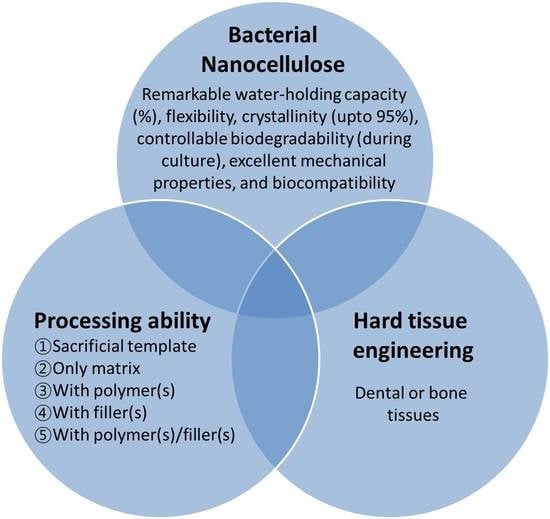
Efficacy of Bacterial Nanocellulose in Hard Tissue Regeneration: A Review
Abstract
:1. Introduction
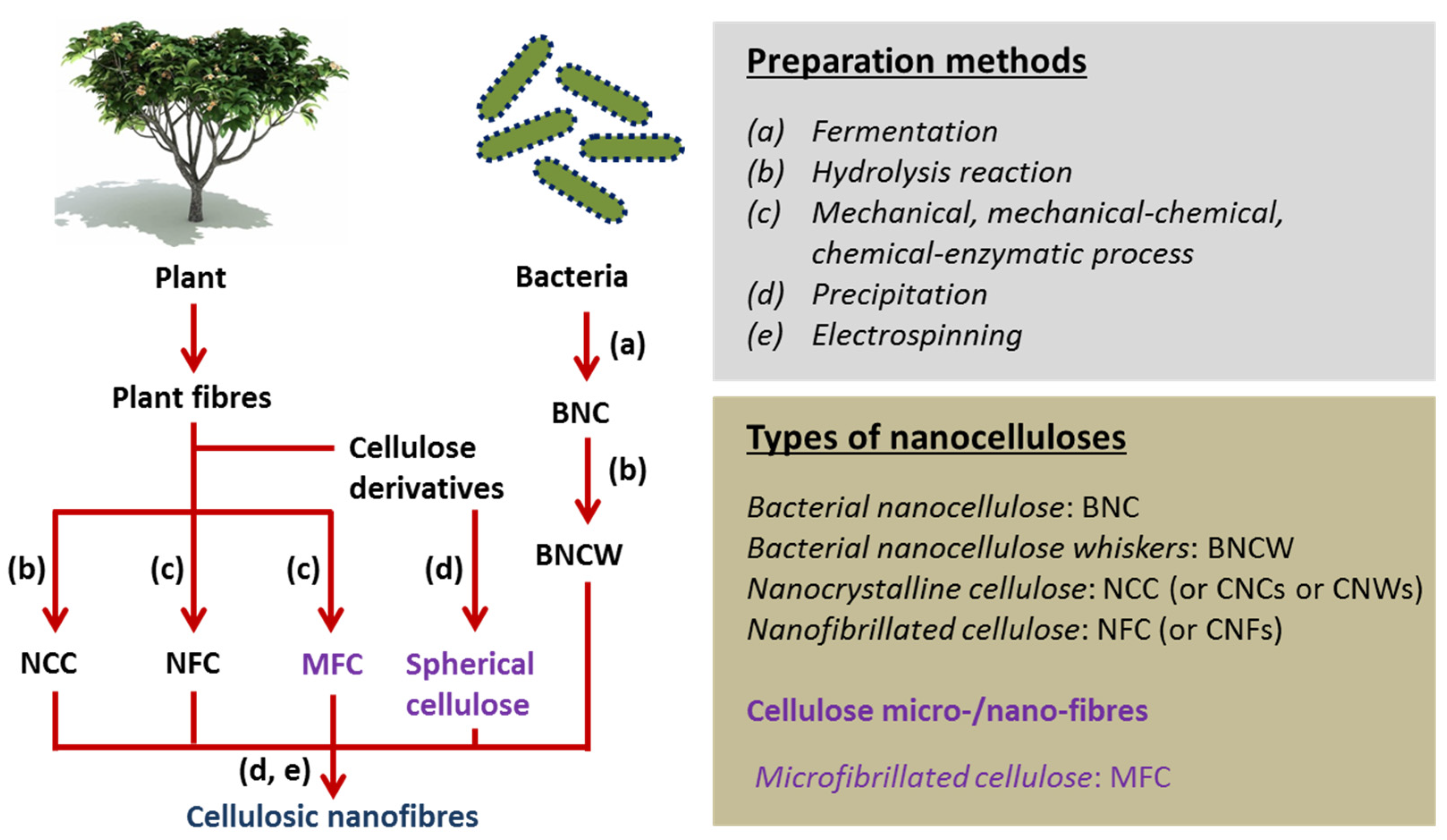
2. Nanocellulose in Tissue Engineering
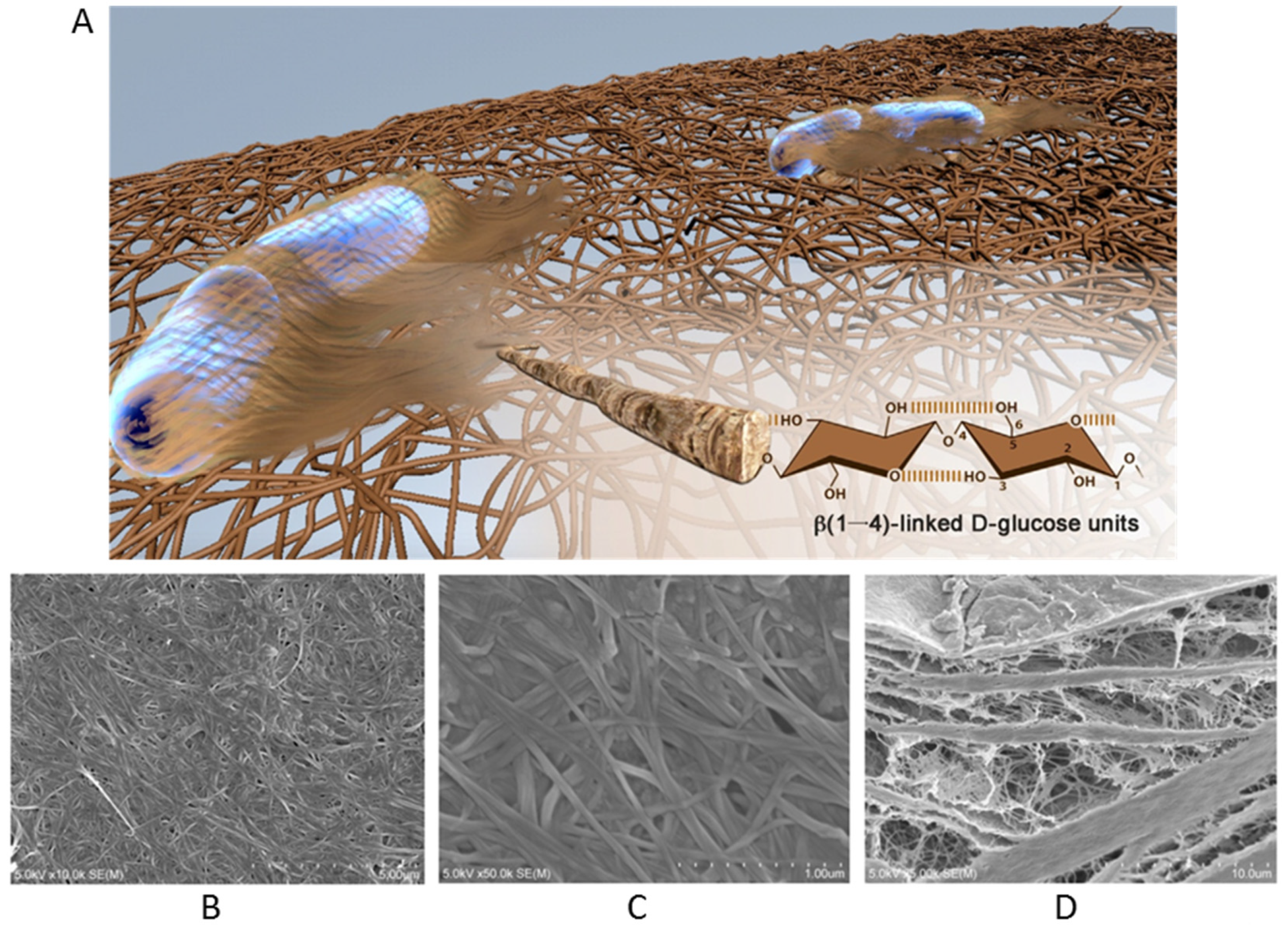
3. Bacterial Nanocellulose
3.1. Synthesis
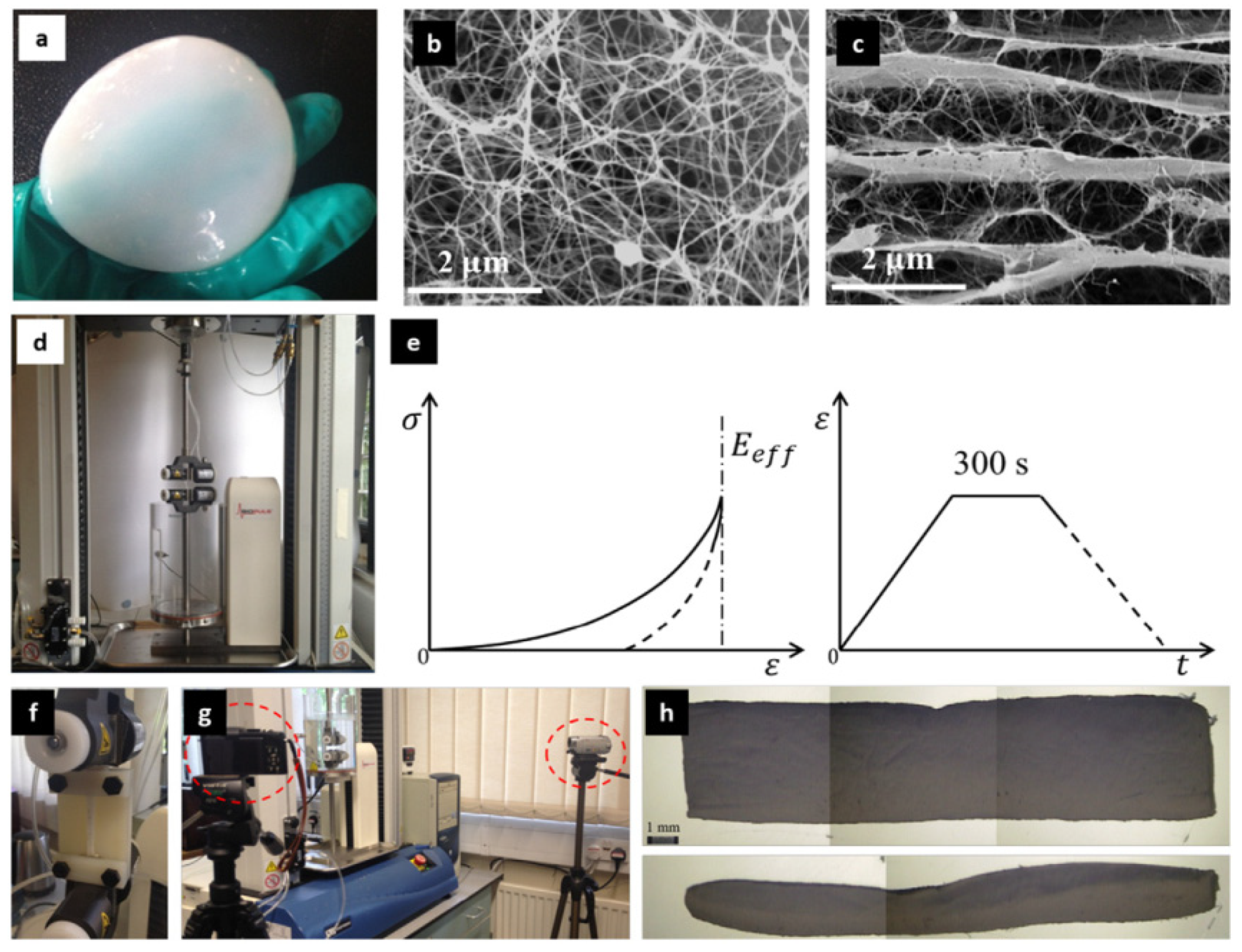
3.2. Properties
3.2.1. Solubility, Biodegradation, and Thermal Stability
3.2.2. Antimicrobial Ability
3.2.3. Toxicity and Cellular Response
4. Surface Modification of Bacterial Nanocellulose
5. Application in Hard Tissue Regeneration
5.1. Bacterial Nanocellulose (as Sacrificial Template)
5.2. Bacterial Nanocellulose (as Only Matrix)
5.3. Bacterial Nanocellulose/Polymer-Based Biomaterials
5.4. Bacterial Nanocellulose/Filler-Based Biomaterials
5.5. Bacterial Nanocellulose/Polymer/Filler-Based Biomaterials
6. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Investigating the potential of combined growth factors delivery, from non-mulberry silk fibroin grafted poly (ɛ-caprolactone)/hydroxyapatite nanofibrous scaffold, in bone tissue engineering. Appl. Mater. Today 2016, 5, 52–67. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Microstructural and mechanical properties of porous biocomposite scaffolds based on polyvinyl alcohol, nano-hydroxyapatite and cellulose nanocrystals. Cellulose 2014, 21, 3409–3426. [Google Scholar] [CrossRef]
- Ruka, D.R.; Simon, G.P.; Dean, K.M. Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr. Polym. 2012, 89, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Otoni, C.G.; Kevin, J.; Barud, H.S.; Lona, L.M.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Halib, N.; Ahmad, I.; Grassi, M.; Grassi, G. The remarkable three-dimensional network structure of bacterial cellulose for tissue engineering applications. Int. J. Pharm. 2019, 566, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Han, S.S. Effect of crosslinking functionality on microstructure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Han, S.S. Synthesis of mechanically stiff and bioactive hybrid hydrogels for bone tissue engineering applications. Chem. Eng. J. 2017, 317, 119–131. [Google Scholar] [CrossRef]
- Kumar, A.; Matari, I.A.I.; Choi, H.; Kim, A.; Suk, Y.J.; Kim, J.Y.; Han, S.S. Development of halloysite nanotube/carboxylated-cellulose nanocrystal-reinforced and ionically-crosslinked polysaccharide hydrogels. Mater. Sci. Eng. C 2019, 104, 109983. [Google Scholar] [CrossRef]
- Kuhnt, T.; Camarero-Espinosa, S. Additive manufacturing of nanocellulose based scaffolds for tissue engineering: Beyond a reinforcement filler. Carbohydr. Polym. 2021, 252, 117159. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; Arroyo, J.; Troncoso, O. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.R.; Bajaj, I.B.; Survase, S.A.; Singhal, R.S. Microbial cellulose: Fermentative production and applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Cañas-Gutiérrez, A.; Osorio, M.; Molina-Ramírez, C.; Arboleda-Toro, D.; Castro-Herazo, C. Bacterial cellulose: A biomaterial with high potential in dental and oral applications. Cellulose 2020, 27, 9737–9754. [Google Scholar] [CrossRef]
