Selective Laser Melting of Hydroxyapatite: Perspectives for 3D Printing of Bioresorbable Ceramic Implants
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
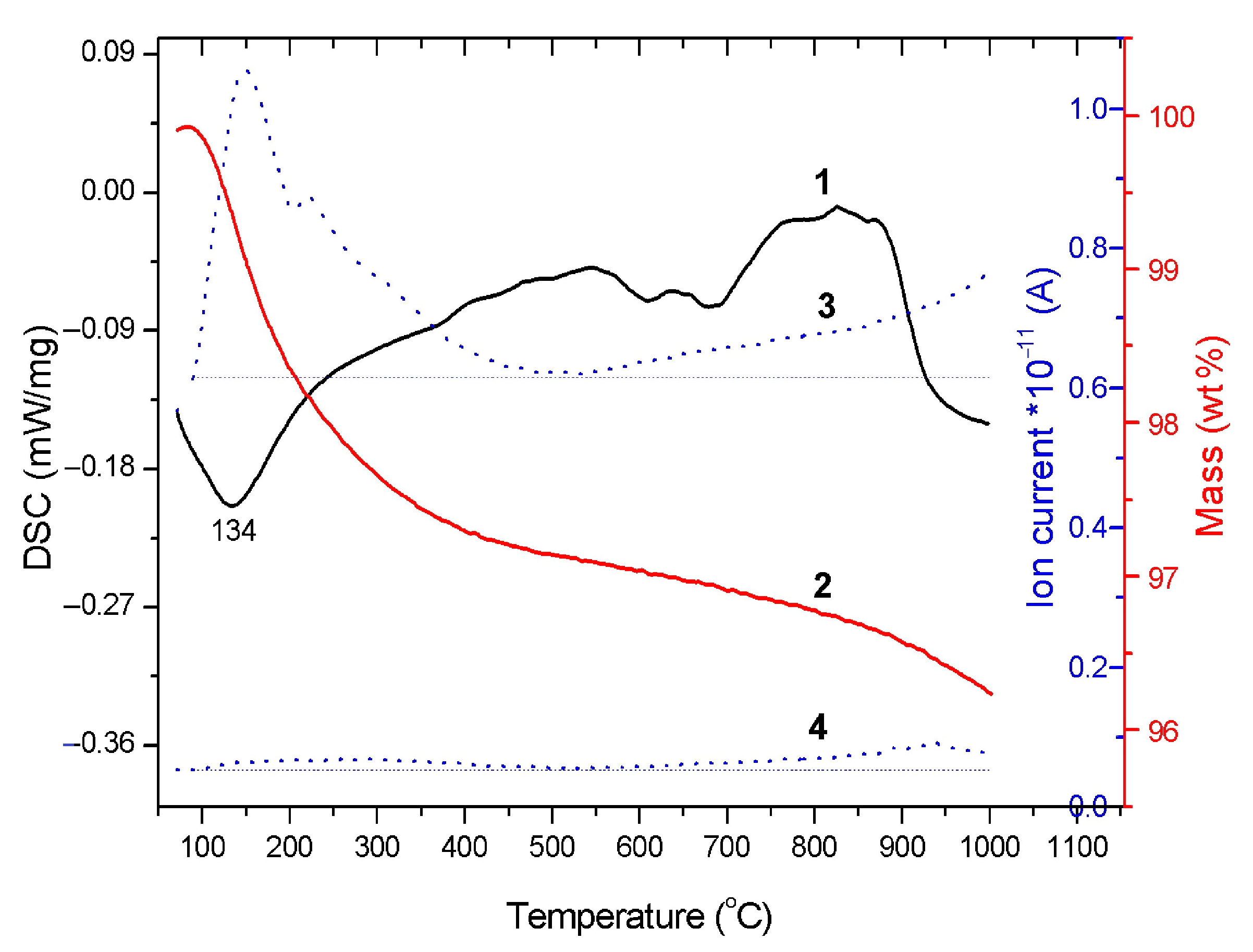
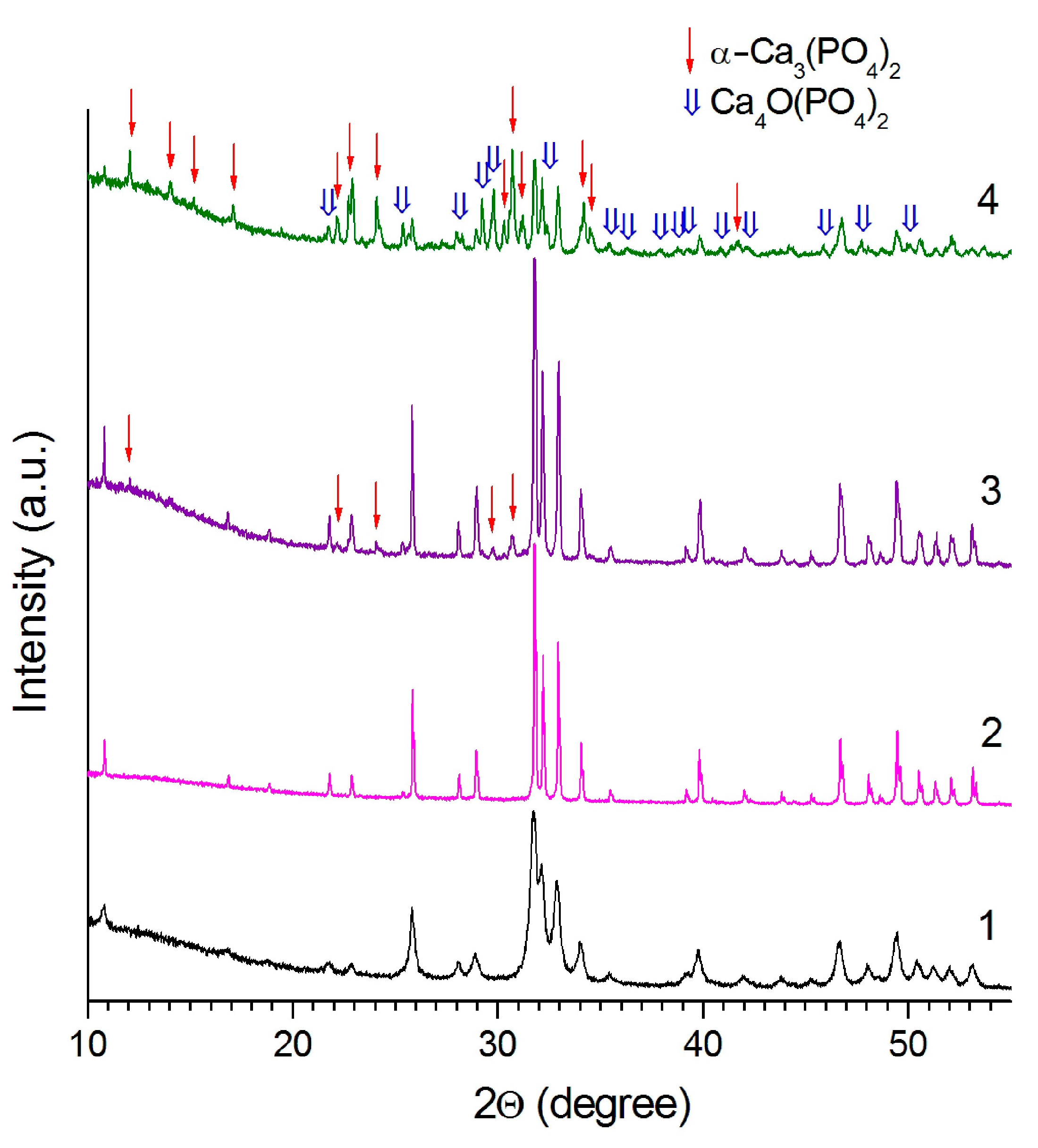
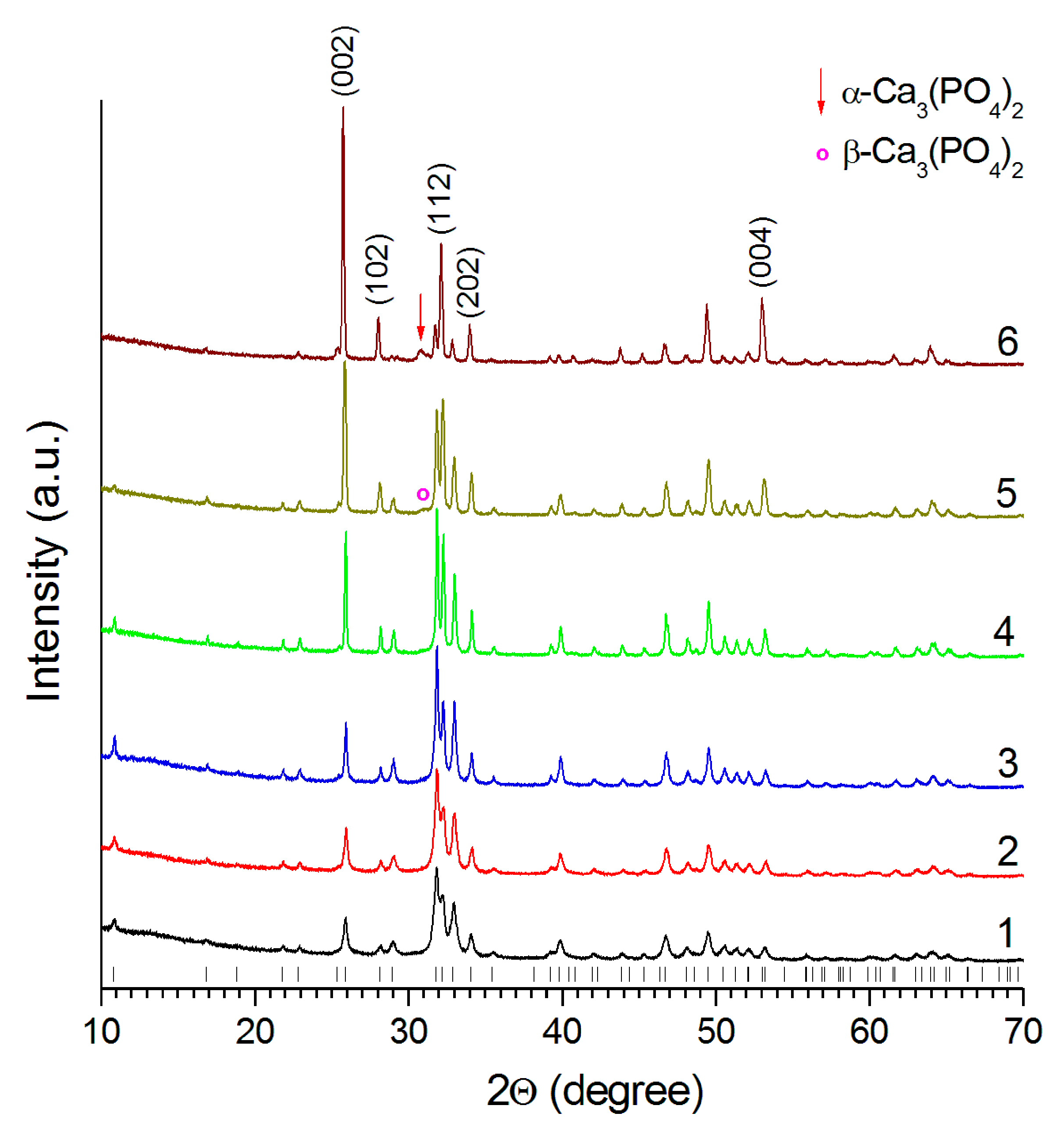
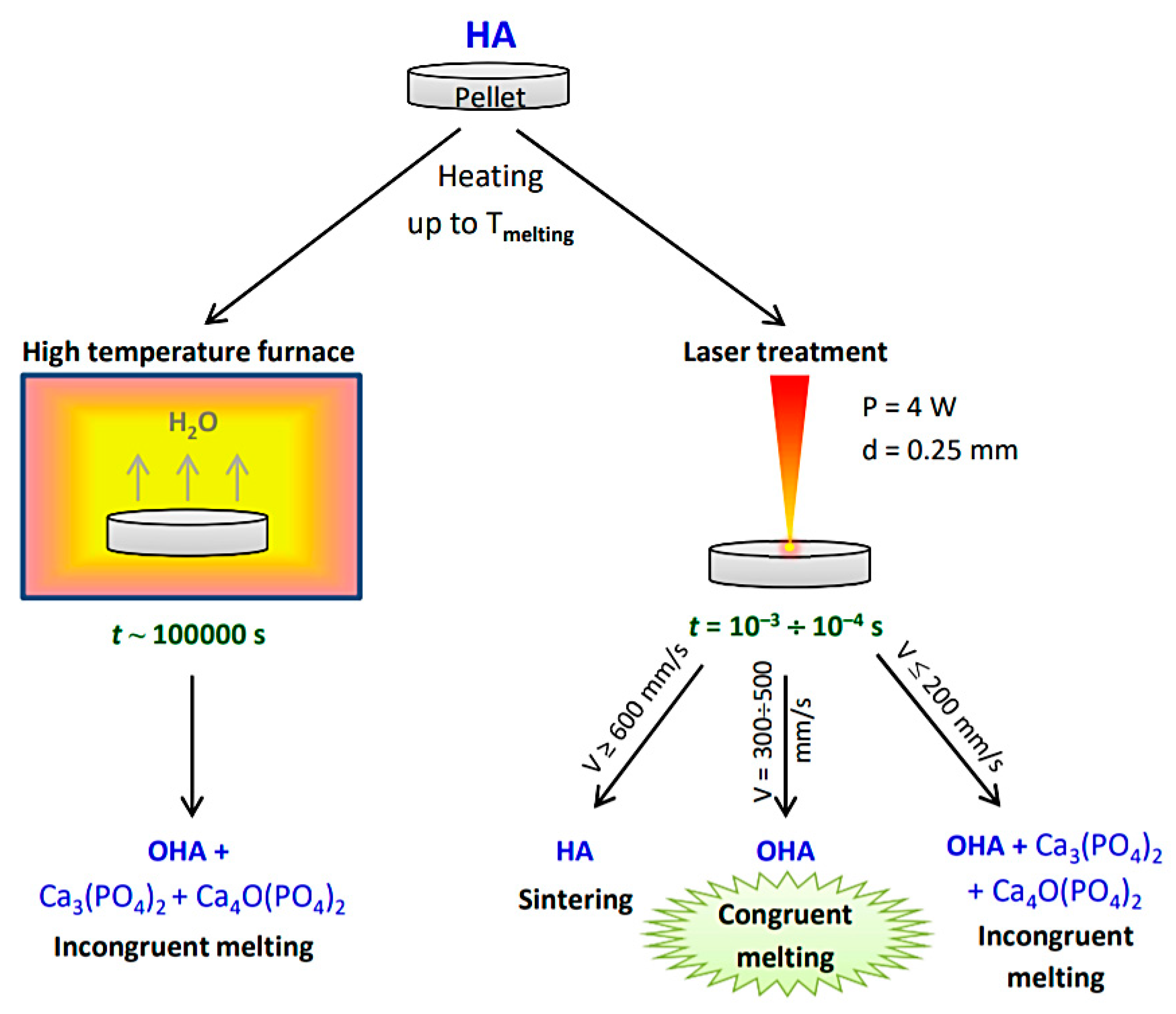
3.1. Sintering in a High Temperature Furnace
3.2. Laser Treatment
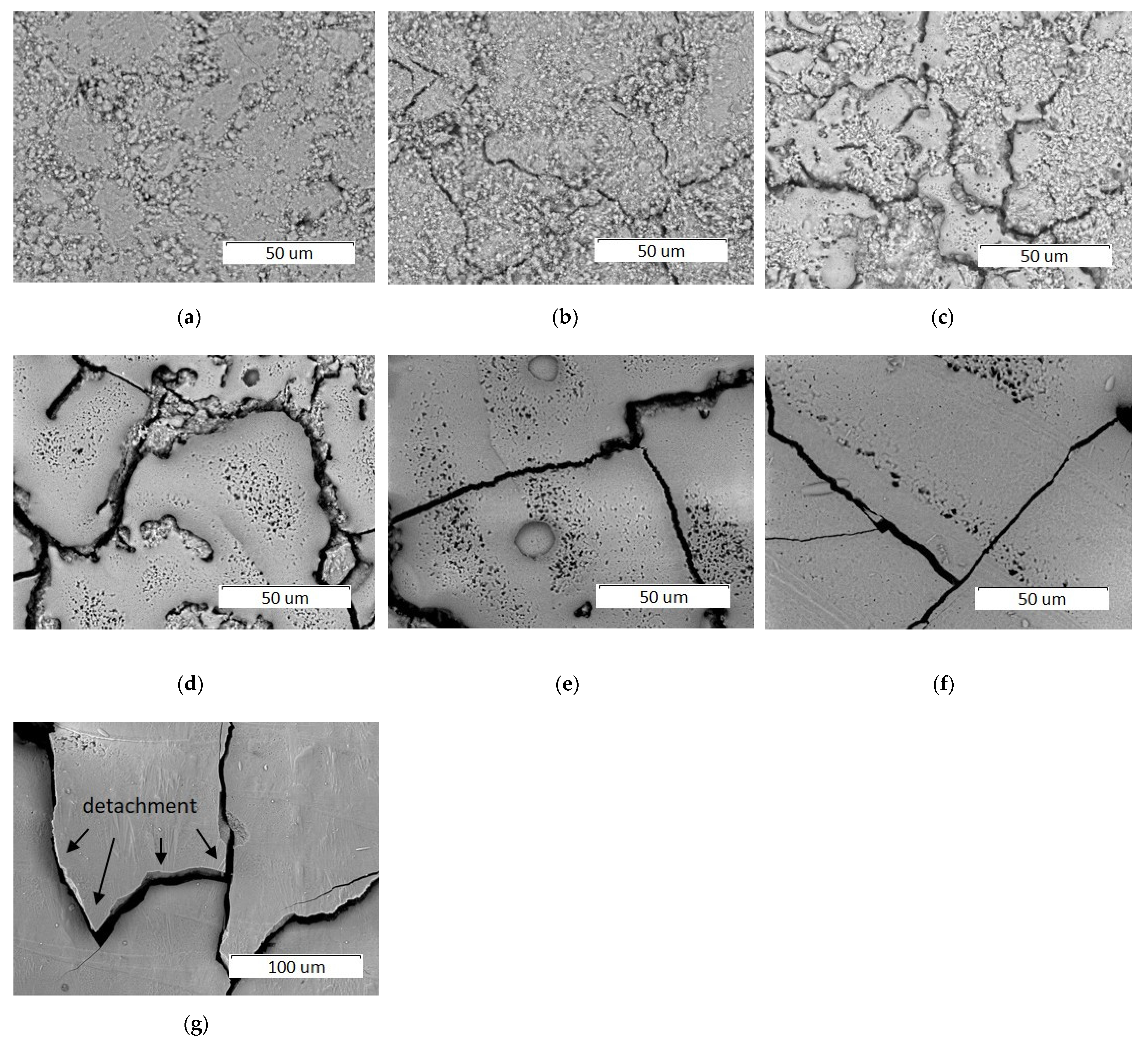
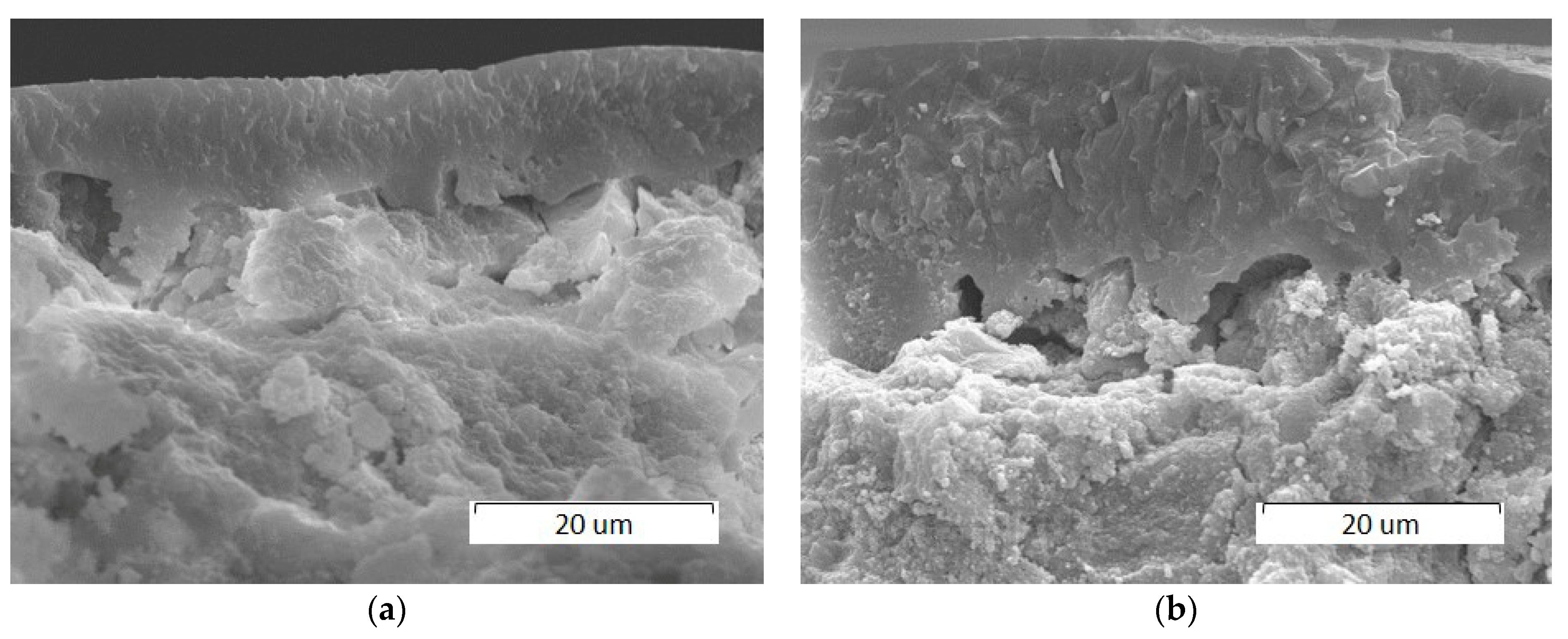
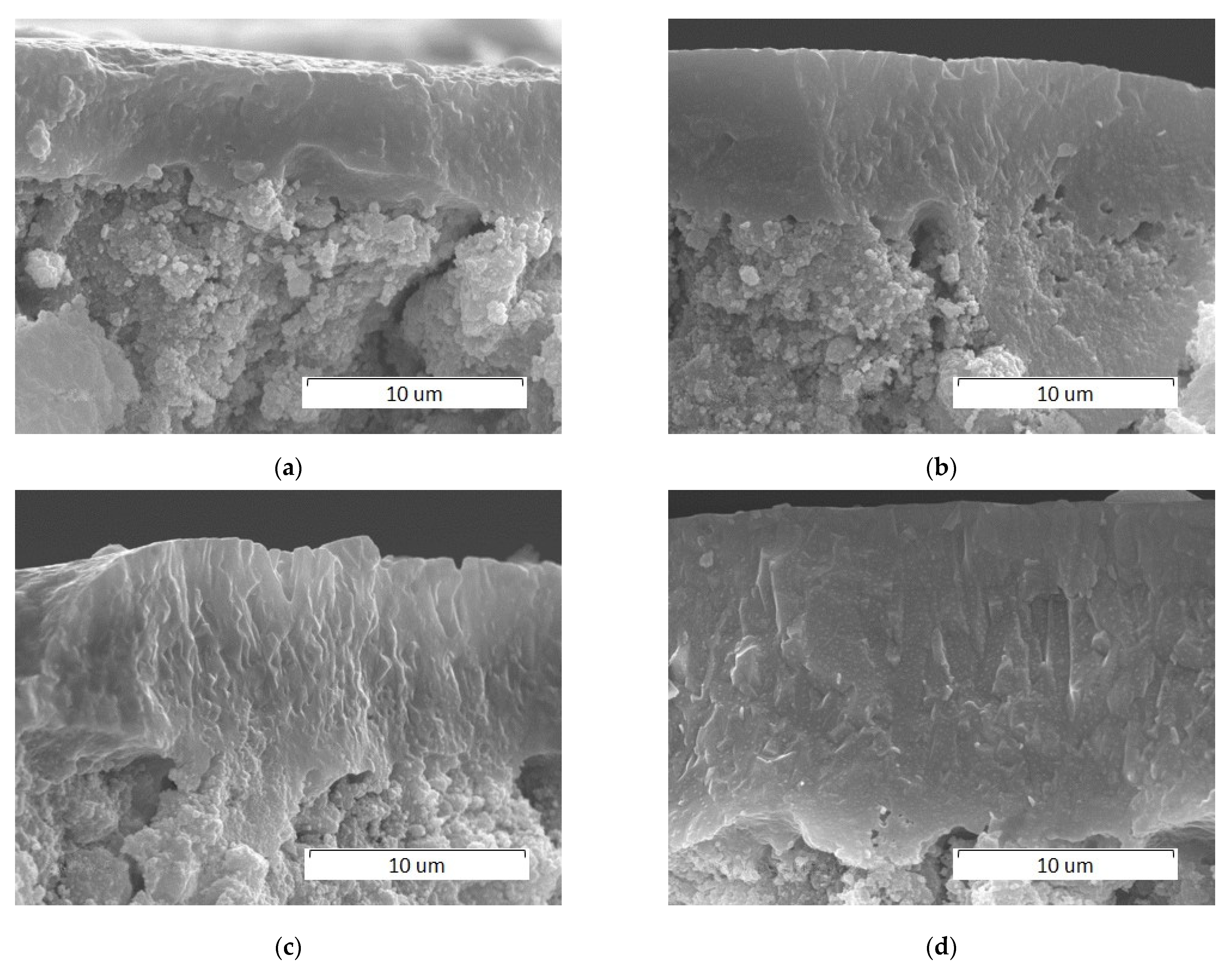
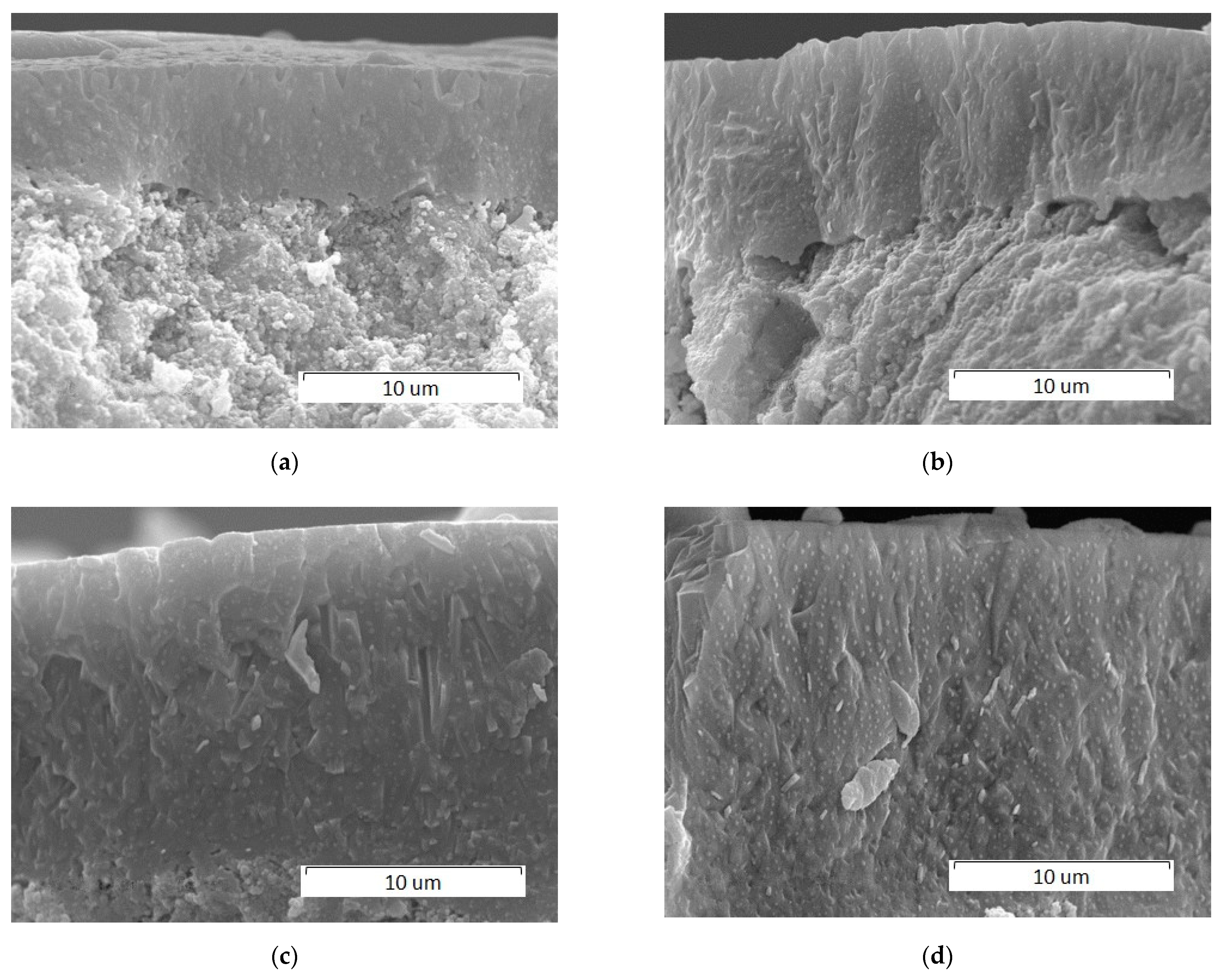
3.2.1. Effect of Scanning Speed
3.2.2. Effect of Laser Spot Diameter
3.2.3. Effect of Laser Power
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Vallet-Regı, M.; Gonzalez-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Current State of Bioceramics. J. Ceram. Sci. Technol. 2018, 9, 353–370. [Google Scholar]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Bio. Eng. Comp. 2013, 51, 245–256. