Particle Deposition in Drying Porous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drying Test
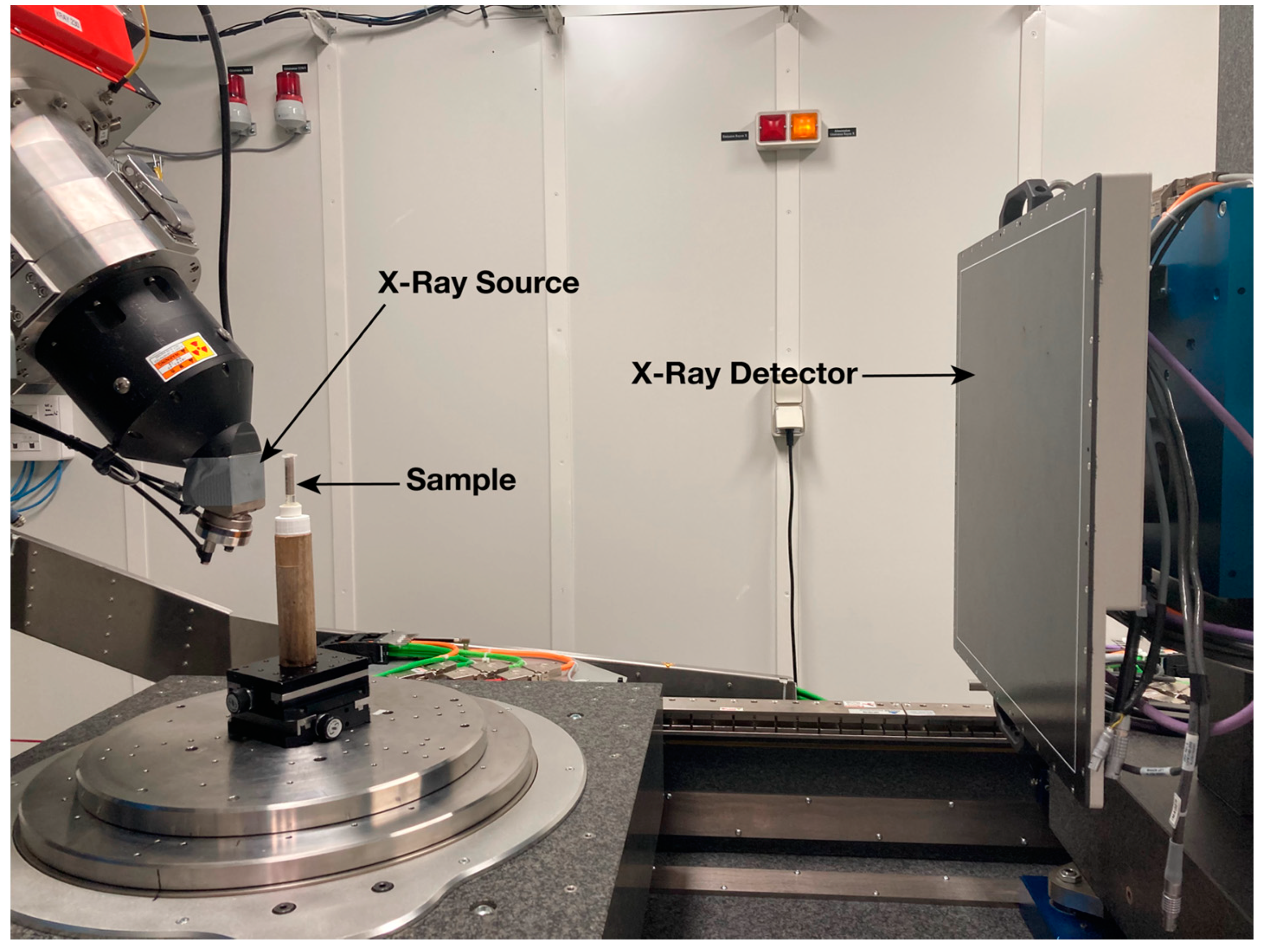
2.3. X-ray Microtomography
2.4. Scanning Electron Microscopy
3. Results
3.1. Drying Kinetics
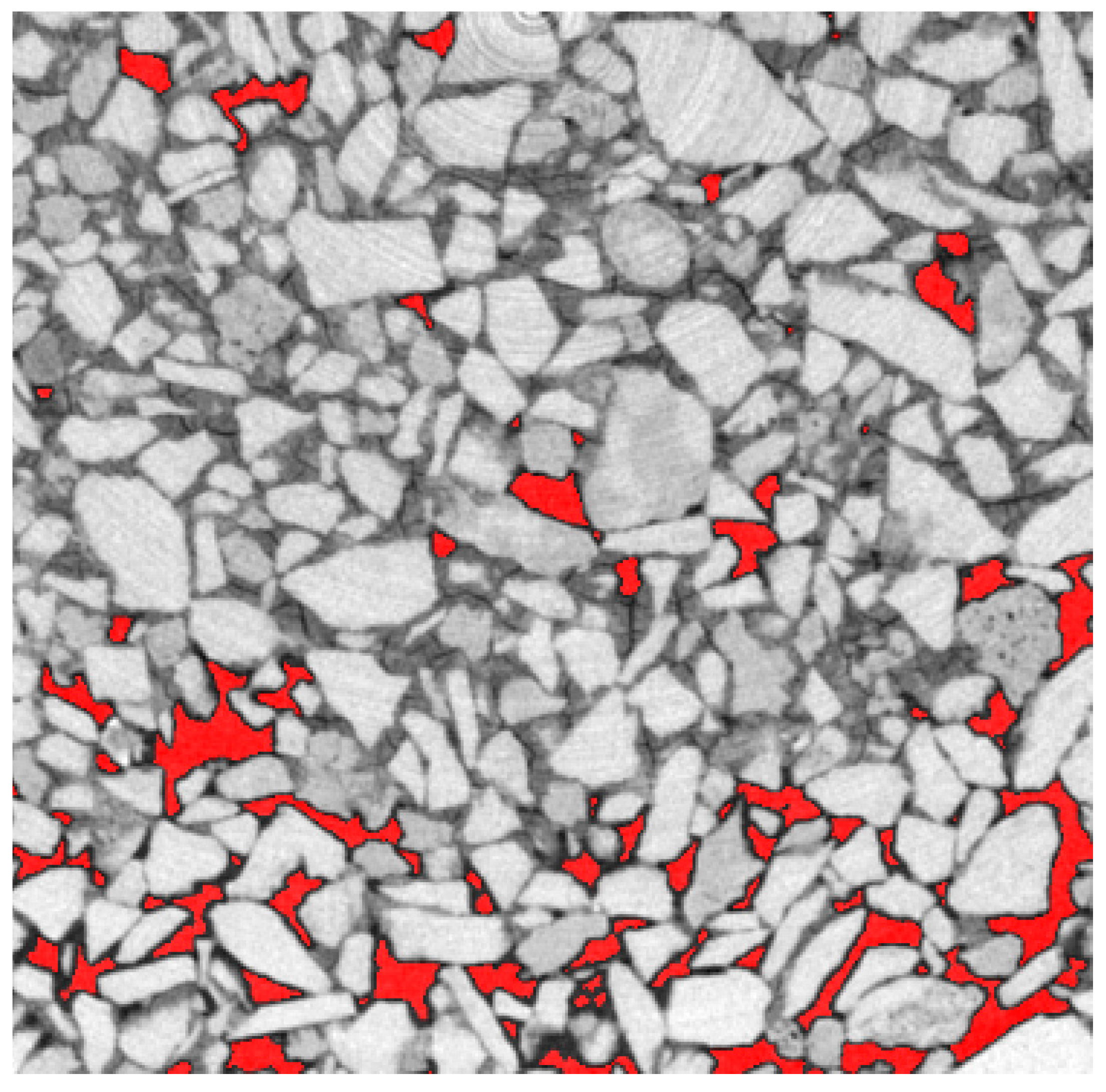
3.2. Local Observations
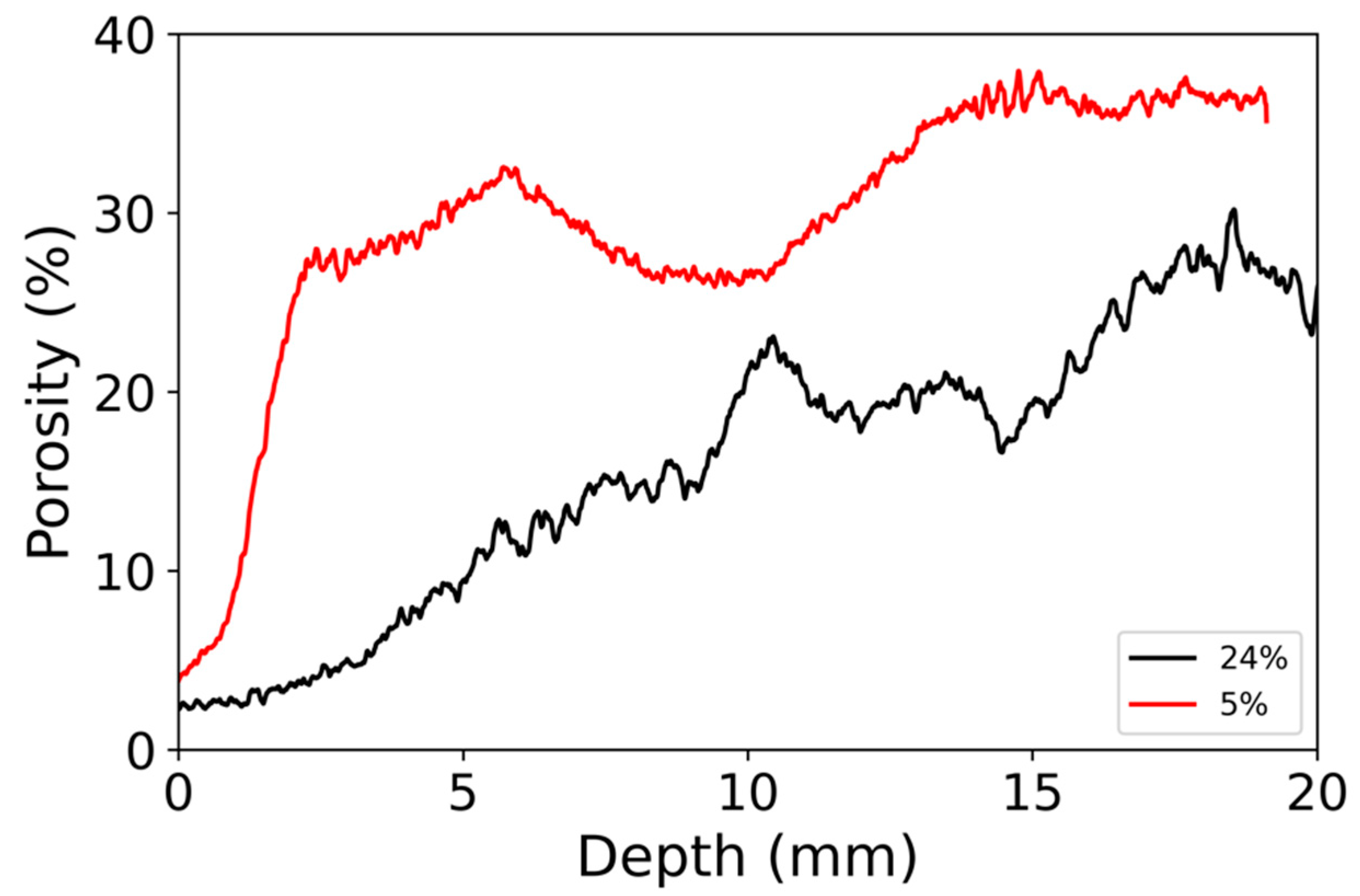
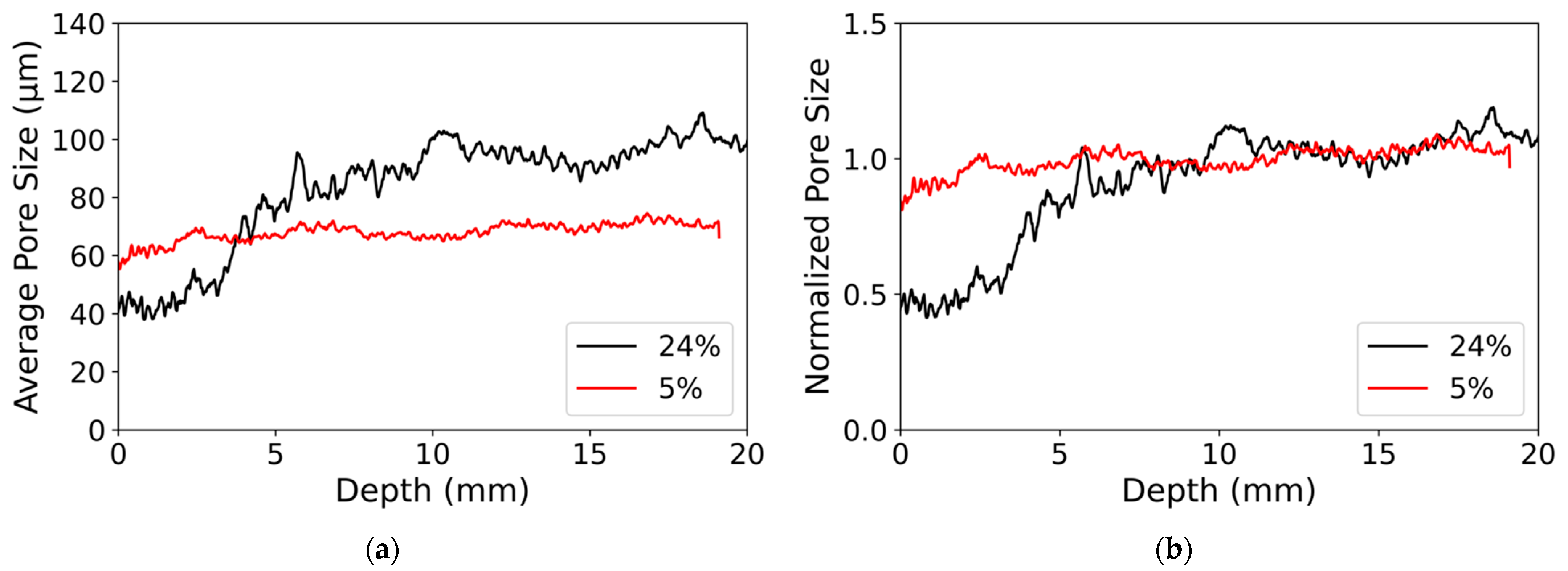
3.3. Modifications of the Porous Structure
4. Discussion
4.1. Advection of Nanoparticles
4.2. Gradient of Nanoparticle Deposit
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corapcioglu, M.Y.; Choi, H. Modeling Colloid Transport in Unsaturated Porous Media and Validation with Laboratory Column Data. Water Resour. Res. 1996, 32, 3437–3449. [Google Scholar] [CrossRef]
- McCarthy, J.F.; McKay, L.D. Colloid Transport in the Subsurface: Past, Present, and Future Challenges. Vadose Zone J. 2004, 3, 326–337. [Google Scholar] [CrossRef]
- Kanti Sen, T.; Khilar, K.C. Review on Subsurface Colloids and Colloid-Associated Contaminant Transport in Saturated Porous Media. Adv. Colloid Interface Sci. 2006, 119, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.N.; Elimelech, M. Colloid Mobilization and Transport in Groundwater. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 1–56. [Google Scholar] [CrossRef]