- El-Hoseny, S.M.; Basmaji, P.; de Olyveira, G.M.; Costa, L.M.M.; Alwahedi, A.M.; da Costa Oliveira, J.D.; Francozo, G.B. Natural ECM-bacterial cellulose wound healing—Dubai Study. J. Biomater. Nanobiotechnol. 2015, 6, 237. [Google Scholar] [CrossRef] [Green Version]
- Da Gama, F.M.P.; Dourado, F. Bacterial NanoCellulose: What future? BioImpacts BI 2018, 8, 1. [Google Scholar]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef]
- Bae, S.; Sugano, Y.; Shoda, M. Improvement of bacterial cellulose production by addition of agar in a jar fermentor. J. Biosci. Bioeng. 2004, 97, 33–38. [Google Scholar] [CrossRef]
- Chao, Y.; Ishida, T.; Sugano, Y.; Shoda, M. Bacterial cellulose production by Acetobacter xylinum in a 50-L internal-loop airlift reactor. Biotechnol. Bioeng. 2000, 68, 345–352. [Google Scholar] [CrossRef]
- Krystynowicz, A.; Czaja, W.; Wiktorowska-Jezierska, A.; Gonçalves-Miśkiewicz, M.; Turkiewicz, M.; Bielecki, S. Factors affecting the yield and properties of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2002, 29, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Dahman, Y. Improvements in the production of bacterial synthesized biocellulose nanofibres using different culture methods. J. Chem. Technol. Biotechnol. 2010, 85, 151–164. [Google Scholar] [CrossRef]
- Brown, A.J. XLIII.—On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Mol. Biol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef] [Green Version]
- Koike, T.; Sha, J.; Bai, Y.; Matsuda, Y.; Hideshima, K.; Yamada, T.; Kanno, T. Efficacy of Bacterial Cellulose as a Carrier of BMP-2 for Bone Regeneration in a Rabbit Frontal Sinus Model. Materials 2019, 12, 2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira Junior, O.B.; Ribeiro, S.J. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef] [Green Version]
- Gama, M.; Dourado, F.; Bielecki, S. Bacterial Nanocellulose: From Biotechnology to Bio-Economy; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Pometto, A.L., III; Demirci, A.; Johnson, K.E. Immobilization of Microorganisms on a Support Made of Synthetic Polymer and Plant Material. U.S. Patent 5,595,893, 21 January 1997. [Google Scholar]
- Keshk, S.M.; El-Kott, A.F. Natural bacterial biodegradable medical polymers: Bacterial cellulose. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 295–319. [Google Scholar]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods–A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.U.; Ullah, M.W.; Khan, S.; Shah, N.; Park, J.K. Strategies for cost-effective and enhanced production of bacterial cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Jung, J.Y.; Park, Y.H. Cellulose production by Gluconacetobacter hansenii in a medium containing ethanol. Biotechnol. Lett. 2003, 25, 2055–2059. [Google Scholar] [CrossRef]
- Lee, K.Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than meets the eye in bacterial cellulose: Biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef] [Green Version]
