Oxidation Mechanism of Al-Sn Bearing Alloys
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussions
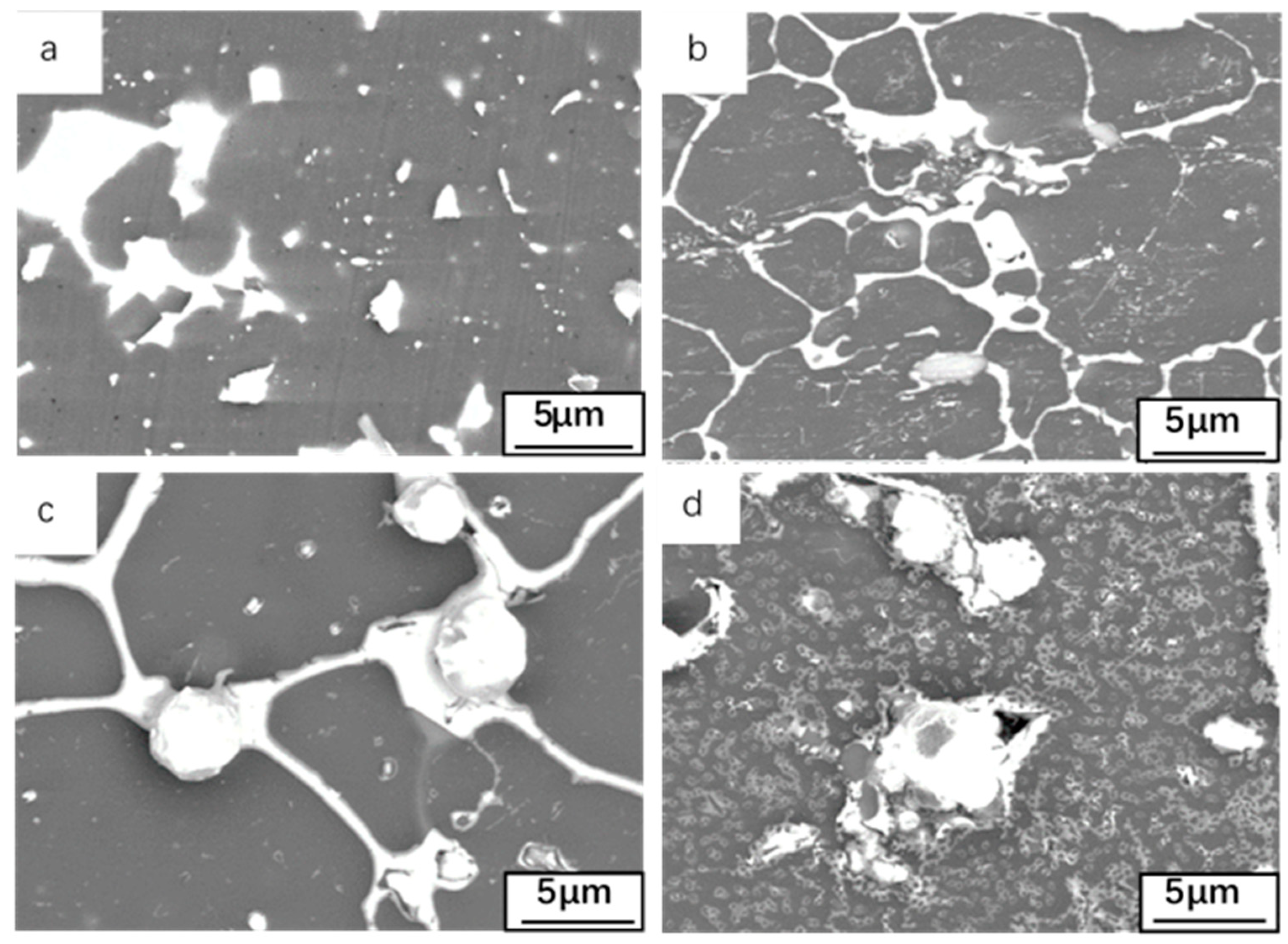
3.1. Microstructures of Al-Sn Alloy
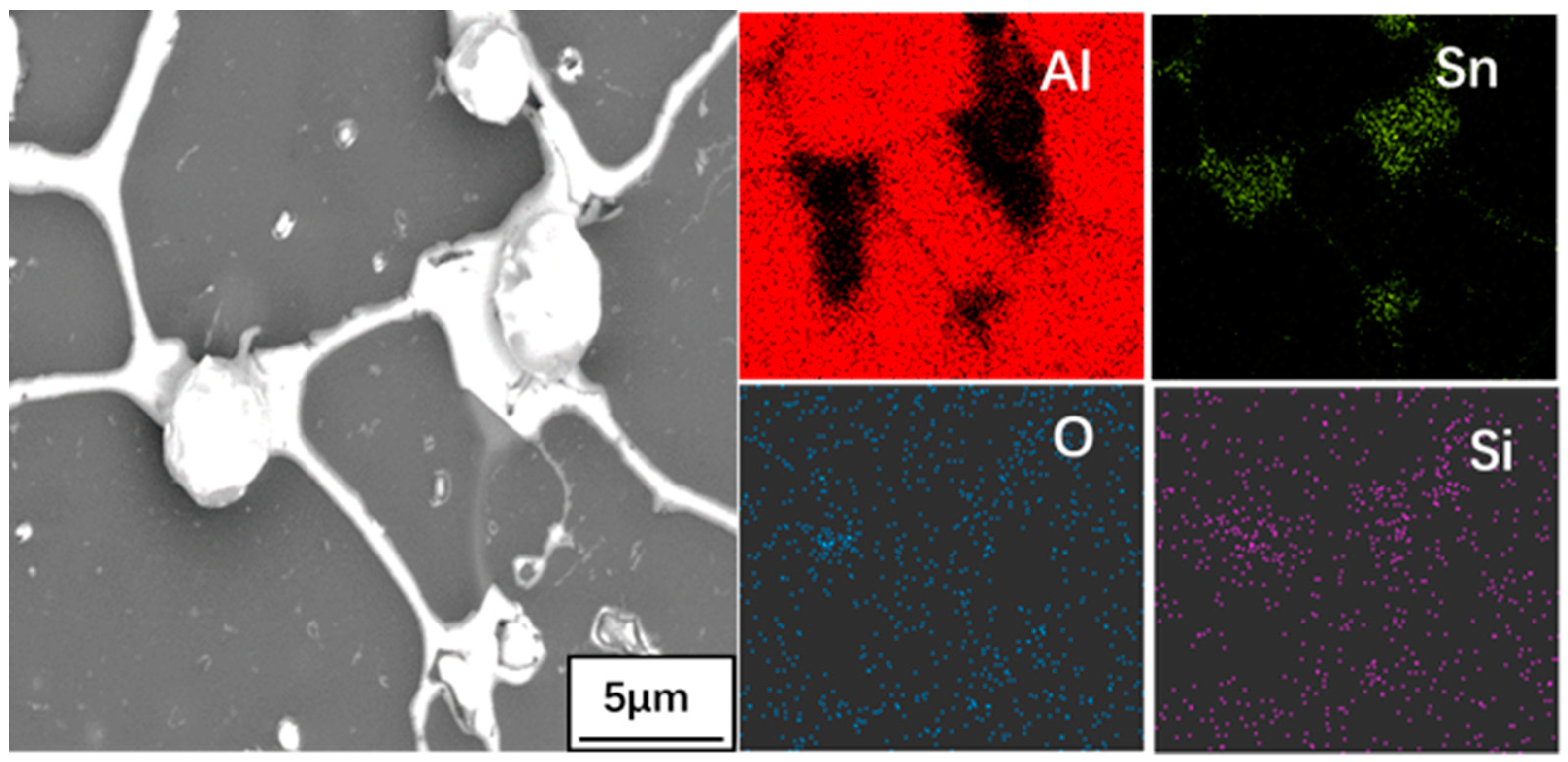
3.2. Phase Composition Analysis
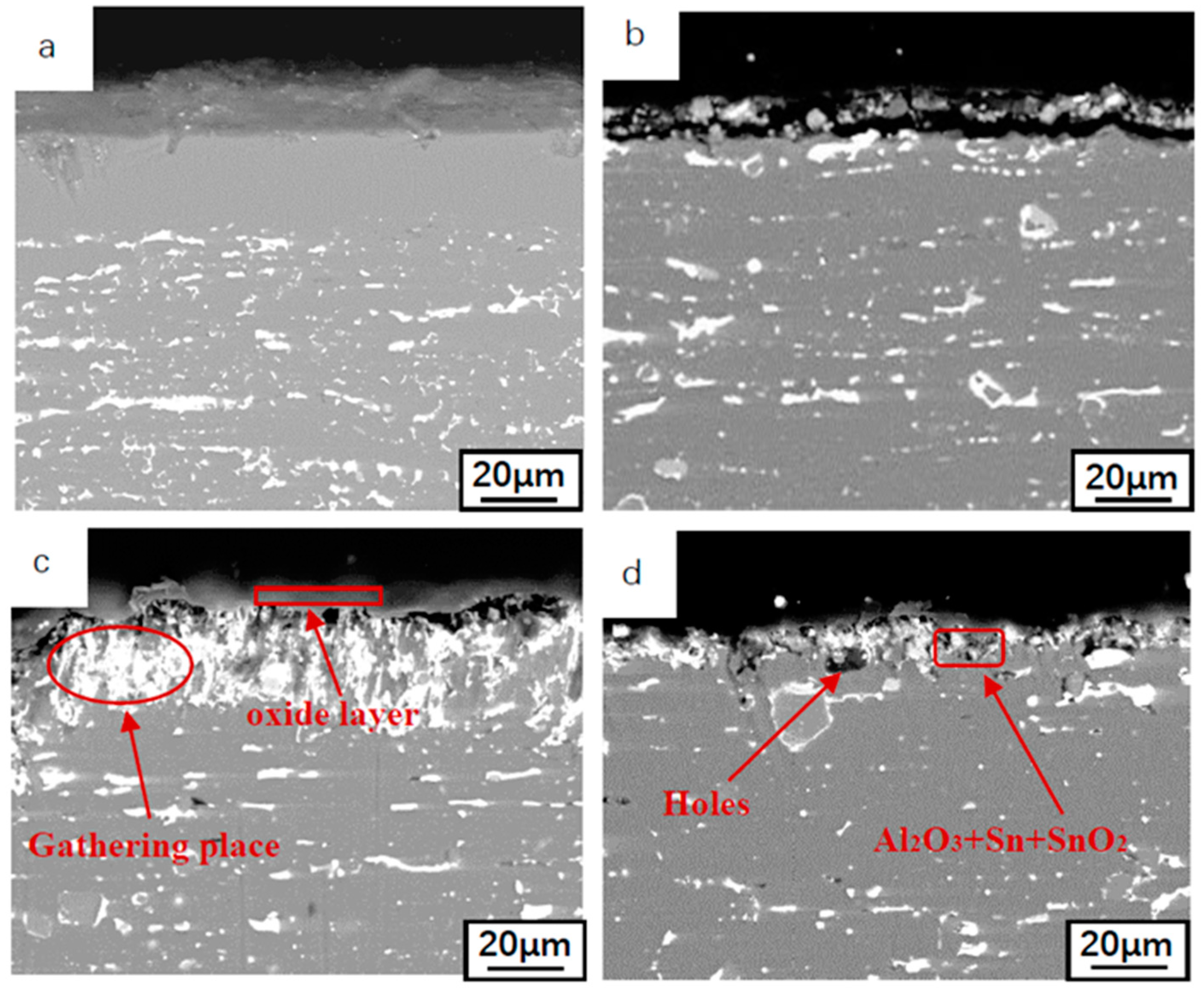
3.3. Oxidation Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, P.; Zhang, H.X.; Xiao, R.Q.; Shi, J.Z. Simulation Analysis of Engine-Oil inside the Clearance between Crankshaft Journal and Bearing Shells in the Ideal Stable Conditions. Appl. Mech. Mater. 2014, 635–637, 532–536. [Google Scholar] [CrossRef]
- Chernets, M.V. Prediction of the life of a sliding bearing based on a cumulative wear model taking into account the lobing of the shaft contour. J. Frict. Wear 2015, 36, 163–169. [Google Scholar] [CrossRef]
- Goryacheva, I.G.; Mezrin, A.M. Simulation of combined wearing of the shaft and bush in a heavily loaded sliding bearing. J. Frict. Wear 2011, 32, 1–7. [Google Scholar] [CrossRef]
