The Application of Spray-Dried and Reconstituted Flaxseed Oil Cake Extract as Encapsulating Material and Carrier for Probiotic Lacticaseibacillus rhamnosus GG
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation, Fermentation and Spray Drying of Flaxseed Oil Cake Extract with LGG (FOCE-LGG)
2.3. Powders Characterization
2.4. FOCE-LGG Reconstitution, Determination of pH, Free Amino Acids, Sulfhydryl Groups (-SH) and Disulfide Bonds (-S-S-) Contents
2.5. Enumeration of LGG Counts
2.6. Gastrointestinal Tract Simulator (GITS)
2.6.1. Simulated Digestion
Saliva Preparation
Gastric Medium Preparation
Intestinal Juice Preparation
Mucin Agar Preparation
2.6.2. Characterization of Survival in GITS
2.7. Determination of Probiotic Properties of LGG on GITS Stages
2.7.1. Cholesterol Binding Activity
2.7.2. Surface Hydrophobicity Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Powders
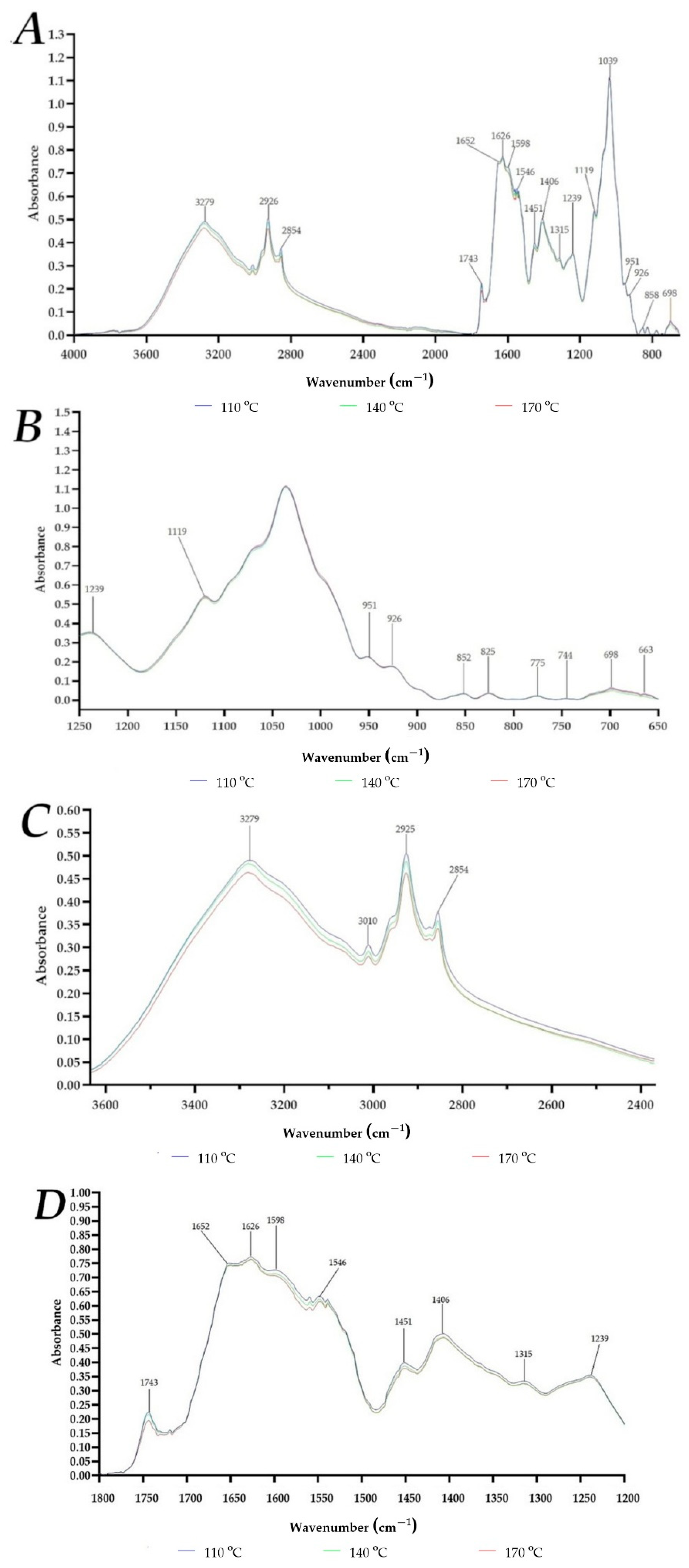
3.2. LGG Survivability after Spray Drying, FOCE Acidity, Free Amino Acids, Sulfydryl Groups and Disulfide Bonds Contents
3.3. LGG Survivability in Saliva Juice, Gastric Juice and Intestinal Juice
3.4. Maintenance of LGG Probiotic Properties—Hydrophobicity, Adhesion to Mucin and Cholesterol Reduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akanny, E.; Bourgeois, S.; Bonhommé, A.; Commun, C.; Doleans-Jordheim, A.; Bessueille, F.; Bordes, C. Development of enteric polymer-based microspheres by spray-drying for colonic delivery of Lactobacillus rhamnosus GG. Int. J. Pharm. 2020, 584, 119414. [Google Scholar] [CrossRef] [PubMed]
- Westerik, N.; Kort, R.; Sybesma, W.; Reid, G. Lactobacillus rhamnosus probiotic food as a tool for empowerment across the value chain in Africa. Front. Microbiol. 2018, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Doron, S.; Hibberd, P.L.; Goldin, B.; Thorpe, C.; McDermott, L.; Snydman, D.R. Effect of Lactobacillus rhamnosus GG administration on vancomycin-resistant Enterococcus colonization in adults with comorbidities. Antimicrob. Agents Chemother. 2015, 59, 4593–4599. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Polk, D.B. Lactobacillus rhamnosus GG: An Updated Strategy to Use Microbial Products to Promote Health. Funct. Food Rev. 2012, 4, 77–84. [Google Scholar]
- De Keersmaecker, S.C.J.; Verhoeven, T.L.A.; Desair, J.; Marchal, K.; Vanderleyden, J.; Nagy, I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol. Lett. 2006, 259, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Princivalli, M.S.; Paoletti, C.; Magi, G.; Palmieri, C.; Ferrante, L.; Facinelli, B. Lactobacillus rhamnosus GG inhibits invasion of cultured human respiratory cells by prtF1-positive macrolide-resistant group A streptococci. Lett. Appl. Microbiol. 