Multiple Porous Synthetic Bone Graft Comprising EngineeredMicro-Channel for Drug Carrier and Bone Regeneration
Abstract
:1. Introduction
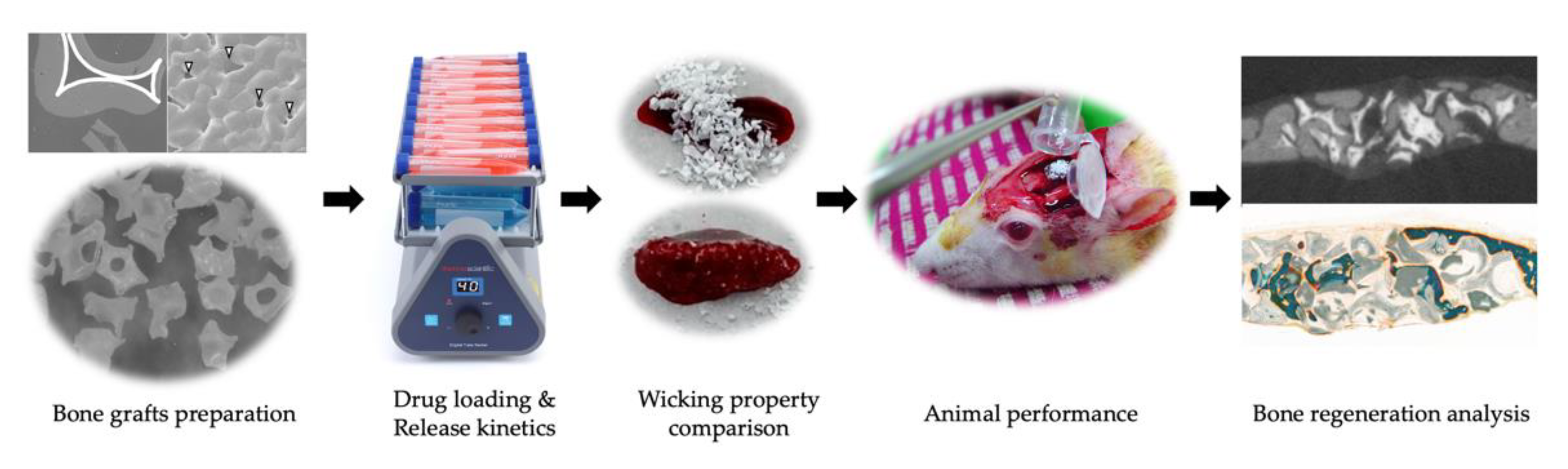
2. Materials and Methods
2.1. Preparation of Alendronate (Aln) Loaded Bone Grafts
2.2. Characterization of Aln-Loaded Bone Grafts (BGs)
2.3. Release Kinetic of Aln from Bone Grafts
2.4. Animals
- Group I: Aln (0 mg)/Bio-Oss;
- Group II: Aln (1 mg)/Bio-Oss;
- Group III: Aln (5 mg)/Bio-Oss
- Group IV: Aln (0 mg)/InRoad;
- Group V: Aln (1 mg)/InRoad;
- Group VI: Aln (5 mg)/InRoad.
2.5. Ovariectomize (Ovx) Operation and Defect Surgery
2.6. Analysis of Bone Formation
2.7. Histological Evaluation
2.8. Statistical Analysis
3. Results
3.1. Clinical Observation
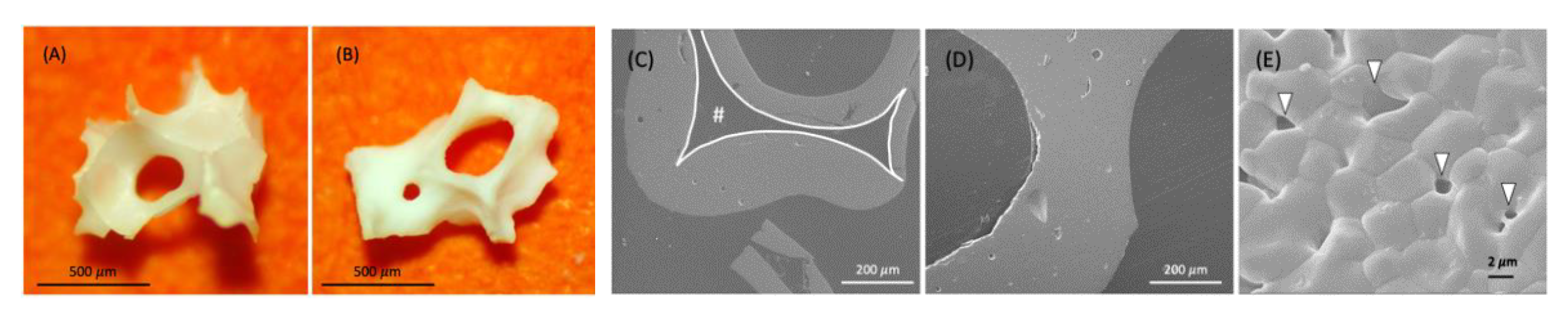
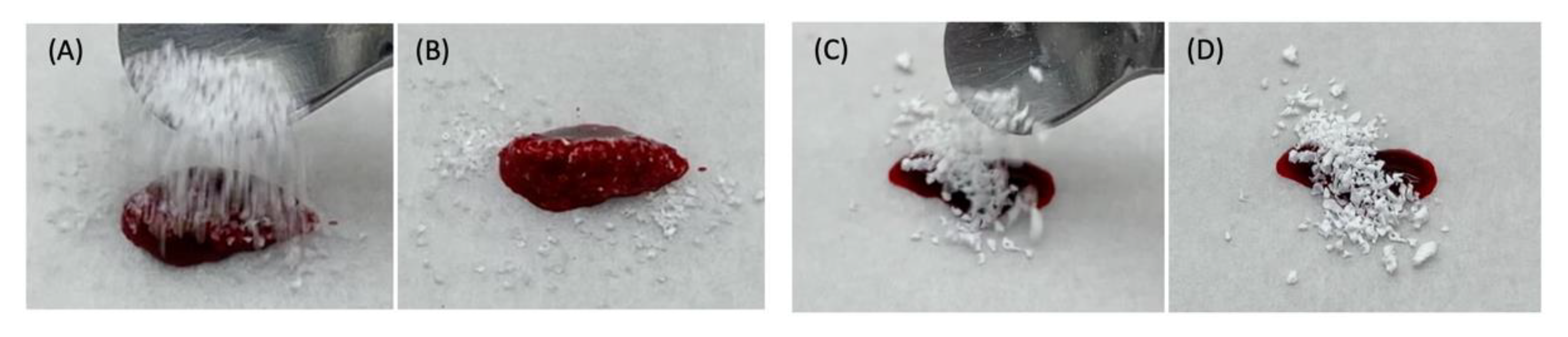
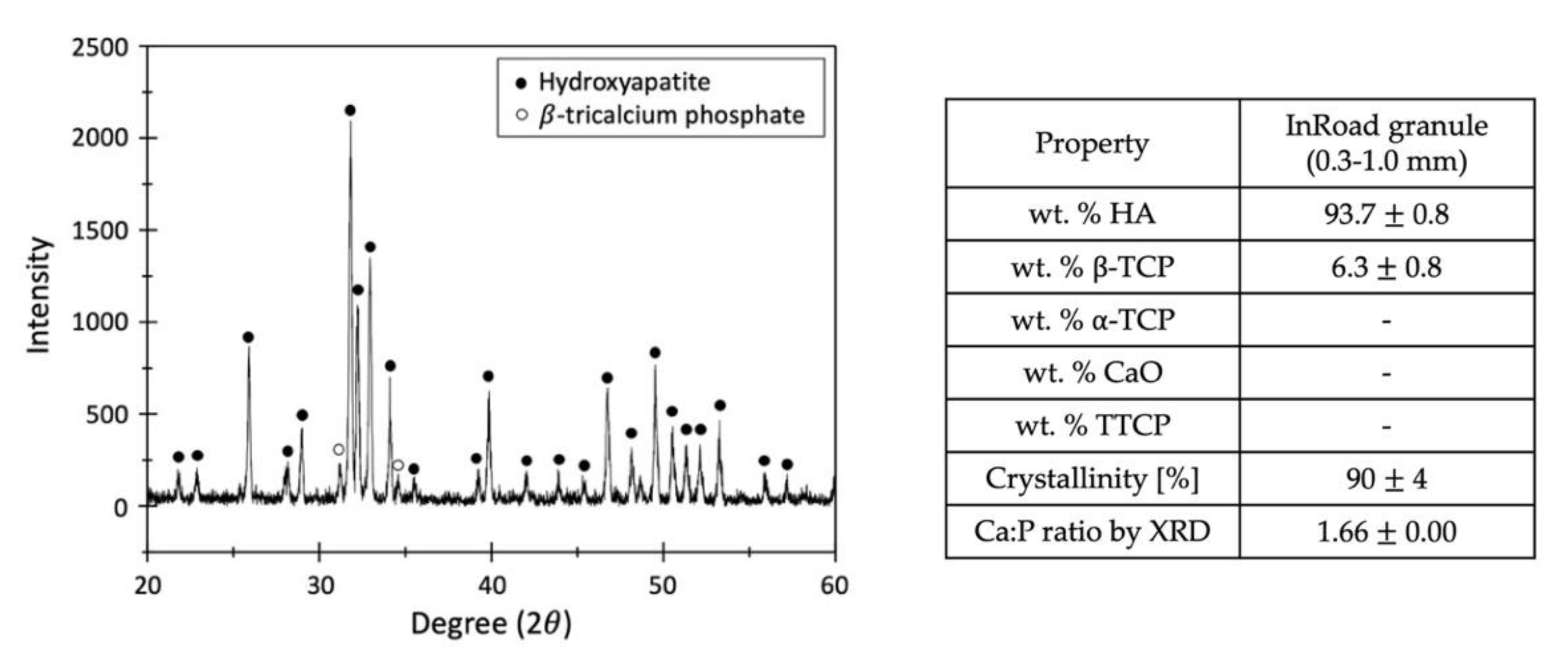
3.2. Characterization of Aln-Loaded Bone Graft
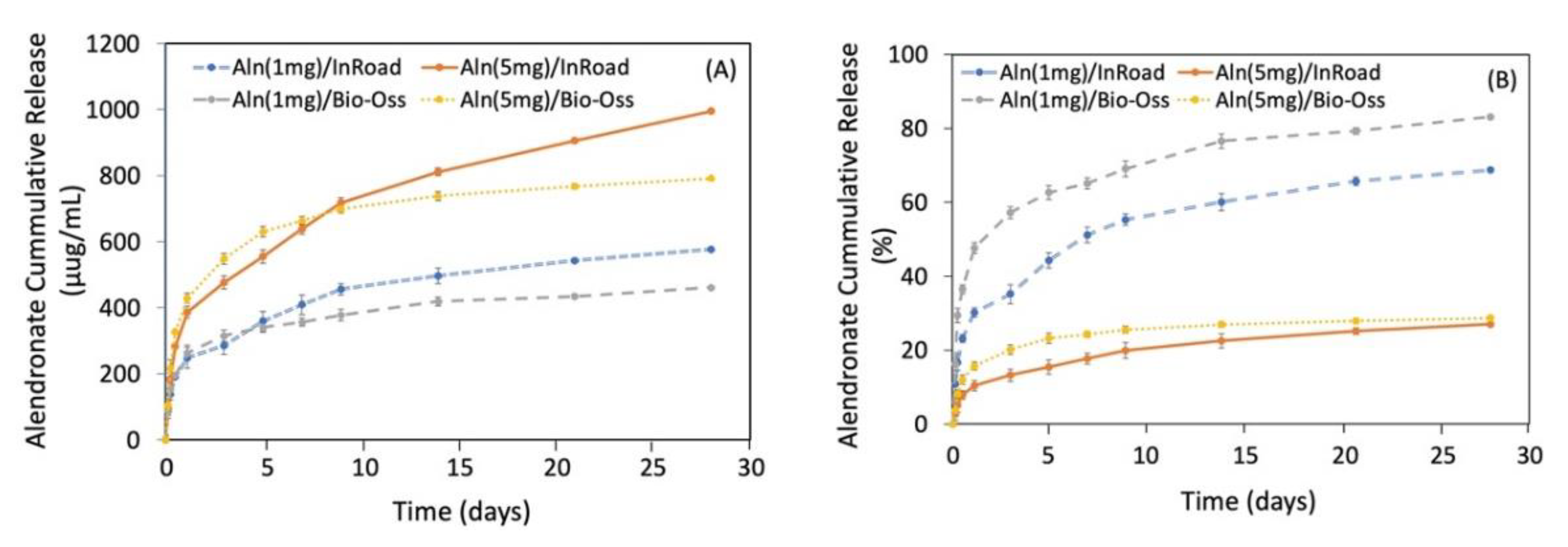
3.3. Release Kinetic of Aln from Bone Grafts
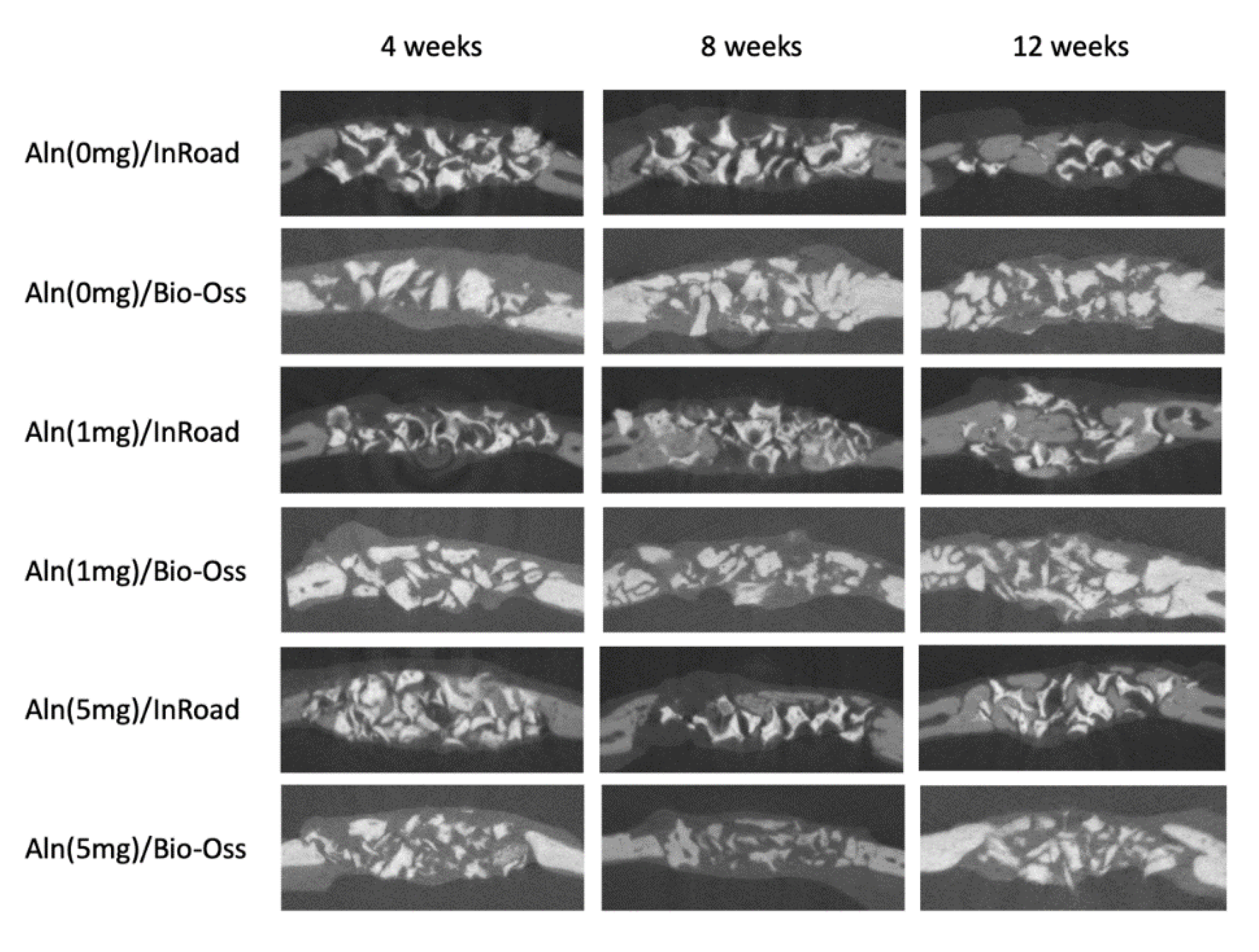
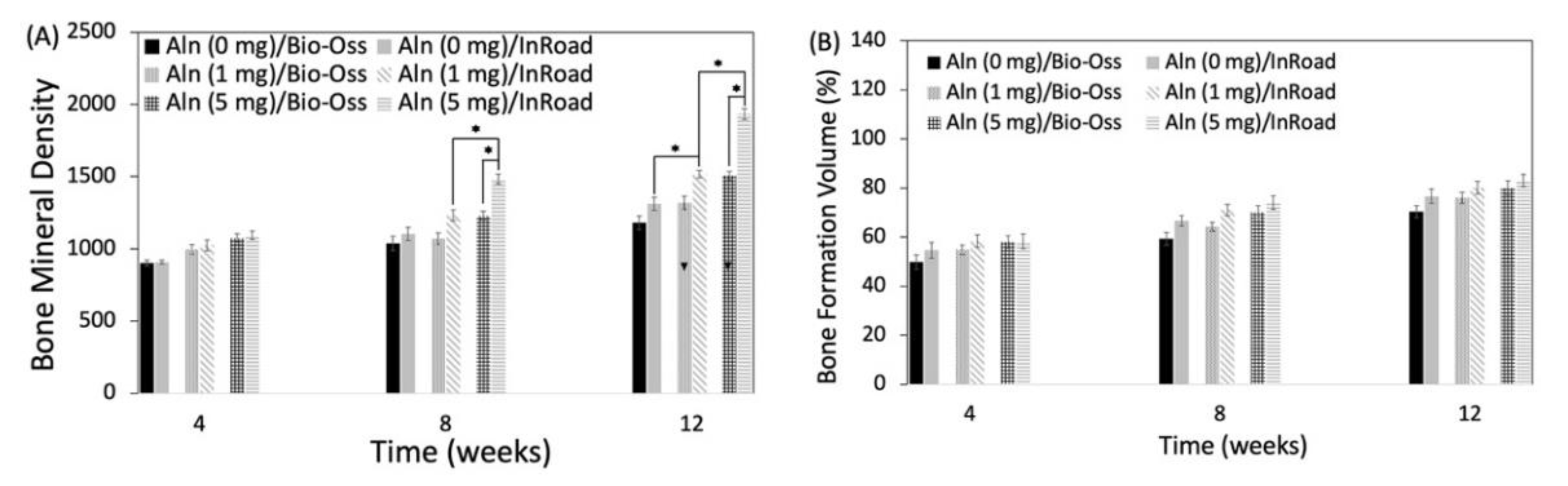
3.4. Analysis of Bone Formation
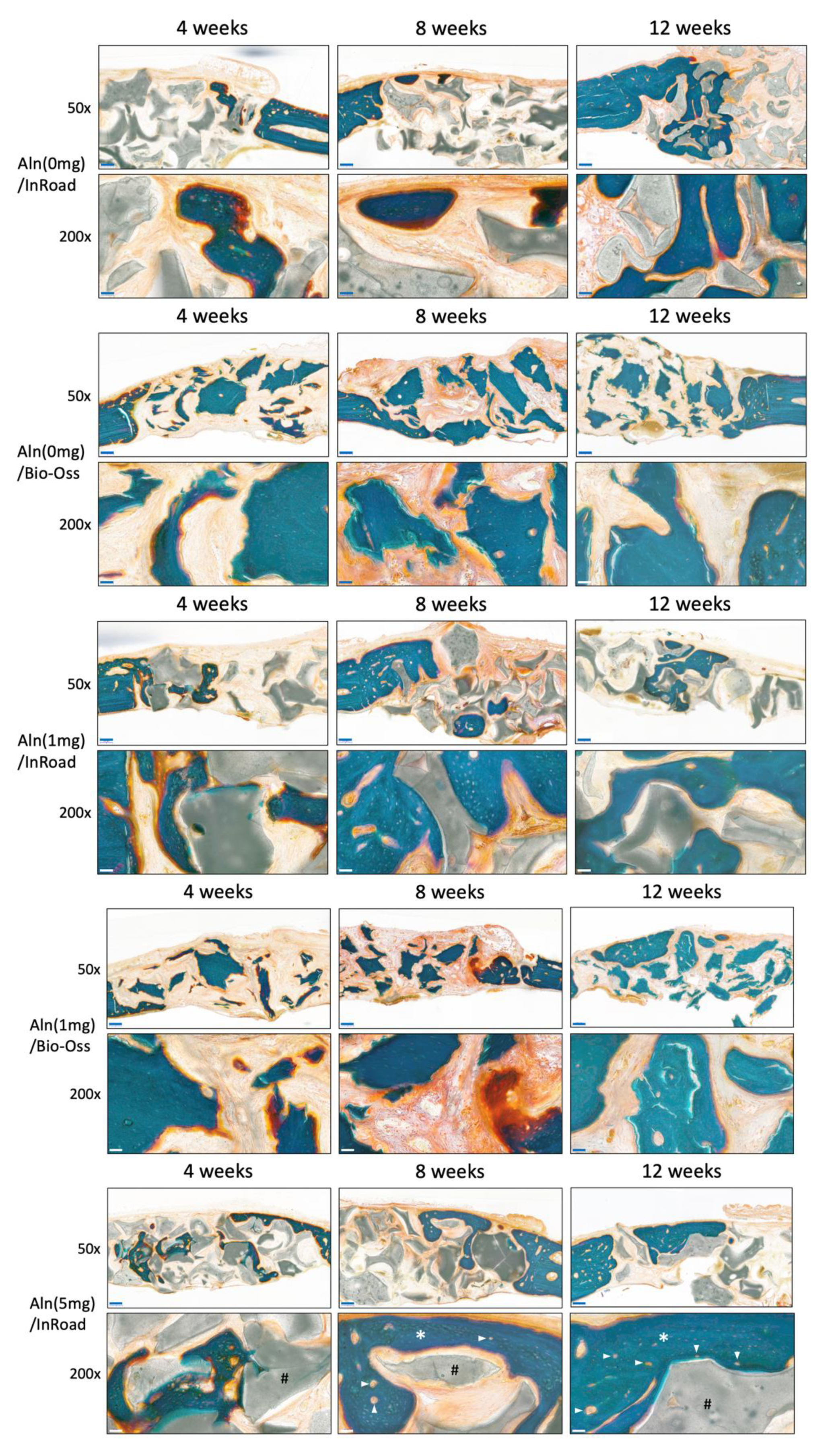
3.5. Histological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Muschler, G.F. Bone graft materials: An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Transmission of HIV through bone transplantation: Case report and public health recommendations. MMWR Morb. Mortal. Wkly. Rep. 1988, 37, 597. [Google Scholar]
