Effect of Free Volume on Curcumin Release from Various Polymer-Based Composite Films Analyzed Using Positron Annihilation Lifetime Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Curcumin-Added Films
2.2.1. Natural Biopolymer-Based Films
2.2.2. Bioplastic-Based Films
2.3. Curcumin Release Test
2.4. Positron Annihilation Spectroscopy Analysis
3. Results and Discussion
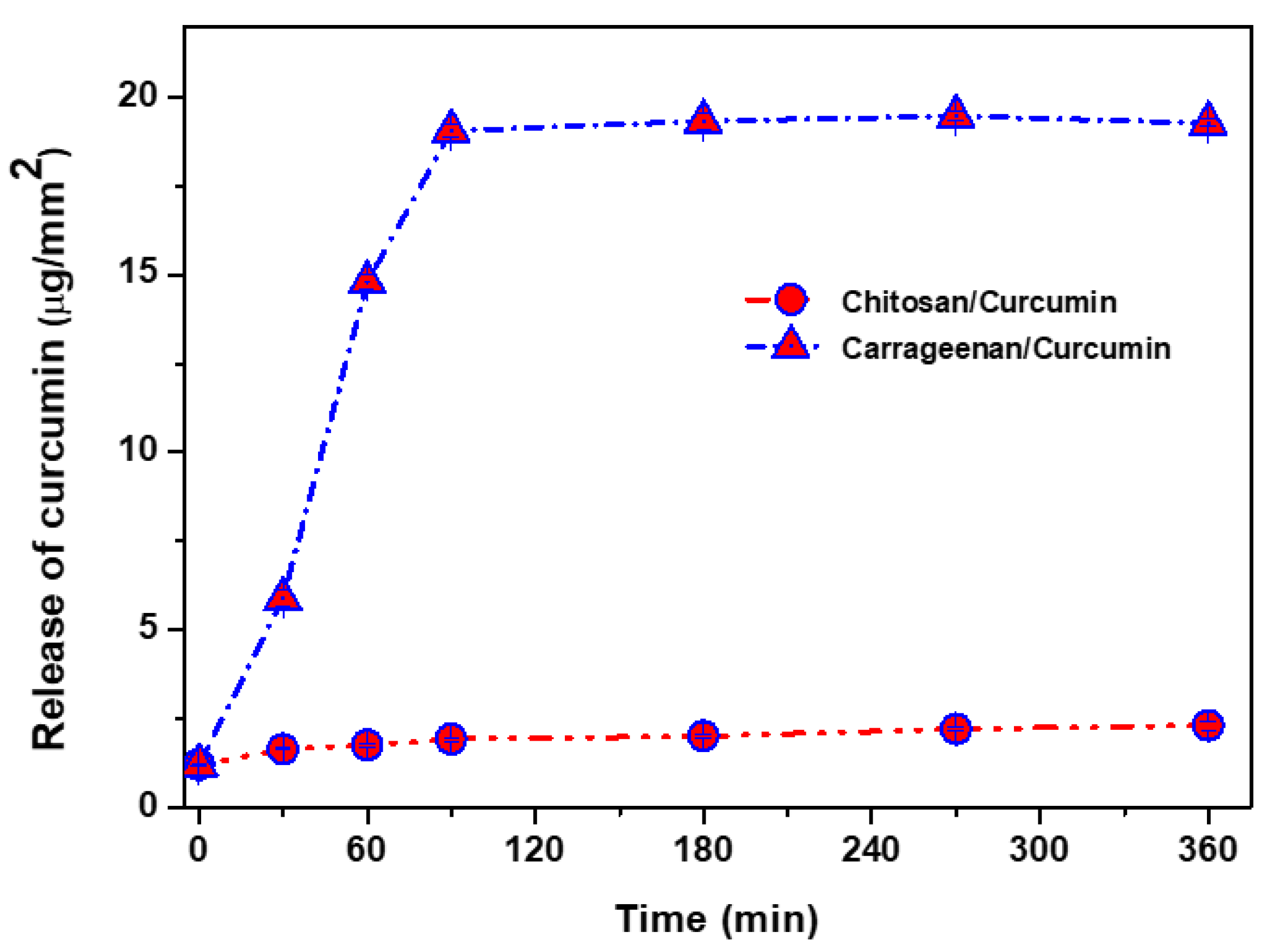
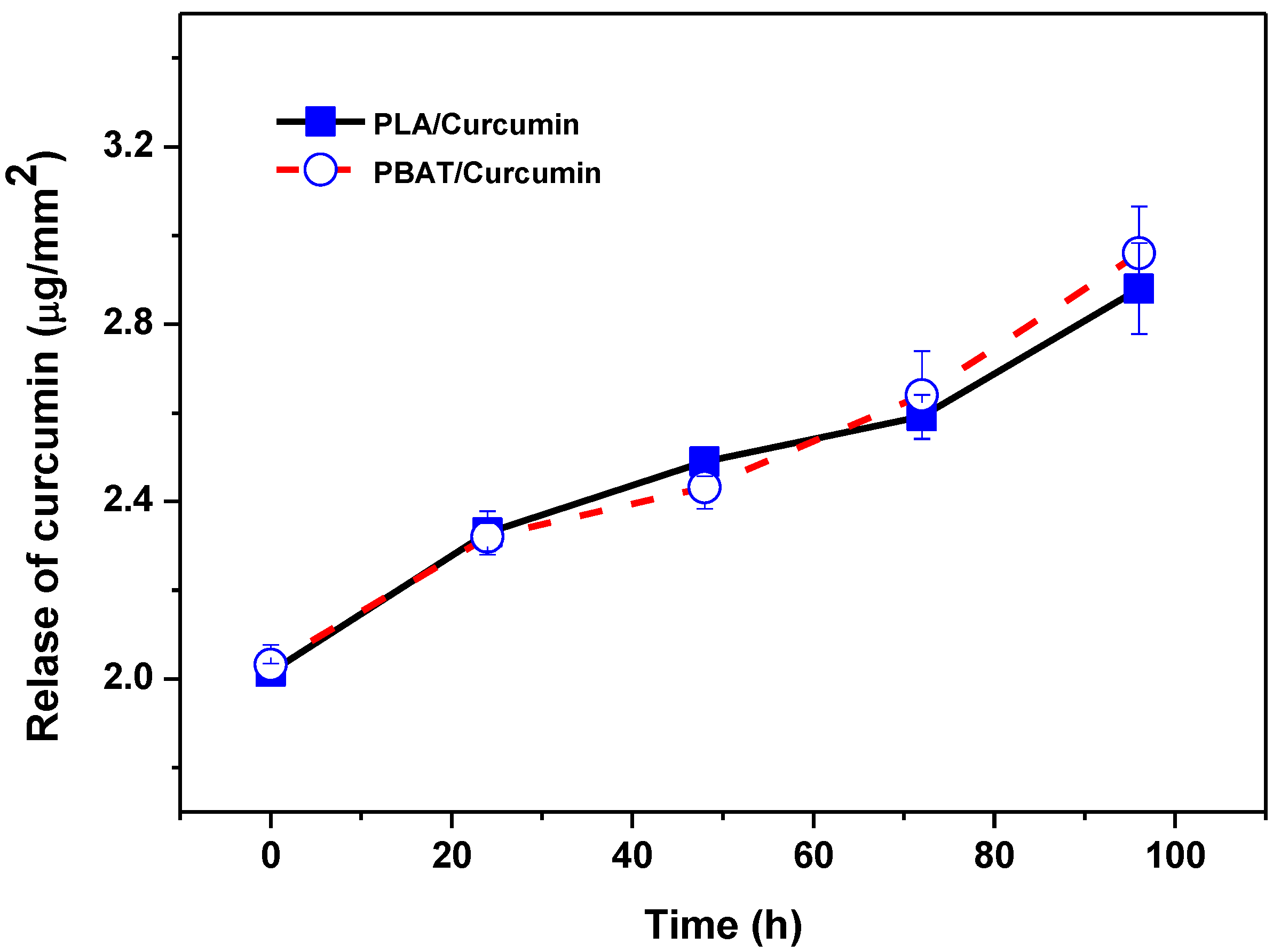
3.1. Curcumin Release Test
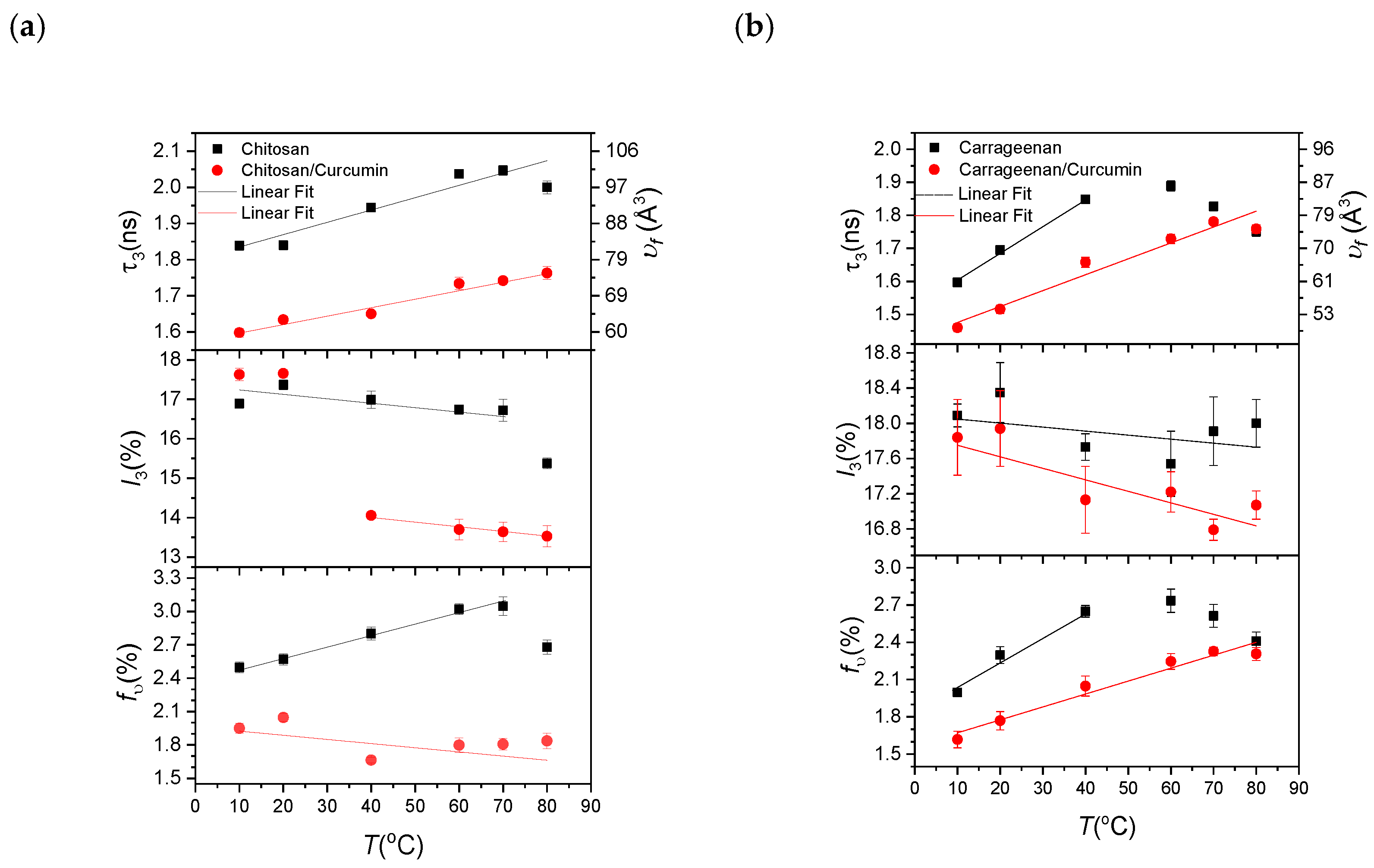
3.2. PALS Results
3.2.1. Chitosan- and Carrageenan-Based Films as Natural Biopolymers
3.2.2. PLA- and PBAT-Based Films as Bioplastic Polymers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Rhim, J.-W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the gap for bioplastic use in food packaging: An update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Antioxidant and antimicrobial poly(vinyl alcohol)-based films incorporated with grapefruit seed extract and curcumin. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Zia, J.; Paul, U.C.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Low-density polyethylene/curcumin melt extruded composites with enhanced water vapor barrier and antioxidant properties for active food packaging. Polymer 2019, 175, 137–145. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H.; Abd Rahman, N.; Nai-Shang, L. pH-responsive poly(lactic acid)/sodium carboxymethyl cellulose film for enhanced delivery of curcumin. J. Drug Deliv. Sci. Technol. 2020, 58, 101787. [Google Scholar] [CrossRef]
- Pan, J.; Cao, D.; Ma, X.; Yang, J. Preparation, characterization and in vitro release properties of pectin-based curcumin film. Korean J. Chem. Eng. 2019, 36, 822–827. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of bioactive functional poly (lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Curcumin incorporated poly(butylene adipate-co-terephthalate) film with improved water vapor barrier and antioxidant properties. Materials 2020, 13, 4369. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of antimicrobial and antioxidant gelatin/curcumin composite films for active food packaging application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-González, L.; Cháfer, M.; González-Martínez, C.; Chiralt, A.; Desobry, S. Study of the release of limonene present in chitosan films enriched with bergamot oil in food simulants. J. Food Eng. 2011, 105, 138–143. [Google Scholar] [CrossRef]
- Akdeniz, G.; Yahsi, U.; Tav, C. Viscous behavior of PS, PP, and ABS in terms of temperature and pressure-dependent hole fraction. J. Appl. Polym. Sci. 2010, 117, 110–113. [Google Scholar] [CrossRef]
- Sahin, F.; Tav, C.; Yahsi, U. Linking the viscous and vacancy behavior of mixtures of high-molecular-weight hydrocarbons. Int. J. Thermophys. 2006, 27, 1501–1514. [Google Scholar] [CrossRef]
- Dinc, F.S.; Yahsi, U.; Sedlacek, T. Interrelationships of Pressure-Dependent Hole Fraction and Elongational Viscosity in Polymer Melts. Adv. Polym. Technol. 2019, 2019, 9493769. [Google Scholar] [CrossRef]
- Sahin-Dinc, F. Hole fraction dependence on linear viscosity of PS, PP, and ABS. Int. Polym. Proc. 2015, 30, 585–592. [Google Scholar] [CrossRef]
- Sahin-Dinc, F.; Sorrentino, A.; Tav, C.; Yahsi, U. The effect of hole fraction on viscosity in atactic and syndiotactic polystyrenes. Int. J. Thermophys. 2015, 36, 3239–3254. [Google Scholar] [CrossRef]
- Yahsi, U. Interrelationships between P-V-T and flow behavior of linear and non linear hydrocarbons. Polym. Eng. Sci. 1998, 38, 464–470. [Google Scholar] [CrossRef]
- Yahsi, U.; Sahin, F. Linking the viscous and vacancy behavior of high molecular weight hydrocarbons. Rheol. Acta 2004, 43, 159–167. [Google Scholar]
- Utracki, L.A.; Sedlacek, T. Free volume dependence of polymer viscosity. Rheol. Acta 2006, 46, 479–494. [Google Scholar] [CrossRef] [Green Version]