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Nair, L.S. Nanotechnology and Regenerative Engineering: The Scaffold, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Jariwala, S.H.; Lewis, G.S.; Bushman, Z.J.; Adair, J.H.; Donahue, H.J. 3D printing of personalized artificial bone scaffolds. 3D Print. Addit. Manuf. 2015, 2, 56–64. [Google Scholar] [CrossRef]
- Kokubo, T. Bioceramics and Their Clinical Applications; Woodhead Publishing: Cambridge, UK, 2008; p. 784. [Google Scholar]
- Jazayeri, H.E.; Rodriguez-Romero, M.; Razavi, M.; Tahriri, M.; Ganjawalla, K.; Rasoulianboroujeni, M.; Malekoshoaraie, M.H.; Khoshroo, K.; Tayebi, L. The cross-disciplinary emergence of 3D printed bioceramic scaffolds in orthopedic bioengineering. Cer. Int. 2018, 44, 1–9. [Google Scholar] [CrossRef]
- Kalsoom, U.; Nesterenko, P.N.; Paull, B. Recent developments in 3D printable composite materials. RSC Adv. 2016, 6, 60355–60371. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Cer. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive Manufacturing Methods for Producing Hydroxyapatite and Hydroxyapatite-Based Composite Scaffolds: A Review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D printing of bioceramic scaffolds—Barriers to the clinical translation: From promise to reality, and future perspectives. Materials 2019, 12, 2660. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.; Niu, M.; Gao, C.; Peng, S.; Shuai, C. A novel two-step sintering for nano-hydroxyapatite scaffolds for bone tissue engineering. Sci. Rep. 2014, 4, 5599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuai, C.; Gao, C.; Nie, Y.; Hu, H.; Zhou, Y.; Peng, S. Structure and properties of nano-hydroxypatite scaffolds for bone tissue engineering with a selective laser sintering system. Nanotechnology 2011, 22, 285703. [Google Scholar] [CrossRef]
- Shuai, C.; Nie, Y.; Gao, C.; Feng, P.; Zhuang, J.; Zhou, Y.; Peng, S. The microstructure evolution of nanohydroxapatite powder sintered for bone tissue engineering. J. Exp. Nanosci. 2013, 8, 762–773. [Google Scholar] [CrossRef] [Green Version]