- Flatt, R.; Aly Mohamed, N.; Caruso, F.; Derluyn, H.; Desarnaud, J.; Lubelli, B.; Espinosa Marzal, R.M.; Pel, L.; Rodriguez-Navarro, C.; Scherer, G.W.; et al. Predicting Salt Damage in Practice: A Theoretical Insight into Laboratory Tests. RILEM Tech. Lett. 2017, 2, 108–118. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical Chloride Content in Reinforced Concrete—A Review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Derluyn, H.; Moonen, P.; Carmeliet, J. Deformation and Damage Due to Drying-Induced Salt Crystallization in Porous Limestone. J. Mech. Phys. Solids 2014, 63, 242–255. [Google Scholar] [CrossRef]
- Wilkinson, D.; Willemsen, J.F. Invasion Percolation: A New Form of Percolation Theory. J. Phys. A Math. Gen 1983, 16, 3365–3376. [Google Scholar] [CrossRef]
- Sinha, P.K.; Wang, C. Pore-Network Modeling of Liquid Water Transport in Gas Diffusion Layer of a Polymer Electrolyte Fuel Cell. Electrochim. Acta 2007, 52, 7936–7945. [Google Scholar] [CrossRef]
- Chapuis, O.; Prat, M.; Quintard, M.; Chane-Kane, E.; Guillot, O.; Mayer, N. Two-Phase Flow and Evaporation in Model Fibrous Media. J. Power Sources 2008, 178, 258–268. [Google Scholar] [CrossRef]
- Papafotiou, A.; Schütz, C.; Lehmann, P.; Vontobel, P.; Or, D.; Neuweiler, I. Measurement of Preferential Flow during Infiltration and Evaporation in Porous Media. Bull. Geol. Soc. Greece 2010, 43, 1831–1839. [Google Scholar] [CrossRef][Green Version]
- Xu, L.; Davies, S.; Schofield, A.B.; Weitz, D.A. Dynamics of Drying in 3D Porous Media. Phys. Rev. Lett. 2008, 101, 094502. [Google Scholar] [CrossRef] [PubMed]