- Jagannath, A.; Kalaiselvan, A.; Manjunatha, S.; Raju, P.; Bawa, A. The effect of pH, sucrose and ammonium sulphate concentrations on the production of bacterial cellulose (Nata-de-coco) by Acetobacter xylinum. World J. Microbiol. Biotechnol. 2008, 24, 2593–2599. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Effects of CMC addition on bacterial cellulose production in a biofilm reactor and its paper sheets analysis. Biomacromolecules 2011, 12, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sun, D.; Hu, L.; Li, Y.; Yang, J. Effect of addition of sodium alginate on bacterial cellulose production by Acetobacter xylinum. J. Ind. Microbiol. Biotechnol. 2007, 34, 483. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, J.; Singh, R. Biotransformation of sweet lime pulp waste into high-quality nanocellulose with an excellent productivity using Komagataeibacter europaeus SGP37 under static intermittent fed-batch cultivation. Bioresour. Technol. 2018, 247, 73–80. [Google Scholar] [CrossRef]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Troncoso, O.P.; Canet-Ferrer, J.; Martínez-Pastor, J. Development of self-assembled bacterial cellulose–starch nanocomposites. Mater. Sci. Eng. C 2009, 29, 1098–1104. [Google Scholar] [CrossRef]
- Gao, X.; Shi, Z.; Kuśmierczyk, P.; Liu, C.; Yang, G.; Sevostianov, I.; Silberschmidt, V.V. Time-dependent rheological behaviour of bacterial cellulose hydrogel. Mater. Sci. Eng. C 2016, 58, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent advances in modified cellulose for tissue culture applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wan, Y.; Li, L.; Liang, H.; Wang, J. Preparation and characterization of 2, 3-dialdehyde bacterial cellulose for potential biodegradable tissue engineering scaffolds. Mater. Sci. Eng. C 2009, 29, 1635–1642. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Biodegradation and thermal stability of bacterial cellulose as biomaterial: The relevance in biomedical applications. Polym. Degrad. Stab. 2020, 179, 109232. [Google Scholar] [CrossRef]
- Liyaskina, E.; Revin, V.; Paramonova, E.; Nazarkina, M.; Pestov, N.; Revina, N.; Kolesnikova, S. Nanomaterials from Bacterial Cellulose for Antimicrobial Wound Dressing. J. Phys. Conf. Ser. 2017, 784, 012034. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Barud, H.d.S.; de Araújo Júnior, A.M.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; De Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; Vaz, M.M.d.O.L.L. Antimicrobial Brazilian propolis (EPP-AF) containing biocellulose membranes as promising biomaterial for skin wound healing. Evid.-Based Complement. Altern. Med. 2013, 2013, 703024. [Google Scholar] [CrossRef]
- Ciechanska, D. Multifunctional bacterial cellulose/chitosan composite materials for medical applications. Fibres Text East Eur. 2004, 12, 69–72. [Google Scholar]