- Stevenson, S.; Horowitz, M. The response to bone allografts. J. Bone Jt. Surg. 1992, 74, 939–950. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2017, 29, 1–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papkov, M.S.; Agashi, K.; Olaye, A.; Shakesheff, K.; Domb, A.J. Polymer carriers for drug delivery in tissue engineering. Adv. Drug. Deliv. Rev. 2007, 59, 187–206. [Google Scholar] [CrossRef]
- Khang, G.; Lee, S.J.; Kim, M.S.; Lee, H.B. Biomaterials: Tissue engineering and scaffold. In Encyclopedia of Medical Devices and Instrumentation; Webster, J., Ed.; Wiley: Hoboken, NJ, USA, 2006; Volume 2, pp. 366–383. [Google Scholar]
- Mandal, B.B.; Kundu, S.C. Non-bioengineered high strength three-dimensional gland fibroin scaffolds from tropical non-mulberry silkworm for potential tissue engineering applications. Macromol. Biosci. 2008, 8, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Osteogenic and adipogenic differentiation of rat bone marrow cells on nonmulberry and mulberry silk gland fibroin 3D scaffolds. Biomaterials 2009, 30, 5019–5030. [Google Scholar] [CrossRef] [PubMed]
- Teofilo, J.M.; Brentegani, L.G.; Lamano-Carvalho, T.L. Bone healing in osteoporotic female rats following intra-alveolar grafting of bioactive glass. Arch. Oral. Biol. 2004, 49, 755–762. [Google Scholar] [CrossRef]
- Okazaki, A.; Koshino, T.; Saito, T.; Takagi, T. Osseous tissue reaction around hydroxyapatite block implanted into proximal metaphysis of tibia of rat with collagen-induced arthritis. Biomaterials 2000, 21, 483–487. [Google Scholar] [CrossRef]
