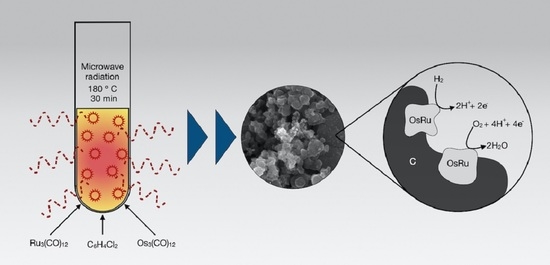
Hydrogen Oxidation and Oxygen Reduction Reactions on an OsRu-Based Electrocatalyst Synthesized by Microwave Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microwave-Assisted Synthesis of OsRu
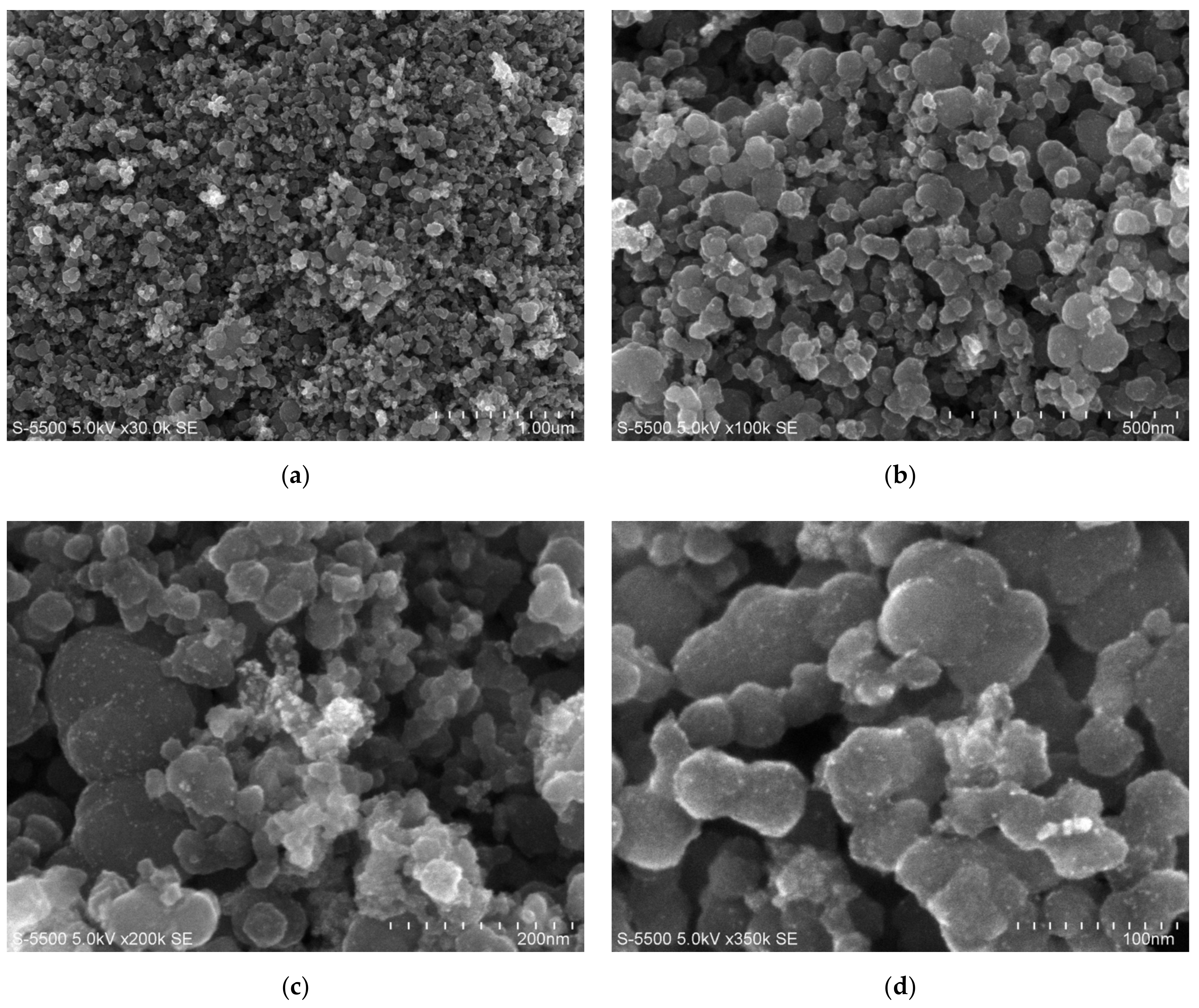
2.2. Morphological and Structural Characterization
2.3. Electrode Preparation
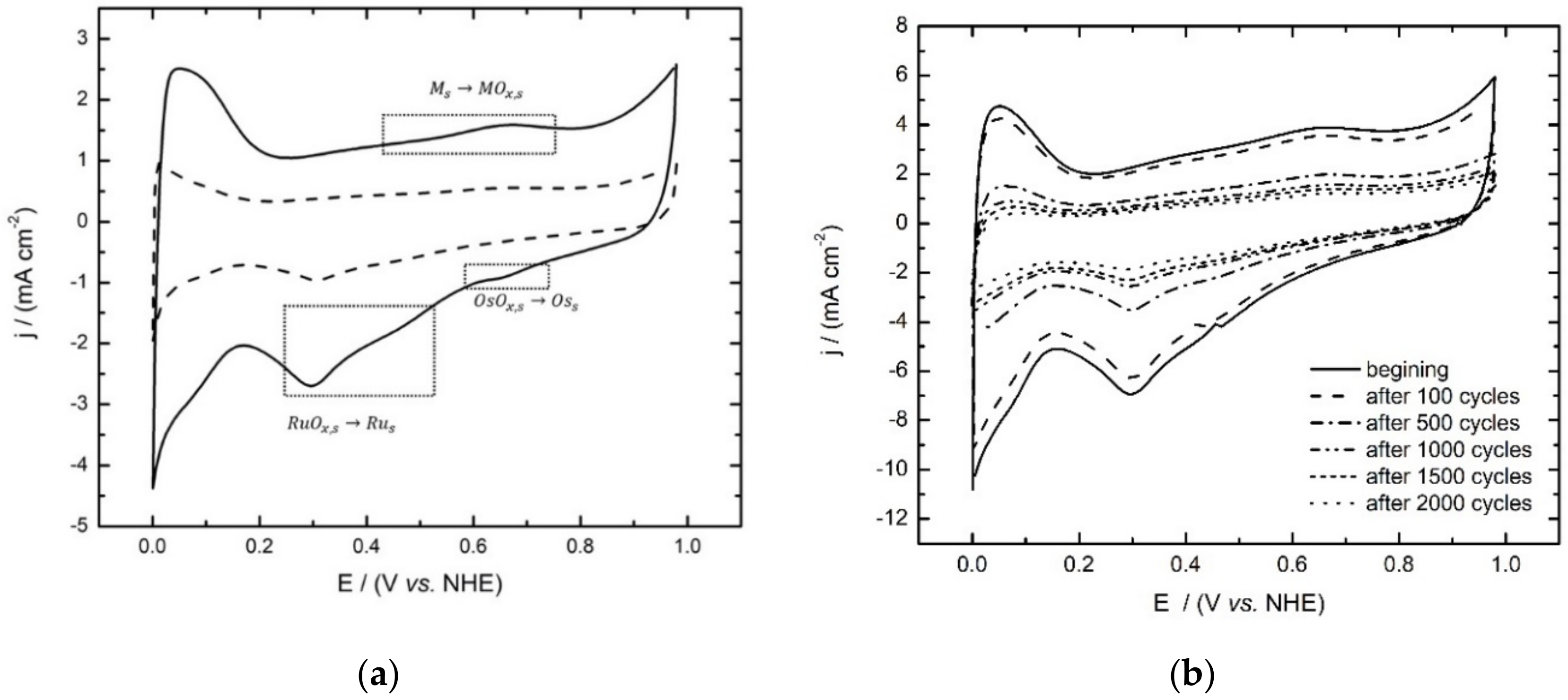
2.4. Cyclic Voltammetry
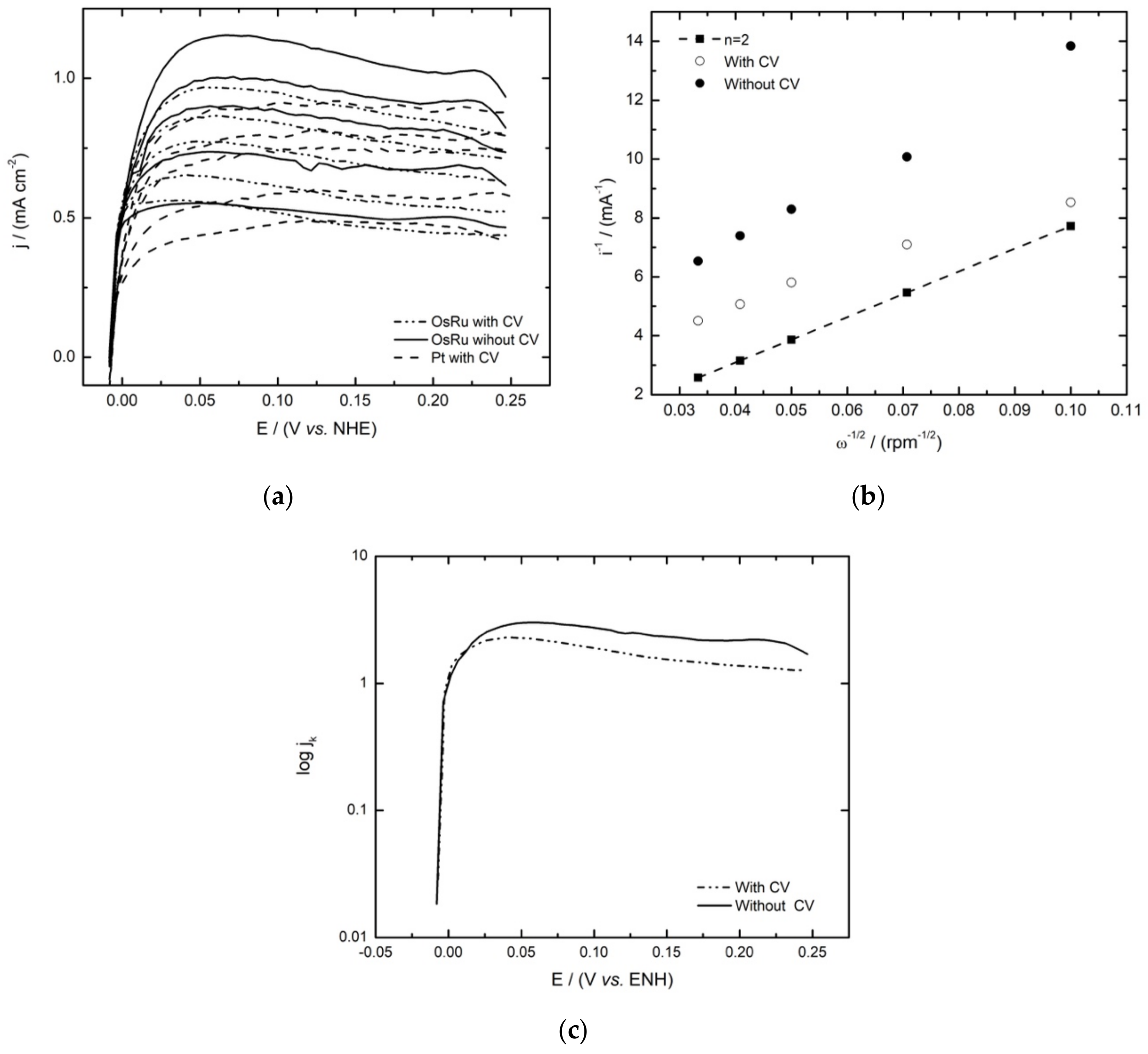
2.5. Hydrogen Oxidation Reaction
2.6. Oxygen Reduction Reaction
3. Results and Discussion
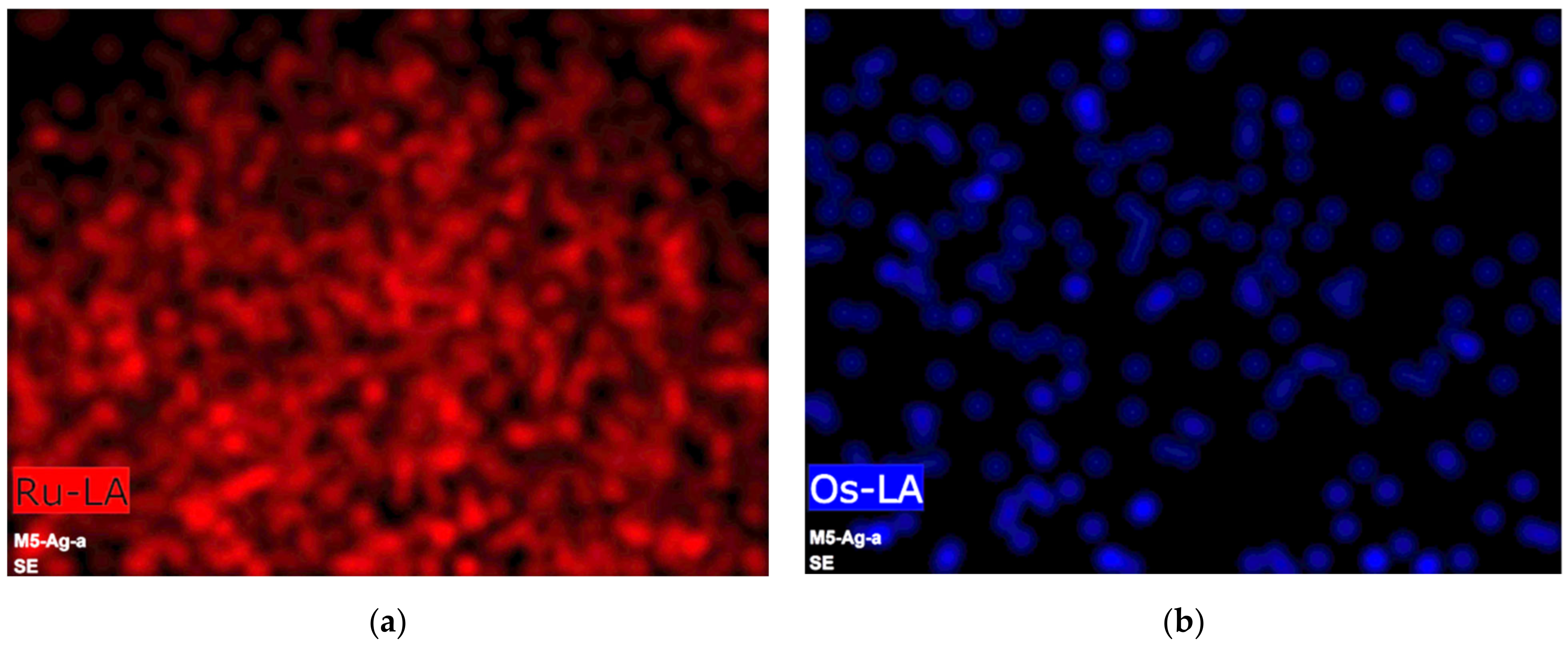
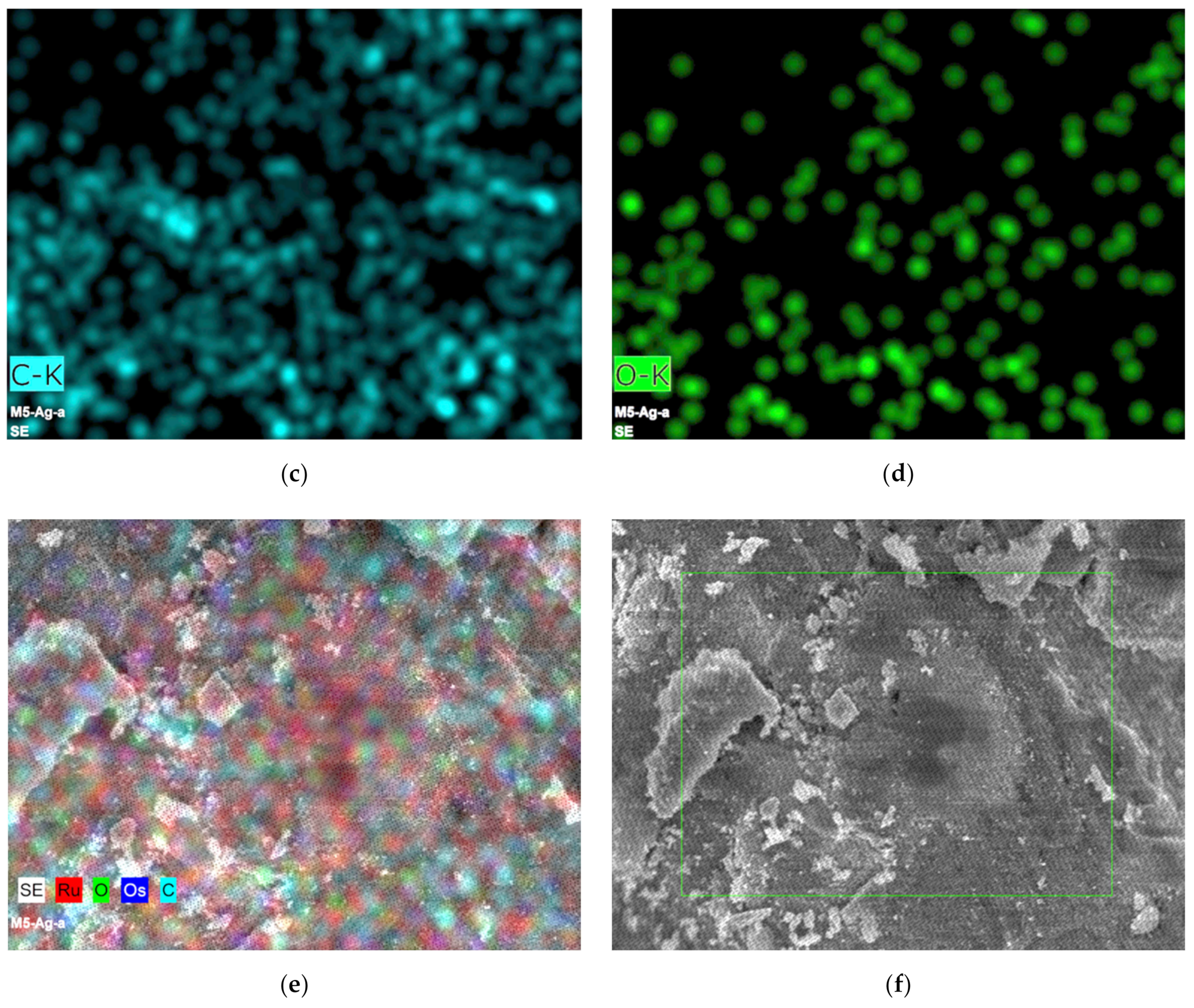
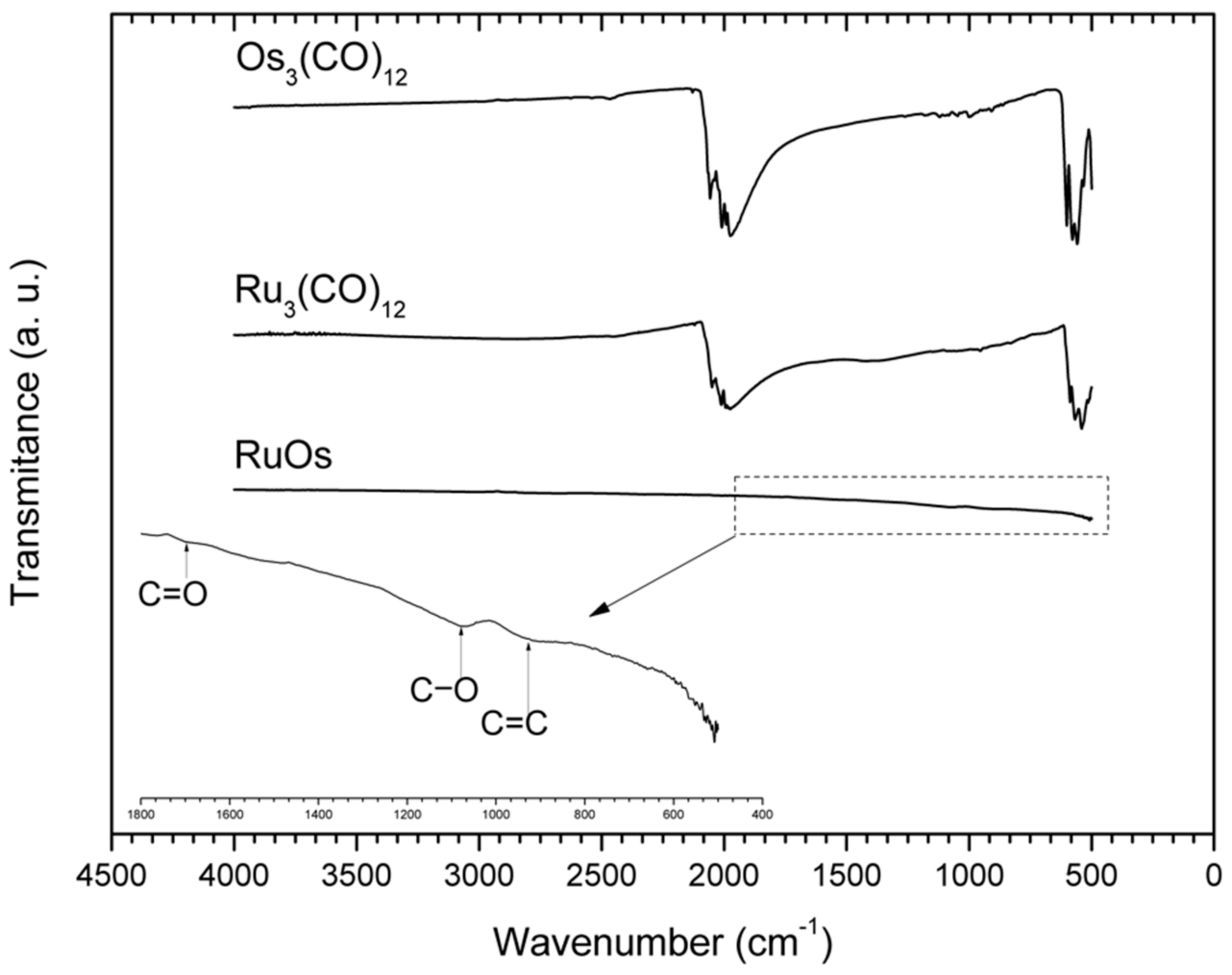
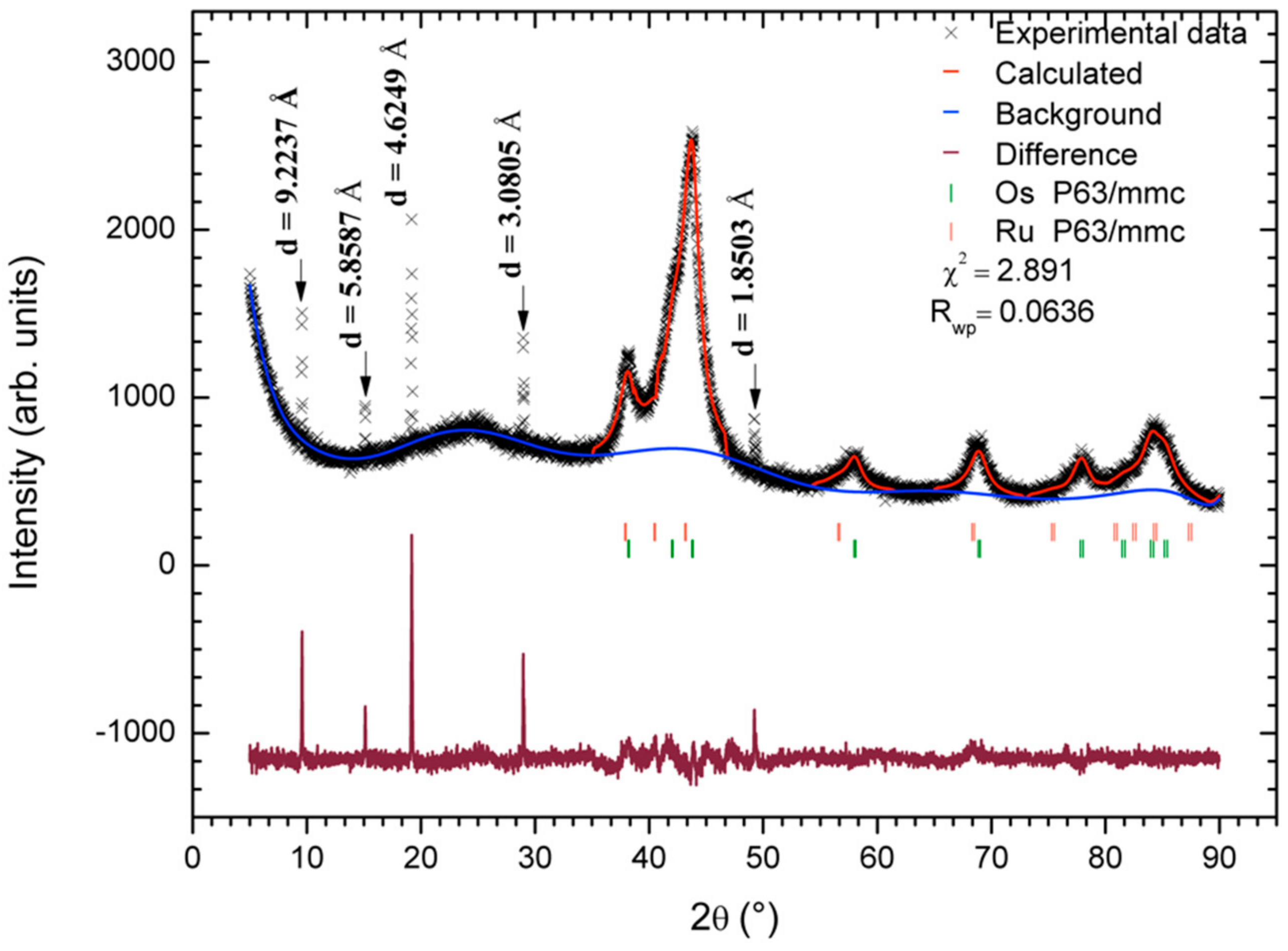
3.1. Morphological and Structural Characterization
3.2. Cyclic Voltammetry
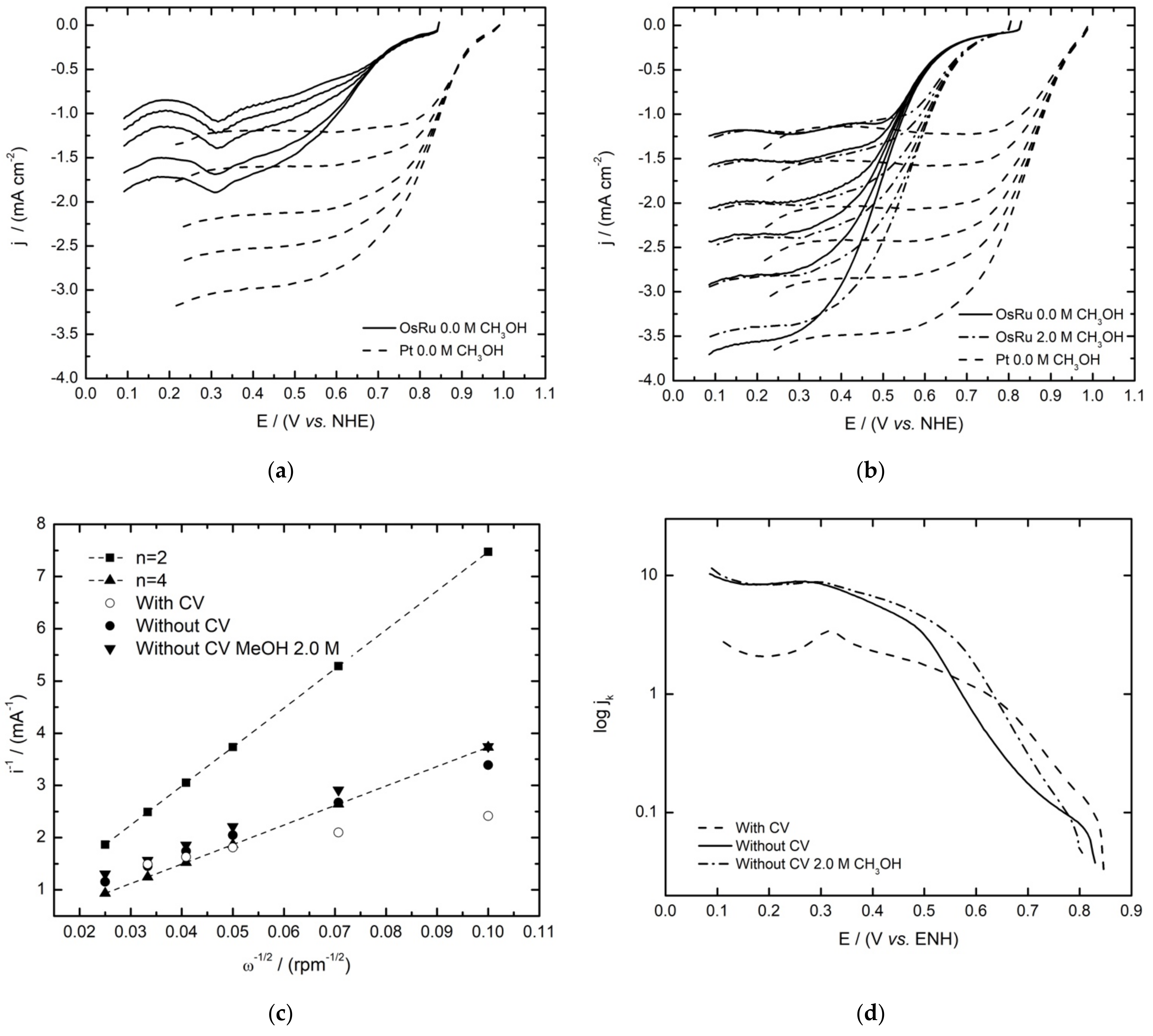
3.3. Hydrogen Oxidation Reaction
3.4. Oxygen Reduction Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durst, J.; Simon, C.; Hasché, F.; Gasteiger, H.A. Hydrogen Oxidation and Evolution Reaction Kinetics on Carbon Supported Pt, Ir, Rh, and Pd Electrocatalysts in Acidic Media. J. Electrochem. Soc. 2015, 162, F190–F203. [Google Scholar] [CrossRef]