- Yahsi, U.; Deligöz, H.; Tav, C.; Ulutaş, K.; Değer, D.; Yılmaztürk, S.; Erdemci, G.; Yilmazoglu, M.; Yakut, Ş. Ionic conductivity of PVdF-co-HFP/LiClO4 in terms of free volume defects probed by positron annihilation lifetime spectroscopy. Radiat. Eff. Defects Solids 2019, 174, 214–228. [Google Scholar] [CrossRef]
- Yahsi, U.; Ulutas, K.; Tav, C.; Deger, D. On the ionic conductivity of polymer electrolytes in terms of hole fraction. J. Polym. Sci. B Polym. Phys. 2008, 46, 2249–2254. [Google Scholar] [CrossRef]
- Yahsi, U.; Coskun, B.; Yumak, A.; Boubaker, K.; Tav, C. Relaxation time of polypropylene glycol and polypropylene glycol dimethyl ether-like polymers in terms of fluid-phase temperature and pressure-dependent hole fraction. Eur. Polym. J. 2015, 68, 226–232. [Google Scholar] [CrossRef]
- Sharma, S.K.; Prakash, J.; Sudarshan, K.; Sen, D.; Mazumder, S.; Pujari, P.K. Structure at interphase of poly(vinyl alcohol)-SiC nano Fi Ber composite and its impact on mechanical properties: Positron annihilation and small-angle X-ray scattering studies. Macromolecules 2015, 48, 5706–5713. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sudarshan, K.; Sahu, M.; Pujari, P.K. Investigation of free volume characteristics of the interfacial layer in poly(methyl methacrylate)-alumina nanocomposite and its role in thermal behavior. RSC Adv. 2016, 6, 67997–68004. [Google Scholar] [CrossRef]
- Dryzek, E.; Wróbel, M.; Juszyńska-gałązka, E. Free-volume and tensile properties of glass fibre reinforced polyamide 6 composites. Acta Phys. Pol. A. 2017, 132, 1501–1505. [Google Scholar] [CrossRef]
- Jean, Y.C.; Mallon, P.E.; Schrader, D.M. (Eds.) Principles and Applications of Positron and Positronium Chemistry; World Scientific: Singapore, 2003; p. 424. [Google Scholar]
- Kansy, J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers. Detect. Assoc. Equip. 1996, 374, 235–244. [Google Scholar] [CrossRef]
