Effect of Cerium on the Microstructure and Inclusion Evolution of C-Mn Cryogenic Vessel Steels
Abstract
:1. Introduction
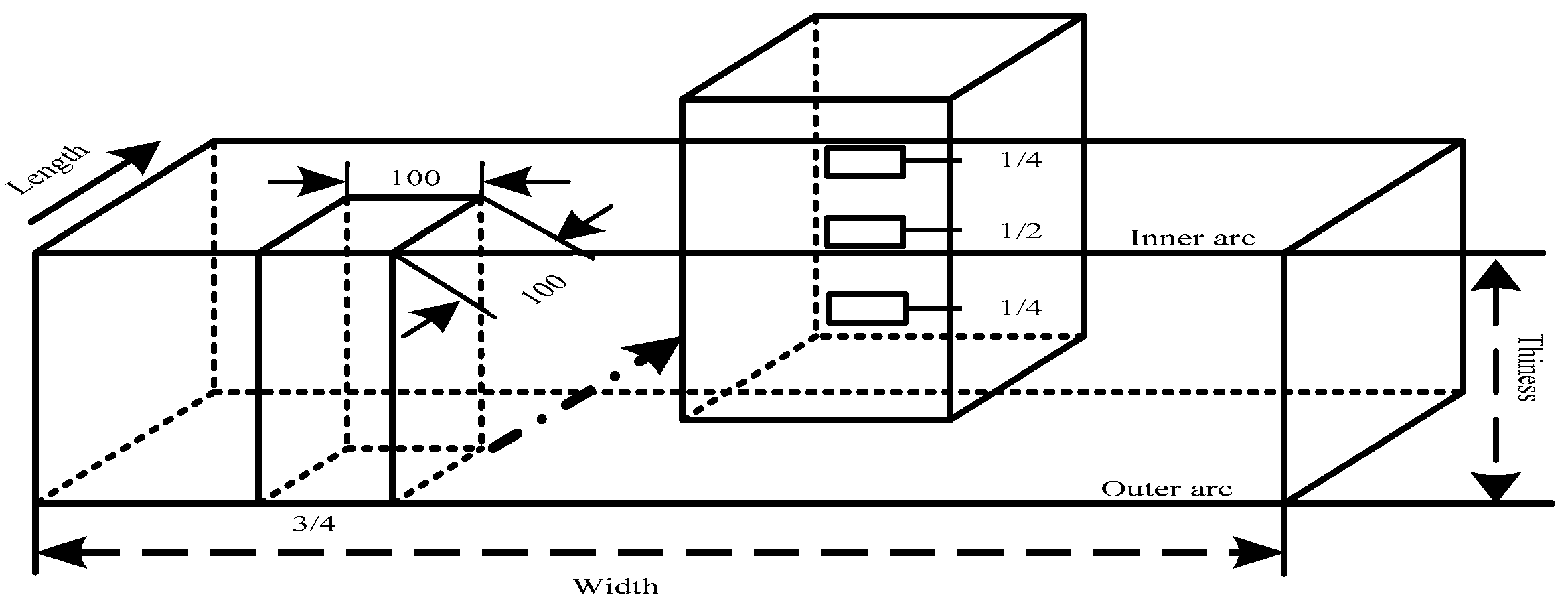
2. Experimental
3. Results and Discussion
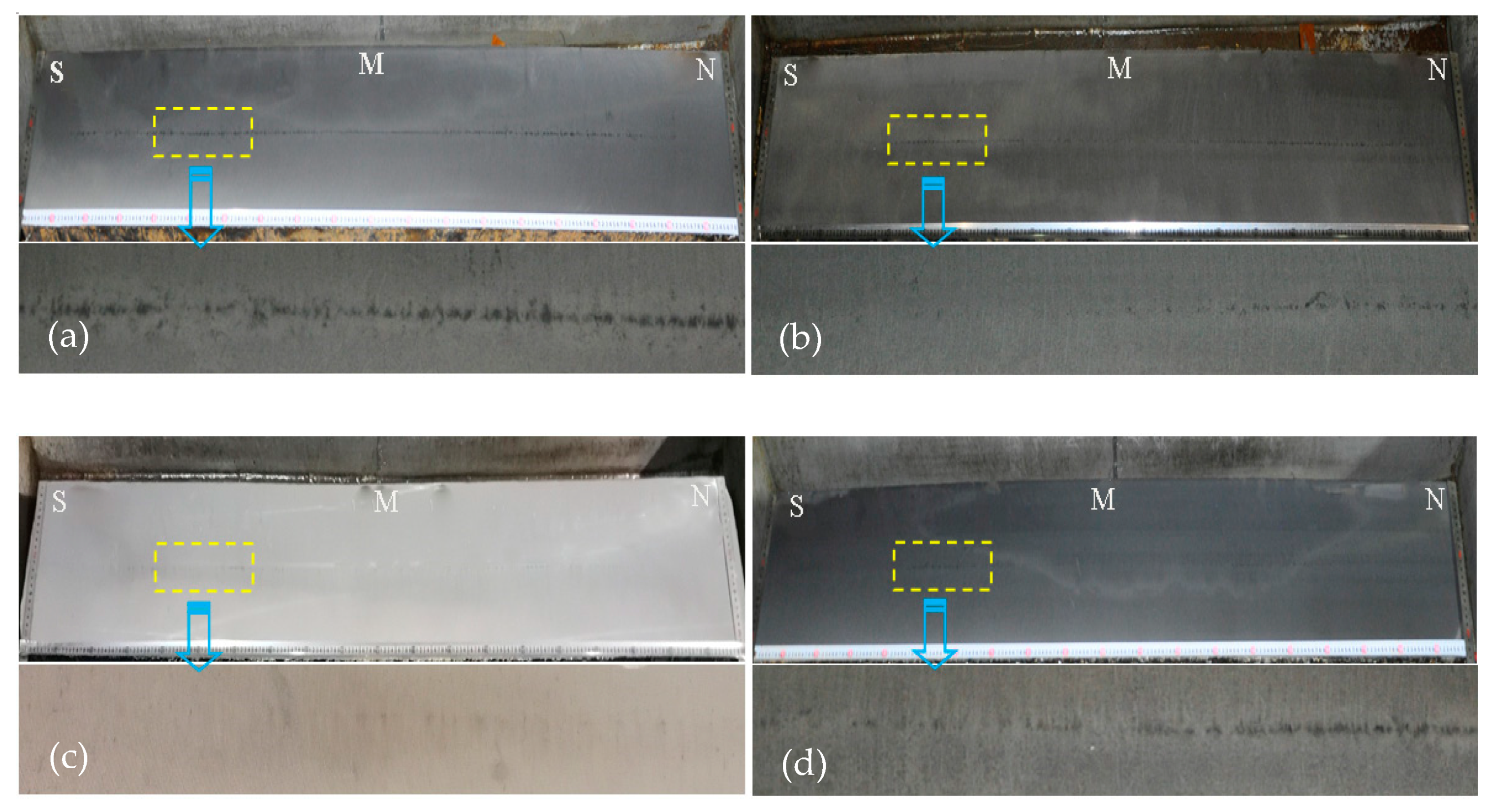
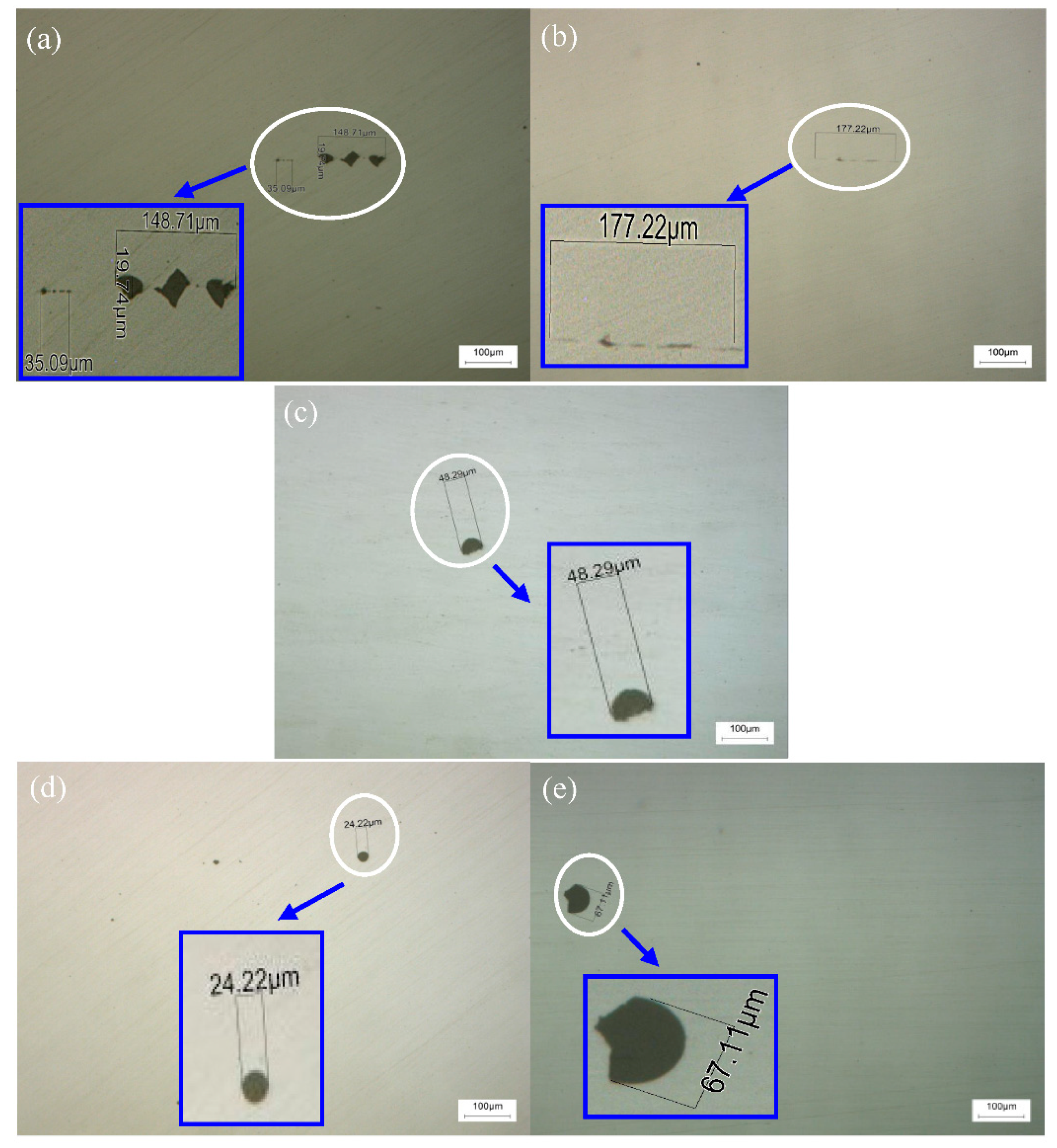
3.1. Effect of Ce on the Casting Slab Quality
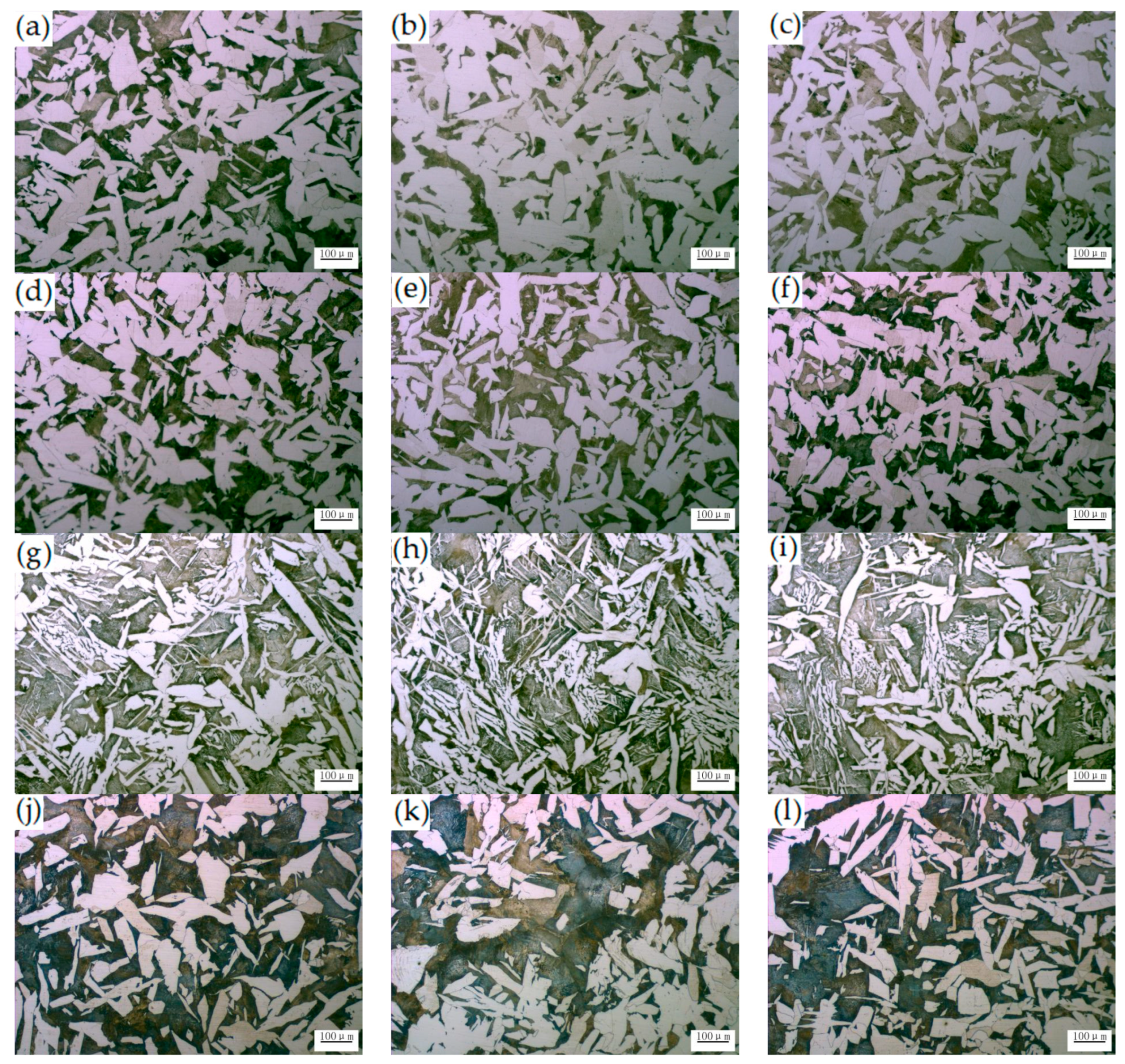
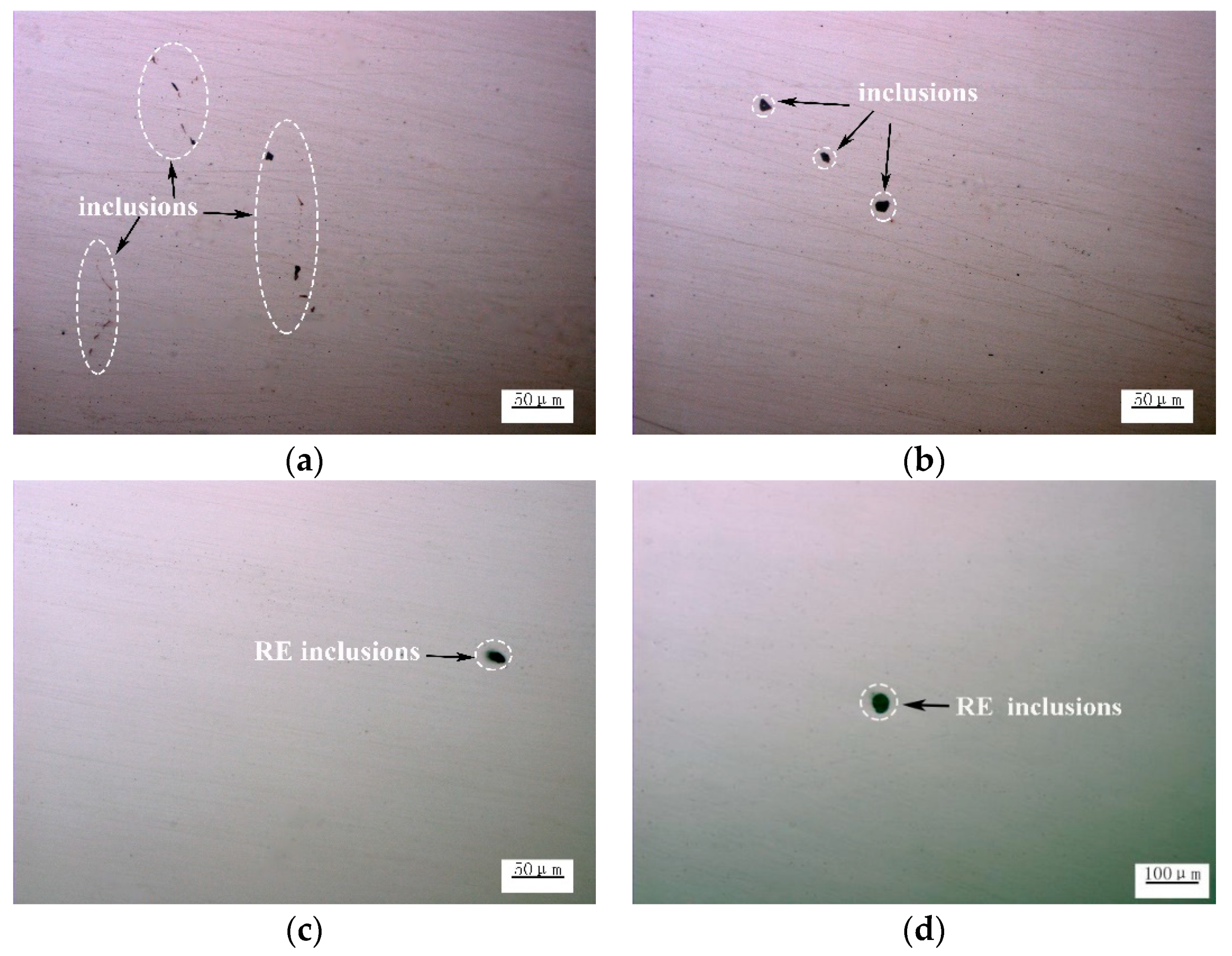
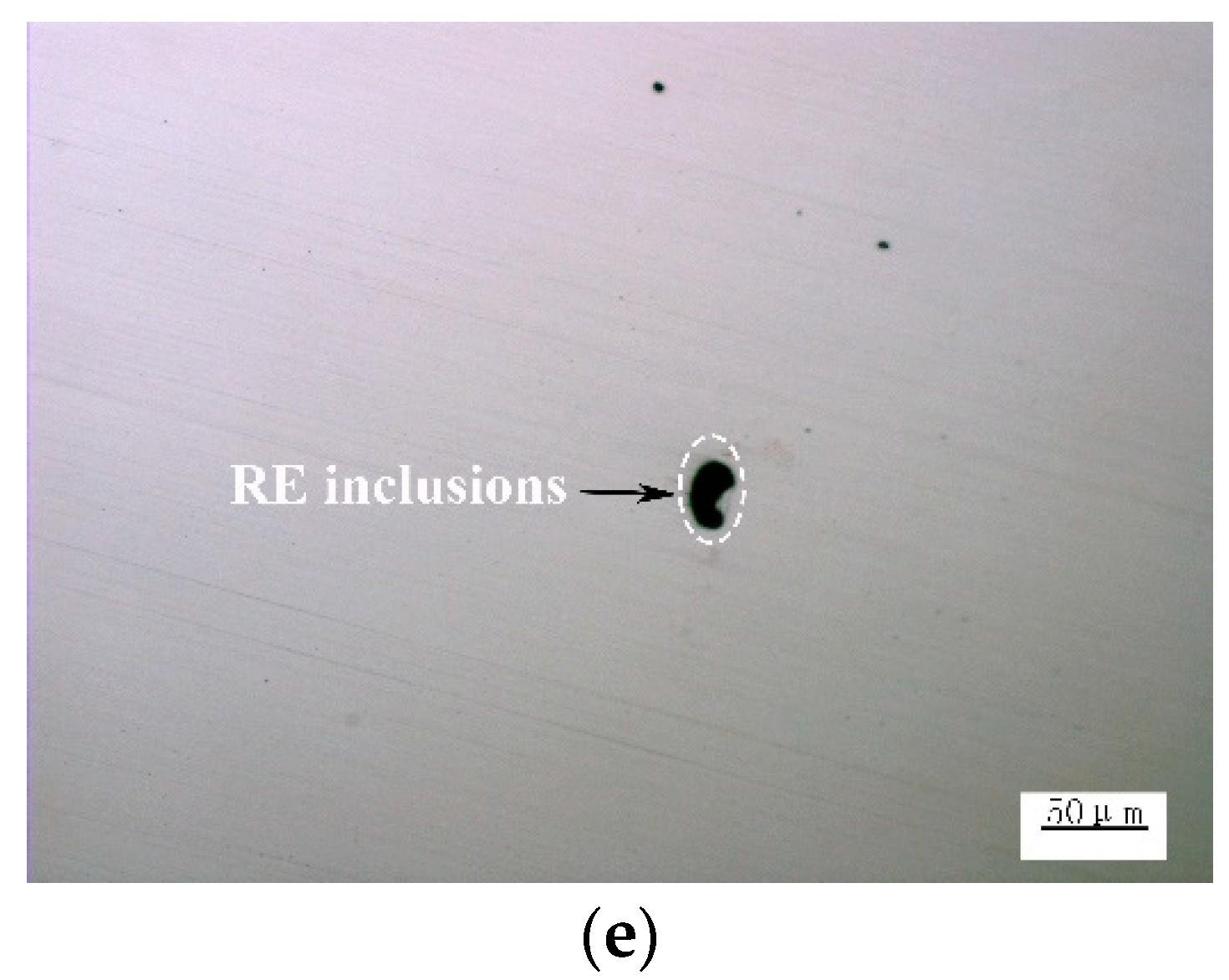
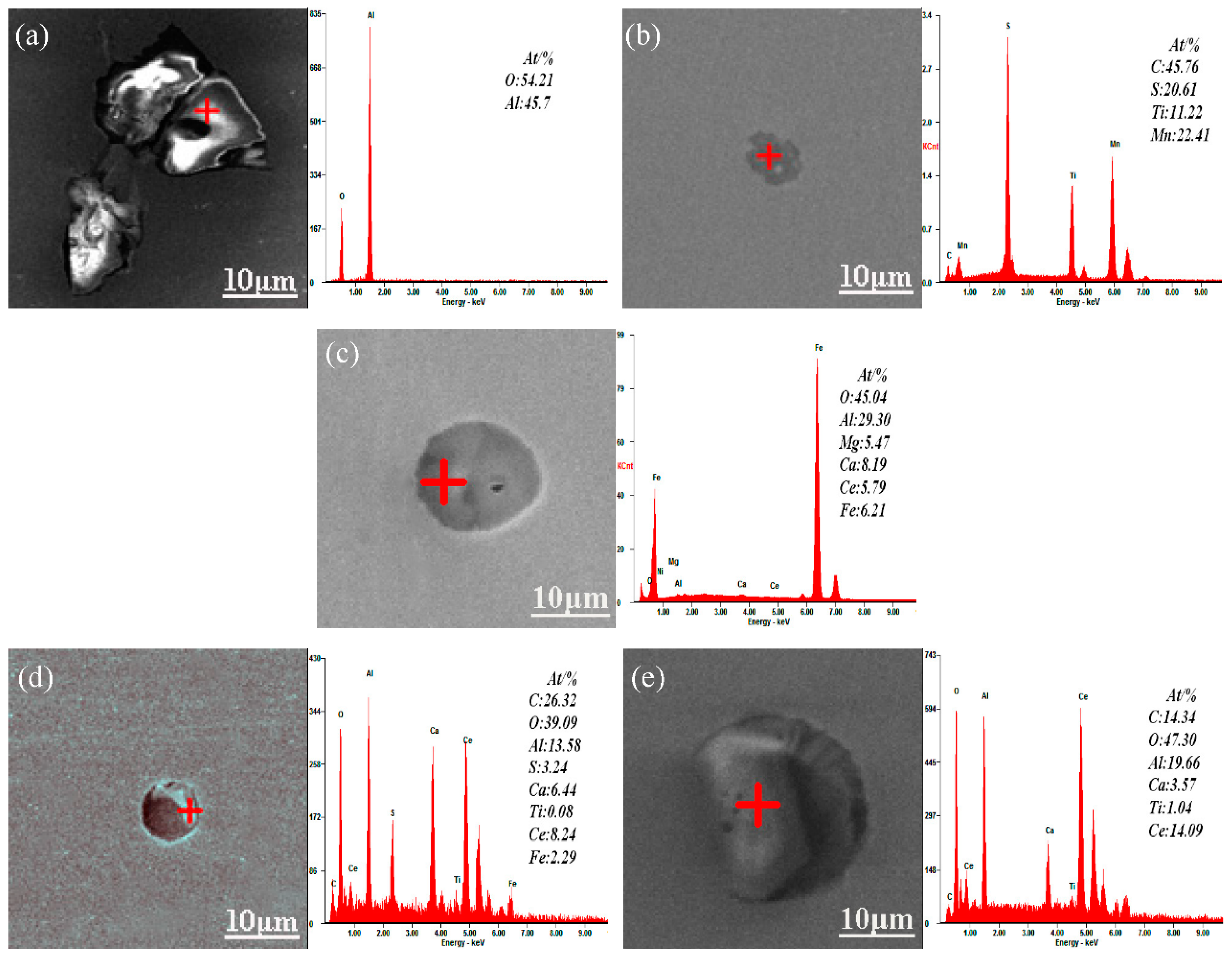
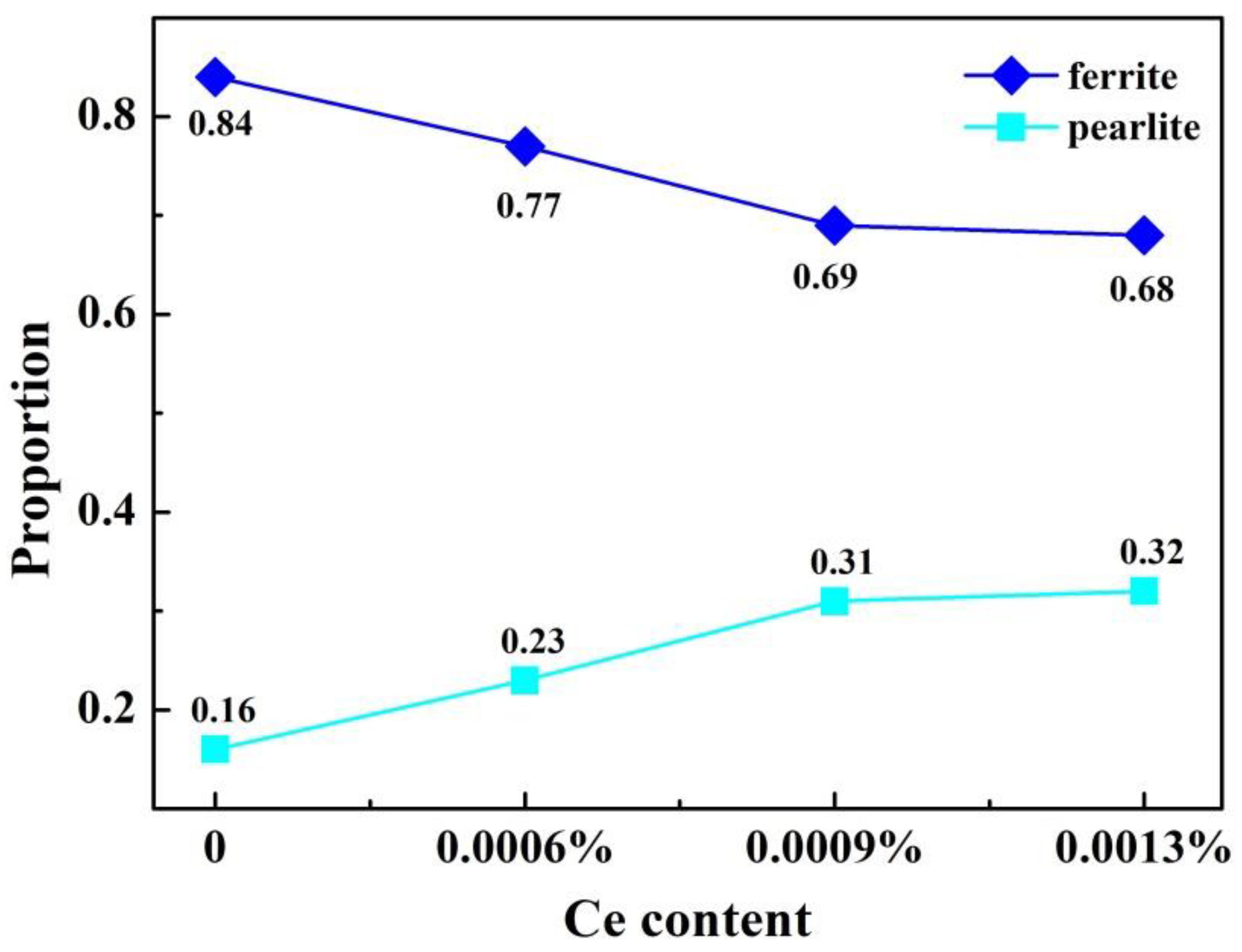
3.2. Effect of Ce on Microstructure and Inclusions in Steel Strips
3.3. Ce Inclusions Formed Thermodynamic Mechanisms
3.4. Inclusion Formation Mechanisms with the Addition of Ce
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Pan, J.H.; Jin, T.; Hong, Z.Y.; Wu, Y.C. Estimation of fracture toughness of 16MnDR steel using Master Curve method and Charpy V-notch impact energy. Theor. Appl. Fract. Mech. 2018, 96, 443–451. [Google Scholar] [CrossRef]
- Liu, D.F.; Yang, X.L.; Hou, L.F.; Cui, T.X.; Hu, Y.T.; Wei, Y.H. Research and Application of Ultralow Temperature 9Ni Steel for LNG Storage Tank. J. Iron Steel Res. 2009, 21, 1–5. [Google Scholar]
- Xu, L.; Li, Z.C.; Zhang, J.; Xu, Y.S. LNG land storage and transmission in China. Nat. Gas Ind. 2002, 22, 89–91. [Google Scholar]
- Barsouma, I.; Lawala, S.A.; Simmonsb, R.J.; Rodrigues, C.C. Failure analysis of a pressure vessel subjected to an internal blast load. Eng. Fail. Anal. 2018, 91, 354–369. [Google Scholar] [CrossRef]
