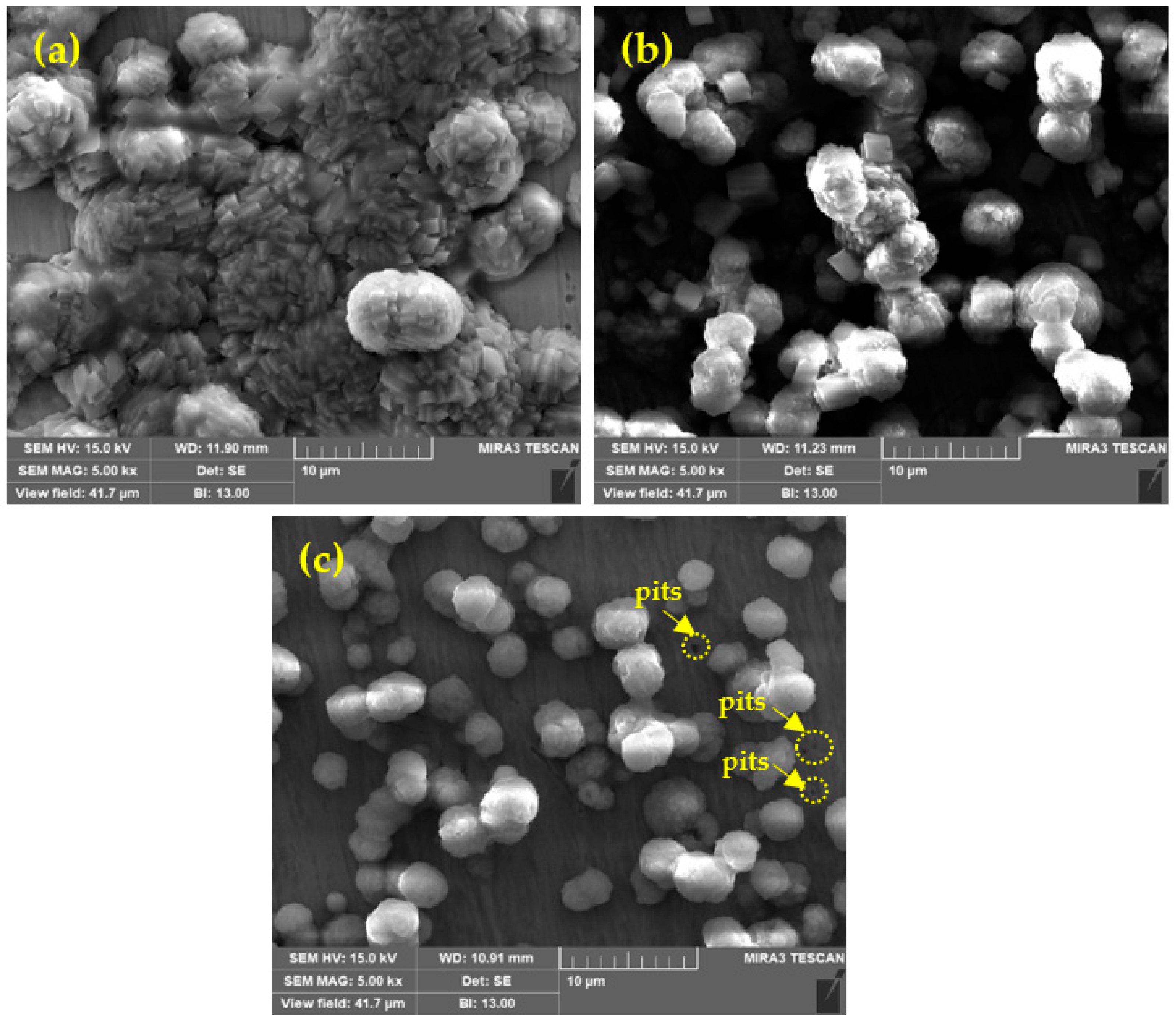
Surface Morphology of Passive Film by SEM
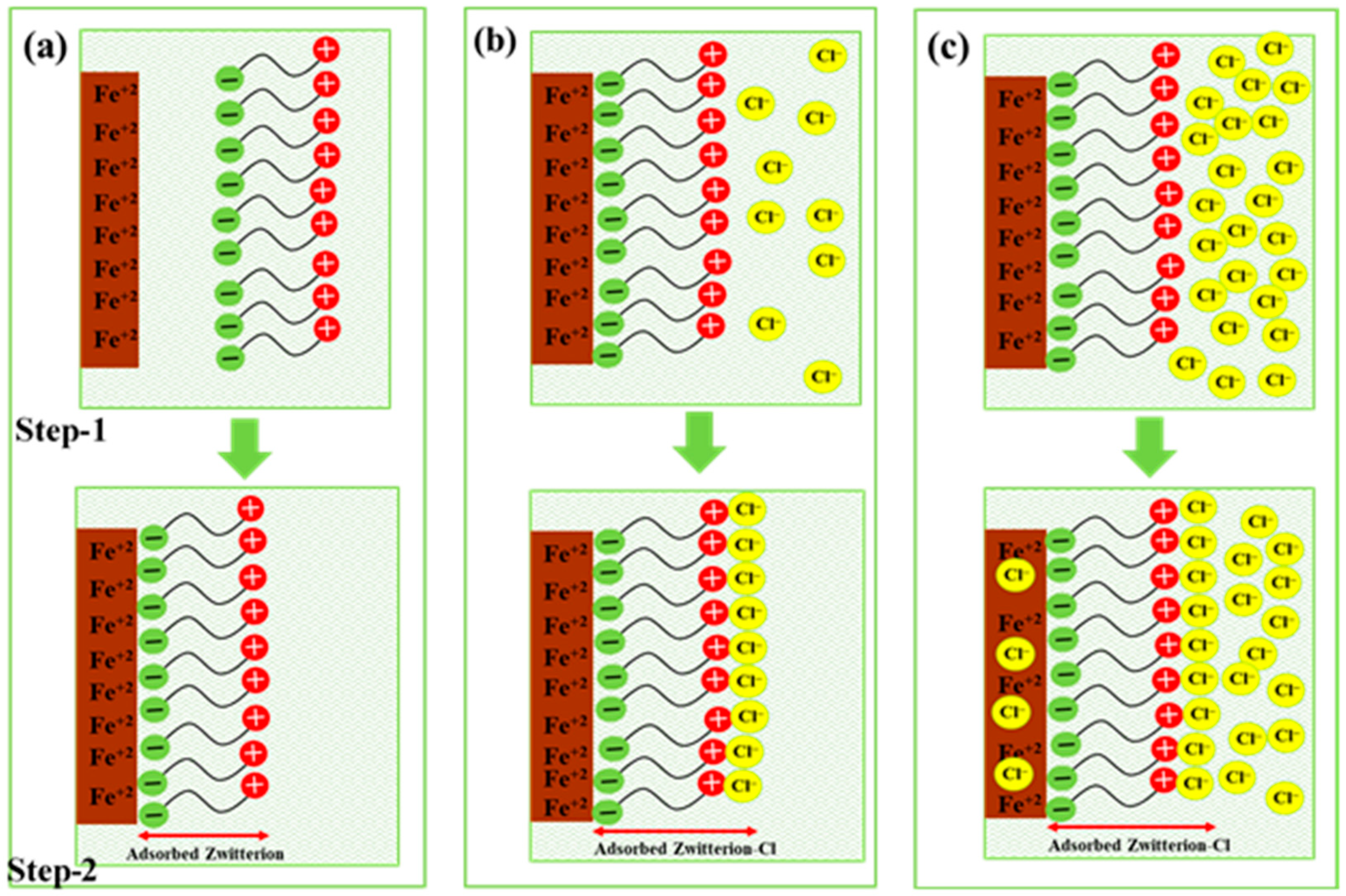
3.3. Plausible Mechanism for Initiation of the Corrosion Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penttala, V. Causes and mechanisms of deterioration in reinforced concrete. In Failure, Distress and Repair of Concrete Structures; Elsevier: Amsterdam, The Netherlands, 2009; pp. 3–31. [Google Scholar]
- Poursaee, A. Corrosion of steel in concrete structures. In Corrosion of Steel in Concrete Structures; Elsevier: Amsterdam, The Netherlands, 2016; pp. 19–33. [Google Scholar]
- Garcés, P.; Saura, P.; Méndez, A.; Zornoza, E.; Andrade, C. Effect of nitrite in corrosion of reinforcing steel in neutral and acid solutions simulating the electrolytic environments of micropores of concrete in the propagation period. Corros. Sci. 2008, 50, 498–509. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C.; Castellote, M.; Castro, P. Chloride threshold values to depassivate reinforcing bars embedded in a standardized OPC mortar. Cem. Concr. Res. 2000, 30, 1047–1055. [Google Scholar] [CrossRef]
- Hausmann, D. Steel corrosion in concrete—How does it occur? Mat. Prot. 1967, 11, 19–23. [Google Scholar]
- Gouda, V. Corrosion and corrosion inhibition of reinforcing steel: I. Immersed in alkaline solutions. Br. Corros. J. 1970, 5, 198–203. [Google Scholar] [CrossRef]
- Vassie, P.; TRRL. Reinforcement corrosion and the durability of concrete bridges. Proc. Inst. Civ. Eng. 1984, 76, 713–723. [Google Scholar] [CrossRef]
- Standard, B. 8110: Part 1, Structural use of concrete—Code of practice for design and construction. Br. Stand. Inst. Lond. UK 1985, 3–8. [Google Scholar]
- Hooton, R.D.; Geiker, M.R.; Bentz, E.C. Effects of curing on chloride ingress and implications on service life. Mater. J. 2002, 99, 201–206. [Google Scholar]
- Youping, L.; Weyers Richard, E. Time to cracking for chloride-induced corrosion in reinforced concrete. Corros. Reinf. Concr. Constr. R. Soc. Chem. Lond. 1996, 183, 88–104. [Google Scholar]
- Funahashi, M. Predicting corrosion-free service life of a concrete structure. ACI Mater. J. 1990, 87, M62. [Google Scholar]
- Clear, K.C. Time-to-Corrosion of Reinforcing Steel in Concrete Slabs. Volume 3: Performance after 830 Daily Salt Applications; The National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 1976. [Google Scholar]
- West, R.E.; Hime, W.G. Chloride profiles in salty concrete. Mater. Perform. 1985, 24, 29–36. [Google Scholar]