- Ul-Islam, M.; Khan, T.; Khattak, W.A.; Park, J.K. Bacterial cellulose-MMTs nanoreinforced composite films: Novel wound dressing material with antibacterial properties. Cellulose 2013, 20, 589–596. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Fabrication of natural-origin antibacterial nanocellulose films using bio-extracts for potential use in biomedical industry. Int. J. Biol. Macromol. 2020, 145, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; Regiani, T.; Marques, R.F.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J. Antimicrobial bacterial cellulose-silver nanoparticles composite membranes. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.J.; Neves, M.C.; Neto, C.P.; Trindade, T. Growth and chemical stability of copper nanostructures on cellulosic fibers. Eur. J. Inorg. Chem. 2012, 2012, 5043–5049. [Google Scholar] [CrossRef]
- Gutierrez, J.; Tercjak, A.; Algar, I.; Retegi, A.; Mondragon, I. Conductive properties of TiO2/bacterial cellulose hybrid fibres. J. Colloid Interface Sci. 2012, 377, 88–93. [Google Scholar] [CrossRef]
- Dydak, K.; Junka, A.; Szymczyk, P.; Chodaczek, G.; Toporkiewicz, M.; Fijałkowski, K.; Dudek, B.; Bartoszewicz, M. Development and biological evaluation of Ti6Al7Nb scaffold implants coated with gentamycin-saturated bacterial cellulose biomaterial. PLoS ONE 2018, 13, e0205205. [Google Scholar] [CrossRef] [Green Version]
- Jinga, S.I.; Draghici, A.D.; Mocanu, A.; Nicoara, A.I.; Iordache, F.; Busuioc, C. Bacterial cellulose–assisted synthesis of glass-ceramic scaffolds with TiO2 crystalline domains. Int. J. Appl. Ceram. Technol. 2020, 17, 2017–2024. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Prateek, R.S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and antiviral functional materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics. ACS Pharmacol. Transl. Sci. 2020, 4, 8–54. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Mcclements, D.J.; Moore, M.D.; Williams, L. Development of nanoparticle-delivery systems for antiviral agents: A review. J. Control. Release 2021, 331, 30–44. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 76, 431–438. [Google Scholar] [CrossRef]
- Dourado, F.; Gama, M.; Rodrigues, A.C. A review on the toxicology and dietetic role of bacterial cellulose. Toxicol. Rep. 2017, 4, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Pelham, R.J.; Wang, Y.-L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, C.-M.; Wang, H.-B.; Dembo, M.; Wang, Y.-L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Taokaew, S.; Phisalaphong, M.; Newby, B.-M.Z. In vitro behaviors of rat mesenchymal stem cells on bacterial celluloses with different moduli. Mater. Sci. Eng. C 2014, 38, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sozumert, E.; Shi, Z.; Yang, G.; Silberschmidt, V.V. Assessing stiffness of nanofibres in bacterial cellulose hydrogels: Numerical-experimental framework. Mater. Sci. Eng. C 2017, 77, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Aki, D.; Ulag, S.; Unal, S.; Sengor, M.; Ekren, N.; Lin, C.-C.; Yılmazer, H.; Ustundag, C.B.; Kalaskar, D.M.; Gunduz, O. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater. Des. 2020, 196, 109094. [Google Scholar] [CrossRef]