- Miao, C.J.; Zheng, J.Y.; Gao, X.Z.; Huang, Z.; Guo, A.B.; Ye, D.Y.; Ma, L. Investigation of low-cycle fatigue behavior of austenitic stainless steel for cold-stretched pressure vessels. J. Zhejiang Univ. Sci. A 2013, 14, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Xin, W.B.; Zhang, J.; Luo, G.P.; Wang, R.F.; Meng, Q.Y.; Song, B. Improvement of hot ductility of C-Mn Steel containing arsenic by rare earth Ce. Metall. Res. Technol. 2018, 115, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Garrison, W.M.; Maloney, J.L. Lanthanum additions and the toughness of ultra-high strength steels and the determination of appropriate lanthanum additions. Mater. Sci. Eng. A 2005, 403, 299–310. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, J.; Chen, H.L.; Su, Y.H.; Kuo, C.L.; Su, Y.H.; Chen, S.H.; Lin, K.J.; Hsieh, P.H. Effects of Rare Earth Metals on Steel Microstructures. Materials 2016, 9, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Chen, W.Q.; Wu, S.J. Effect of lanthanum content on microstructure and magnetic properties of non-oriented electrical steels. J. Rare Earths 2013, 31, 727–733. [Google Scholar] [CrossRef]
- Liu, H.J.; Liu, H.J.; Chen, Y.Z. Effect of RE on the structure and property of steel 45. Chin. Rare Earths 2012, 33, 24–27. [Google Scholar]
- Lin, Q.; Ye, W.; Chen, N.; Liu, Y.H.; Guo, Y.; Chen, Z.Q.; Zhu, Q.H. Effect of rare earths on extra-low sulfur microalloy steel. J. Chin. Rare Earth Soc. 1997, 22, 228–233. [Google Scholar]
- Xiao, J.G.; Cheng, H.J.; Wang, F.M. Effects of rare earth in ship plate steel on its microstructure and low temperature toughness. Chin. Rare Earths 2010, 31, 52–57. [Google Scholar]
