An Evaluation of the Physical and Chemical Stability of Dry Bottom Ash as a Concrete Light Weight Aggregate
Abstract
:1. Introduction
2. Preceding Research on the dBA
2.1. The Discharging Process of dBA
2.2. Pore Properties of dBA
2.3. Physical Properties of dBA
2.4. Feasibility of dBA as a Construction Material in Relation to its Physical Properties
3. Experimental Plan and Method
3.1. Experimental Plan
3.2. Shapes of Various Artificial Aggregates
3.3. Test Method
3.3.1. Oxide Composition by XRF
3.3.2. Mineralogical Analysis by XRD
3.3.3. Chloride Content
3.3.4. Unburned carbon
3.3.5. Potential of Hydrogen
3.3.6. Heavy Metal Leaching Test
3.3.7. Minor Inorganic Compounds by ICP
3.4. The Physical Properties of Various Artificial Aggregates
4. Test Results and Discussion
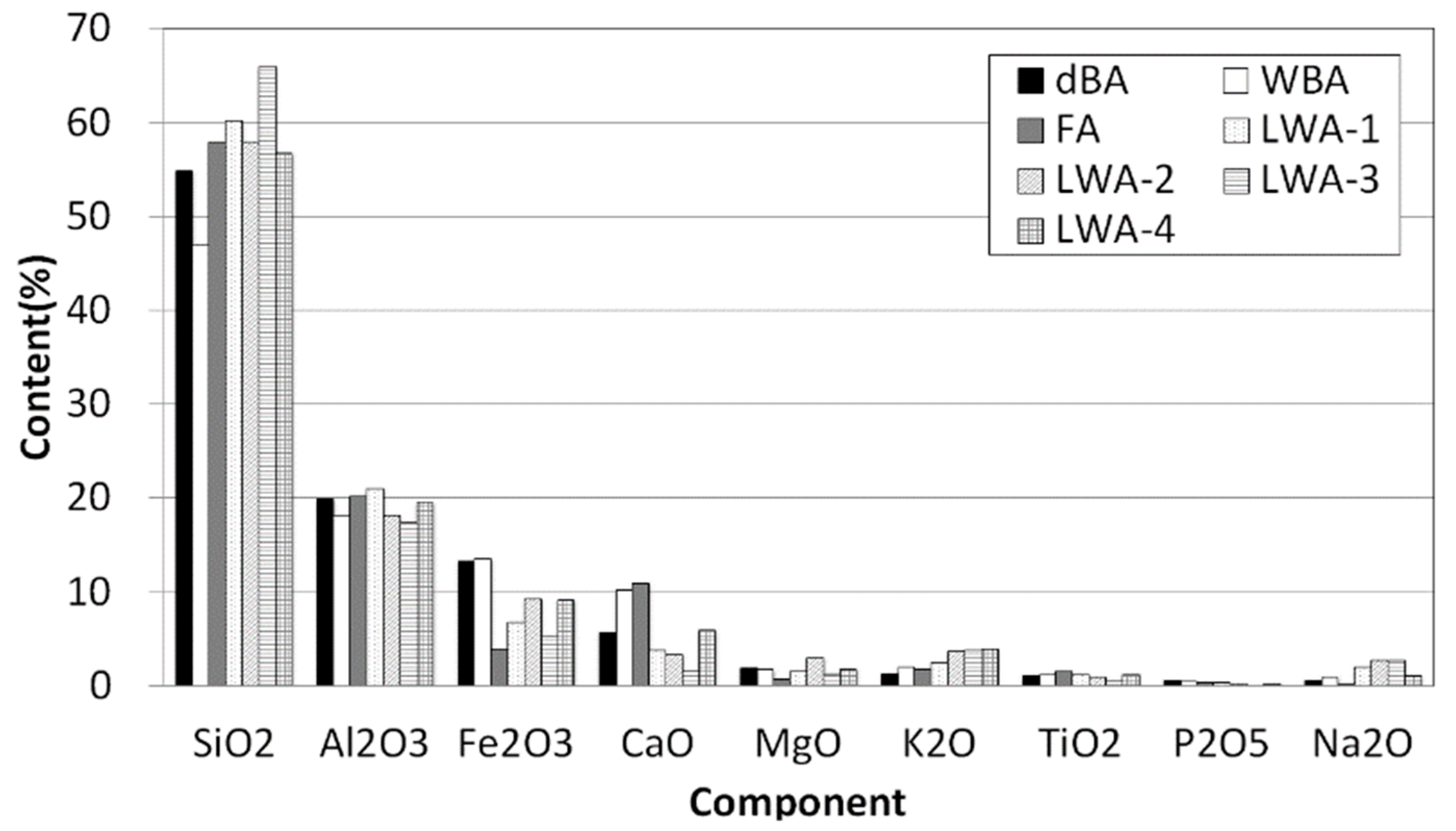
4.1. Oxide Composition
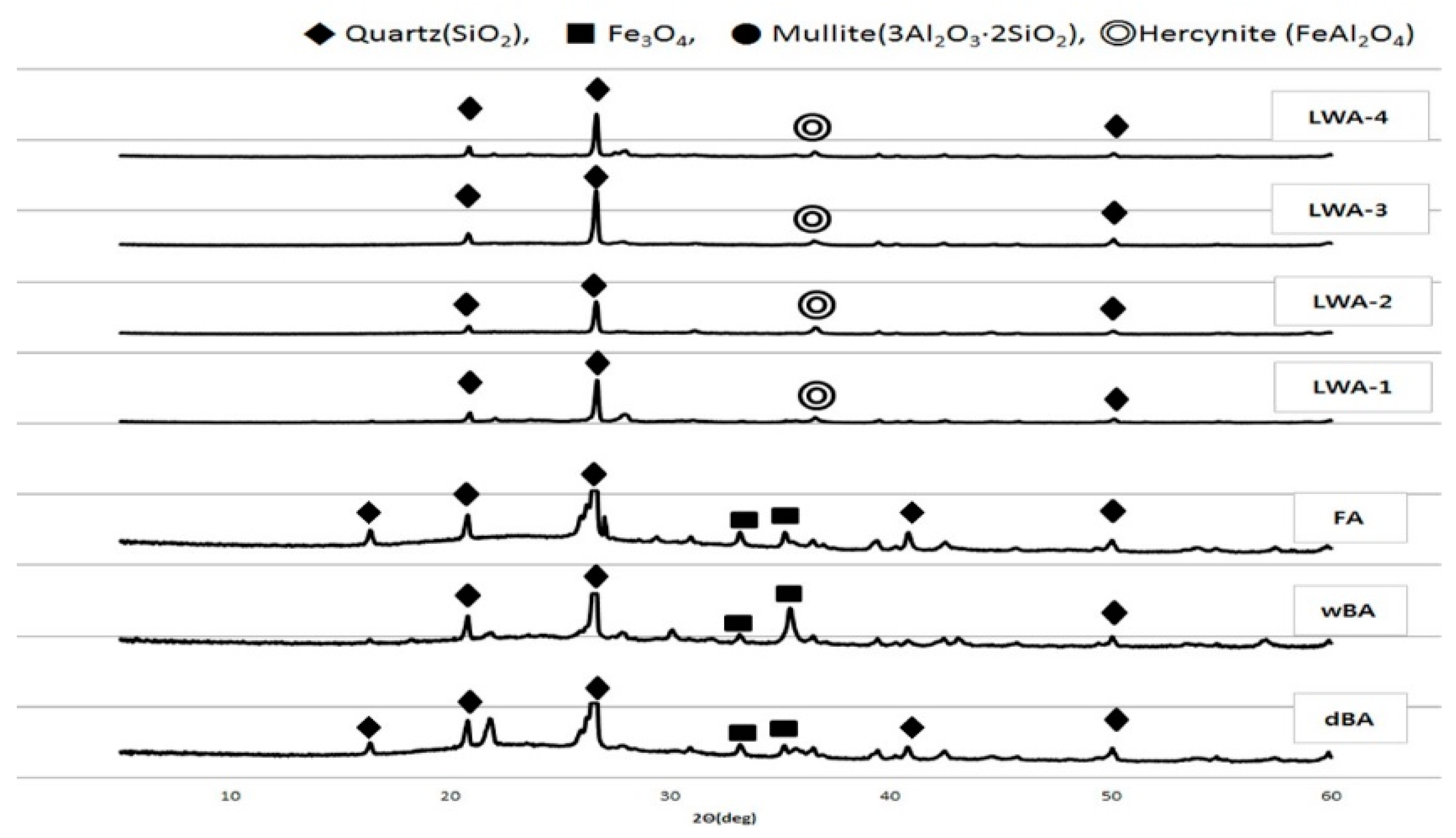
4.2. Mineralogical Analysis
4.2.1. Quartz