- Zimmermann, K.A.; LeBlanc, J.M.; Sheets, K.T.; Fox, R.W.; Gatenholm, P. Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C 2011, 31, 43–49. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, J. Preparation and characterization of novel bacterial cellulose/gelatin scaffold for tissue regeneration using bacterial cellulose hydrogel. J. Nanotechnol. Eng. Med. 2010, 1. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Shin, S.; Hyun, J. Effect of negatively charged cellulose nanofibers on the dispersion of hydroxyapatite nanoparticles for scaffolds in bone tissue engineering. Colloids Surf. B Biointerfaces 2015, 130, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Chen, X.; Feng, M.; Shi, Z.; Zhang, D.; Lin, Q. Layer-by-layer assembly of 3D alginate-chitosan-gelatin composite scaffold incorporating bacterial cellulose nanocrystals for bone tissue engineering. Mater. Lett. 2017, 209, 492–496. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Li, Z.; Wu, Z.; Zhang, M.; Sheng, N.; Liang, Q.; Wang, H.; Chen, S. A 3D-printable TEMPO-oxidized bacterial cellulose/alginate hydrogel with enhanced stability via nanoclay incorporation. Carbohydr. Polym. 2020, 238, 116207. [Google Scholar] [CrossRef] [PubMed]
- Luz, E.P.C.G.; Chaves, P.H.S.; Vieira, L.d.A.P.; Ribeiro, S.F.; de Fátima Borges, M.; Andrade, F.K.; Muniz, C.R.; Infantes-Molina, A.; Rodríguez-Castellón, E.; de Freitas Rosa, M. In vitro degradability and bioactivity of oxidized bacterial cellulose-hydroxyapatite composites. Carbohydr. Polym. 2020, 237, 116174. [Google Scholar] [CrossRef] [PubMed]
- Luz, E.P.C.G.; das Chagas, B.S.; de Almeida, N.T.; de Fátima Borges, M.; Andrade, F.K.; Muniz, C.R.; Castro-Silva, I.I.; Teixeira, E.H.; Popat, K.; de Freitas Rosa, M. Resorbable bacterial cellulose membranes with strontium release for guided bone regeneration. Mater. Sci. Eng. C 2020, 116, 111175. [Google Scholar] [CrossRef] [PubMed]
- Abdelraof, M.; Hasanin, M.S.; Farag, M.M.; Ahmed, H.Y. Green synthesis of bacterial cellulose/bioactive glass nanocomposites: Effect of glass nanoparticles on cellulose yield, biocompatibility and antimicrobial activity. Int. J. Biol. Macromol. 2019, 138, 975–985. [Google Scholar] [CrossRef]
- Xiao, J.; Luo, H.; Ao, H.; Huang, Y.; Yao, F.; Zhang, Q.; Wan, Y. A rhBMP-2-loaded three-dimensional mesoporous bioactive glass nanotubular scaffold prepared from bacterial cellulose. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123838. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Haider, A.; Abd Razak, S.I.; Kadir, M.R.A.; Shah, S.A.; Javed, A.; Shakir, I.; Al-Zahrani, A.A. Development of porous, antibacterial and biocompatible GO/n-HAp/bacterial cellulose/β-glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem. 2021, 14, 102924. [Google Scholar] [CrossRef]
- Noh, Y.K.; Da Costa, A.D.S.; Park, Y.S.; Du, P.; Kim, I.-H.; Park, K. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesenchymal stem cells. Carbohydr. Polym. 2019, 219, 210–218. [Google Scholar] [CrossRef]
- Andrade, F.K.; Moreira, S.M.G.; Domingues, L.; Gama, F. Improving the affinity of fibroblasts for bacterial cellulose using carbohydrate-binding modules fused to RGD. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-K.; Chen, K.-H.; Ou, K.-L.; Liu, M. Effects of different extracellular matrices and growth factor immobilization on biodegradability and biocompatibility of macroporous bacterial cellulose. J. Bioact. Compat. Polym. 2011, 26, 508–518. [Google Scholar] [CrossRef]