- Tami, A.E.; Leitner, M.M.; Baucke, M.G.; Mueller, T.L.; van Lenthe, G.H.; Muller, R.; Ito, K. Hydroxyapatite particles maintain peri-implant bone mantle during osseointegration in osteoporoticbone. Bone 2009, 45, 1117–1124. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, T.; Han, M.H.; Bae, C.; Oh, D. Wicking Property of Graft Material Enhanced Bone Regenerationin the Ovariectomized Rat Model. Tissue Eng. Regen. Med. 2018, 15, 503–510. [Google Scholar] [CrossRef]
- Xuan, F.; Lee, C.U.; Son, J.S.; Jeong, S.M.; Choi, B.H. A comparative study of the regenerative effect of sinus bone grafting with platelet-rich fibrin-mixed Bio-Oss and commercial fibrin-mixed Bio-Oss: An experimental study. J. Cranio-Maxillofacial Surg. 2014, 42, 47–50. [Google Scholar] [CrossRef]
- Ribeiro, C.C.; Barrias, C.C.; Barbosa, M.A. Preparation and characterisation of calcium-phosphate porous microspheres with a uniform size for biomedical applications. J. Mater. Sci. Mater. Med. 2006, 17, 455–463. [Google Scholar] [CrossRef]
- Schlapp, M.; Friess, W. Collagen/PLGA microparticle composites for local controlled delivery of gentamicin. J. Pharm. Sci. 2003, 92, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- NIH Consensus development panel on osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [CrossRef]
- Poole, K.E.; Treece, G.M.; Ridgway, G.R.; Mayhew, P.M.; Borggrefe, J.; Gee, A.H. Targeted regeneration of bone in the osteoporotichuman femur. PLoS ONE 2011, 6, e16190. [Google Scholar] [CrossRef] [PubMed]