- Tritthart, J. Chloride binding in cement II. The influence of the hydroxide concentration in the pore solution of hardened cement paste on chloride binding. Cem. Concr. Res. 1989, 19, 683–691. [Google Scholar] [CrossRef]
- Yu, H.; Chiang, K.-T.K.; Yang, L. Threshold chloride level and characteristics of reinforcement corrosion initiation in simulated concrete pore solutions. Constr. Build. Mater. 2012, 26, 723–729. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, J.; Zhu, Y.; Mo, L. Influence of chloride salt type on threshold level of reinforcement corrosion in simulated concrete pore solutions. Constr. Build. Mater. 2012, 30, 516–521. [Google Scholar] [CrossRef]
- Xu, J.-x.; Jiang, L.-h.; Wang, W.-l.; Tang, L.; Cui, L. Effectiveness of inhibitors in increasing chloride threshold value for steel corrosion. Water Sci. Eng. 2013, 6, 354–363. [Google Scholar]
- Cabrini, M.; Fontana, F.; Lorenzi, S.; Pastore, T.; Pellegrini, S. Effect of organic inhibitors on chloride corrosion of steel rebars in alkaline pore solution. J. Chem. 2015, 2015, 521507. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Song, Z.; Wang, W.; Jiang, L.; Zhang, Y.; Guo, M.; Song, F.; Xu, N. Effect of ginger extract as green inhibitor on chloride-induced corrosion of carbon steel in simulated concrete pore solutions. J. Clean. Prod. 2019, 214, 298–307. [Google Scholar] [CrossRef]
- Hassoune, M.; Bezzar, A.; Sail, L.; Ghomari, F. Chloride threshold value to initiate steel corrosion in simulated concrete pore solution, and the effectiveness of DMEA as an amino-alcohol-based corrosion inhibitor. J. Adhes. Sci. Technol. 2020, 35, 504–521. [Google Scholar] [CrossRef]
- Nahali, H.; Dhouibi, L.; Idrissi, H. Effect of phosphate based inhibitor on the threshold chloride to initiate steel corrosion in saturated hydroxide solution. Constr. Build. Mater. 2014, 50, 87–94. [Google Scholar] [CrossRef]
- Uwah, I.; Okafor, P.; Ebiekpe, V. Inhibitive action of ethanol extracts from Nauclea latifolia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arab. J. Chem. 2013, 6, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.R.; Gupta, P.; Gupta, K. The litchi (Litchi Chinensis) peels extract as a potential green inhibitor in prevention of corrosion of mild steel in 0.5 M H2SO4 solution. Arab. J. Chem. 2019, 12, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- M′hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Phytochemical characteristics of citrus peel and effect of conventional and nonconventional processing on phenolic compounds: A review. Food Rev. Int. 2017, 33, 587–619. [Google Scholar] [CrossRef]