- Bulina, N.V.; Titkov, A.I.; Baev, S.G.; Makarova, S.V.; Khusnutdinov, V.R.; Bessmeltsev, V.P.; Lyakhov, N.Z. Selective laser sintering of hydroxyapatite for fabrication of bioceramic scaffolds. Mater. Today Proc. 2020, 37, 4022–4026. [Google Scholar] [CrossRef]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef]
- Bessmeltsev, V.P.; Goloshevsky, N.V.; Smirnov, K.K. Specific features of controlling laser systems for micromachining of moving carriers. Optoelectron. Instrum. Data Process 2010, 4, 79–86. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Gross, K.A.; Plūduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim. 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Muralithran, G.; Ramesh, S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram. Int. 2000, 26, 221–230. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Solid-phase conversion of nonstoichiometric hydroxyapatite into two-phase calcium phosphate. Russ. J. Appl. Chem. 2002, 75, 1897–1902. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameter | Laser Power (W) | Scanning Speed (mm/s) | Scan-Line Pitch (mm) | Laser Spot Size (mm) |
|---|---|---|---|---|
| Values | 4–14 | 50–700 | 0.05 | 0.2, 0.25 |
| Temperature (oC) | Content (wt%) | Structural Parameters of the HA Phase | ||||
|---|---|---|---|---|---|---|
| α- Ca3(PO4)2 | Сa4O(PO4)2 | HA | a (Å) | c (Å) | Crystallite Size (nm) | |
| – | – | – | 100 | 9.4336(12) | 6.8932(9) | 29.0(4) |
| 900 | – | – | 100 | 9.4248(3) | 6.8818(2) | 116(2) |
| 1000 | – | – | 100 | 9.4247(1) | 6.8812(1) | 245(5) |
| 1200 | – | – | 100 | 9.4220(1) | 6.8832(1) | 345(5) |
| 1300 | – | – | 100 | 9.4173(2) | 6.8880(2) | 256(8) |
| 1400 | 10 | – | 90 | 9.4123(4) | 6.8902(4) | 138(4) |
| 1500 | 42 | 30 | 28 | 9.4153(8) | 6.8916(10) | 116(10) |
| Scanning Speed (mm/s) | Diameter of Laser Spot (mm) | |||
|---|---|---|---|---|
| 0.25 | Process | 0.2 | Process | |
| 700 | 11.4 | S | 11.4 | M |
| 600 | 13.3 | S | 13.3 | M |
| 500 | 16.0 | M | 16.0 | M |