- Leppanen, O.V.; Sievanen, H.; Jokihaara, J.; Pajamaki, I.; Kannus, P.; Jarvinen, T.L. Pathogenesis of age-related osteoporosis: Impaired mechano-responsiveness of bone is not the culprit. PLoS ONE 2008, 3, 2540. [Google Scholar] [CrossRef]
- Khosla, S.; Westendorf, J.J.; Oursler, M.J. Building bone to reverseosteoporosis and repair fractures. J. Clin. Investig. 2008, 118, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, L.J.; Scalisi, R.; Barbagallo, M. Therapeutic options in osteoporosis. Acta Biomed. 2010, 81, 55–65. [Google Scholar] [PubMed]
- Black, D.M.; Schwartz, A.V.; Ensrud, K.E.; Cauley, J.A.; Levis, S.; Quandt, S.A.; Satterfield, S.; Wallace, R.B.; Bauer, D.C.; Palermo, L.; et al. Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial Long-term Extension (FLEX): A randomized trial. JAMA 2006, 296, 2927. [Google Scholar] [CrossRef] [PubMed]
- Yates, J. A meta-analysis characterizing the dose-response relationships for three oral nitrogen-containing bisphosphonates in postmenopausal women. Osteoporos. Int. 2013, 24, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, R.; Zhao, Y.L.; Sun, X.H.; Zhao, H.X.; Tan, L.; Chen, D.C.; Xu, B.H. Efficacy of intravenous zoledronic acid in the prevention and treatment of osteoporosis: A meta-analysis. Asian Pac. J. Trop. Med. 2012, 5, 743. [Google Scholar] [CrossRef] [Green Version]