- Muthukrishnan, A.; Nabae, Y.; Hayakawa, T.; Okajima, T.; Ohsaka, T. Fe-containing polyimide-based high-performance ORR catalysts in acidic medium: A kinetic approach to study the durability of catalysts. Catal. Sci. Technol. 2015, 5, 475–483. [Google Scholar] [CrossRef]
- Si, F.; Zhang, Y.; Yan, L.; Zhu, J.; Xiao, M.; Liu, C.; Xing, W.; Zhang, J. Electrochemical Oxygen Reduction Reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalyst; Xing, W., Yin, G., Zhand, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 133–170. [Google Scholar]
- Uribe-Godínez, J.; Altamirano-Gutiérrez, A. Systematic study of iridium-based catalysts derived from Ir4(CO)12 capable to perform the ORR and HOR. Catal. Today 2020, 374, 124–134. [Google Scholar] [CrossRef]
- Tzorbatzoglou, F.; Brouzgou, A.; Tsiakaras, P. Electrocatalytic activity of Vulcan-XC-72 supported Pd, Rh and PdxRhy toward HOR and ORR. Appl. Catal. B Environ. 2015, 174–175, 203–211. [Google Scholar] [CrossRef]
- Uribe-Godínez, J.; Castellanos, R.H.; Borja-Arco, E.; Altamirano-Gutiérrez, A.; Jiménez-Sandoval, O. Novel osmium-based electrocatalysts for oxygen reduction and hydrogen oxidation in acid conditions. J. Power Sources 2008, 177, 286–295. [Google Scholar] [CrossRef]
- Bernal-López, M.; Selva-Ochoa, A.; Borja-Arco, E.; Magallón-Cacho, L.; Su-Gallegos, J. Microwave assisted synthesis of metallic Ru for the HOR and ORR. Mater. Res. Express 2020, 7, 025503. [Google Scholar] [CrossRef]