- Huang, Y.; Cheng, G.G.; Xie, Y. Modification Mechanism of Cerium on the Inclusions in Drill Steel. Acta Metall. Sin. 2018, 54, 1253–1261. [Google Scholar]
- Imashuku, S.; Wagatsuma, K. Cathodoluminescence Analysis of Nonmetallic Inclusions in Steel Deoxidized and Desulfurized by Rare-Earth Metals (La, Ce, Nd). Metall. Mater. Trans. B 2019, 51, 79–84. [Google Scholar] [CrossRef]
- Gao, S.; Wang, M.; Guo, J.L.; Wang, H.; Zhi, J.G.; Bao, Y.P. Characterization Transformation of Inclusions Using Rare Earth Ce Treatment on Al-Killed Titanium Alloyed Interstitial Free Steel. Steel Res. Int. 2019, 90, 1900194. [Google Scholar] [CrossRef]
- Fan, L.F.; Zhu, R.; He, J.Z.; Lu, B. Effect of Rare Earth Element La on Texture and Inclusion of Nonoriented Electrical Steel Produced by Thin Slab Casting and Rolling Process. ISIJ Int. 2018, 58, 2348–2353. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Jin, Z.L.; Ren, H.P.; Song, W.Z. Effect of rare earth on microstructure and non-metallicinclusions in thin slab producted by CSP. J. Chin. Rare Earth Soc. 2010, 28, 601–606. [Google Scholar]
- Wang, L.M.; Zhu, G.L.; Xun, J.; Qi, G.P.; Zhu, J.X.; Chen, D.X. Study of Rare Earth Effect and Mechanism in Ferrite Stainless Steel(430). Chin. Rare Earths 2008, 28, 67–71. [Google Scholar]
- Wang, H.P.; Jiang, S.L.; Yu, P.; Bai, B.; Sun, L.F.; Wang, Y. Distribution of Arsenic Inclusions in Rare Earth Steel Ingots. Metals 2020, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.X.; Li, P.Z. Influence of RE addition on the central segregation in 16Mn concast slabs and the fractograph of finished products. Iron Steel 1986, 21, 13–19. [Google Scholar]