- Crandall, C.J.; Newberry, S.J.; Diamant, A.; Lim, Y.W.; Gellad, W.F.; Booth, M.J.; Motala, A.; Shekelle, P.G. Comparative effectiveness of pharmacologic treatments to prevent fractures: An updated systematic review. Ann. Intern. Med. 2014, 161, 711. [Google Scholar] [CrossRef] [Green Version]
- Liberman, U.A.; Weiss, S.R.; Bröll, J.; Minne, H.W.; Quan, H.; Bell, N.H.; Rodriguez-Portales, J.; Downs, R.W.; Dequeker, J.; Favus, M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N. Engl. J. Med. 1995, 333, 1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, L.M.; Rauch, F.; Whyte, M.P.; D’Astous, J.; Gates, P.E.; Grogan, D.; Lester, E.L.; McCall, R.E.; Pressly, T.A.; Sanders, J.O.; et al. Alendronate for the Treatment of Pediatric Osteogenesis Imperfecta: A Randomized Placebo-Controlled Study. J. Clin. Endocrinol. Metab. 2011, 96, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Tsuchimoto, M.; Azuma, Y.; Higuchi, O.; Sugimoto, I.; Hirata, N.; Kiyoki, M.; Yamamoto, I. Alendronate Modulates osteogenesis of Human Osteoglastic Cells in Vitro. Jpn. J. Pharmacol. 1994, 66, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, M.H.; Son, J.S.; Kim, K.M.; Han, M.; Oh, D.S.; Lee, Y.K. Drug-loaded porous spherical hydroxyapatite granules for bone regeneration. J. Mater. Sci. Mater. Med. 2011, 22, 349–355. [Google Scholar] [CrossRef]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; Hong, L.; Ling, Y.; Pang, J.; Fang, Y.; Wei, K.; Gao, X. Synthesis, characterization and osteoconductivity properties of bone fillers based on