- Eldrup, M.; Lightbody, D.; Sherwood, J.N. The temperature dependence of positron lifetimes in solid pivalic acid. Chem. Phys. 1981, 63, 51–58. [Google Scholar] [CrossRef]
- Tao, S.J. Positronium annihilation in molecular substances. J. Chem. Phys. 1972, 56, 5499–5510. [Google Scholar] [CrossRef]
- Yu, Z.; Yahsi, U.; McGervey, J.; Jamieson, A.M.; Simha, R. Molecular weight-dependence of free volume in polystyrene studied by positron annihilation measurements. J. Polym. Sci. B Polym. Phys. 1994, 32, 2637–2644. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Zheng, W.; Meyer, E.F.; McGervey, J.D.; Jamieson, A.M.; Simha, R. Free volume and physical aging of poly(vinyl acetate) studied by positron annihilation. Macromolecules 2020, 22, 2302–2306. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of chitosan-based functional nanocomposite films: Effect of quercetin-loaded chitosan nanoparticles. Food Hydrocoll. 2021, 121, 107065. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Effect of chitosan modified halloysite on the physical and functional properties of pullulan/chitosan biofilm integrated with rutin. Appl. Clay Sci. 2021, 211, 106205. [Google Scholar] [CrossRef]
- Roussenova, M.; Hughes, D.; Enrione, J.; Diaz-Calderon, P.; Sivaniah, E.; Song, Q.; Ubbink, J.; Beavis, P.; Swain, A.; Alam, M. Free volume, molecular mobility, and polymer structure: Towards the rational design of multifunctional materials. Acta Phys. Pol. A 2014, 125, 801–805. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A.D.; Schut, H.; Picken, S.J. The free volume in acrylic resin/laponite nanocomposite coatings. Eur. Polym. J. 2011, 47, 264–272. [Google Scholar] [CrossRef]
- Dickmann, M.; Tarter, S.; Egger, W.; Pegoretti, A.; Rigotti, D.; Brusa, R.S.; Checchetto, R. Interface nanocavities in poly(lactic acid) membranes with dispersed cellulose nanofibrils: Their role in the gas barrier performances. Polymer 2020, 202, 122729. [Google Scholar] [CrossRef]