- Mammoliti, L.; Hansson, C.; Hope, B. Corrosion inhibitors in concrete Part II: Effect on chloride threshold values for corrosion of steel in synthetic pore solutions. Cem. Concr. Res. 1999, 29, 1583–1589. [Google Scholar] [CrossRef]
- Ormellese, M.; Bolzoni, F.; Lazzari, L.; Pedeferri, P. Effect of corrosion inhibitors on the initiation of chloride-induced corrosion on reinforced concrete structures. Mater. Corros. 2008, 59, 98–106. [Google Scholar] [CrossRef]
- Singh, J.K.; Yang, H.-M.; Lee, H.-S.; Mandal, S.; ASLAM, F.; Alyousef, R. Role of L-arginine on the formation and breakdown of passive film onto the steel rebars surface in chloride contaminated concrete pore solution. J. Mol. Liq. 2021, 337, 116454. [Google Scholar] [CrossRef]
- Lee, H.-S.; Yang, H.-M.; Singh, J.K.; Prasad, S.K.; Yoo, B. Corrosion mitigation of steel rebars in chloride contaminated concrete pore solution using inhibitor: An electrochemical investigation. Constr. Build. Mater. 2018, 173, 443–451. [Google Scholar] [CrossRef]
- Mandal, S.; Singh, J.K.; Lee, D.-E.; Park, T. Effect of phosphate-based inhibitor on corrosion kinetics and mechanism for formation of passive film onto the steel rebar in chloride-containing pore solution. Materials 2020, 13, 3642. [Google Scholar] [CrossRef]
- Mandal, S.; Singh, J.K.; Lee, D.-E.; Park, T. Ammonium phosphate as inhibitor to mitigate the corrosion of steel rebar in chloride contaminated concrete pore solution. Molecules 2020, 25, 3785. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Ghosh, R. Corrosion resistance performance of fusion bonded epoxy coated rebars used as reinforcement in concrete structures. J. Metall. Mater. Sci. 2003, 45, 73–83. [Google Scholar]
- Ghosh, R.; Singh, D. Kinetics, mechanism and characterisation of passive film formed on hot dip galvanized coating exposed in simulated concrete pore solution. Surf. Coat. Technol. 2007, 201, 7346–7359. [Google Scholar] [CrossRef]
- Yang, H.-M.; Myung, N.V.; Lee, H.-S.; Singh, J.K. L-arginine-incorporated cement mortar as sustainable artificial reefs. Sustainability 2020, 12, 6346. [Google Scholar] [CrossRef]
- Badawy, W.A.; Ismail, K.M.; Fathi, A.M. Effect of Ni content on the corrosion behavior of Cu–Ni alloys in neutral chloride solutions. Electrochim. Acta 2005, 50, 3603–3608. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sadovski, A.; Melo, A.P.; Pereira, E.V. Chloride threshold value to initiate reinforcement corrosion in simulated concrete pore solutions: The influence of surface finishing and pH. Constr. Build. Mater. 2017, 141, 183–200. [Google Scholar] [CrossRef]