4.2.2. Sulfate
4.2.3. Chloride
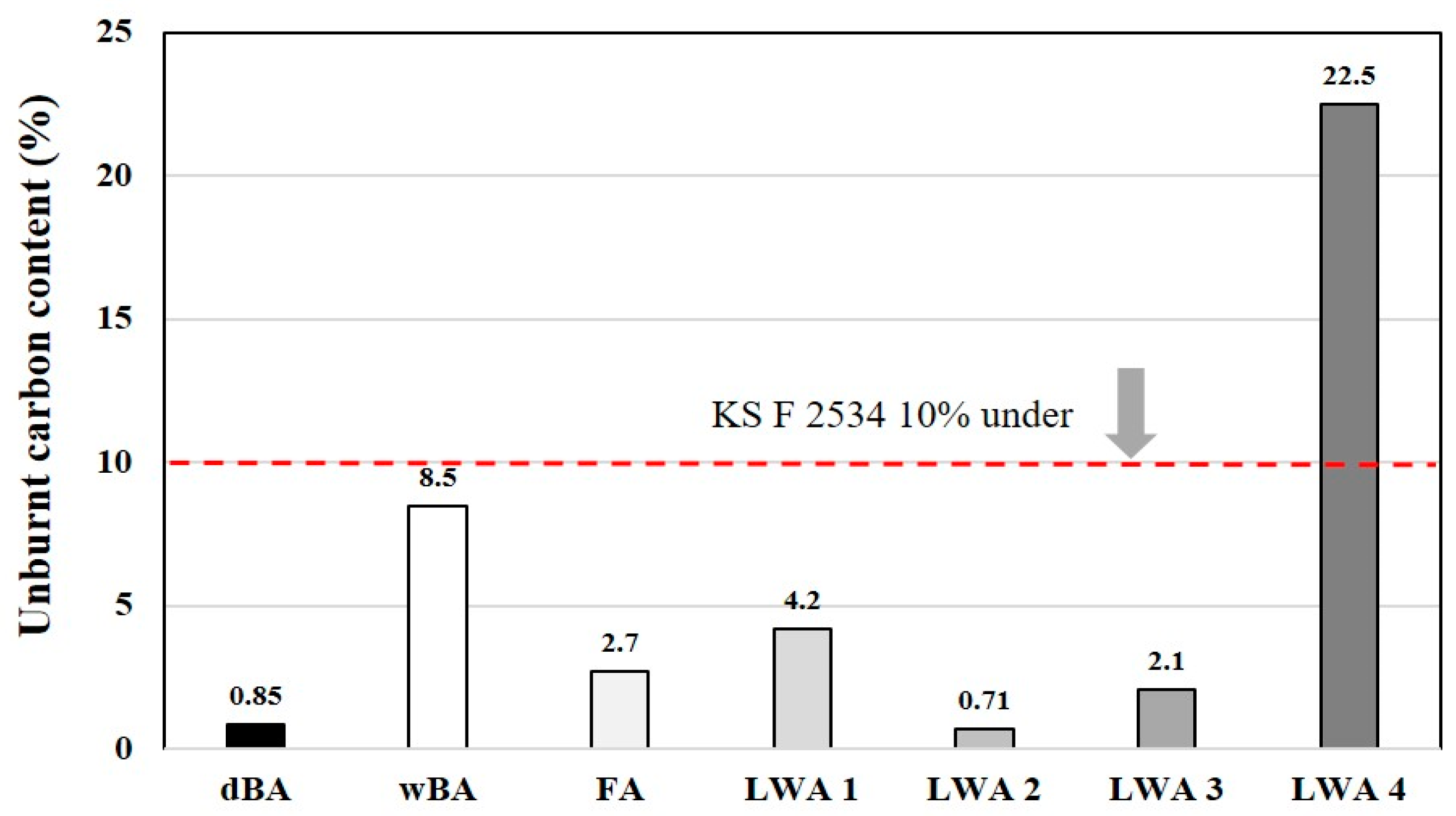
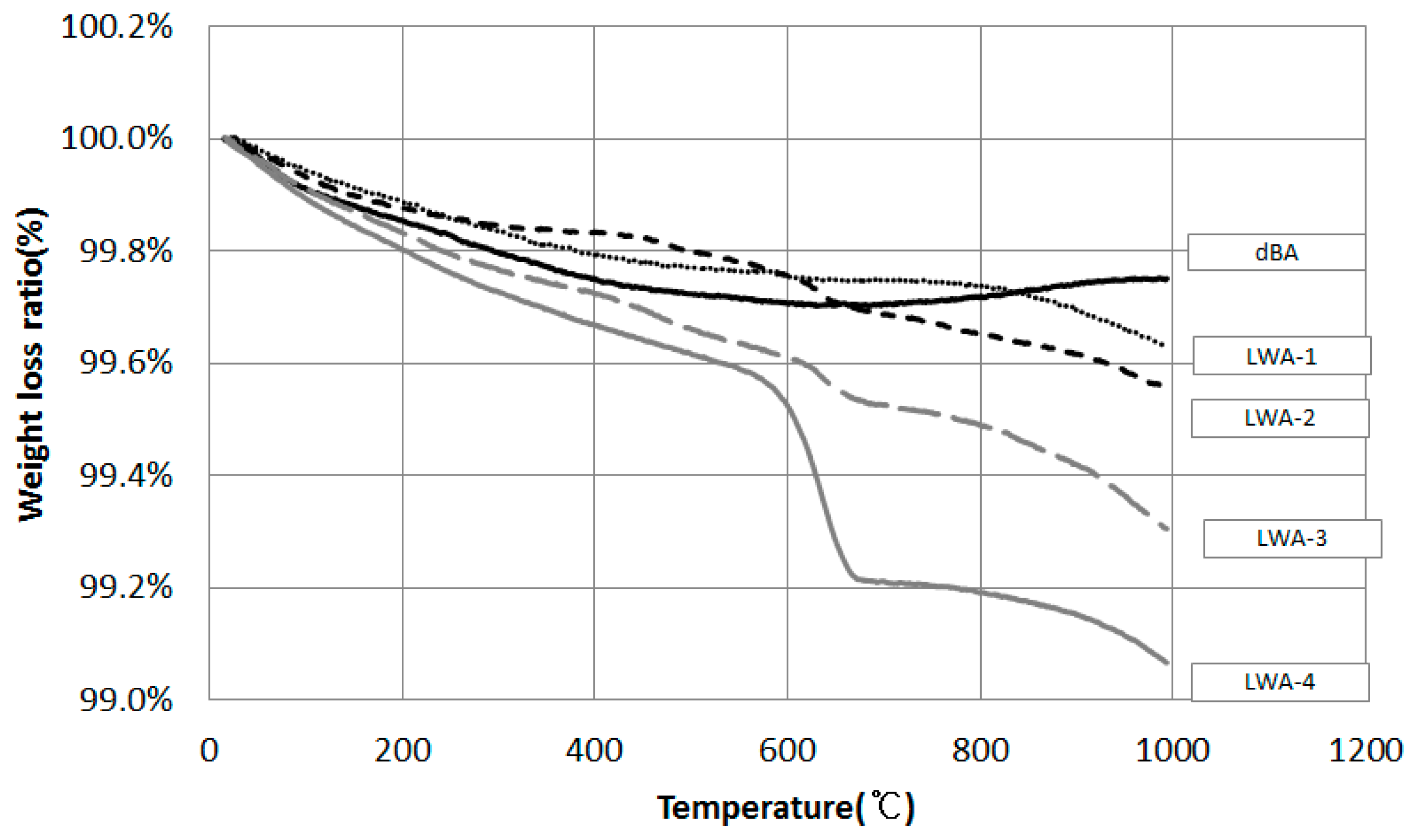
4.2.4. Unburned Carbon
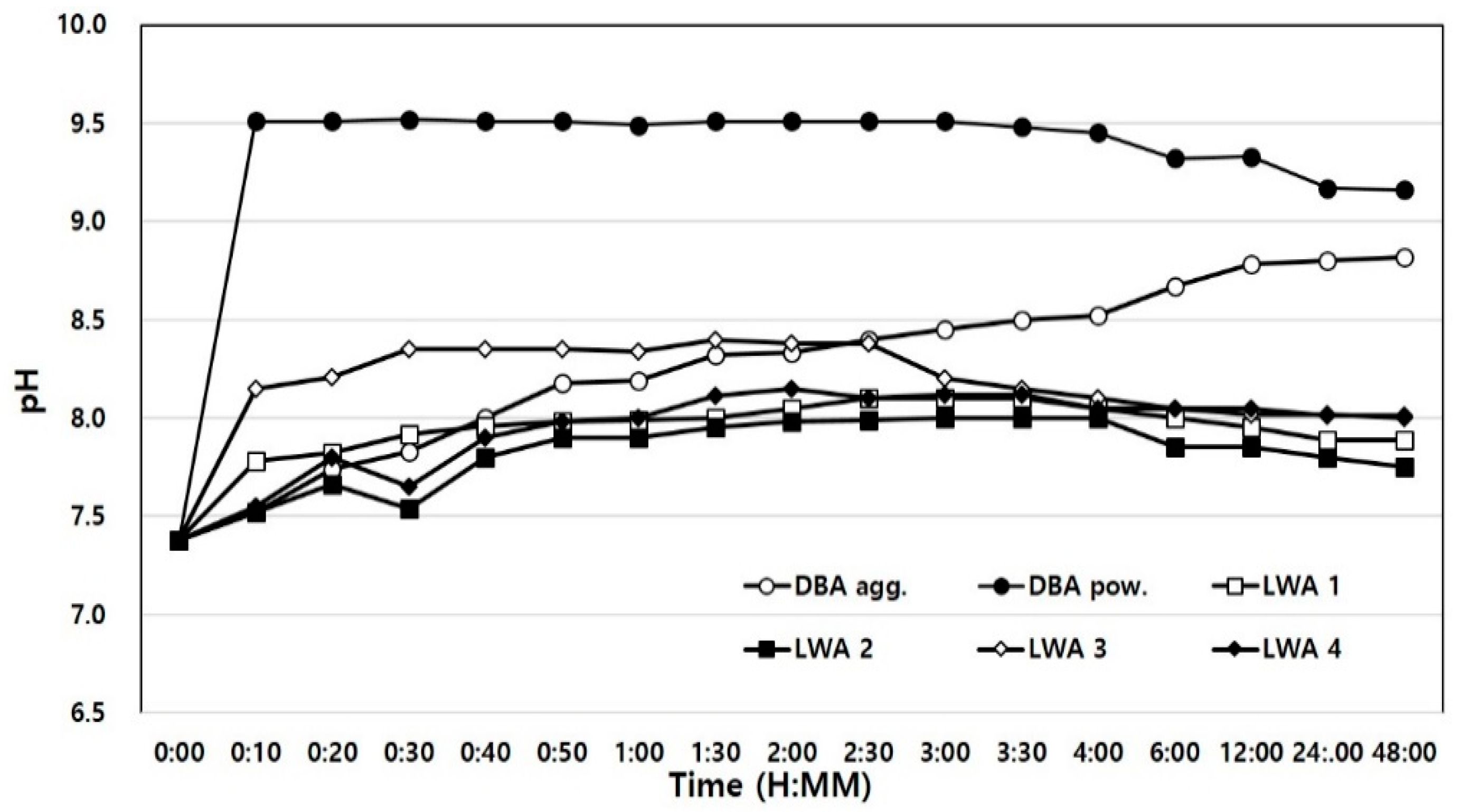
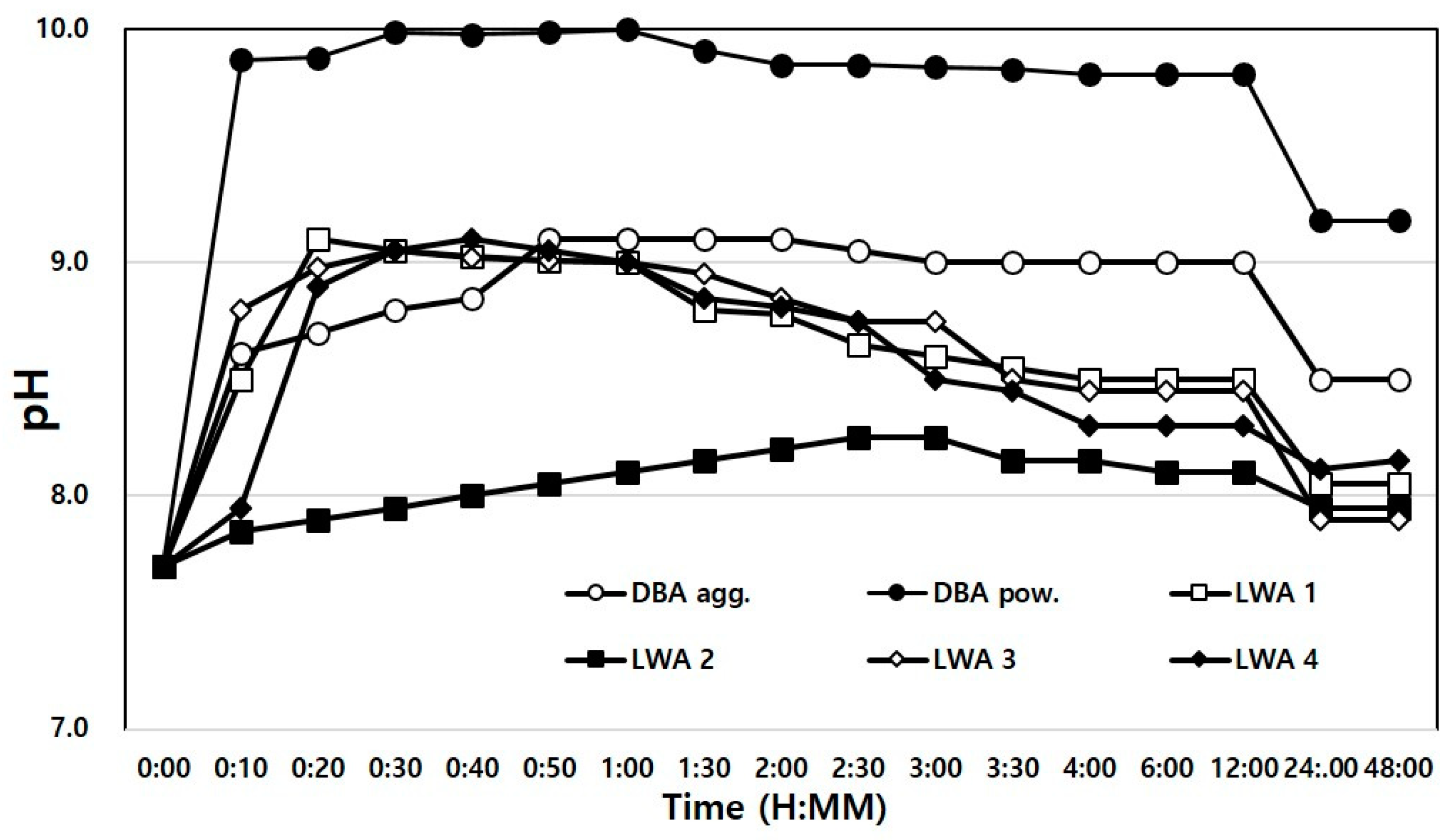
4.2.5. Hydrogen Exponent (pH)
4.2.6. Heavy Metal Leaching Test
4.2.7. Minor Inorganic Compounds by ICP
5. Conclusions
- (1)
- An oxide analysis of dBA showed a composition similar to that of wBA, FA, and four types of LWA, and a mineral analysis showed that the primary component was quartz (SiO2), and in the case of dBA, wBA, and FA, the presence of Fe3O4 was clearer.
- (2)
- As for the SO3 content, it was found in significantly lower amounts in dBA compared to wBA, FA, and LWA-1 to 4, and the chloride content was also lower in dBA compared to the existing artificial LWAs. Thus, it is believed that there will not be any concerns of expansion caused by SO3 or corrosion of rebar by chloride.