2009, 48, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.; Vidanarachchi, J.; Rocha, R.; Cruz, A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.J.; Frutos, M.J. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Szparaga, A.; Tabor, S.; Kocira, S.; Czerwińska, E.; Kuboń, M.; Płóciennik, B.; Findura, P. Survivability of probiotic bacteria in model systems of non-fermented and fermented coconut and hemp milks. Sustainability 2019, 11, 6093. [Google Scholar] [CrossRef] [Green Version]
- Lipan, L.; Rusu, B.; Sendra, E.; Hernández, F.; Vázquez-Araújo, L.; Vodnar, D.C.; Carbonell-Barrachina, Á.A. Spray drying and storage of probiotic-enriched almond milk: Probiotic survival and physicochemical properties. J. Sci. Food Agric. 2020, 100, 3697–3708. [Google Scholar] [CrossRef]
- Su, Y.; Zheng, X.; Zhao, Q.; Fu, N.; Xiong, H.; Wu, W.D.; Chen, X.D. Spray drying of Lactobacillus rhamnosus GG with calcium-containing protectant for enhanced viability. Powder Technol. 2019, 358, 87–94. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of flaxseed oil cake residual from cold-press oil production as a material for preparation of spray-dried functional powders for food applications as emulsion stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Łopusiewicz, Ł. Formulation and Evaluation of Spray-Dried Reconstituted Flaxseed Oil-In-Water Emulsions Based on Flaxseed Oil Cake Extract as Emulsifying and Stabilizing Agent. Foods 2021, 10, 256. [Google Scholar] [CrossRef]
- Azam, M.; Saeed, M.; Pasha, I.; Shahid, M. A prebiotic-based biopolymeric encapsulation system for improved survival of Lactobacillus rhamnosus. Food Biosci. 2020, 37, 100679. [Google Scholar] [CrossRef]
- Lai, K.; How, Y.; Pui, L. Microencapsulation of Lactobacillus rhamnosus GG with flaxseed mucilage using co-extrusion technique. J. Microencapsul. 2021, 38, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Drusch, S.; Brodkorb, A. Advances of plant-based structured food delivery systems on the in vitro digestibility of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2021, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shim, Y.Y.; Tse, T.J.; Wang, Y.; Reaney, M.J.T. Flaxseed gum a versatile natural hydrocolloid for food and non-food applications. Trends Food Sci. Technol. 2018, 75, 146–157. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Azhar, M.D.; Hashib, S.A.; Ibrahim, U.K.; Rahman, N.A. Development of carrier material for food applications in spray drying technology: An overview. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Petrović, T.; Dimitrijević-Branković, S.; Lević, S.; Nedović, V.; Kourkoutas, Y. Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT Food Sci. Technol. 2016, 71, 169–174. [Google Scholar] [CrossRef]