- Meng, G.; Chang, Z.; Cui, X.; Tian, H.; Ma, Z.; Peng, L.; Chen, Y.; Chen, C.; Shi, J. SnO2/CeO2 nanoparticle-decorated mesoporous ZSM-5 as bifunctional electrocatalyst for HOR and ORR. Chem. Eng. J. 2020, 417, 127913. [Google Scholar] [CrossRef]
- Choi, J.; Cho, J.; Roh, C.W.; Kim, B.S.; Choi, M.S.; Jeong, H.; Ham, H.C.; Lee, H. Au-doped PtCo/C catalyst preventing Co leaching for proton exchange membrane fuel cells. Appl. Catal. B Environ. 2019, 247, 142–149. [Google Scholar] [CrossRef]
- Sorsa, O.; Romar, H.; Lassi, U.; Kallio, T. Co-electrodeposited Mesoporous PtM (M=Co, Ni, Cu) as an Active Catalyst for Oxygen Reduction Reaction in a Polymer Electrolyte Membrane Fuel Cell. Electrochim. Acta 2017, 230, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Thompsett, D. Recent Developments in Electrocatalyst Activity and Stability for Proton Exchange Membrane Fuel Cells. In Proton Exchange Membrane Fuel Cells. Materials Properties and Performance; Wilkinson, D.P., Zhang, J., Hui, R., Fergus, J., Li, X., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–60. [Google Scholar]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem.—Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Borja-Arco, E.; Castellanos, R.H.; Uribe-Godínez, J.; Altamirano-Gutiérrez, A.; Jiménez-Sandoval, O. Osmium-ruthenium carbonyl clusters as methanol tolerant electrocatalysts for oxygen reduction. J. Power Sources 2009, 188, 387–396. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report; LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2004; pp. 86–748. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Mochalov, A.G.; Dmitrenko, G.G.; Rudashevsky, N.S.; Zhernovsky, I.V.; Boldyreva, M.M. Hexaferrum (Fe,Ru), (Fe,Os), (Fe,Ir)—A new mineral. Zap. Vseross. Mineral. Obs. 1998, 127, 41–51. [Google Scholar]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectros. Relat. Phenomena 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.W.; Jung, W.G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Jagiełło, J.; Chlanda, A.; Baran, M.; Gwiazda, M.; Lipińska, L. Synthesis and characterization of graphene oxide and reduced graphene oxide composites with inorganic nanoparticles for biomedical applications. Nanomaterials 2020, 10, 1846. [Google Scholar] [CrossRef]
- Saxena, M.; Sarkar, S. Fluorescence imaging of human erythrocytes by carbon nanoparticles isolated from food stuff and their fluorescence enhancement by blood plasma. Mater. Express 2013, 3, 201–209. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, O.H.; Park, I.S.; Cho, Y.H.; Sung, Y.E. Excellent Performances of Modified RuOs Bimetallic Materials as Anode Catalysts for Polymer Electrolyte Membrane Fuel Cells. Electrocatalysis 2017, 9, 352–358. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Roh, J.S. Analysis of activation process of carbon black based on structural parameters obtained by XRD analysis. Crystals 2021, 11, 153. [Google Scholar] [CrossRef]
- Borja-Arco, E.; Jiménez-Sandoval, O.; Magallón-Cacho, L.; Sebastian, P.J. Ruthenium based electrocatalysts for hydrogen oxidation, prepared by a microwave assisted method. J. Power Sources 2014, 246, 438–442. [Google Scholar] [CrossRef]
- Altamirano-Gutiérrez, A.; Jiménez-Sandoval, O.; Uribe-Godínez, J.; Castellanos, R.H.; Borja-Arco, E.; Olivares-Ramírez, J.M. Methanol resistant ruthenium electrocatalysts for oxygen reduction synthesized by pyrolysis of Ru3(CO)12 in different atmospheres. Int. J. Hydrog. Energy 2009, 34, 7983–7994. [Google Scholar] [CrossRef]
- Gileadi, E. Electrode Kinetics for Chemists, Chemical Engineers and Materials Scientists; VCH Publishers Inc.: New York, NY, USA, 1993. [Google Scholar]