| Films | T (°C) | τ2 (ns) (±0.003) | I2 (%) (±0.8) | τ3 (ns) (±0.01) | I3 (%) (±0.3) | υf (Å3) (±1.4) | fυ (%) (±0.11) |
|---|---|---|---|---|---|---|---|
| Chitosan (chitosan/curcumin) | 10 | 0.378 (0.358) | 68.4 (77.0) | 1.84 (1.60) | 16.9 (17.6) | 82.1 (61.4) | 2.50 (1.95) |
| 20 | 0.372 (0.352) | 70.9 (79.9) | 1.84 (1.63) | 17.4 (17.7) | 82.2 (64.4) | 2.57 (2.05) | |
| 40 | 0.368 (0.336) | 72.5 (70.0) | 1.94 (1.65) | 17.0 (14.1) | 91.6 (65.7) | 2.80 (1.66) | |
| 60 | 0.372 (0.343) | 70.8 (66.1) | 2.05 (1.73) | 16.7 (13.7) | 100 (72.6) | 3.02 (1.80) | |
| 70 | 0.361 (0.341) | 76.1 (67.8) | 2.05 (1.74) | 16.7 (13.6) | 101 (73.6) | 3.05 (1.81) | |
| 80 | 0.375 (0.342) | 69.6 (68.2) | 2.00 (1.76) | 15.4 (13.5) | 96.8 (75.4) | 2.68 (1.84) | |
| Carrageenan (carrageenan/curcumin) | 10 | 0.359 (0.352) | 75.9 (75.9) | 1.60 (1.46) | 18.1 (17.8) | 61.3 (50.4) | 2.00 (1.62) |
| 20 | 0.354 (0.352) | 76.7 (76.5) | 1.70 (1.52) | 18.4 (17.9) | 69.5 (54.8) | 2.30 (1.77) | |
| 40 | 0.358 (0.361) | 76.8 (74.9) | 1.85 (1.66) | 17.7 (17.1) | 83.0 (66.4) | 2.65 (2.05) | |
| 60 | 0.361 (0.357) | 76.7 (76.7) | 1.89 (1.73) | 17.5 (17.2) | 86.6 (72.6) | 2.73 (2.25) | |
| 70 | 0.358 (0.366) | 74.7 (73.6) | 1.83 (1.78) | 17.9 (16.8) | 81.0 (77.0) | 2.61 (2.33) | |
| 80 | 0.352 (0.361) | 77.7 (75.0) | 1.75 (1.76) | 18.0 (17.1) | 74.4 (75.1) | 2.41 (2.31) |
| Films | T (°C) | τ2 (ns) (±0.003) | I2 (%) (±0.8) | τ3 (ns) (±0.01) | I3 (%) (±0.3) | υf (Å3) (±1.6) | fυ (%) (±0.11) |
|---|---|---|---|---|---|---|---|
| PLA (PLA/curcumin) | 10 | 0.382 (0.376) | 75.7 (71.8) | 2.07 (1.82) | 15.3 (14.7) | 103 (80.3) | 2.84 (2.13) |
| 20 | 0.384 (0.379) | 74.6 (71.5) | 2.09 (1.83) | 15.0 (14.7) | 105 (80.8) | 2.84 (2.14) | |
| 40 | 0.374 (0.379) | 80.0 (73.1) | 2.17 (1.89) | 15.3 (14.7) | 113 (86.8) | 3.10 (2.29) | |
| 60 | 0.378 (0.402) | 78.4 (61.9) | 2.31 (2.20) | 14.6 (13.4) | 127 (116) | 3.34 (2.80) | |