- (3)
- As a result of measuring the unburned carbon content, it was found that dBA would be a stable aggregate for concrete production, as it has a relatively very low unburned carbon content compared to wBA, FA, and artificial LWAs.
- (4)
- In a leaching test carried out in accordance with the Official Waste Test Standards, the six major heavy metals were not detected. In contrast, Pb, Cd, Du, Hg, and As were detected when the test was conducted according to the Official Soil Pollution Test Standards, but they were all below the baseline.
- (5)
- An ICP analysis showed that some of the alkali metals and alkaline earth metals were found in larger amounts in dBA than in artificial LWAs, wBA, and FA, thereby resulting in higher pH levels, and Pb, Cr, and Cu were detected in a heavy metal leaching test. However, the heavy metals detected were found at levels below the baseline, based on which it was judged that there will not be any problems in real-life application.
- (6)
- Based on the above results, it is believed that there are no significant physical and chemical problems in using dBA as a lightweight aggregate.
- (7)
- This paper physically and chemically examined the possibility of using dBA as aggregate for concrete and it is intended that its characteristics by using it in mortar and concrete in the future will be examined.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korea Energy Economics. Yearbook of Energy Statistic; Notification on 2011; Korea Energy Economics Institute: Seoul, Korea, 2011; pp. 172–179. [Google Scholar]
- Korea Energy Economics. Yearbook of Energy Statistic; Notification on 2011; Korea Energy Economics Institute: Seoul, Korea, 2013; pp. 345–349. [Google Scholar]
- Choi, S.-J.; Kim, M.-H. A Study on the Durabilities of High Volume Coal Ash Concrete by the Kinds of Coal Ash. J. Korean Inst. Build. Constr. 2009, 9, 73–78. [Google Scholar] [CrossRef]
- Abis, M.; Bruno, M.; Simon, F.-G.; Grönholm, R.; Hoppe, M.; Kuchta, K.; Fiore, S. A Novel Dry Treatment for Municipal Solid Waste Incineration Bottom Ash for the Reduction of Salts and Potential Toxic Elements. Materials 2021, 14, 3133. [Google Scholar] [CrossRef]
- Kim, J.M.; Choi, H.B.; Lee, M.J.; Yu, J.S.; Sun, J.S. Properties of Fresh Concrete with Dry Bottom Ash Processed by Various Method. J. Korean Inst. Build. Const. 2014, l14, 92–93. [Google Scholar]
- Kim, M.H.; Yeon, N.K. Properties of Mortar Using Lightweight Fine Aggregate Made by Bottom Ash Discharged Air Cooling Process according to Grading. J. Korean Inst. Build. Const. 2016, l14, 92–93. [Google Scholar]
- Kim, J.M. Development for Environment Friendly Construction Material Having Low Density Using the Dry Processed Bottom Ash; Notification on 06-2011; Ministry of Environment: Seoul, Korea, 2016.
- Standard Test Method for Density and Absorption of Coarse Aggregate; KS F 2503; Korea Standard Committee: Seoul, Korea, 2019.
- Standard Test Method for Density and Absorption of Fine Aggregate; KS F 2504; Korea Standard Committee: Seoul, Korea, 2020.
- Standard Test Method for Bulk Density and Percentage of Absolute Volume in Aggregate; KS F 2505; Korea Standard Committee: Seoul, Korea, 2017.
- U.S. Environmental Protection Agency (EPA). Wastes from the Combustion of Coal by Electric Utility Power Plants; Notification on February 1988; U.S. Environmental Protection Agency: Washington, DC, USA, 1988.
- Cai, Y. Laboratory Investigation of Pulverized Coal Combustion Bottom Ash as a Fine Aggregate in Roller Compacted Concretes. Master’s Thesis, Department of Civil Engineering, Southern Illinois University, Carbondale, IL, USA, 1996. [Google Scholar]
- Kim, J.M.; Choi, S.M.; Sung, J.H.; Bok, Y.J.; Sun, J.S. Engineering Properties of Lightweight Aggregate Concrete Using Dry Bottom Ash as Coarse Aggregate. J. Korea Instit. Build. Constr. 2013, 13, 166–167. [Google Scholar]
- Kim, H.-S.; Kim, J.-M.; Kim, B. Quality improvement of recycled fine aggregate using steel ball with the help of acid treatment. J. Mater. Cycles Waste Manag. 2017, 20, 754–765. [Google Scholar] [CrossRef]
- Testing Method for Analysis of Chloride in Concrete and Concrete Raw Materials; KS F 2713; Korea Standard Committee: Seoul, Korea, 2002.
- Fly Ash; KS L 5405; Korea Standard Committee: Seoul, Korea, 2009.
- Surface Active Agents—Determination of pH of Aqueous Solutions—Potentiometric Method; KS M ISO 4316; Korea Standard Committee: Seoul, Korea, 2009.
- Korea Ministry of Environment Regulation. Official Wastes Test Method; Notification on 2011-160; Ministry of Environment: Seoul, Korea, 2011.
- Korea Ministry of Environment Regulation. Official Wastes Test Method; Notification on 2013-113; Ministry of Environment: Seoul, Korea, 2013.
- Ministry of Trade. Development of Adsorbent Using Highly Unburned Carbon; Notification on 2000; Ministry of Industry & Energy: Seoul, Korea, 2000.
- Jung, M.Y. Beneficiation of Domestic Anthracite Fly Ash by Reverse Flotation. J. Korean Inst. Miner. Energy Resour. Eng. 2000, 37, 72–79. [Google Scholar]
- Ministry of Trade. Coal Ash Ceramic Body Manufactured by Low Temperature Processing; Notification on 2002; Ministry of Industry & Energy: Seoul, Korea, 2002.