- Coussot, P. Scaling Approach of the Convective Drying of a Porous Medium. Eur. Phys. J. B-Condens. Matter Complex Syst. 2000, 15, 557–566. [Google Scholar] [CrossRef]
- Sawdy, A.; Heritage, A.; Pel, L. A Review of Salt Transport in Porous Media: Assessment Methods and Salt Reduction Treatments. In Proceedings of the Salt Weathering on Buildings and Stone Sculptures, Copenhagen, Denmark, 22–24 October 2008; pp. 1–27. [Google Scholar]
- Petković, J.; Huinink, H.P.; Pel, L.; Kopinga, K.; van Hees, R.P.J. Salt Transport in Plaster/Substrate Layers. Mater. Struct. 2007, 40, 475–490. [Google Scholar] [CrossRef]
- Saidov, T.A.; Pel, L.; Van Der Heijden, G.H.A. Crystallization of Sodium Sulfate in Porous Media by Drying at a Constant Temperature. Int. J. Heat Mass Transf. 2015, 83, 621–628. [Google Scholar] [CrossRef]
- Gupta, S.; Huinink, H.P.; Prat, M.; Pel, L.; Kopinga, K. Paradoxical Drying of a Fired-Clay Brick Due to Salt Crystallization. Chem. Eng. Sci. 2014, 109, 204–211. [Google Scholar] [CrossRef]
- Shahidzadeh-Bonn, N.; Desarnaud, J.; Bertrand, F.; Chateau, X.; Bonn, D. Damage in Porous Media Due to Salt Crystallization. Phys. Rev. E 2010, 81, 066110. [Google Scholar] [CrossRef]
- Derluyn, H.; Boone, M.A.; Boone, M.N.; De Kock, T.; Desarnaud, J.; Peetermans, S.; Molari, L.; de Miranda, S.; Shahidzadeh, N.; Cnudde, V. Salt Crystallization Dynamics in Building Rocks: A 4D Study Using Laboratory X-ray Micro-CT. In Proceedings of the 2nd International Conference on Tomography of Materials and Structures (ICTMS 2015), Quebec City, QC, Canada, 29 June–3 July 2015; pp. 565–568. [Google Scholar]