- Litwin, W. Experimental research on water Lubricated three layer sliding bearing with lubrication Grooves in the upper part of the bush and its comparison with a rubber bearing. Tribol. Int. 2014, 82, 153–161. [Google Scholar] [CrossRef]
- Whitney, W.J.; Yun-Shan, S. Al-based bearings and bushings for long engine life. Foreign Locomot. Roll. Stock Technol. 2001, 2, 27–30. [Google Scholar]
- Mei-qin, Z.; Zhong-chen, L.; Jin-tian, C.; Kai-qiang, S.; Ren-zong, H.; Min, Z. Research Progress on Al-based Sliding Bearing Alloys. Mech. Eng. Mater. 2018, 42, 7–14. [Google Scholar]
- Qin, Z.; Hong-tao, H.; Ming-zhi, W. Research progress of metal-based sliding bearing materials. J. Yan Shan Univ. 2016, 40, 1–8. [Google Scholar]
- Chong-cai, Z.; Wei-xing, W.; Quan, W.; Long, W. Study on Microstructure and Properties of New Al-Based Sliding Bearing Alloy. Adv. Mater. Res. 2011, 1368, 603–607. [Google Scholar]
- Cruz, K.S.; Meza, E.S.; Fernandes, F.A.P.; Quaresma, J.M.V.; Casteletti, L.C.; Garcia, A. Dendritic Arm Spacing Affecting Mechanical Properties and Wear Behavior of Al-Sn and Al-Si Alloys Directionally Solidified under Unsteady-State Conditions. Metall. Mater. Trans. A 2010, 41, 972–984. [Google Scholar] [CrossRef]