- Kheiry, E.V.; Parivar, K.; Baharara, J.; Bazzaz, B.S.F.; Iranbakhsh, A. The osteogenesis of bacterial cellulose scaffold loaded with fisetin. Iran. J. Basic Med. Sci. 2018, 21, 965. [Google Scholar]
- Chu, P.K.; Chen, J.; Wang, L.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef] [Green Version]
- Fontana, J.; De Souza, A.; Fontana, C.; Torriani, I.; Moreschi, J.; Gallotti, B.; De Souza, S.; Narcisco, G.; Bichara, J.; Farah, L. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Shi, Q.; Li, Y.; Sun, J.; Zhang, H.; Chen, L.; Chen, B.; Yang, H.; Wang, Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 2012, 33, 6644–6649. [Google Scholar] [CrossRef]
- Luo, H.; Ji, D.; Li, W.; Xiao, J.; Li, C.; Xiong, G.; Zhu, Y.; Wan, Y. Constructing a highly bioactive 3D nanofibrous bioglass scaffold via bacterial cellulose-templated sol-gel approach. Mater. Chem. Phys. 2016, 176, 1–5. [Google Scholar] [CrossRef]
- Wen, C.; Hong, Y.; Wu, J.; Luo, L.; Qiu, Y.; Ye, J. The facile synthesis and bioactivity of a 3D nanofibrous bioglass scaffold using an amino-modified bacterial cellulose template. RSC Adv. 2018, 8, 14561–14569. [Google Scholar] [CrossRef] [Green Version]
- Busuioc, C.; Ghitulica, C.; Stoica, A.; Stroescu, M.; Voicu, G.; Ionita, V.; Averous, L.; Jinga, S. Calcium phosphates grown on bacterial cellulose template. Ceram. Int. 2018, 44, 9433–9441. [Google Scholar] [CrossRef]
- Draghici, A.-D.; Busuioc, C.; Mocanu, A.; Nicoara, A.-I.; Iordache, F.; Jinga, S.-I. Composite scaffolds based on calcium phosphates and barium titanate obtained through bacterial cellulose templated synthesis. Mater. Sci. Eng. C 2020, 110, 110704. [Google Scholar] [CrossRef]
- Zang, S.; Zhuo, Q.; Chang, X.; Qiu, G.; Wu, Z.; Yang, G. Study of osteogenic differentiation of human adipose-derived stem cells (HASCs) on bacterial cellulose. Carbohydr. Polym. 2014, 104, 158–165. [Google Scholar] [CrossRef]
- Dubey, S.; Mishra, R.; Roy, P.; Singh, R. 3-D macro/microporous-nanofibrous bacterial cellulose scaffolds seeded with BMP-2 preconditioned mesenchymal stem cells exhibit remarkable potential for bone tissue engineering. Int. J. Biol. Macromol. 2021, 167, 934–946. [Google Scholar] [CrossRef]
- An, S.-J.; Lee, S.-H.; Huh, J.-B.; Jeong, S.I.; Park, J.-S.; Gwon, H.-J.; Kang, E.-S.; Jeong, C.-M.; Lim, Y.-M. Preparation and characterization of resorbable bacterial cellulose membranes treated by electron beam irradiation for guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 2236. [Google Scholar] [CrossRef] [PubMed]