- Shokri, N. Pore-Scale Dynamics of Salt Transport and Distribution in Drying Porous Media. Phys. Fluids 2014, 26, 012106. [Google Scholar] [CrossRef]
- Saidov, T.A.; Pel, L.; Kopinga, K. Sodium Sulfate Salt Weathering of Porous Building Materials Studied by NMR. Mater. Struct. 2017, 50, 1–11. [Google Scholar] [CrossRef]
- Norouzi Rad, M.; Shokri, N.; Sahimi, M. Pore-Scale Dynamics of Salt Precipitation in Drying Porous Media. Phys. Rev. E. 2013, 88, 032404. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.N.; Shokri, N.; Keshmiri, A.; Withers, P.J. Effects of Grain and Pore Size on Salt Precipitation During Evaporation from Porous Media. Transp. Porous Media 2015, 110, 281–294. [Google Scholar] [CrossRef]
- Pauchard, L.; Parisse, F.; Allain, C. Influence of Salt Content on Crack Patterns Formed through Colloidal Suspension Desiccation. Phys. Rev. E 1999, 59, 3737–3740. [Google Scholar] [CrossRef]
- Lazarus, V.; Pauchard, L. From Craquelures to Spiral Crack Patterns: Influence of Layer Thickness on the Crack Patterns Induced by Desiccation. Soft Matter 2011, 7, 2552–2559. [Google Scholar] [CrossRef]
- Bradford, S.A.; Torkzaban, S.; Walker, S.L. Coupling of Physical and Chemical Mechanisms of Colloid Straining in Saturated Porous Media. Water Res. 2007, 41, 3012–3024. [Google Scholar] [CrossRef]