- Wang, B.J.; Wu, W.H.; Liu, J.M.; Zhai, W. Ultrasonic cavitation and acoustic streaming effects during liquid phase separation and dynamic solidification of ternary Al-Sn–Si immiscible alloy. Appl. Phys. A Mater. Sci. Process. 2020, 126, 2202–2211. [Google Scholar] [CrossRef]
- Lu, Z.C.; Zeng, M.Q.; Gao, Y.; Xing, X.J.; Zhu, M. Improving wear performance of dual-scale Al-Sn alloys by adding nano-Si@Sn: Effects of Sn nanophase lubrication and nano-Si polishing. Wear 2015, 338–339, 258–267. [Google Scholar] [CrossRef]
- Qin, D.; Zhong-wei, Y.; Hu-lin, L.; Geng-yuan, G.; Ning, Z.; Yong-hong, C. Simulation and Experimental Verification of Fatigue Strength Evaluation of Journal Bearing Bush. Eng. Fail. Anal. 2019, 109, 104275. [Google Scholar]
- Qiao-qin, G.; Yong-chun, G.; Ding, G.; Zhong, Y.; Jian-ping, L.; Wei, Y.; Yu-ting, Y.; Pei-hu, G.; Ya-ping, B. Microstructure and properties of the Cp/AlSn coatings deposited by magnetron sputtering/multi-arc ion plating. Surf. Coat. Technol. 2020, 384, 125303. [Google Scholar]