- Jeon, D.H.; Yum, W.S.; Song, H.M.; Yoon, S.Y.; Bae, Y.H.; Oh, J.E. Use of Coal Bottom Ash and CaO-CaCl2-Activated GGBFS Binder in the Manufacturing of Artificial Fine Aggregates through Cold-Bonded Pelletization. Materials 2020, 13, 5598. [Google Scholar] [CrossRef] [PubMed]
- Risdanareni, P.; Villagran, Y.; Schollbach, K.; Wang, J.; De Belie, N. Properties of Alkali Activated Lightweight Aggregate Generated from Sidoarjo Volcanic Mud (Lusi), Fly Ash, and Municipal Solid Waste Incineration Bottom Ash. Materials 2020, 13, 2528. [Google Scholar] [CrossRef]
- Kurda, R.; Vasco Silva, R.; de Brito, J. Incorporation of Alkali-Activated Municipal Solid Waste Incinerator Bottom Ash in Mortar and Concrete: A Critical Review. Materials 2020, 13, 3428. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.H.; Park, J.H. A Study on the Thermal Properties of High-Strength Concrete Containing CBA Fine Aggregates. Materials 2020, 13, 1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Wei, X.; Li, T.; Zhang, L. Strengthening Behavior of Cemented Paste Backfill Using Alkali-Activated Slag Binders and Bottom Ash Based on the Response Surface Method. Materials 2020, 13, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menéndez, E.; Argiz, C.; Sanjuán, M.Á. Chloride Induced Reinforcement Corrosion in Mortars Containing Coal Bottom Ash and Coal Fly Ash. Materials 2019, 12, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Heede, P.; Ringoot, N.; Beirnaert, A.; Van Brecht, A.; Van den Brande, E.; De Schutter, G.; De Belie, N. Sustainable High Quality Recycling of Aggregates from Waste-to-Energy, Treated in a Wet Bottom Ash Processing Installation, for Use in Concrete Products. Materials 2016, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holm, T.A. Performance of structural lightweight concrete in a marine environment. J. Am. Concr. Inst. 1980, 65, 589–608. [Google Scholar]
- Tuan, B.L.A.; Tesfamariam, M.G.; Chen, Y.-Y.; Hwang, C.-L.; Lin, K.-L.; Young, M.-P. Production of Lightweight Aggregate from Sewage Sludge and Reservoir Sediment for High-Flowing Concrete. J. Constr. Eng. Manag. 2014, 140, 04014005. [Google Scholar] [CrossRef]
- Lightweight Aggregate for Structural Concrete; KS F 2534; Korea Standard Committee: Seoul, Korea, 2009.
- Concrete Aggregate; KS F 2526; Korea Standard Committee: Seoul, Korea, 2007.
- Bottom Ash Aggregate for the Precast Concrete Product; KS F 4570; Korea Standard Committee: Seoul, Korea, 2007.
- Berry, E.E.; Hemming, R.T. Beneficiation of Fly Ash. An Overview of a Resource. In Proceedings of the 2nd International Conference on the Use of Fly Ash, Silica Fume, Slag and Natural Pozzolans in Concrete, Madrid, Spain, 21–25 April 1986; pp. 241–274. [Google Scholar]