- Bradford, S.A.; Yates, S.R.; Bettahar, M.; Simunek, J. Physical Factors Affecting the Transport and Fate of Colloids in Saturated Porous Media. Water Resour. Res. 2002, 38, 63-1–63-12. [Google Scholar] [CrossRef]
- Crist, J.T.; McCarthy, J.F.; Zevi, Y.; Baveye, P.C.; Throop, J.A.; Steenhuis, T.S. Pore-Scale Visualization of Colloid Transport and Retention in Partly Saturated Porous Media. Vadose Zo. J. 2004, 3, 444–450. [Google Scholar] [CrossRef]
- Allaire, S.E.; Roulier, S.; Cessna, A.J. Quantifying Preferential Flow in Soils: A Review of Different Techniques. J. Hydrol. 2009, 378, 179–204. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Siwek, B.; Zembala, M.; Belouschek, P. Kinetics of Localized Adsorption of Colloid Particles. Adv. Colloid Interface Sci. 1994, 48, 151–280. [Google Scholar] [CrossRef]
- Gerber, G.; Bensouda, M.; Weitz, D.A.; Coussot, P. Self-Limited Accumulation of Colloids in Porous Media. Phys. Rev. Lett. 2019, 123, 158005. [Google Scholar] [CrossRef]
- Dufresne, E.; Corwin, E.; Greenblatt, N.; Ashmore, J.; Wang, D.; Dinsmore, A.; Cheng, J.; Xie, X.; Hutchinson, J.; Weitz, D. Flow and Fracture in Drying Nanoparticle Suspensions. Phys. Rev. Lett. 2003, 91, 1–4. [Google Scholar] [CrossRef]
- Allain, C.; Limat, L. Regular Patterns of Cracks Formed by Directional Drying of a Collodial Suspension. Phys. Rev. Lett. 1995, 74, 2981–2984. [Google Scholar] [CrossRef]