- Yu-qin, J. Development status and trend of automotive plain bearing materials at home and abroad. Automot. Technol. Mater. 2009, 3, 10–13. [Google Scholar]
- Ya-jun, G. Research on Magnetron Sputtering Coating Technology and Mechanical Properties of Materials; Harbin Engineering University: Harbin, China, 2002. [Google Scholar]
- Qiao-qin, G.; Yong-chun, G.; Zhong, Y.; Jian-ping, L.; Guang-tao, X. Study on microstructure and tribological properties of AlSn films deposited via magnetron sputtering ion plating. Appl. Surf. Sci. 2019, 483, 123–132. [Google Scholar]
- Shu-tong, Y.; Qing-fen, L.; Ya-jun, G.; Zheng-yi, R.; Jun, W. Research on Magnetron Sputtering Coating Process of Sliding Bearing. China Surf. Eng. 2002, 2, 39–42. [Google Scholar]
- Yong-jing, S.; Si-yuan, L.; Jie, W.; Fu-sheng, P. Advance of direct current magnetron sputtering. Mater. Guide 2008, 1, 65–69. [Google Scholar]
- Zhen-hua, L.; Yong, H.; Jun, C. The Oxidation of Pure Copper in High Heat. China Foundry Equip. Technol. 2011, 6, 11–13. [Google Scholar]
- Zhen-hua, L. Study on the Oxidation Behavior of Single Crystal Copper; Lanzhou University of Technolog: Lanzhou, China, 2010. [Google Scholar]
- Zhi-hui, P.; Gao, W.; Wei-ping, G.; Xu-fan, S. Research of dynamics of surface oxidization of 4004 alloy at a constant temperature. J. Cent. South Univ. Technol. 1998, 5, 479–482. [Google Scholar]
- Feng-Shan, Y. Study on High Temperature Oxidation Behavior of Rare Earth Al Alloy; Institute of Metal Research, Chinese Academy of Sciences: Shenyang, China, 1998; pp. 74–77. [Google Scholar]
- Cheng-long, Y.; Qing, S.; Hong-tao, J.; Li-li, W. Transformation and Morphology of the Surface Oxides during High-temperature Oxidation of aluminum. J. Synth. Cryst. 2010, 39, 1308–1312, 1324. [Google Scholar]
- Er-lin, Z.; Jun-gang, L.; Mu-qin, L.; Shou-fan, R. Preparation and Microstructure of Semi-solid Al-20Sn-1Cu Bearing Alloy. Spec. Cast. Nonferrous Alloys 2008, 4, 284–286, 243. [Google Scholar]