- Farnezi Bassi, A.P.; Bizelli, V.F.; Brasil, L.F.d.M.; Pereira, J.C.; Al-Sharani, H.M.; Momesso, G.A.C.; Faverani, L.P.; Lucas, F.d.A. Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property? Membranes 2020, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Weyell, P.; Beekmann, U.; Küpper, C.; Dederichs, M.; Thamm, J.; Fischer, D.; Kralisch, D. Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morello, K.C.; Wurz, G.T.; DeGregorio, M.W. Pharmacokinetics of selective estrogen receptor modulators. Clin. Pharmacokinet. 2003, 42, 361–372. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Abdelkhalek, A.A.; Elkasabgy, N.A. Nanofibrillated cellulose/cyclodextrin based 3D scaffolds loaded with raloxifene hydrochloride for bone regeneration. Int. J. Biol. Macromol. 2020, 156, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Inoue, B.S.; Streit, S.; dos Santos Schneider, A.L.; Meier, M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020, 148, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Klinthoopthamrong, N.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 2020, 149, 51–59. [Google Scholar] [CrossRef]
- Wan, Y.; Zuo, G.; Yu, F.; Huang, Y.; Ren, K.; Luo, H. Preparation and mineralization of three-dimensional carbon nanofibers from bacterial cellulose as potential scaffolds for bone tissue engineering. Surf. Coat. Technol. 2011, 205, 2938–2946. [Google Scholar] [CrossRef]
- Costa, L.M.M.; Olyveira, G.M.d.; Basmaji, P.; Valido, D.P.; Gois, P.B.P.; Junior, R.L.A.C. Novel otoliths/bacterial cellulose nanocomposites as a potential natural product for direct dental pulp capping. J. Biomater. Tissue Eng. 2012, 2, 48–53. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, T.; Zhao, Z.; Ren, H.; Zhang, Q.; Yan, Y.; Lv, G. Preparation and characterization of bacterial cellulose microfiber/goat bone apatite composites for bone repair. J. Appl. Polym. Sci. 2013, 129, 595–603. [Google Scholar] [CrossRef]
- Voicu, G.; Jinga, S.-I.; Drosu, B.-G.; Busuioc, C. Improvement of silicate cement properties with bacterial cellulose powder addition for applications in dentistry. Carbohydr. Polym. 2017, 174, 160–170. [Google Scholar] [CrossRef]
- Maia, M.T.; Luz, É.P.C.G.; Andrade, F.K.; Rosa, M.d.F.; Borges, M.d.F.; Arcanjo, M.R.A.; Vieira, R.S. Advances in Bacterial Cellulose/Strontium Apatite Composites for Bone Applications. Polym. Rev. 2021, 1–29. [Google Scholar] [CrossRef]
- Sousa, R.B.; Dametto, A.C.; Sábio, R.M.; de Carvalho, R.A.; Vieira, E.G.; do Amaral Oliveira, A.F.; Ribeiro, L.K.; Barud, H.S.; Silva-Filho, E.C. Cerium-doped calcium phosphates precipitated on bacterial cellulose platform by mineralization. Ceram. Int. 2020, 46, 26985–26990. [Google Scholar] [CrossRef]
- Ito, A.; Kamihira, M. Tissue engineering using magnetite nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011, 104, 355–395. [Google Scholar] [PubMed]
- Torgbo, S.; Sukyai, P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019, 237, 121868. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Shahin, K.; Mirström, M.M.; Li, D. Cellulose-reinforced bioglass composite as flexible bioactive bandage to enhance bone healing. Ceram. Int. 2021, 47, 416–423. [Google Scholar] [CrossRef]
- Sukyai, P.; Lam, N.T.; Niamsap, T. In situ biosynthesis of bacterial nanocellulose incorporated with hydroxyapatite/cellulose nanocrystals. New Biotechnol. 2018, 44, S11. [Google Scholar] [CrossRef]