- Borja Arco, E.J. Desarrollo de Nuevos Electrocatalizadores Para su Aplicación como Cátodos y Ánodos en Celdas de Combustible Tipo PEM de Hidrógeno y Metanol. Ph.D. Thesis, CINVESTAV, Querétaro, Mexico, 2009. [Google Scholar]
- Mello, R.; Ticianelli, E.A. Kinetic study of the hydrogen oxidation reaction on platinum and Nafion covered platinum electrodes. Electrochem. Acta 1997, 42, 1031–1039. [Google Scholar] [CrossRef]
- Borja-Arco, E.; Jiménez-Sandoval, O.; Escalante-García, J.; Sandoval-González, A.; Sebastian, P.J. Microwave assisted synthesis of ruthenium electrocatalysts for oxygen reduction reaction in the presence and absence of aqueous methanol. Int. J. Hydrog. Energy 2011, 36, 103–110. [Google Scholar] [CrossRef]
- Borja-Arco, E.; Jiménez-Sandoval, O.; Escalante-García, J.; Magallón-Cacho, L.; Sebastian, P.J. Microwave Assisted Synthesis of Osmium Electrocatalysts for the Oxygen Reduction Reaction in the Absence and Presence of Aqueous Methanol. Int. J. Electrochem. 2011, 2011, 830541. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, K. Electrochemical Oxygen Technology; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
| Ink | Electrocatalyst | Nafion | Isopropyl Alcohol |
|---|---|---|---|
| HOR | 1 mg | 10 µL | 90 µL |
| 153 µg cm−2 | 255 µg cm−2 | --- | |
| ORR | 2 mg | 20 µL | 20 µL |
| 764 µg cm−2 | 1274 µg cm−2 | --- |
| Element | Ru | Os | O |
|---|---|---|---|