alendronate-loaded poly(epsilon-caprolactone)/hydroxyapatite microspheres. J. Mater. Sci. Mater. Med. 2011, 22, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Fini, M.; Bigi, A. The effect of alendronate doped calcium phosphates on bone cells activity. Bone 2012, 51, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.; Vidal, C.; Rivas, D. Protein isoprenylation regulates osteogenic differentiation of mesenchymal stem cells: Effect of alendronate, and farnesyl and geranylgeranyl transferase inhibitors. Br. J. Pharmacol. 2011, 162, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, K.; Shimada, A.; Shibata, T.; Wada, S.; Ideno, H.; Nakashima, K.; Amizuka, N.; Noda, M.; Nifuji, A. Alendronate promotes bone formation by inhibiting protein prenylation in osteoblasts in rat tooth replantation model. J. Endocrinol. 2013, 219, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Peter, B.; Pioletti, D.P.; Laib, S.; Bujoli, B.; Pilet, P.; Janvier, P.; Guicheux, J.; Zambelli, P.Y.; Bouler, J.M.; Gauthier, O. Calcium phosphate drug delivery system: Influence of local zoledronate release on bone implant osteointegration. Bone 2005, 36, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josse, S.; Faucheux, C.; Soueidan, A.; Grimandi, G.; Massiot, D.; Alonso, B.; Janvier, P.; Laib, S.; Pilet, P.; Gauthier, O.; et al. Novel biomaterials for bisphosphonate delivery. Biomaterials 2005, 26, 2073–2080. [Google Scholar] [CrossRef]