- Tuttle, R.B. Study of Rare Earth Additions Effect on the Solidification and Properties of 4130 Steel. JMEPG 2019, 28, 6720–6727. [Google Scholar] [CrossRef]
- Fruehan, R.J. The Free Energy of Formation of Ce2O2S and the Nonstoichiometry of Cerium Oxides. Metall. Trans. B 1979, 10, 143–148. [Google Scholar] [CrossRef]
- Guo, H.H.; Song, B.; Mao, J.H.; Zhao, P. Effect of rare earth elements on macrosegregation in weather-resisting steel. J. Univ. Sci. Technol. Beijing 2010, 32, 44–49. [Google Scholar]
- Lv, W.; Zheng, D.H.; Xu, Z.Y. Influence of Rare earth on TTT Diagram for 0.27C-1Cr Steel. Acta Metall. Sin. 1993, 29, 308–311. [Google Scholar]
- Ji, Y.P.; Kang, L.; Song, Y.Q.; Qu, W.; Ren, H.P. Crystallographic Calculation about Heterogeneous Nucleation Potency of RE2O3 in Liquid Steel. Rare Met. Mater. Eng. 2017, 46, 2889–2894. [Google Scholar]
- Gao, X.Y.; Wang, H.Y.; Xing, L.; Ma, C.N.; Ren, H.P. Evolution of local atomic structure during solidification of Fe-RE (RE = La, Ce) alloy. J. Non-Cryst. Solids 2020, 542, 120109. [Google Scholar] [CrossRef]
- Bao, X.R.; Chen, L.; Guo, Y.P. Effect of RE on Dynamic Recrystallization and Pearlite Spacing of Heavy Rail Steel. Iron Steel 2012, 47, 76–78. [Google Scholar]
- Jiang, J.K.; Xiao, L.F.; You, D.L.; Shu, J.S.; Ren, L.E. Effect of Rare Earth addition on Properties of Controlled Rolling Low Carbon MnMoNb Ferrite Steel. Acta Metall. Sin. 1987, 23, 165–167. [Google Scholar]