| 400 | 20.0 | M | 20.0 | D |
| 300 | 26.7 | M | 26.7 | D |
| 200 | 40.0 | D | 40.0 | D |
| Process | Power (W)/ Speed (mm/s) | Content (wt%) | Structural Parameters of the HA Phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β-TCP | α-TCP | TTCP | HA | a (Å) | c (Å) | Crystallite Size (nm) | Preferred Orientation | ||
| 0.25 mm laser spot | |||||||||
| S | 4/600 | – | – | – | 100 | 9.4253(10) | 6.8875(8) | 33.0(4) | – |
| M | 4/500 | – | – | – | 100 | 9.4224(8) | 6.8858(6) | 47.0(4) | – |
| M | 4/400 | – | – | – | 100 | 9.4225(6) | 6.8861(6) | 57.6(8) | – |
| M | 4/300 | – | – | – | 100 | 9.4162(6) | 6.8869(4) | 90(2) | 0.87 |
| D | 4/200 | 3 | – | – | 97 | 9.4088(7) | 6.8884(5) | 80(2) | 0.74 |
| D | 4/100 | 7 | 10 | – | 83 | 9.4105(10) | 6.8949(6) | 117(5) | 0.57 |
| D | 4/50 | – | 25 | 13 | 62 | 9.3977(12) | 6.9034(8) | 120(6) | 0.65 |
| D | 6/600 | 2 | – | – | 98 | 9.4106(12) | 6.8895(8) | 64(1) | 0.8 |
| D | 10/600 | 6 | – | – | 94 | 9.4134(8) | 6.8911(5) | 115(4) | 0.6 |