- Li, L.; Sagues, A. Chloride corrosion threshold of reinforcing steel in alkaline solutions—Open-circuit immersion tests. Corrosion 2001, 57, 19–28. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, H.; Chu, H.; Zhu, C.; Xiong, C.; You, L.; Xu, J.; Zhang, Y.; Qin, Y. Influence of compression fatigue on chloride threshold value for the corrosion of steels in simulated concrete pore. Constr. Build. Mater. 2014, 73, 699–704. [Google Scholar] [CrossRef]
- Kim, K.; Chang, H.; Lim, B.; Park, H.; Kim, Y. Effect of ethanolamines on corrosion inhibition of ductile cast Iron in nitrite containing solutions. Corros. Sci. Technol. 2016, 15, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.R.; Bhrara, K.; Singh, G. The inhibitory effect of diethanolamine on corrosion of mild steel in 0.5 M sulphuric acidic medium. Port Electrochim. Acta 2008, 26, 479–492. [Google Scholar] [CrossRef]
- Garcia-Arriaga, V.; Alvarez-Ramirez, J.; Amaya, M.; Sosa, E. H2S and O2 influence on the corrosion of carbon steel immersed in a solution containing 3 M diethanolamine. Corros. Sci. 2010, 52, 2268–2279. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Singh, J.K.; Lee, H.-S.; Ismail, M.A.; Park, W.-J. Effect of LiNO2 inhibitor on corrosion characteristics of steel rebar in saturated Ca(OH)2 solution containing NaCl: An electrochemical study. Constr. Build. Mater. 2017, 133, 387–396. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Singh, J.K.; Lee, H.-S.; Park, W.-J. An electrochemical study to evaluate the effect of calcium nitrite inhibitor to mitigate the corrosion of reinforcement in sodium chloride contaminated Ca(OH)2 solution. Adv. Mater. Sci. Eng. 2017, 2017, 6265184. [Google Scholar] [CrossRef] [Green Version]
- Yohai, L.; Vázquez, M.; Valcarce, M. Phosphate ions as corrosion inhibitors for reinforcement steel in chloride-rich environments. Electrochim. Acta 2013, 102, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.-j.; Sun, W. Electrochemical and analytical characterization of three corrosion inhibitors of steel in simulated concrete pore solutions. Int. J. Miner. Metall. Mater. 2012, 19, 38–47. [Google Scholar] [CrossRef]
- Monticelli, C.; Frignani, A.; Balbo, A.; Zucchi, F. Influence of two specific inhibitors on steel corrosion in a synthetic solution simulating a carbonated concrete with chlorides. Mater. Corros. 2011, 62, 178–186. [Google Scholar] [CrossRef]