- Wang, L.M. Application Prospects and Behavior of RE in New Generation High Strength Steels with Superior Toughness. J. Chin. Rare Earth Soc. 2004, 22, 48–53. [Google Scholar]
- Li, X.; Jiang, Z.H.; Geng, X.; Chen, M.J.; Peng, L.Z. Evolution Mechanism of Inclusions in H13 Steel with Rare Earth Magnesium Alloy Addition. ISIJ Int. 2019, 59, 1552–1561. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.F. Non-Metallic Inclusions in Steel; Metallurgical Industry Press: Beijing, China, 2019; Volume 26. [Google Scholar]
- Meng, Y.; Yan, C.L.; Yang, X.; Ju, X.H. A Statistical Analysis on the Complex Inclusions in Rare Earth Element Treated Steel. ISIJ Int. 2020, 60, 534–538. [Google Scholar] [CrossRef]
- Zang, Q.Y.; YJin, Y.F.; Zhang, T.; Yang, Y.T. Effect of yttrium addition on microstructure, mechanical and corrosion properties of 20Cr13 martensitic stainless steel. J. Iron Steel Res. Int. 2020, 27, 451–460. [Google Scholar] [CrossRef]
- Pang, F.X.; Yin, S.C.; Li, J.J.; Wang, S.B. Study on deoxidization thermodynamics of trace rare earth element and formation mechanism of inclusions in LZ50 molten steel. J. Taiyuan Univ. Technol. 2011, 42, 646–649. [Google Scholar]
- Wang, H.P.; Yu, P.; Jiang, S.L.; Wang, Y. Effect of Heterogeneous Nucleation on Removal of Arsenic from Molten Steel by Rare Earth Addition. Metals 2020, 10, 664. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, C.J. Agglomeration Characteristics of Various Inclusions in Al-killed Molten Steel Containing Rare Earth Element. Metall. Mater. Trans. B 2020, 51, 2585–2595. [Google Scholar] [CrossRef]