- Ying, D.; Sun, J.; Sanguansri, L.; Weerakkody, R.; Augustin, M.A. Enhanced survival of spray-dried microencapsulated Lactobacillus rhamnosus GG in the presence of glucose. J. Food Eng. 2012, 109, 597–602. [Google Scholar] [CrossRef]
- Avila-Reyes, S.V.; Garcia-Suarez, F.J.; Jiménez, M.T.; San Martín-Gonzalez, M.F.; Bello-Perez, L.A. Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the viability. Carbohydr. Polym. 2014, 102, 423–430. [Google Scholar] [CrossRef]
- Alvarado-Reveles, O.; Fernández-Michel, S.; Jiménez-Flores, R.; Cueto-Wong, C.; Vázquez-Moreno, L.; Montfort, G.R.C. Survival and Goat Milk Acidifying Activity of Lactobacillus rhamnosus GG Encapsulated with Agave Fructans in a Buttermilk Protein Matrix. Probiotics Antimicrob. Proteins 2019, 11, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Sunny-Roberts, E.O.; Knorr, D. The protective effect of monosodium glutamate on survival of Lactobacillus rhamnosus GG and Lactobacillus rhamnosus E-97800 (E800) strains during spray-drying and storage in trehalose-containing powders. Int. Dairy J. 2009, 19, 209–214. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Hornowska, Ł.; Pobiega, K.; Gniewosz, M.; Witrowa-Rajchert, D. The influence of Lactobacillus bacteria type and kind of carrier on the properties of spray-dried microencapsules of fermented beetroot powders. Int. J. Food Sci. Technol. 2021, 56, 2166–2174. [Google Scholar] [CrossRef]
- Golowczyc, M.A.; Silva, J.; Teixeira, P.; De Antoni, G.L.; Abraham, A.G. Cellular injuries of spray-dried Lactobacillus spp. isolated from kefir and their impact on probiotic properties. Int. J. Food Microbiol. 2011, 144, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Zouari, A.; Briard-Bion, V.; Schuck, P.; Gaucheron, F.; Delaplace, G.; Attia, H.; Ayadi, M.A. Changes in physical and biochemical properties of spray dried camel and bovine milk powders. LWT 2020, 128, 109437. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Rasika, D.M.D.; Vidanarachchi, J.K.; Rocha, R.S.; Balthazar, C.F.; Cruz, A.G.; Sant’Ana, A.S.; Ranadheera, C.S. Plant-based milk substitutes as emerging probiotic carriers. Curr. Opin. Food Sci. 2021, 38, 8–20. [Google Scholar] [CrossRef]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Mahdi Jafari, S.; Sarabandi, K.; Mohammadi, M.; Khakbaz Heshmati, M.; Pezeshki, A. Influence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Łopusiewicz, Ł. Characterization of flaxseed oil bimodal emulsions prepared with flaxseed oil cake extract applied as a natural emulsifying agent. Polymers 2020, 12, 2207. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, X.; Zhen, W.; Li, Z.; Kang, J.; Sun, X.; Wang, S.; Cui, S.W. Rheological properties and stabilizing effects of high-temperature extracted flaxseed gum on oil/water emulsion systems. Food Hydrocoll. 2021, 112, 106289. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Bienkiewicz, G.; Łopusiewicz, Ł. The influence of flaxseed oil cake extract on oxidative stability of microencapsulated flaxseed oil in spray-dried powders. Antioxidants 2021, 10, 211. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, Characterization, and Bioactivity of Non-Dairy Kefir-Like Fermented Beverage Based on Flaxseed Oil Cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of flaxseed oil cake into bioactive camembert-analogue using lactic acid bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms 2020, 8, 1266. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Bartkowiak, A.; Kwiatkowski, P. The development of novel probiotic fermented plant milk alternative from flaxseed oil cake using Lactobacillus rhamnosus GG acting as a preservative agent against pathogenic bacteria during short-term refrigerated storage. Emir. J. Food Agric. 2021, 33, 266–276. [Google Scholar]
- Khalloufi, S.; Corredig, M.; Goff, H.D.; Alexander, M. Flaxseed gums and their adsorption on whey protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2009, 23, 611–618. [Google Scholar] [CrossRef]
- Bustamante, M.; Villarroel, M.; Rubilar, M.; Shene, C. Lactobacillus acidophilus La-05 encapsulated by spray drying: Effect of mucilage and protein from flaxseed (Linum usitatissimum L.). LWT Food Sci. Technol. 2015, 62, 1162–1168. [Google Scholar] [CrossRef]
- Verruck, S.; de Liz, G.R.; Dias, C.O.; de Mello Castanho Amboni, R.D.; Prudencio, E.S. Effect of full-fat goat’s milk and prebiotics use on Bifidobacterium BB-12 survival and on the physical properties of spray-dried powders under storage conditions. Food Res. Int. 2019, 119, 643–652. [Google Scholar] [CrossRef]
- Fang, S.; Qiu, W.; Mei, J.; Xie, J. Effect of Sonication on the Properties of Flaxseed Gum Films Incorporated with Carvacrol. Int. J. Mol. Sci. 2020, 21, 1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, C.; Rubilar, M.; Jara, C.; Verdugo, M.; Sineiro, J.; Shene, C. Flaxseed and flaxseed cake as a source of compounds for food industry. J. Soil Sci. Plant Nutr. 2010, 10, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Oomah, B.D.; Mazza, G. Optimization of a spray drying process for flaxseed gum. Int. J. Food Sci. Technol. 2001, 36, 135–143. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Microencapsulating polymers for probiotics delivery systems: Preparation, characterization, and applications. Food Hydrocoll. 2021, 120, 106882. [Google Scholar] [CrossRef]