- Baumann, T.; Werth, C.J. Visualization of Colloid Transport through Heterogeneous Porous Media Using Magnetic Resonance Imaging. Colloids Surf. A Physicochem. Eng. Asp. 2005, 265, 2–10. [Google Scholar] [CrossRef]
- Levich, V.G. Physicochemical Hydrodynamics; Prentice-Hall: Englewood Cliffs, NJ, USA, 1962. [Google Scholar]
- Keita, E.; Faure, P.; Rodts, S.; Coussot, P. MRI Evidence for a Receding-Front Effect in Drying Porous Media. Phys. Rev. E 2013, 87, 062303. [Google Scholar] [CrossRef]
- Keita, E.; Kodger, T.E.; Faure, P.; Rodts, S.; Weitz, D.A.; Coussot, P. Water Retention against Drying with Soft-Particle Suspensions in Porous Media. Phys. Rev. E 2016, 94, 033104. [Google Scholar] [CrossRef]
- Qin, F.; Mazloomi Moqaddam, A.; Kang, Q.; Derome, D.; Carmeliet, J. LBM Simulation of Self-Assembly of Clogging Structures by Evaporation of Colloidal Suspension in 2D Porous Media. Transp. Porous Media 2019, 128, 929–943. [Google Scholar] [CrossRef]
- Tufenkji, N.; Elimelech, M. Correlation Equation for Predicting Single-Collector Efficiency in Physicochemical Filtration in Saturated Porous Media. Environ. Sci. Technol. 2004, 38, 529–536. [Google Scholar] [CrossRef]
- Lehoux, A.P.; Faure, P.; Michel, E.; Courtier-Murias, D.; Rodts, S.; Coussot, P. Transport and Adsorption of Nano-Colloids in Porous Media Observed by Magnetic Resonance Imaging. Transp. Porous Media 2017, 119, 403–423. [Google Scholar] [CrossRef]