- Cheeseman, C.; Makinde, A.; Bethanis, S. Properties of lightweight aggregate produced by rapid sintering of incinerator bottom ash. Resour. Conserv. Recycl. 2005, 43, 147–162. [Google Scholar] [CrossRef]
- Bhatty, J.I.; Reid, K.J. Lightweight aggregates from incinerated sludge ash. J. Waste Manag. Res. 1986, 7, 363–376. [Google Scholar] [CrossRef]
- Yip, W.; Tay, J. Aggregate Made from Incinerated Sludge Residue. J. Mater. Civ. Eng. 1990, 2, 84–93. [Google Scholar] [CrossRef]
- Park, J.-B.; Lee, B.-C.; Jang, M.-H.; Na, H.-H. Environmental Characteristics of Leachates from Steel Slag. J. Korean Geosynth. Soc. 2012, 11, 31–38. [Google Scholar] [CrossRef]
- Test Method for pH of Soils; KS F 2103; Korea Standard Committee: Seoul, Korea, 2013.
- Wang, W.; Qin, Y.; Song, D.; Wang, K. Column leaching of coal and its combustion residues, Shizuishan, China. Int. J. Coal Geol. 2008, 75, 81–87. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Kim, J.-W.; Kim, H.-S.; Lee, S.-P.; Yang, J.-E.; Kim, S.-C. Bottom Ash Modification via Sintering Process for Its Use as a Potential Heavy Metal Adsorbent: Sorption Kinetics and Mechanism. Materials 2021, 14, 3060. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Trade. Quality Standard for Drinking Water; Notification on 2012; Ministry of Environment: Seoul, Korea, 2012.
- Brigatti, M.F.; Corradini, F.; Franchini, G.C.; Mazzoni, S.; Medici, L.; Poppi, L. Interaction between montmorillonite and polluants from industrial waste-water exchange of Zn2+ and Pb2+ from aqueous solution. Appl. Clay Sci. 1995, 9, 383–395. [Google Scholar] [CrossRef]



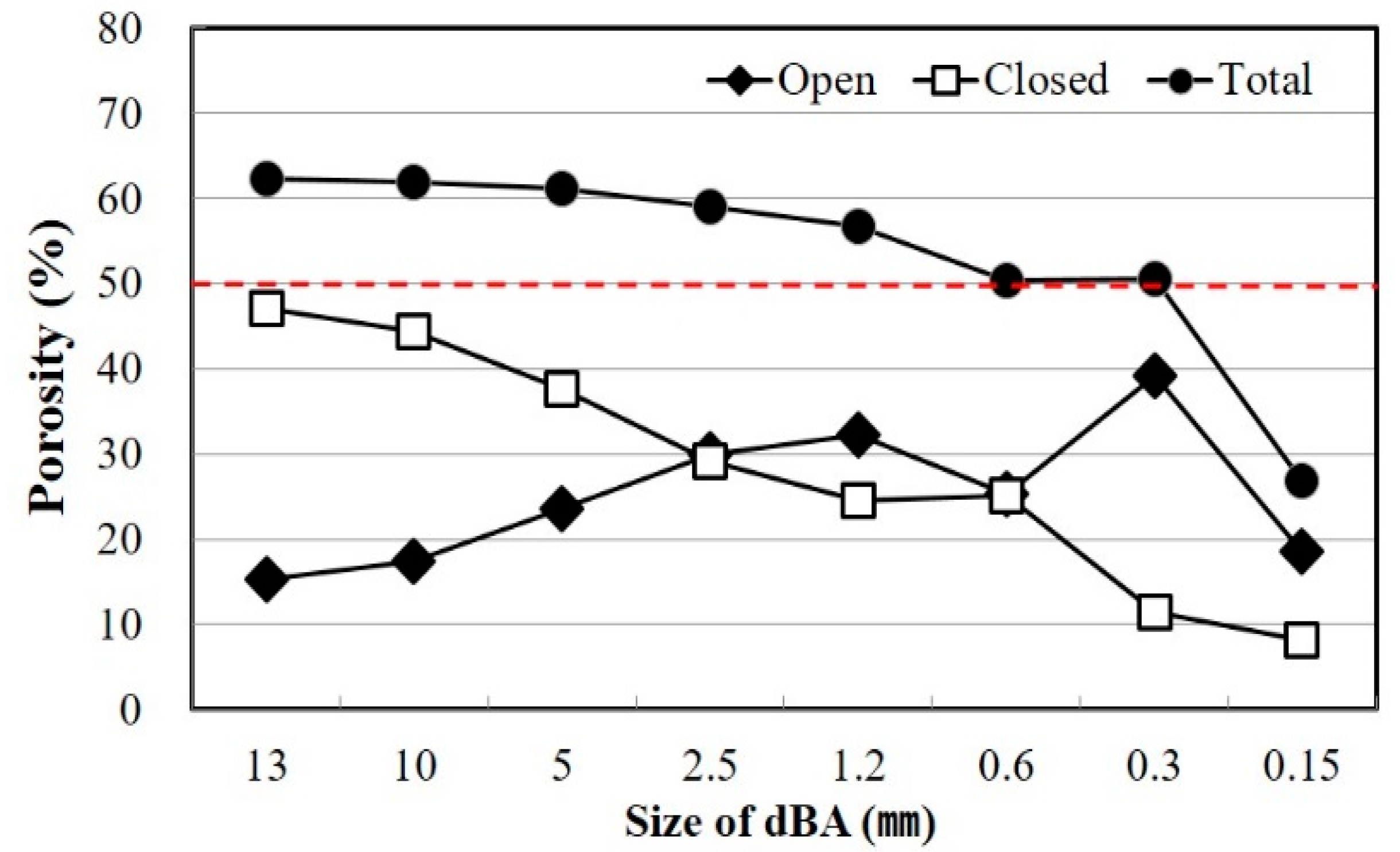
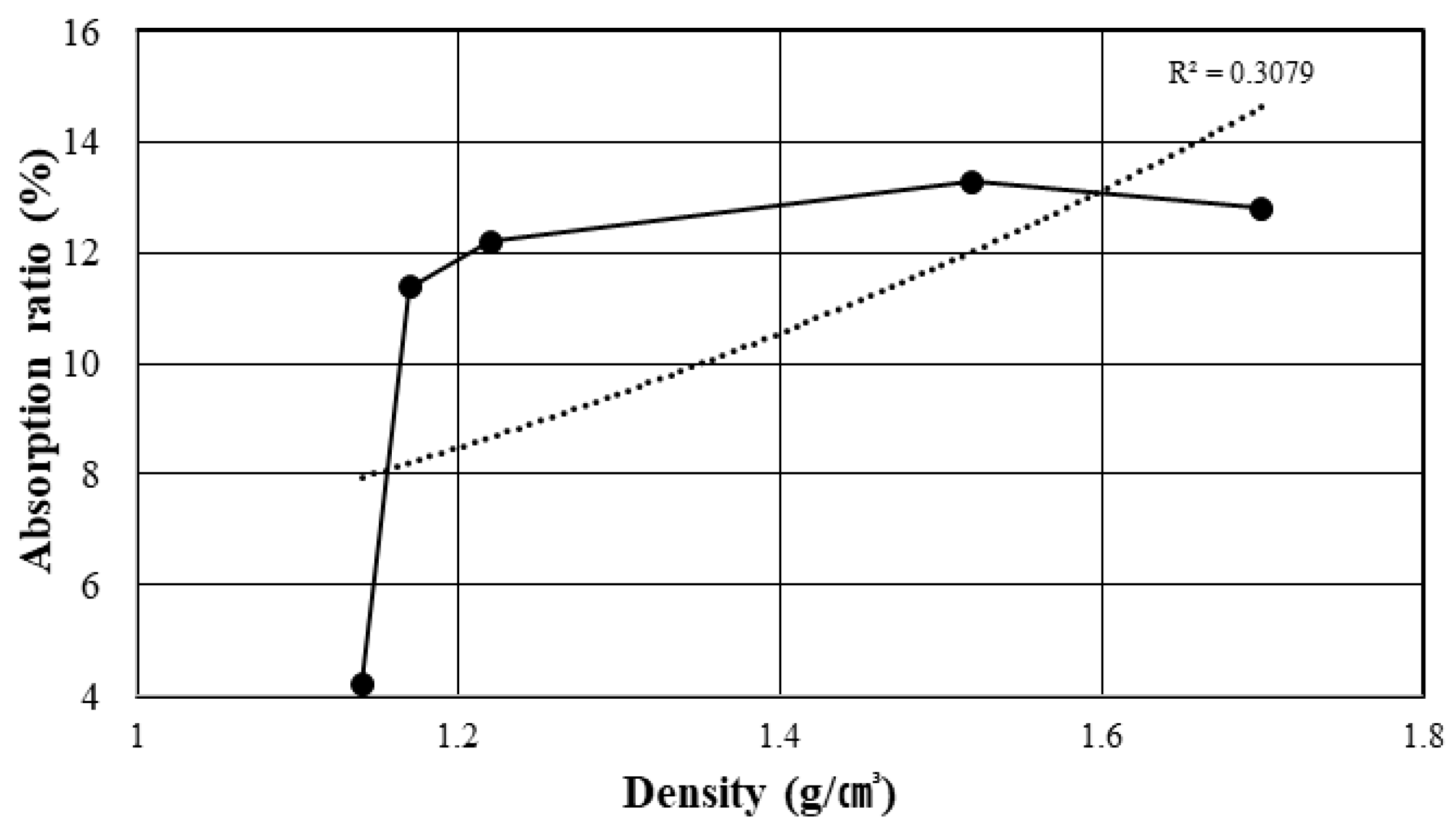




| Aggregate Size (mm) | Density (g/cm3) | Absorption (%) | Bulk Density (kg/m3) | Percentage of Absolute Volume (%) | |
|---|---|---|---|---|---|
| OD * | SSD ** | ||||
| 13 | 1.09 | 1.14 | 4.21 | 437.7 | 40.1 |
| 10 | 1.05 | 1.17 | 11.38 | 437.2 | 41.6 |
| 5 | 1.08 | 1.22 | 12.20 | 432.0 | 39.8 |
| 2.5 | 1.34 | 1.52 | 13.29 | 497.1 | 37.1 |
| 1.2 | 1.51 | 1.70 | 12.79 | 585.1 | 38.8 |
| Type of Aggregates | Advantages | Drawbacks |
|---|---|---|
| dBA |
|
|
| wBA | - |
|
| Artificial light weight aggregate |
|
|
| Natural aggregate |
|
|
| ID | Full Name of Light Weight Aggregate | Test Items |
|---|---|---|
| dBA | Bottom ash discharged from dry process |
|
| wBA | Bottom ash discharged from wet process | |
| FA | Fly ash | |
| LWA-1 | Artificial light aggregate from Korea | |
| LWA-2 | Artificial light aggregate from USA | |
| LWA-3 | Artificial light aggregate from Japan | |
| LWA-4 | Artificial light aggregate from China |
| ID | Density (g/cm3) | Absorption (%) | Bulk Density (kg/m3) | Percentage of Absolute Volume (%) | |
|---|---|---|---|---|---|
| OD | SSD | ||||
| dBA | 1.72 | 1.76 | 12.11 | 732.8 | 45.52 |
| wBA | 1.67 | 1.73 | 12.34 | ||
| FA | 2.10 | - | - | - | - |