- Bustamante, M.; Laurie-Martínez, L.; Vergara, D.; Campos-Vega, R.; Rubilar, M.; Shene, C. Effect of three polysaccharides (inulin, and mucilage from chia and flax seeds) on the survival of probiotic bacteria encapsulated by spray drying. Appl. Sci. 2020, 10, 4623. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Rubilar, M.; Shene, C. Effective Lactobacillus plantarum and Bifidobacterium infantis encapsulation with chia seed (Salvia hispanica L.) and flaxseed (Linum usitatissimum L.) mucilage and soluble protein by spray drying. Food Chem. 2017, 216, 97–105. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- European Pharmacopoeia. Flow Powder, 7.1 ed.; Council of Europe: Strasbourg, France, 2011. [Google Scholar]
- Gong, K.J.; Shi, A.M.; Liu, H.Z.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2015, 170, 33–40. [Google Scholar] [CrossRef]
- Joly, C.; Gay-Quéheillard, J.; Léké, A.; Chardon, K.; Delanaud, S.; Bach, V.; Khorsi-Cauet, H. Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) and in the rat. Environ. Sci. Pollut. Res. 2013, 20, 2726–2734. [Google Scholar] [CrossRef]
- Sumeri, I.; Arike, L.; Adamberg, K.; Paalme, T. Single bioreactor gastrointestinal tract simulator for study of survival of probiotic bacteria. Appl. Microbiol. Biotechnol. 2008, 80, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, M.; Vanhoecke, B.; De Ryck, T.; Sadaghian Sadabad, M.; Pinheiro, I.; Possemiers, S.; Van Den Abbeele, P.; Derycke, L.; Bracke, M.; Pieters, J.; et al. The HMITM module: A new tool to study the Host-Microbiota Interaction in the human gastrointestinal tract in vitro. BMC Microbiol. 2014, 14, 133. [Google Scholar] [CrossRef] [Green Version]
- Oomen, A.G.; Rompelberg, C.J.M.; Bruil, M.A.; Dobbe, C.J.G.; Pereboom, D.P.K.H.; Sips, A.J.A.M. Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants. Arch. Environ. Contam. Toxicol. 2003, 44, 281–287. [Google Scholar] [CrossRef]
- Bondue, P.; Lebrun, S.; Taminiau, B.; Everaert, N.; LaPointe, G.; Crevecoeur, S.; Daube, G.; Delcenserie, V. A toddler SHIME® model to study microbiota of young children. FEMS Microbiol. Lett. 2020, 367, 16. [Google Scholar] [CrossRef]