- Lehoux, A.P.; Faure, P.; Lafolie, F.; Rodts, S.; Courtier-Murias, D.; Coussot, P.; Michel, E. Combined Time-Lapse Magnetic Resonance Imaging and Modeling to Investigate Colloid Deposition and Transport in Porous Media. Water Res. 2017, 123, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Marcato, A.; Boccardo, G.; Marchisio, D. A Computational Workflow to Study Particle Transport and Filtration in Porous Media: Coupling CFD and Deep Learning. Chem. Eng. J. 2021, 417, 128936. [Google Scholar] [CrossRef]
- Allaire-Leung, S.E.; Gupta, S.C.; Moncrief, J.F. Water and Solute Movement in Soil as Influenced by Macropore Characteristics: 1. Macropore Continuity. J. Contam. Hydrol. 2000, 41, 283–301. [Google Scholar] [CrossRef]
- Giraldez, J.V.; Sposito, G. Infiltration in Swelling Soils. Water Resour. Res. 1985, 21, 33–44. [Google Scholar] [CrossRef]
- Naël-Redolfi, J.; Keita, E.; Roussel, N. Water Absorption Measurement of Fine Porous Aggregates Using an Evaporative Method: Experimental Results and Physical Analysis. Cem. Concr. Res. 2018, 104, 61–67. [Google Scholar] [CrossRef]
- Keita, E.; Rifaai, Y.; Belin, P.; Roussel, N. Influence of Non-Adsorbing Polymers on Drying of Fresh Mortars. Cem. Concr. Res. 2019, 116, 38–44. [Google Scholar] [CrossRef]