- Waudby, P.E. Rare earth additions to steel. Int. Met. Rev. 1978, 2, 74–98. [Google Scholar]
- Kwon, S.K.; Park, J.S.; Park, J.H. Influence of Refractory-Steel Interfacial Reaction on the Formation Behavior of Inclusions in Ce-containing Stainless Steel. ISIJ Int. 2015, 55, 2589–2596. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.Z.; Yu, Y.C.; Li, H.; Ren, X.; Wang, S.B. Effect of Rare Earth Cerium Addition on Microstructures and Mechanical Properties of Low Carbon High Manganese Steels. High Temp. Mater. Process. 2017, 36, 145–153. [Google Scholar] [CrossRef]
- Fan, H.Q.; Xu, W.C.; Wei, L.; Zhang, Z.H.; Liu, Y.B.; Li, Q. Relationship between La and Ce additions on microstructure and corrosion resistance of hot-dip galvanized steel. J. Iron Steel Res. Int. 2020, 27, 1108–1116. [Google Scholar] [CrossRef]
- Wang, L.J.; Liu, Y.Q.; Wang, Q.; Chou, K.C. Evolution mechanisms of MgO•Al2O3 inclusions by cerium in spring steel used in fasteners of high-speed railway. ISIJ Int. 2015, 55, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Bao, Y.P.; Duan, C.Y.; Lu, L.; Liu, Y.; Zhang, Q. Effect of Rare Earth Ce on Deep Stamping Properties of High-Strength Interstitial-Free Steel Containing Phosphorus. Materials 2020, 13, 1473. [Google Scholar] [CrossRef] [Green Version]
- An, Y.Q.; Wang, L.Z.; Li, Y.T.; Song, G.B.; Lei, S.Z.; Tan, J. Effects of Rare Earth Cerium on the Characteristics of Inclusions. Wide Heavy Plate 2020, 26, 11–17. [Google Scholar]