- Van Den Abbeele, P.; Marzorati, M.; Derde, M.; De Weirdt, R.; Joan, V.; Possemiers, S.; Van De Wiele, T. Arabinoxylans, inulin and Lactobacillus reuteri 1063 repress the adherent-invasive Escherichia coli from mucus in a mucosa-comprising gut model. NPJ Biofilms Microbiomes 2016, 2, 16016. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Roos, S.; Eeckhaut, V.; MacKenzie, D.A.; Derde, M.; Verstraete, W.; Marzorati, M.; Possemiers, S.; Vanhoecke, B.; Van Immerseel, F.; et al. Incorporating a mucosal environment in a dynamic gut model results in a more representative colonization by lactobacilli. Microb. Biotechnol. 2012, 5, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Kim, W.S.; Kumura, H.; Shimazaki, K.I. Autoaggregation and surface hydrophobicity of bifidobacteria. World J. Microbiol. Biotechnol. 2008, 24, 1593–1598. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Microencapsulation of Lactobacillus acidophilus LA-5, Bifidobacterium animalis subsp. lactis BB-12 and Propionibacterium jensenii 702 by spray drying in goat’s milk. Small Rumin. Res. 2015, 123, 155–159. [Google Scholar] [CrossRef]
- Ananta, E.; Volkert, M.; Knorr, D. Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int. Dairy J. 2005, 15, 399–409. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Singh, A.K.; Kumar, D.; Sharma, H. Effect of spray and freeze drying on physico-chemical, functional, moisture sorption and morphological characteristics of camel milk powder. LWT 2020, 134, 110117. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT Food Sci. Technol. 2015, 60, 773–780. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Telang, A.M.; Thorat, B.N. Optimization of process parameters for spray drying of fermented soy milk. Dry. Technol. 2010, 28, 1445–1456. [Google Scholar] [CrossRef]
- Maciel, G.M.; Chaves, K.S.; Grosso, C.R.F.; Gigante, M.L. Microencapsulation of Lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J. Dairy Sci. 2014, 97, 1991–1998. [Google Scholar] [CrossRef] [Green Version]
- Dimitrellou, D.; Salamoura, C.; Kontogianni, A.; Katsipi, D.; Kandylis, P.; Zakynthinos, G.; Varzakas, T. Effect of milk type on the microbiological, physicochemical and sensory characteristics of probiotic fermented milk. Microorganisms 2019, 7, 274. [Google Scholar] [CrossRef] [Green Version]
- Safdar, B.; Pang, Z.; Liu, X.; Jatoi, M.A.; Mehmood, A.; Rashid, M.T.; Ali, N.; Naveed, M. Flaxseed gum: Extraction, bioactive composition, structural characterization, and its potential antioxidant activity. J. Food Biochem. 2019, 43, e13014. [Google Scholar] [CrossRef]
- Chung, M.W.Y.; Lei, B.; Li-Chan, E.C.Y. Isolation and structural characterization of the major protein fraction from NorMan flaxseed (Linum usitatissimum L.). Food Chem. 2005, 90, 271–279. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.J.; Xu, W. Comparisons on the Functional Properties and Antioxidant Activity of Spray-Dried and Freeze-Dried Egg White Protein Hydrolysate. Food Bioprocess Technol. 2012, 5, 2342–2352. [Google Scholar] [CrossRef]