- Falahati, A.; Wu, J.; Lang, P.; Ahmadi, M.R.; Povoden-Karadeniz, E. Ernst KOZESCHNIK. Assessment of parameters for precipitation simulation of heat treatable aluminum alloys using differential scanning calorimetry. Trans. Nonferrous Met. Soc. China 2014, 24, 2157–2167. [Google Scholar] [CrossRef]
- Jun, S.; Zhi-guo, C.; Shi-chen, L.; Ji-shuai, Z.; Jie-ke, R. Effect of trace Sn (In) addition on ageing behaviour and microstructure evolution of Al-3.5%Cu alloy. Cent. South Univ. 2012, 22, 2486–2491. [Google Scholar]
- Belov, N.A.; Akopyan, T.K.; Gershman, I.S.; Stolyarova, O.O.; Yakovleva, A.O. Effect of Si and Cu additions on the phase composition, microstructure and properties of Al-Sn alloys. J. Alloy. Compd. 2017, 695, 2730–2739. [Google Scholar] [CrossRef]
- Zhi-hong, J.; Yao-yao, W.; Li-peng, D.; Tao, C.; Ying-ying, L.; Qing, L. Sn Microalloying for Al Alloys: Strengthening Effects and Mechanisms. Mater. Guide. 2017, 31, 123–127. [Google Scholar]