| D | 14/600 | 9 | – | – | 91 | 9.4099(9) | 6.8919(5) | 136(6) | 0.51 |
| 0.2 mm laser spot | |||||||||
| M | 4/700 | – | – | – | 100 | 9.4162(8) | 6.8879(6) | 67(2) | 0.76 |
| M | 4/600 | – | – | – | 100 | 9.4144(8) | 6.8886(6) | 83(2) | 0.74 |
| M | 4/500 | – | – | – | 100 | 9.4124(8) | 6.8895(6) | 103(4) | 0.65 |
| D | 4/400 | 4 | – | – | 96 | 9.4063(8) | 6.8868(6) | 109(4) | 0.58 |
| D | 4/300 | 6 | – | – | 94 | 9.4074(10) | 6.8925(6) | 111(4) | 0.51 |
| D | 4/200 | 3 | 8 | – | 89 | 9.4021(10) | 6.8948(4) | 128(4) | 0.32 |
| D | 4/100 | 0 | 12 | 7 | 81 | 9.4009(9) | 6.9017(7) | 89(3) | 0.61 |
| D | 6/600 | 4 | – | – | 96 | 9.4057(12) | 6.8882(7) | 71(2) | 0.56 |
| D | 10/600 | 5 | 5 | 6 | 84 | 9.4071(9) | 6.8965(5) | 151(6) | 0.48 |
| D | 14/600 | 4 | 7 | 16 | 73 | 9.4064(18) | 6.9014(8) | 67(3) | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulina, N.V.; Baev, S.G.; Makarova, S.V.; Vorobyev, A.M.; Titkov, A.I.; Bessmeltsev, V.P.; Lyakhov, N.Z. Selective Laser Melting of Hydroxyapatite: Perspectives for 3D Printing of Bioresorbable Ceramic Implants. Materials 2021, 14, 5425. https://doi.org/10.3390/ma14185425
Bulina NV, Baev SG, Makarova SV, Vorobyev AM, Titkov AI, Bessmeltsev VP, Lyakhov NZ. Selective Laser Melting of Hydroxyapatite: Perspectives for 3D Printing of Bioresorbable Ceramic Implants. Materials. 2021; 14(18):5425. https://doi.org/10.3390/ma14185425
Chicago/Turabian StyleBulina, Natalia V., Sergey G. Baev, Svetlana V. Makarova, Alexander M. Vorobyev, Alexander I. Titkov, Victor P. Bessmeltsev, and Nikolay Z. Lyakhov. 2021. "Selective Laser Melting of Hydroxyapatite: Perspectives for 3D Printing of Bioresorbable Ceramic Implants" Materials 14, no. 18: 5425. https://doi.org/10.3390/ma14185425
APA StyleBulina, N. V., Baev, S. G., Makarova, S. V., Vorobyev, A. M., Titkov, A. I., Bessmeltsev, V. P., & Lyakhov, N. Z. (2021). Selective Laser Melting of Hydroxyapatite: Perspectives for 3D Printing of Bioresorbable Ceramic Implants. Materials, 14(18), 5425. https://doi.org/10.3390/ma14185425