| wt% | 67.18 | 17.26 | 15.55 |
| at% | 38.48 | 5.25 | 56.27 |
| Phase | Lattice Parameters | wt% | Crystallite Size [nm] | ||
|---|---|---|---|---|---|
| a | b | c | |||
| Ru | 2.748192 | 2.748192 | 4.469018 | 71.953 | 6.21 |
| σ | 0.003019 | 0.003019 | 0.006282 | 0.831 | 0.31 * |
| Os | 2.729110 | 2.729110 | 4.311171 | 28.047 | 6.90 |
| σ | 0.001234 | 0.001234 | 0.002242 | 0.603 | 0.15 * |
| Electrocatalyst | EOC (V/NHE) | b (mV decade−1) | (1 − α) | jo (mA cm−2) |
|---|---|---|---|---|
| * OsRu | 0.0 | 77 | 0.770 | 1.246 |
| ** OsRu | 0.0 | 133 | 0.445 | 1.420 |
| Ru [7] | 0.0 | 49 | 0.601 | 0.113 |
| Os [6] | 0.0 | 43 | 0.416 | 0.031 |
| OsxRuy(CO)n [28] | 0.0 | 41 | 0.544 | 0.153 |
| ** 30 wt% Pt/C | 0.0 | 70 | 0.85 | 0.55 |
| Electrocatalyst | CH3OH [mol L−1] | EOC (V/NHE) | b (mV decade−1) | α | jo × 10−5 (mA cm−2) |
|---|---|---|---|---|---|
| * OsRu | 0.0 | 0.830 | 178 | 0.3261 | 18.0 |
| 2.0 | 0.805 | 145 | 0.4014 | 7.0 | |
| ** OsRu | 0.0 | 0.846 | 184 | 0.3158 | 63.8 |
| Ru [30] | 0.0 | 0.851 | 154 | 0.3828 | 26.9 |
| 2.0 | 0.846 | 160 | 0.3699 | 29.9 | |
| Os [31] | 0.0 | 0.761 | 332 | 0.1805 | 44.9 |
| 2.0 | 0.598 | 262 | 0.2262 | 6.5 | |
| OsxRuy(CO)n [13] | 0.0 | 0.815 | 129 | 0.4601 | 2.02 |
| 2.0 | 0.815 | 132 | 0.4475 | 2.00 | |