- Chun-hua, W.; Hui, L.; Hong-jie, F.; Zheng-feng, L.; Deng-feng, Y. Effect of Sn on microstructure and mechanical properties of 6061 Al alloy. Heat Treat. Met. 2017, 42, 231–235. [Google Scholar]
- Jing-guang, T.; Ji-jun, F.; Jia-wen, Z.; Wen-chang, H. Study on manufacturing defect of tin-aluminum bearings. Phys. Chem. Insp. 2001, 4, 144–147. [Google Scholar]
- Ji-jun, F.; Jing-guang, T. Analysis on Defect in the Manufacturing of Al-Sn Alloy Plain Bearing. Auto Technol. Mater. 2001, 2, 40–43. [Google Scholar]
- Vermaak, N.; Parry, G.; Estevez, R. New insight into crack formation during corrosion of zirconium-based metal-oxide systems. Acta Mater. 2013, 61, 4374–4383. [Google Scholar] [CrossRef]

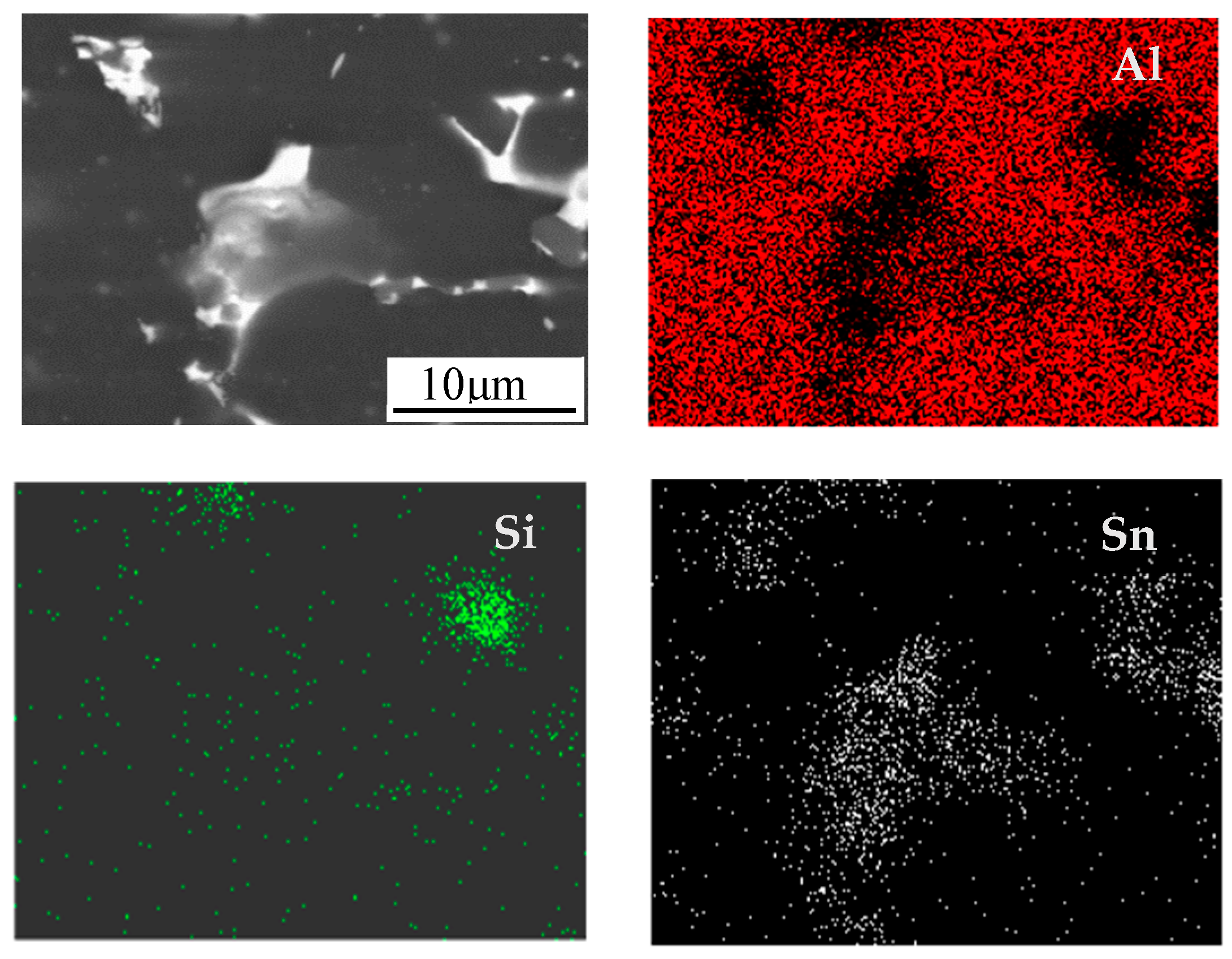
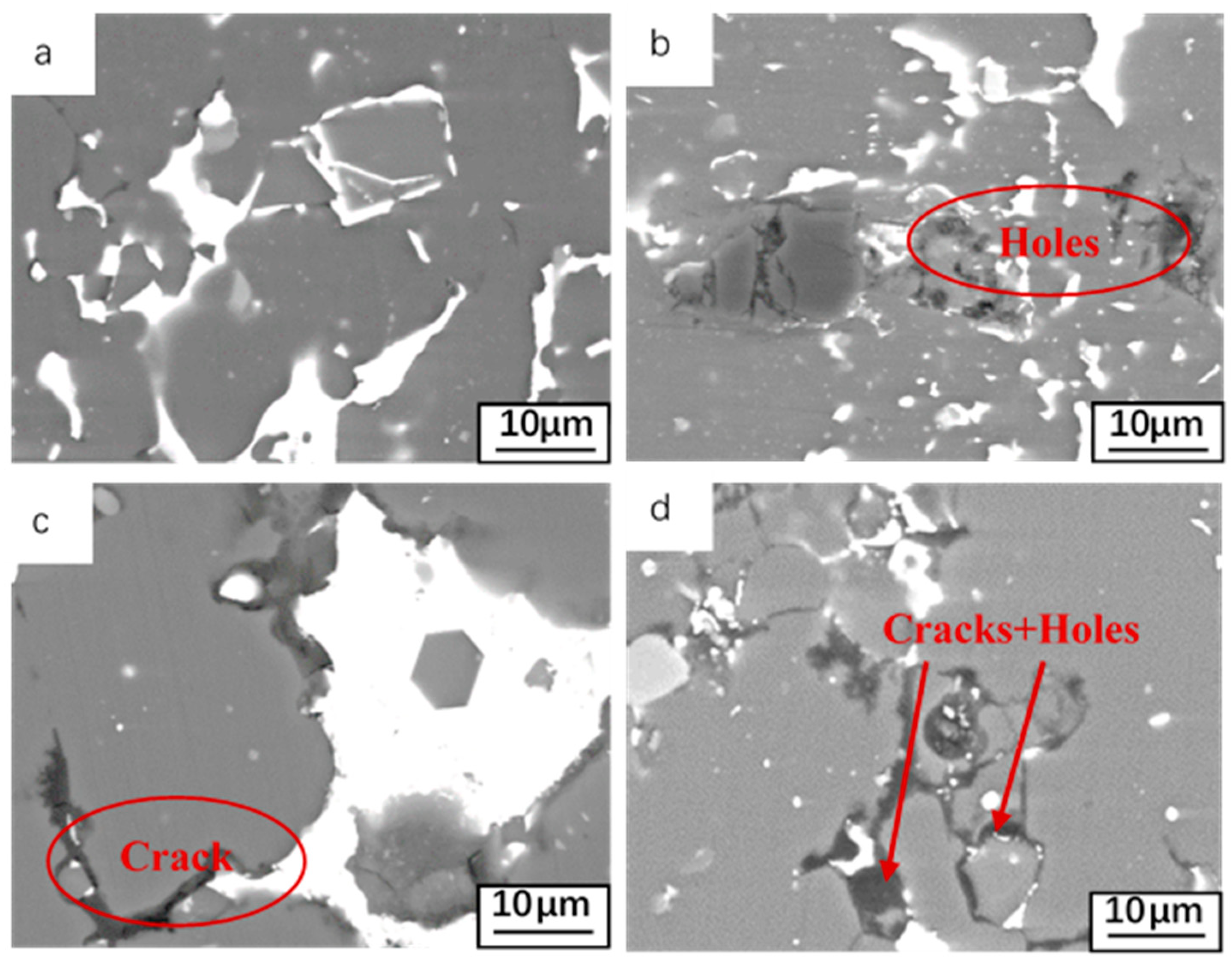

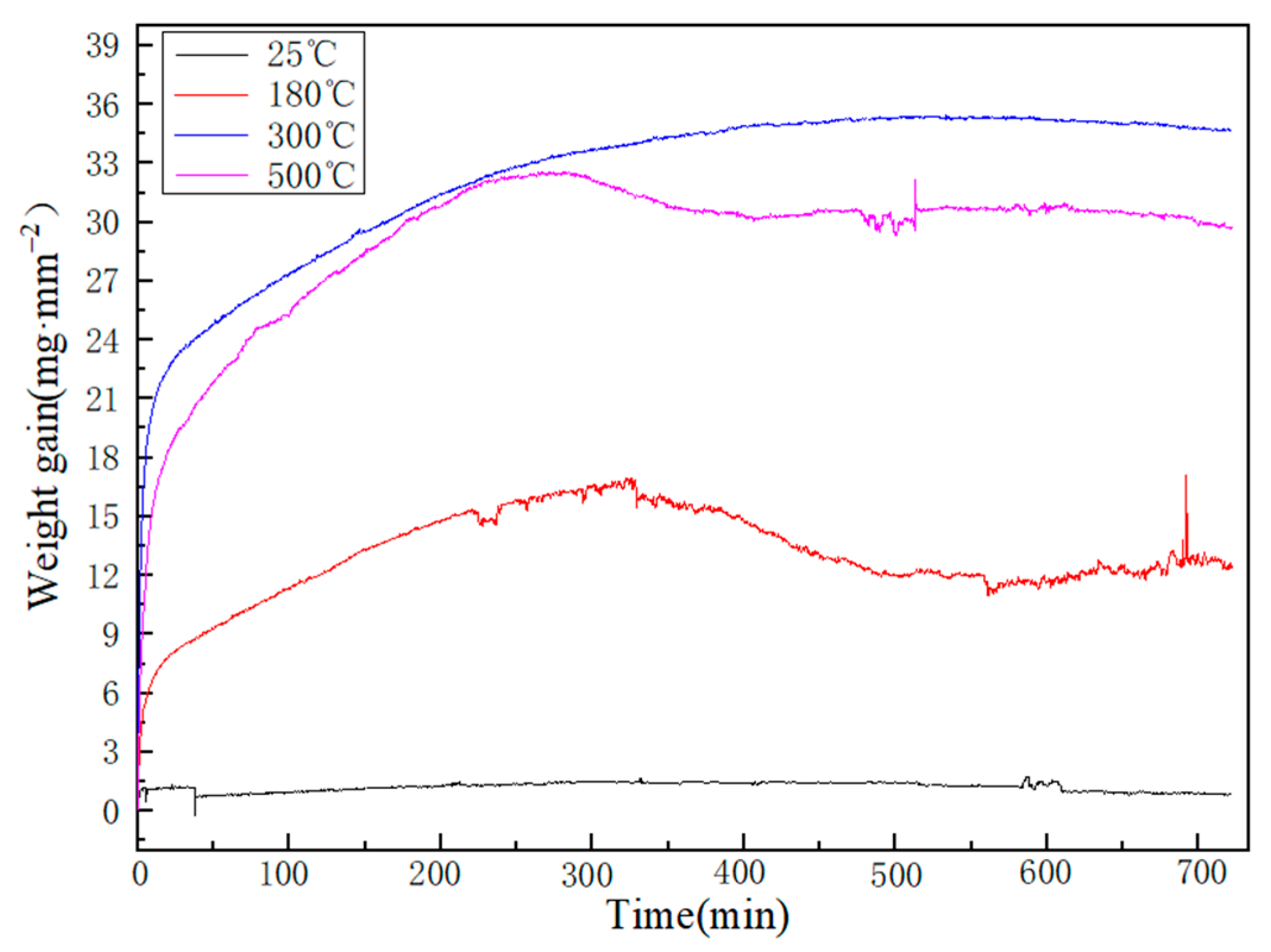
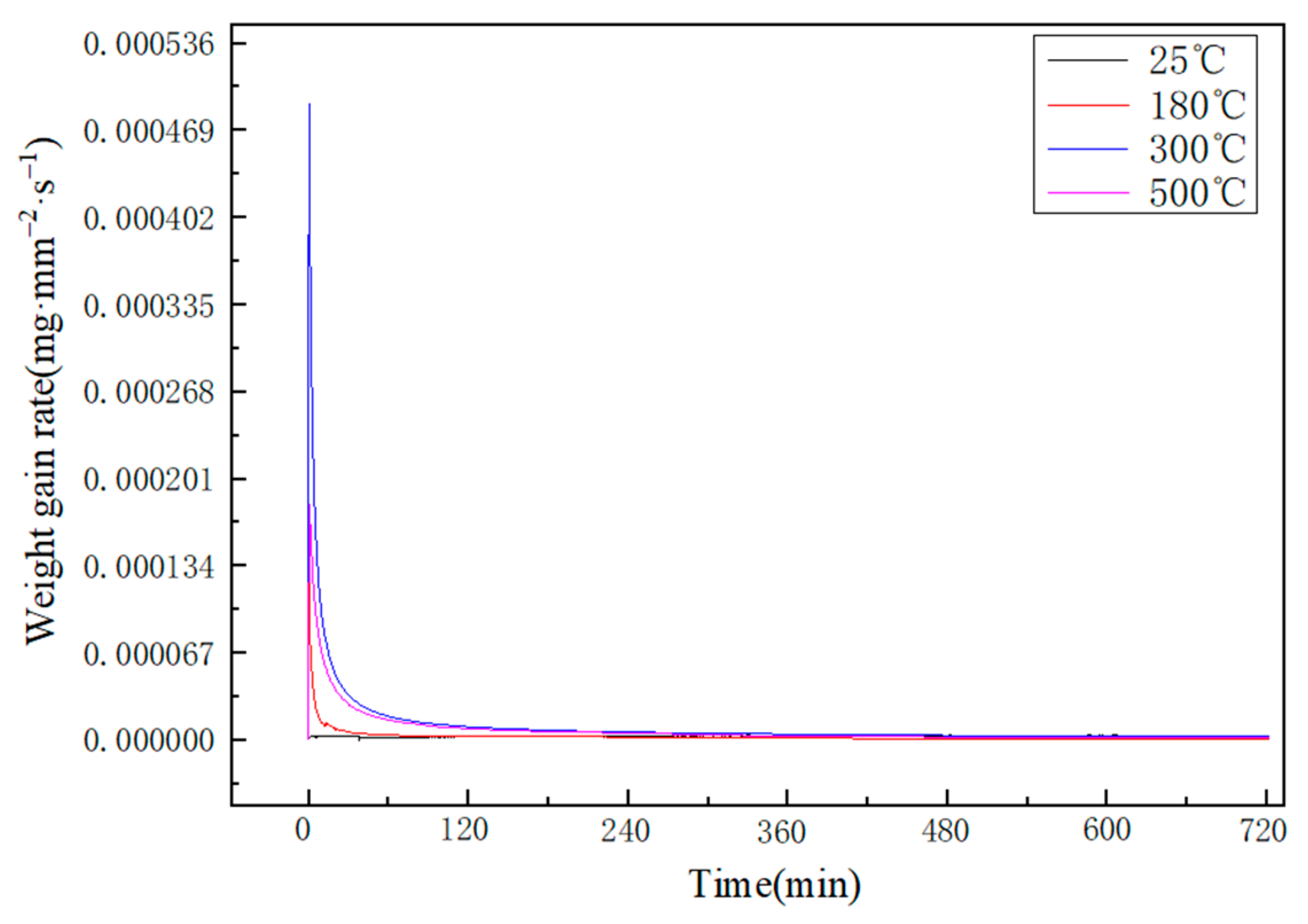

| Elements | Sn | Si | Al |
|---|---|---|---|
| Content (wt %) | 5.9 | 4.0 | balance |
| Elements | C | Si | Mn | Ni | Cu | Fe |
|---|---|---|---|---|---|---|
| Content (wt %) | 0.12 | 0.37 | 0.65 | 0.25 | 0.20 | balance |