- Li, Y.D.; Liu, C.J.; Zhang, T.S.; Jiang, M.F.; Peng, C. Liquid Inclusions in Heat-Resistant Steel Containing Rare Earth Elements. Metall. Mater. Trans. B 2017, 48, 956–965. [Google Scholar] [CrossRef]
- Wang, C.; Han, Y.S.; Zhang, J.S.; Xiao, D.; Yang, J.; Chen, J.; Liu, Q. Behaviour of oxide inclusions and sulphur in ‘two-stage basicity control’ refining method of Si-killed spring steel. Ironmak Steelmak 2020, 48, 466–476. [Google Scholar] [CrossRef]
- Gao, R.Z.; Chen, H.Q. The effect of rare earth elements on the solidification characteristics and crystal structure of steel. Chin. Rare Earths 1985, 3, 27–33. [Google Scholar]
- Yu, Y.C.; Chen, W.Q.; Zheng, H.G. Effect of Cerium and Mischmetal on Solidification Structure of Fe-36Ni Invar Alloy. J. Chin. Rare Earth Soc. 2012, 30, 175–180. [Google Scholar]
- Wang, H.Y. Effect of Rare Earth on NbC Dissolution and Precipitation Behaviors in Microalloy Steels. University of Science and Technology: Beijing, China, 2017; Volume 14. [Google Scholar]

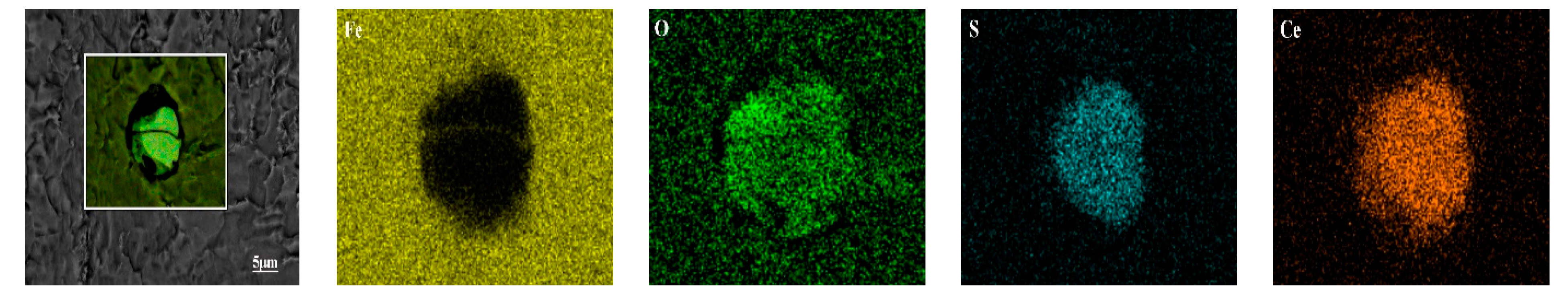
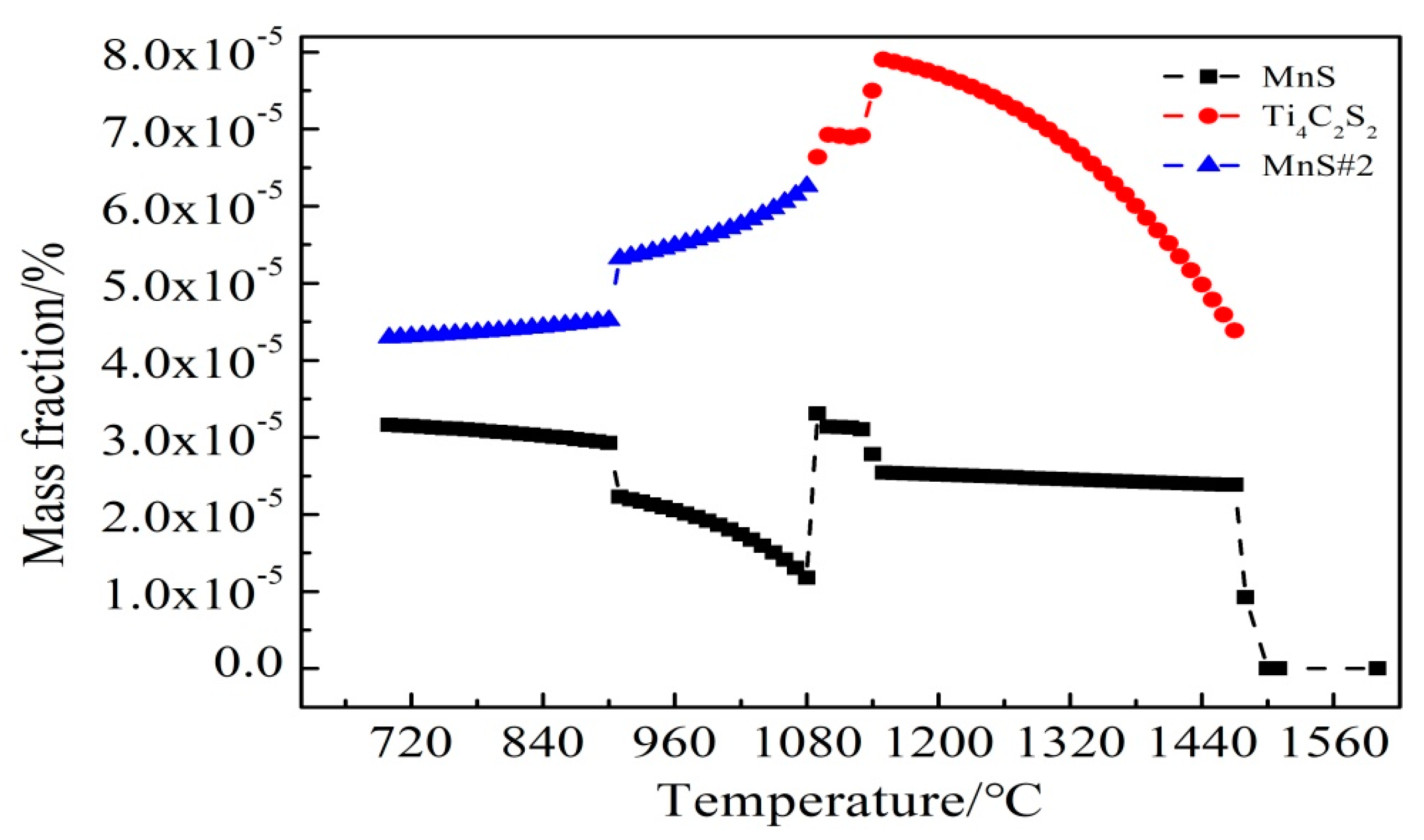
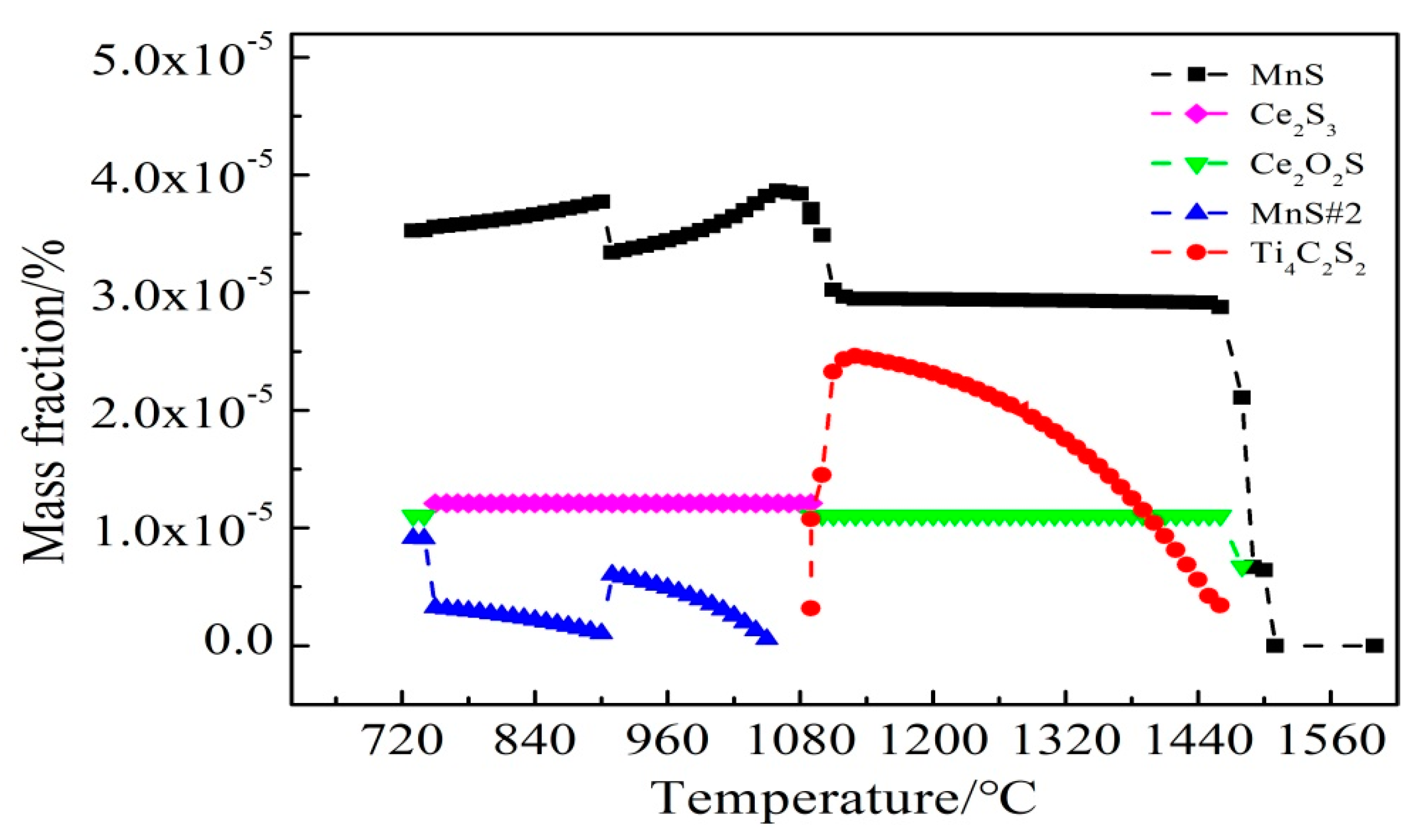
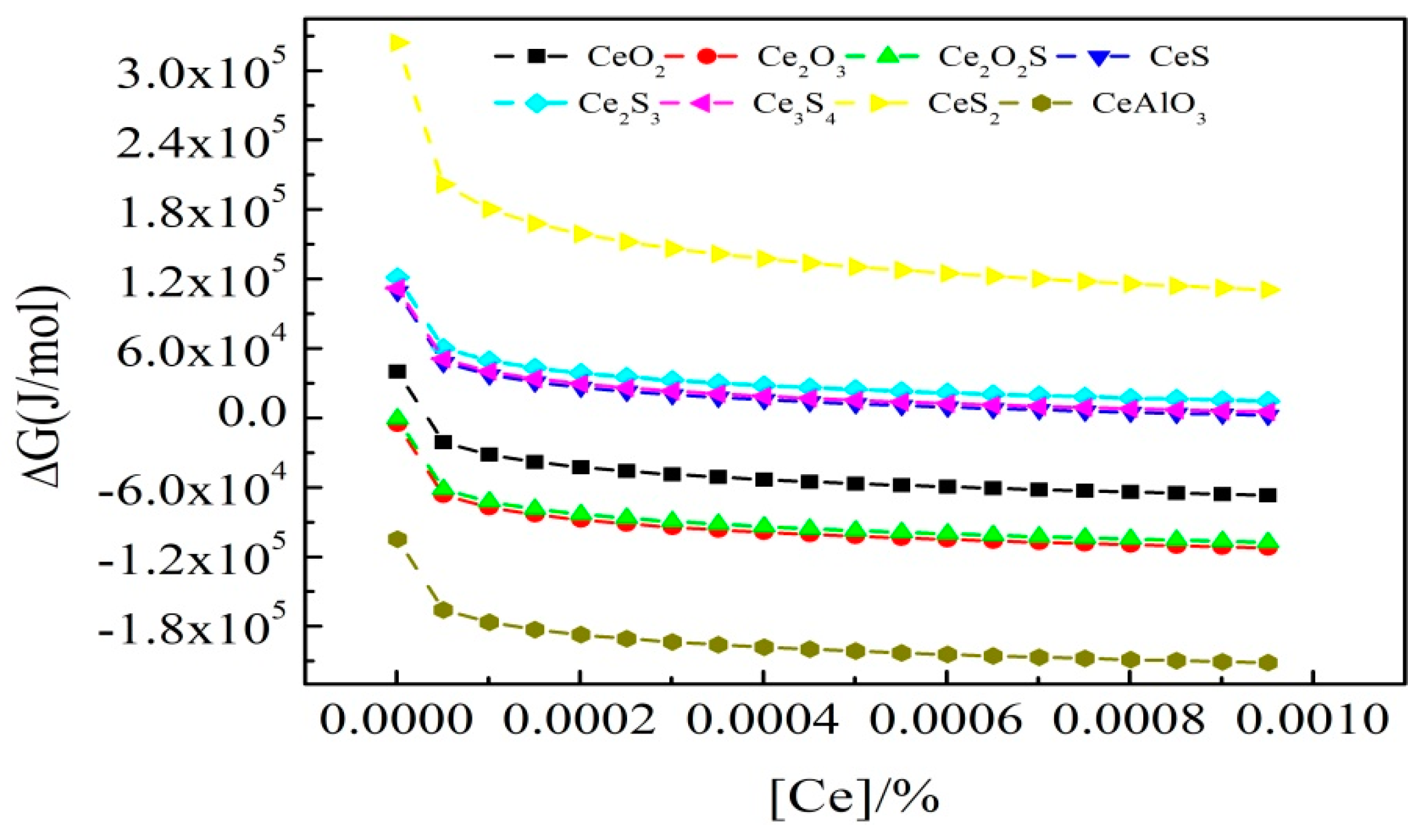


| No. | C | Si | Mn | P | S | Ti | Als 1 | Ca | Ce |
|---|---|---|---|---|---|---|---|---|---|
| I | 0.18 | 0.16 | 1.45 | 0.010 | 0.003 | 0.017 | 0.040 | 0.0020 | 0 |
| II | 0.18 | 0.15 | 1.48 | 0.010 | 0.002 | 0.018 | 0.039 | 0.0020 | 0.0006 |
| III | 0.18 | 0.15 | 1.48 | 0.010 | 0.002 | 0.018 | 0.039 | 0.0020 | 0.0009 |
| IV | 0.18 | 0.15 | 1.48 | 0.010 | 0.002 | 0.018 | 0.039 | 0.0020 | 0.0013 |