- Ralston, S.H.; Hacking, L.; Willocks, L.; Bruce, F.; Pitkeathly, D.A. Clinical, biochemical, and radiographic effects of aminohydroxypropylidene bisphosphonate treatment in rheumatoid arthritis. Ann. Rheum. Dis. 1989, 48, 396–399. [Google Scholar] [CrossRef]
- Eggelmeijer, F.; Papapoulos, S.E.; van Paassen, H.C.; Dijkmans, B.A.; Breedveld, F.C. Clinical and biochemical response to single infusion of pamidronate in patients with active rheumatoid arthritis: A double blind placebo controlled study. J. Rheumatol. 1994, 21, 2016–2020. [Google Scholar]
- Mashiba, T.; Hirano, T.; Turner, C.H.; Forwood, M.R.; Johnston, C.C.; Burr, D.B. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J. Bone Miner. Res. 2000, 15, 613–620. [Google Scholar] [CrossRef]
- Sama, A.A.; Khan, S.N.; Myers, E.R.; Huang, R.C.; Cammisa, F.P., Jr.; Sandhu, H.S.; Lane, J.M. High-dose alendronate uncouples osteoclast and osteoblast function: A study in a rat spine pseudarthrosis model. Clin. Orthop. Related Res. 2004, 425, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Odvina, C.V.; Zerwekh, J.E.; Rao, D.S.; Maalouf, N.; Gottschalk, F.A.; Pak, C.Y. Severely suppressed bone turnover: A potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005, 90, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Li, J.; Follet, H.; Phipps, R.J.; Burr, D.B. Bisphosphonates suppress periosteal osteoblast activity independently of resorption in rat femur and tibia. Bone 2006, 39, 1053–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Samples | Loading Amount (μg) | Loading Efficiency (%) |