- Keita, E.; Koehler, S.A.; Faure, P.; Weitz, D.A.; Coussot, P. Drying Kinetics Driven by the Shape of the Air/Water Interface in a Capillary Channel. Eur. Phys. J. E 2016, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goehring, L.; Clegg, W.J.; Routh, A.F. Plasticity and Fracture in Drying Colloidal Films. Phys. Rev. Lett. 2013, 110, 024301. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A New Method for the Model-independent Assessment of Thickness in Three-dimensional Images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]

| Depth | ||
|---|---|---|
| Top surface | 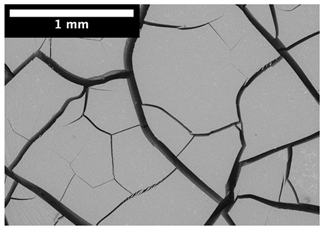 | 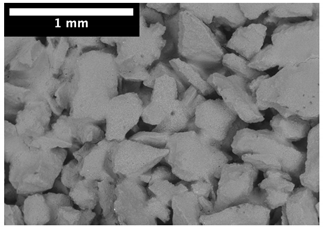 |
| 1 mm |  | 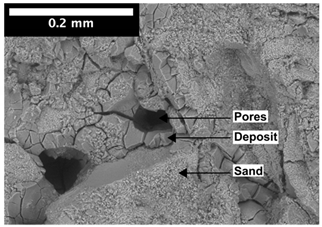 |
| 15 mm | 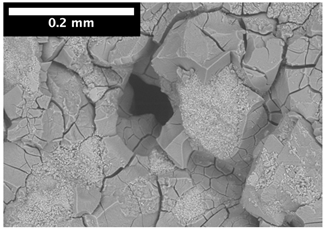 | 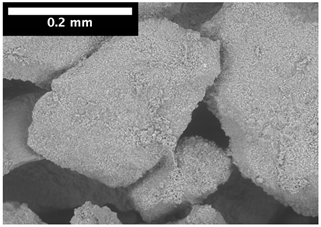 |
| Depth | ||
|---|---|---|
| Top surface | 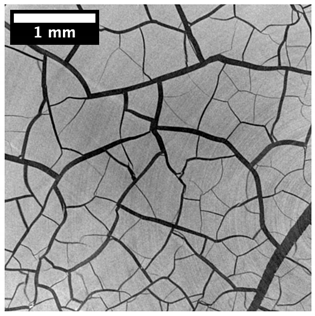 | 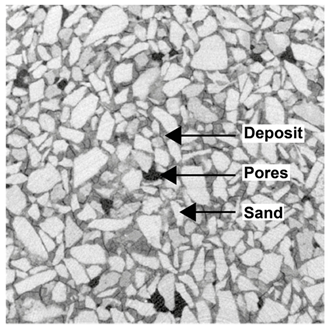 |
| 1 mm | 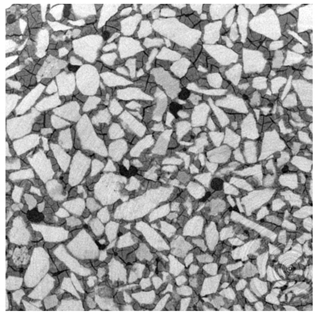 | 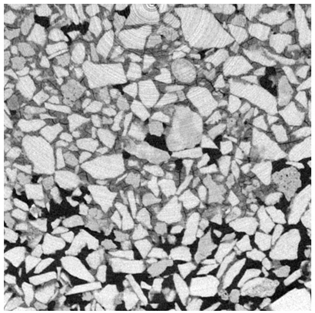 |
| 15 mm |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keita, E. Particle Deposition in Drying Porous Media. Materials 2021, 14, 5120. https://doi.org/10.3390/ma14185120
Keita E. Particle Deposition in Drying Porous Media. Materials. 2021; 14(18):5120. https://doi.org/10.3390/ma14185120
Chicago/Turabian StyleKeita, Emmanuel. 2021. "Particle Deposition in Drying Porous Media" Materials 14, no. 18: 5120. https://doi.org/10.3390/ma14185120
APA StyleKeita, E. (2021). Particle Deposition in Drying Porous Media. Materials, 14(18), 5120. https://doi.org/10.3390/ma14185120