| * 30 wt% Pt/C | 0.0 | 0.987 | 103 | 0.5618 | 48.42 |
| ** 30 wt% Pt/C | 0.0 | 0.990 | 93 | 0.6230 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selva-Ochoa, Á.; Su-Gallegos, J.; Sebastian, P.J.; Magallón-Cacho, L.; Borja-Arco, E. Hydrogen Oxidation and Oxygen Reduction Reactions on an OsRu-Based Electrocatalyst Synthesized by Microwave Irradiation. Materials 2021, 14, 5692. https://doi.org/10.3390/ma14195692
Selva-Ochoa Á, Su-Gallegos J, Sebastian PJ, Magallón-Cacho L, Borja-Arco E. Hydrogen Oxidation and Oxygen Reduction Reactions on an OsRu-Based Electrocatalyst Synthesized by Microwave Irradiation. Materials. 2021; 14(19):5692. https://doi.org/10.3390/ma14195692
Chicago/Turabian StyleSelva-Ochoa, Ángela, Javier Su-Gallegos, Pathiyammattom Joseph Sebastian, Lorena Magallón-Cacho, and Edgar Borja-Arco. 2021. "Hydrogen Oxidation and Oxygen Reduction Reactions on an OsRu-Based Electrocatalyst Synthesized by Microwave Irradiation" Materials 14, no. 19: 5692. https://doi.org/10.3390/ma14195692
APA StyleSelva-Ochoa, Á., Su-Gallegos, J., Sebastian, P. J., Magallón-Cacho, L., & Borja-Arco, E. (2021). Hydrogen Oxidation and Oxygen Reduction Reactions on an OsRu-Based Electrocatalyst Synthesized by Microwave Irradiation. Materials, 14(19), 5692. https://doi.org/10.3390/ma14195692