- Yohai, L.; Schreiner, W.; Valcarce, M.B.; Vázquez, M. Inhibiting steel corrosion in simulated concrete with low phosphate to chloride ratios. J. Electrochem. Soc. 2016, 163, C729. [Google Scholar] [CrossRef]
- Orazem, M.E.; Pébère, N.; Tribollet, B. Enhanced graphical representation of electrochemical impedance data. J. Electrochem. Soc. 2006, 153, B129. [Google Scholar] [CrossRef]
- Brug, G.; van den Eeden, A.L.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Huang, V.M.-W.; Vivier, V.; Orazem, M.E.; Pébère, N.; Tribollet, B. The apparent constant-phase-element behavior of a disk electrode with faradaic reactions: A global and local impedance analysis. J. Electrochem. Soc. 2006, 154, C99. [Google Scholar] [CrossRef] [Green Version]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Ismail, K.M.; Sikora, E. Characterization of the passive state on zinc. J. Electrochem. Soc. 1998, 145, 3141. [Google Scholar] [CrossRef]
- Al-Negheimish, A.; Alhozaimy, A.; Hussain, R.R.; Al-Zaid, R.; Singh, J.; Singh, D. Role of manganese sulfide inclusions in steel rebar in the formation and breakdown of passive films in concrete pore solutions. Corrosion 2014, 70, 74–86. [Google Scholar] [CrossRef]
- ASTM International West. ASTM G102. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International West: Conshohocken, PA, USA, 2010. [Google Scholar]

| Elements (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Si | C | Cr | Ni | Cu | P | Mo | S | Sn |
| 98.501 | 0.90 | 0.25 | 0.235 | 0.037 | 0.028 | 0.018 | 0.014 | 0.009 | 0.006 | 0.002 |
| Sl. No. | Solution | Sample ID |
|---|---|---|
| 1. | SP + 0.115 M LA | SP-0 |
| 2. | SP + 0.115 M LA + 0.51 M NaCl | SP-1 |
| 3. | SP + 0.115 M LA + 0.85 M NaCl | SP-2 |
| Time (h) | Sample ID | Electrochemical Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs (Ω·cm2) | CPE1 | Rct (kΩ·cm2) | CPEct | Warburg (1 × 10−3) (Ω·cm2·s0.5) | tf (nm) | ||||
| Q1 (1 × 10−5) (Ω−1·cm−2·sn) | n1 | Qct (1 × 10−5) (Ω−1·cm−2·sn) | nct | ||||||
| 1 | SP-0 | 28 (±0.13) | 10.3 (±0.06) | 0.90 (±0.02) | 4.66 (±0.27) | 38.35 (±2.72) | |||
| SP-1 | 14 (±0.08) | 14.2 (±0.13) | 0.81 (±0.02) | 4.10 (±0.11) | 12.5 (±1.25) | 0.72 (±0.02) | 45.02 (±2.97) | ||
| SP-2 | 10 (±0.18) | 17.2 (±0.76) | 0.80 (±0.01) | 1.20 (±0.04) | 19.4 (±0.99) | 0.62 (±0.01) | 62.75 (±4.08) | ||
| 48 | SP-0 | 22 (±0.27) | 13.0 (±0.15) | 0.88 (±0.01) | 7.13 (±0.63) | 35.10 (±2.07) | |||