| LWA-1 | 1.70 | 1.88 | 10.16 | 1029.1 | 60.54 |
| LWA-2 | 1.47 | 1.51 | 2.36 | 766.2 | 52.12 |
| LWA-3 | 1.38 | 1.50 | 8.89 | 738.4 | 53.50 |
| LWA-4 | 1.39 | 1.53 | 10.15 | 767.0 | 55.18 |
| MnO | SrO | BaO | Cl | Cr2O3 | ZrO2 | NiO | |
|---|---|---|---|---|---|---|---|
| dBA | 0.209 | 0.181 | 0.129 | 0.066 | 0.066 | 0.018 | |
| wBA | 0.113 | 0.112 | 0.134 | 0.013 | 0.034 | 0.050 | 0.014 |
| FA | 0.190 | 0.025 | 0.081 | 0.038 | 0.024 | 0.009 | |
| LWA-1 | 0.059 | 0.019 | 0.020 | 0.023 | 0.006 | ||
| LWA-2 | 0.143 | 0.029 | 0.062 | 0.009 | 0.033 | 0.037 | 0.013 |
| LWA-3 | 0.145 | 0.099 | 0.140 | 0.038 | 0.042 | 0.035 | 0.031 |
| LWA-4 | 0.061 | 0.098 | 0.076 |
| ID | Test Items (mg/L) | |||||
|---|---|---|---|---|---|---|
| Pb | Cd | Cr6− | Cu | Hg | As | |
| Standard * | 3 | 0.3 | 1.5 | 3 | 0.005 | 1.5 |
| dBA | N.D | N.D | N.D | N.D | N.D | N.D |
| ID | Test Items (mg/L) | |||||
|---|---|---|---|---|---|---|
| Pb | Cd | Cr6− | Cu | Hg | As | |
| Standard | 200 | 4 | 5 | 150 | 4 | 25 |
| dBA—2.5 mm size | 8.04 | 0.27 | N.D | 27.7 | 0.01 | 1.61 |
| dBA—5 mm under | 6.86 | N.D | N.D | 6.23 | N.D | N.D |
| Alkaline Metals and Earth Metals (mg/L) | Heavy Metal (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K | Mg | Ca | Na | Pb | Cd | Cr | Cu | Hg | As | |
| dBA | 1.2 | 34,770 | 10,775 | 22.4 | 0.1 | ND * | 1.0 | 515 | ND | ND |
| wBA | 0.2 | 5552 | 2223 | 6.3 | 1.3 | ND | 0.1 | 56 | ND | ND |
| FA | 0.1 | 9235 | 9552 | 4.9 | 0.0 | ND | 0.3 | 178 | ND | ND |
| LWA-1 | 0.1 | 458 | 134 | 0.9 | 0.1 | ND | 0.6 | 260 | ND | ND |
| LWA-2 | 4.5 | 673 | 2942 | 1.2 | 0.1 | ND | 0.9 | 174 | ND | ND |
| LWA-3 | 5.1 | 4277 | 2419 | 1.3 | 0.1 | ND | 0.4 | 76 | ND | ND |
| LWA-4 | 5.1 | 12,052 | 5880 | 28.1 | 0.2 | ND | 0.9 | 294 | ND | ND |
| ST1 ** | - | - | - | - | 200 | 4 | 5 | 150 | 4 | 25 |
| ST2 *** | - | - | - | - | 400 | 10 | 15 | 500 | 10 | 50 |
| ST3 **** | - | - | - | - | 700 | 60 | 40 | 2000 | 20 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, H.; Shin, S. An Evaluation of the Physical and Chemical Stability of Dry Bottom Ash as a Concrete Light Weight Aggregate. Materials 2021, 14, 5291. https://doi.org/10.3390/ma14185291
Kim J, Kim H, Shin S. An Evaluation of the Physical and Chemical Stability of Dry Bottom Ash as a Concrete Light Weight Aggregate. Materials. 2021; 14(18):5291. https://doi.org/10.3390/ma14185291
Chicago/Turabian StyleKim, Jinman, Haseog Kim, and Sangchul Shin. 2021. "An Evaluation of the Physical and Chemical Stability of Dry Bottom Ash as a Concrete Light Weight Aggregate" Materials 14, no. 18: 5291. https://doi.org/10.3390/ma14185291
APA StyleKim, J., Kim, H., & Shin, S. (2021). An Evaluation of the Physical and Chemical Stability of Dry Bottom Ash as a Concrete Light Weight Aggregate. Materials, 14(18), 5291. https://doi.org/10.3390/ma14185291