| 70 | 0.380 (0.379) | 77.2 (72.9) | 2.34 (2.14) | 14.8 (14.0) | 130 (110) | 3.47 (2.76) | |
| 80 | 0.382 (0.381) | 76.2 (72.1) | 2.36 (2.17) | 14.9 (14.1) | 132 (113) | 3.54 (2.86) | |
| PBAT (PBAT/curcumin) | 10 | 0.357 (0.346) | 67.1 (70.7) | 2.10 (2.03) | 17.8 (15.4) | 106 (99.4) | 3.40 (2.75) |
| 20 | 0.365 (0.350) | 63.2 (69.5) | 2.15 (2.10) | 18.0 (15.5) | 111 (106) | 3.61 (2.96) | |
| 40 | 0.362 (0.360) | 65.7 (66.8) | 2.29 (2.25) | 18.7 (16.4) | 124 (121) | 4.18 (3.57) | |
| 60 | 0.361 (0.360) | 65.6 (69.5) | 2.42 (2.40) | 19.4 (17.3) | 138 (136) | 4.80 (4.25) | |
| 70 | 0.360 (0.351) | 64.8 (68.7) | 2.42 (2.40) | 20.4 (18.7) | 138 (136) | 5.04 (4.57) | |
| 80 | 0.360 (0.367) | 64.9 (61.5) | 2.46 (2.45) | 20.6 (18.8) | 142 (141) | 5.25 (4.76) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhim, J.-W.; Kuzeci, S.; Roy, S.; Akti, N.; Tav, C.; Yahsi, U. Effect of Free Volume on Curcumin Release from Various Polymer-Based Composite Films Analyzed Using Positron Annihilation Lifetime Spectroscopy. Materials 2021, 14, 5679. https://doi.org/10.3390/ma14195679
Rhim J-W, Kuzeci S, Roy S, Akti N, Tav C, Yahsi U. Effect of Free Volume on Curcumin Release from Various Polymer-Based Composite Films Analyzed Using Positron Annihilation Lifetime Spectroscopy. Materials. 2021; 14(19):5679. https://doi.org/10.3390/ma14195679
Chicago/Turabian StyleRhim, Jong-Whan, Saygin Kuzeci, Swarup Roy, Necmettin Akti, Cumali Tav, and Ugur Yahsi. 2021. "Effect of Free Volume on Curcumin Release from Various Polymer-Based Composite Films Analyzed Using Positron Annihilation Lifetime Spectroscopy" Materials 14, no. 19: 5679. https://doi.org/10.3390/ma14195679
APA StyleRhim, J.-W., Kuzeci, S., Roy, S., Akti, N., Tav, C., & Yahsi, U. (2021). Effect of Free Volume on Curcumin Release from Various Polymer-Based Composite Films Analyzed Using Positron Annihilation Lifetime Spectroscopy. Materials, 14(19), 5679. https://doi.org/10.3390/ma14195679