| Temperature/°C | Fitting Equation | Fit/R2 |
|---|---|---|
| 25 | y = 0.0000002 | 0.9931 |
| 180 | y = 0.0001X−0.769 | 0.9243 |
| 300 | y = 0.0006X−0.839 | 0.9961 |
| 500 | y = 0.0006X−0.917 | 0.9753 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Chen, J.; Li, J.; Guo, Y.; Yang, Z.; Yang, W.; Xu, D.; Yang, B. Oxidation Mechanism of Al-Sn Bearing Alloys. Materials 2021, 14, 4845. https://doi.org/10.3390/ma14174845
Guo Q, Chen J, Li J, Guo Y, Yang Z, Yang W, Xu D, Yang B. Oxidation Mechanism of Al-Sn Bearing Alloys. Materials. 2021; 14(17):4845. https://doi.org/10.3390/ma14174845
Chicago/Turabian StyleGuo, Qiaoqin, Jihui Chen, Jianping Li, Yongchun Guo, Zhong Yang, Wei Yang, Dapeng Xu, and Bo Yang. 2021. "Oxidation Mechanism of Al-Sn Bearing Alloys" Materials 14, no. 17: 4845. https://doi.org/10.3390/ma14174845
APA StyleGuo, Q., Chen, J., Li, J., Guo, Y., Yang, Z., Yang, W., Xu, D., & Yang, B. (2021). Oxidation Mechanism of Al-Sn Bearing Alloys. Materials, 14(17), 4845. https://doi.org/10.3390/ma14174845