| SP-1 | 12 (±0.10) | 14.6 (±0.10) | 0.81 (±0.01) | 4.81 (±0.08) | 11.1 (±0.08) | 0.76 (±0.04) | 60.90 (±3.84) | ||
| SP-2 | 7 (±0.04) | 19.8 (±1.56) | 0.76 (±0.05) | 1.24 (±0.11) | 18.8 (±1.50) | 0.63 (±0.04) | 62.75 (±4.20) | ||
| 168 | SP-0 | 22 (±0.30) | 11.9 (±0.78) | 0.89 (±0.01) | 3.79 (±0.45) | 36.33 (±2.62) | |||
| SP-1 | 13 (±0.01) | 15.4 (±0.02) | 0.80 (±0.05) | 5.10 (±0.41) | 10.6 (±0.02) | 0.76 (±0.06) | 64.71 (±3.56) | ||
| SP-2 | 7 (±0.05) | 20.2 (±0.03) | 0.74 (±0.02) | 2.07 (±0.01) | 17.5 (±0.12) | 0.65 (±0.01) | 62.75 (±4.20) | ||
| Sample ID | Electrochemical Parameters | |||
|---|---|---|---|---|
| Ecorr (mV) vs. SCE | icorr (µA·cm−2) | Corrosion Level [19] | C. R. (µm·year−1) | |
| SP-0 | −363 (±1.25) | 0.10 (±0.008) | Passive condition | 1.16 (±0.009) |
| SP-1 | −478 (±1.89) | 0.45 (±0.05) | Low | 5.23 (±0.52) |
| SP-2 | −566 (±1.83) | 1.96 (±0.15) | Severe | 22.77 (±1.59) |
| Sample ID | Elements (Wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| O | Na | Cl | K | Ca | N | C | Fe | |
| SP-0 | 21.87 (±3.82) | 2.12 (±0.26) | - | 0.42 (±0.12) | 18.28 (±1.38) | 2.04 (±0.22) | 11.19 (±1.31) | Balance |
| SP-1 | 19.12 (±2.33) | 3.64 (±0.28) | 2.59 (±0.34) | 0.56 (±0.07) | 17.39 (±1.36) | 1.94 (±0.19) | 11.15 (±1.29) | Balance |
| SP-2 | 17.36 (±2.26) | 3.71 (±0.35) | 4.04 (±0.46) | 0.32 (±0.09) | 14.64 (±1.88) | 1.57 (±0.22) | 11.49 (±0.80) | Balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, J.K.; Mandal, S.; Lee, H.-S.; Yang, H.-M. Effect of Chloride Ions Concentrations to Breakdown the Passive Film on Rebar Surface Exposed to L-Arginine Containing Pore Solution. Materials 2021, 14, 5693. https://doi.org/10.3390/ma14195693
Singh JK, Mandal S, Lee H-S, Yang H-M. Effect of Chloride Ions Concentrations to Breakdown the Passive Film on Rebar Surface Exposed to L-Arginine Containing Pore Solution. Materials. 2021; 14(19):5693. https://doi.org/10.3390/ma14195693
Chicago/Turabian StyleSingh, Jitendra Kumar, Soumen Mandal, Han-Seung Lee, and Hyun-Min Yang. 2021. "Effect of Chloride Ions Concentrations to Breakdown the Passive Film on Rebar Surface Exposed to L-Arginine Containing Pore Solution" Materials 14, no. 19: 5693. https://doi.org/10.3390/ma14195693
APA StyleSingh, J. K., Mandal, S., Lee, H.-S., & Yang, H.-M. (2021). Effect of Chloride Ions Concentrations to Breakdown the Passive Film on Rebar Surface Exposed to L-Arginine Containing Pore Solution. Materials, 14(19), 5693. https://doi.org/10.3390/ma14195693