| Content | Central Segregation | Center Porosity |
|---|---|---|
| 0 Ce | B 1.0 | 1.0 |
| 0.0006 wt.% Ce | B 1.0 | 1.0 |
| 0.0009 wt.% Ce | C 1.0 | 1.0 |
| 0.0013 wt.% Ce | B 1.0 | 1.0 |
| Reactions | Equilibrium Constant | The Relation between a[O] and a[S] |
|---|---|---|
| 2CeO2 = Ce2O3 + [O] | 0.12866 | a[O] = 0.12866 |
| Ce2O3 + [S] = Ce2O2S + [O] | 0.079 | a[S] = 12.67 × a[O] |
| 2CeO2 + [S] = Ce2O2S + 2[O] | 0.01017 | a[S] = 98.3284 × a[O]2 |
| Ce2O2S + [S] = 2CeS + 2[O] | 0.8505 × 10−5 | a[S] = 1.1758 × 105 × a[O]2 |
| Ce2O2S + 2[S] = Ce2S3 + 2[O] | 1.452 × 10−5 | a[S] = 262.433a[O] |
| Ce2S3 = 2CeS + [S] | 0.5857 | a[S] = 0.5857 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Zhi, J.; Zhang, J.; Zhao, B.; Liu, Q. Effect of Cerium on the Microstructure and Inclusion Evolution of C-Mn Cryogenic Vessel Steels. Materials 2021, 14, 5262. https://doi.org/10.3390/ma14185262
Wu L, Zhi J, Zhang J, Zhao B, Liu Q. Effect of Cerium on the Microstructure and Inclusion Evolution of C-Mn Cryogenic Vessel Steels. Materials. 2021; 14(18):5262. https://doi.org/10.3390/ma14185262
Chicago/Turabian StyleWu, Liping, Jianguo Zhi, Jiangshan Zhang, Bo Zhao, and Qing Liu. 2021. "Effect of Cerium on the Microstructure and Inclusion Evolution of C-Mn Cryogenic Vessel Steels" Materials 14, no. 18: 5262. https://doi.org/10.3390/ma14185262
APA StyleWu, L., Zhi, J., Zhang, J., Zhao, B., & Liu, Q. (2021). Effect of Cerium on the Microstructure and Inclusion Evolution of C-Mn Cryogenic Vessel Steels. Materials, 14(18), 5262. https://doi.org/10.3390/ma14185262