|---|---|---|
| Aln (1 mg)/InRoad | 825.72 ± 8.03 | 82.57 ± 0.80 |
| Aln (1 mg)/Bio-Oss | 547.60 ± 7.86 | 54.76 ± 0.79 |
| Aln (5 mg)/InRoad | 3601.05 ± 7.86 | 72.02 ± 0.16 |
| Ain (5 mg)/Bio-Oss | 2752.35 ± 7.00 | 55.05 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, C.-S.; Kim, S.-H.; Ahn, T.; Kim, Y.; Kim, S.-E.; Kang, S.-S.; Kwon, J.-S.; Kim, K.-M.; Kim, S.-G.; Oh, D. Multiple Porous Synthetic Bone Graft Comprising EngineeredMicro-Channel for Drug Carrier and Bone Regeneration. Materials 2021, 14, 5320. https://doi.org/10.3390/ma14185320
Bae C-S, Kim S-H, Ahn T, Kim Y, Kim S-E, Kang S-S, Kwon J-S, Kim K-M, Kim S-G, Oh D. Multiple Porous Synthetic Bone Graft Comprising EngineeredMicro-Channel for Drug Carrier and Bone Regeneration. Materials. 2021; 14(18):5320. https://doi.org/10.3390/ma14185320
Chicago/Turabian StyleBae, Chun-Sik, Seung-Hyun Kim, Taeho Ahn, Yeonji Kim, Se-Eun Kim, Seong-Soo Kang, Jae-Sung Kwon, Kwang-Mahn Kim, Sahng-Gyoon Kim, and Daniel Oh. 2021. "Multiple Porous Synthetic Bone Graft Comprising EngineeredMicro-Channel for Drug Carrier and Bone Regeneration" Materials 14, no. 18: 5320. https://doi.org/10.3390/ma14185320
APA StyleBae, C.-S., Kim, S.-H., Ahn, T., Kim, Y., Kim, S.-E., Kang, S.-S., Kwon, J.-S., Kim, K.-M., Kim, S.-G., & Oh, D. (2021). Multiple Porous Synthetic Bone Graft Comprising EngineeredMicro-Channel for Drug Carrier and Bone Regeneration. Materials, 14(18), 5320. https://doi.org/10.3390/ma14185320