- Ritter, P.; Kohler, C.; Von Ah, U. Evaluation of the passage of Lactobacillus gasseri K7 and bifidobacteria from the stomach to intestines using a single reactor model. BMC Microbiol. 2009, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basiri, S.; Haidary, N.; Shekarforoush, S.S.; Niakousari, M. Flaxseed mucilage: A natural stabilizer in stirred yogurt. Carbohydr. Polym. 2018, 187, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium. Lett. Appl. Microbiol. 2009, 49, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Vij, R.; Kapila, S.; Khan, S.H.; Kumar, N.; Meena, S.; Kapila, R. Milk fermented with probiotic strains Lactobacillus rhamnosus MTCC: 5957 and Lactobacillus rhamnosus MTCC: 5897 ameliorates the diet-induced hypercholesterolemia in rats. Ann. Microbiol. 2019, 69, 483–494. [Google Scholar] [CrossRef]
- Sivieri, K.; Morales, M.L.V.; Adorno, M.A.T.; Sakamoto, I.K.; Saad, S.M.I.; Rossi, E.A. Lactobacillus acidophilus CRL 1014 improved “gut health” in the SHIME® reactor. BMC Gastroenterol. 2013, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Lin, P.P.; Hsieh, Y.M.; Zhang, Z.Y.; Wu, H.C.; Huang, C.C. Cholesterol-Lowering Potentials of Lactic Acid Bacteria Based on Bile-Salt Hydrolase Activity and Effect of Potent Strains on Cholesterol Metabolism In Vitro and In Vivo. Sci. World J. 2014, 690752, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
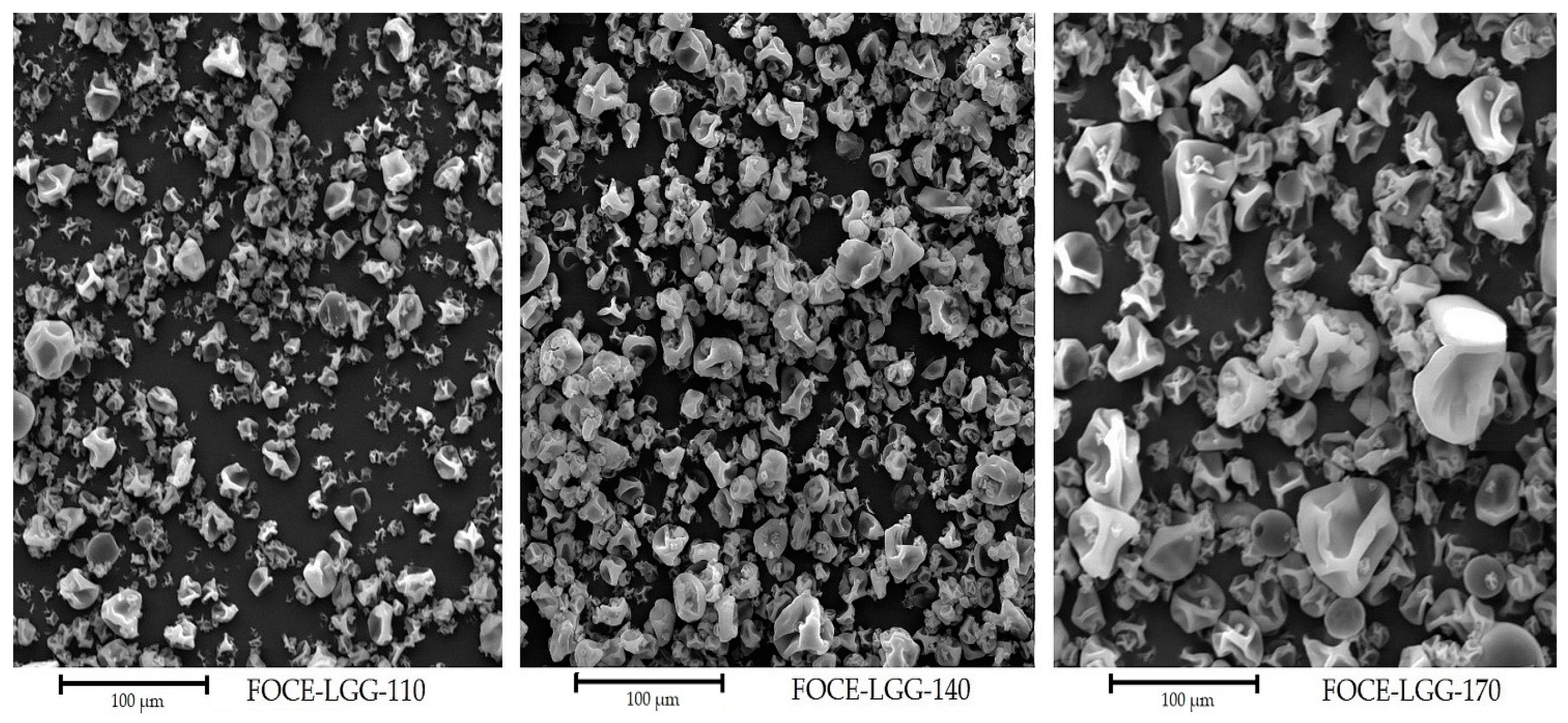

| Carr’s Index | Hausner Ratio | |
|---|---|---|
| Excellent | 0–10% | 1.00–1.11 |
| Good | 10–15% | 1.12–1.18 |
| Fair | 16–20% | 1.19–1.25 |
| Possible | 21–25% | 1.26–1.34 |
| Poor | 26–31% | 1.35–1.45 |
| Very poor | 32–37% | 1.46–1.59 |
| Very very poor | >38% | >1.60 |
| Sample | TSC (%) | D4;3 (µm) | aw | ρb (g/cm3) | ρt (g/cm3) | HR | CI (%) |
|---|---|---|---|---|---|---|---|
| FOCE | 3.11 ± 0.05 d | - | - | - | - | - | - |
| FOCE-LGG-110 | 89.01 ± 0.01 c | 32.91 ± 0.05 a | 0.453 ± 0.002 a | 0.32 ± 0.01 a | 0.37 ± 0.01 a | 1.09 ± 0.01 c | 8.57 ± 0.21 c |
| FOCE-LGG-140 | 90.81 ± 0.03 b | 35.22 ± 0.18 b | 0.307 ± 0.001 b | 0.29 ± 0.02 b | 0.35 ± 0.01 b | 1.26 ± 0.05 a | 20.05 ± 0.16 b |
| FOCE-LGG-170 | 91.13 ± 0.10 a | 42.91 ± 0.03 c | 0.297 ± 0.005 c | 0.27 ± 0.01 c | 0.34 ± 0.02 c | 1.27 ± 0.03 b | 21.62 ± 0.15 a |
| Sample | LGG Counts (log CFU/mL) | Survivability Rate (%) | pH | FAA (mg Gly/mL) | -SH (µmol/g) | -S-S- (µmol/g) |
|---|---|---|---|---|---|---|
| FOCE-LGG | 8.97 ± 0.50 a | - | 4.22 ± 0.01 d | 6.48 ± 0.11 a | 41.11 ± 0.33 a | 15.92 ± 0.17 d |
| FOCE-LGG-110 | 8.64 ± 0.32 a | 96.32 | 4.36 ± 0.01 c | 8.91 ± 0.23 b | 98.48 ± 0.92 a | 27.50 ± 0.22 c |
| FOCE-LGG-140 | 8.60 ± 0.27 a | 95.88 | 4.39 ± 0.01 b | 9.37 ± 0.17 c | 95.33 ± 0.56 b | 29.03 ± 0.15 b |
| FOCE-LGG-170 | 8.02 ± 3.41 a | 89.41 | 4.43 ± 0.01 a | 10.02 ± 0.14 d | 93.25 ± 0.33 c | 29.87 ± 0.05 a |
| FOCE-LGG | FOCE-LGG-110 | FOCE-LGG-140 | FOCE-LGG-170 | ||
|---|---|---|---|---|---|
| log CFU/mL | |||||
| SURsj | start | −0.002 ± 0.41 Aa | −0.04 ± 0.37 Aa | −0.20 ± 0.52 ABa | −0.02 ± 0.56 Aa |
| finish | −0.14 ± 0.12 ABa | −0.11 ± 0.82 Aa | −0.62 ± 0.36 ABa | −0.40 ± 1.41 ABa | |
| SURgj | start | −0.76 ± 0.71 ABa | −1.35 ± 0.34 Ba | −1.08 ± 0.48 ABa | −1.20 ± 0.14 ABa |
| finish | −3.50 ± 0.53 Ab | −4.12 ± 0.15 ABb | −4.18 ± 0.24 ABb | −4.28 ± 2.61 ABb | |
| SORij | start | −4.21 ± 0.36 Aa | −5.48 ± 0.35 Ba | −5.35 ± 0.65 ABa | −5.16 ± 0.71 Ba |
| finish | −4.39 ± 0.19 ADb | −5.83 ± 0.12 Bb | −5.97 ±0.89 BDb | −5.42 ± 0.14 BCDb | |
| Sample | SH% |
|---|---|
| FOCE-LGG | 25.77 ± 4.69 a |
| FOCE-LGG-110 | 28.95 ± 8.74 a |
| FOCE-LGG-140 | 26.75 ± 5.87 a |
| FOCE-LGG-170 | 27.56 ± 8.12 a |
| FOCE-LGG | FOCE-LGG-110 | FOCE-LGG-140 | FOCE-LGG-170 | |
|---|---|---|---|---|
| CHb—before SHIME | 23.21 ± 0.65 Aa | 22.05 ± 0.72 ABa | 21.84 ± 1.12 Ba | 20.73 ± 0.58 Ba |
| CHb—after SHIME | 8.17 ± 0.70 Ab | 7.94 ± 0.98 Ab | 7.20 ± 0.65 Ab | 5.26 ± 0.4 Bb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Bogusławska-Wąs, E.; Drozłowska, E.; Trocer, P.; Dłubała, A.; Mazurkiewicz-Zapałowicz, K.; Bartkowiak, A. The Application of Spray-Dried and Reconstituted Flaxseed Oil Cake Extract as Encapsulating Material and Carrier for Probiotic Lacticaseibacillus rhamnosus GG. Materials 2021, 14, 5324. https://doi.org/10.3390/ma14185324
Łopusiewicz Ł, Bogusławska-Wąs E, Drozłowska E, Trocer P, Dłubała A, Mazurkiewicz-Zapałowicz K, Bartkowiak A. The Application of Spray-Dried and Reconstituted Flaxseed Oil Cake Extract as Encapsulating Material and Carrier for Probiotic Lacticaseibacillus rhamnosus GG. Materials. 2021; 14(18):5324. https://doi.org/10.3390/ma14185324
Chicago/Turabian StyleŁopusiewicz, Łukasz, Elżbieta Bogusławska-Wąs, Emilia Drozłowska, Paulina Trocer, Alicja Dłubała, Kinga Mazurkiewicz-Zapałowicz, and Artur Bartkowiak. 2021. "The Application of Spray-Dried and Reconstituted Flaxseed Oil Cake Extract as Encapsulating Material and Carrier for Probiotic Lacticaseibacillus rhamnosus GG" Materials 14, no. 18: 5324. https://doi.org/10.3390/ma14185324
APA StyleŁopusiewicz, Ł., Bogusławska-Wąs, E., Drozłowska, E., Trocer, P., Dłubała, A., Mazurkiewicz-Zapałowicz, K., & Bartkowiak, A. (2021). The Application of Spray-Dried and Reconstituted Flaxseed Oil Cake Extract as Encapsulating Material and Carrier for Probiotic Lacticaseibacillus rhamnosus GG. Materials, 14(18), 5324. https://doi.org/10.3390/ma14185324