- Niamsap, T.; Lam, N.T.; Sukyai, P. Production of hydroxyapatite-bacterial nanocellulose scaffold with assist of cellulose nanocrystals. Carbohydr. Polym. 2019, 205, 159–166. [Google Scholar] [CrossRef]
- De Olyveira, G.M.; Basmaji, P.; Costa, L.M.M.; Dos Santos, M.L.; dos Santos Riccardi, C.; Guastaldi, F.P.S.; Scarel-Caminaga, R.M.; de Oliveira Capote, T.S.; Pizoni, E.; Guastaldi, A.C. Surface physical chemistry properties in coated bacterial cellulose membranes with calcium phosphate. Mater. Sci. Eng. C 2017, 75, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Kumbhar, J.V.; Jadhav, S.H.; Bodas, D.S.; Barhanpurkar-Naik, A.; Wani, M.R.; Paknikar, K.M.; Rajwade, J.M. In vitro and in vivo studies of a novel bacterial cellulose-based acellular bilayer nanocomposite scaffold for the repair of osteochondral defects. Int. J. Nanomed. 2017, 12, 6437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Zhen, W.; Chen, H.; Shan, Z. Biomimetic design of oxidized bacterial cellulose-gelatin-hydroxyapatite nanocomposites. J. Bionic Eng. 2016, 13, 631–640. [Google Scholar] [CrossRef]
- Ran, J.; Jiang, P.; Liu, S.; Sun, G.; Yan, P.; Shen, X.; Tong, H. Constructing multi-component organic/inorganic composite bacterial cellulose-gelatin/hydroxyapatite double-network scaffold platform for stem cell-mediated bone tissue engineering. Mater. Sci. Eng. C 2017, 78, 130–140. [Google Scholar] [CrossRef]
- Chen, K.Y.; Shyu, P.C.; Chen, Y.S.; Yao, C.H. Novel Bone Substitute Composed of Oligomeric Proanthocyanidins-Crosslinked Gelatin and Tricalcium Phosphate. Macromol. Biosci. 2008, 8, 942–950. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Y.; Han, J.; Lei, X.; Yan, T.; Gao, C. Nanocomposite prepared by immobilising gelatin and hydroxyapatite on bacterial cellulose nanofibres. Micro Nano Lett. 2011, 6, 133–136. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Atila, D.; Karataş, A.; Evcin, A.; Keskin, D.; Tezcaner, A. Bacterial cellulose-reinforced boron-doped hydroxyapatite/gelatin scaffolds for bone tissue engineering. Cellulose 2019, 26, 9765–9785. [Google Scholar] [CrossRef]
- Kumar, A.; Matari, I.A.I.; Han, S.S. 3D printable carboxylated cellulose nanocrystal-reinforced hydrogel inks for tissue engineering. Biofabrication 2020, 12, 025029. [Google Scholar] [CrossRef]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Bhushan, S.; Gupta, M.K.; Kumar, A.; Mishra, N.C. Fabrication of graphene oxide and nanohydroxyapatite reinforced gelatin–alginate nanocomposite scaffold for bone tissue regeneration. Front. Mater. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Development of sodium alginate-xanthan gum based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polym. Test. 2017, 63, 214–225. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.-C.; Li, X.-Y.; Jiang, F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020, 236, 116043. [Google Scholar] [CrossRef]
- Hong, L.; Wang, Y.; Jia, S.; Huang, Y.; Gao, C.; Wan, Y. Hydroxyapatite/bacterial cellulose composites synthesized via a biomimetic route. Mater. Lett. 2006, 60, 1710–1713. [Google Scholar] [CrossRef]
- Wan, Y.; Hong, L.; Jia, S.; Huang, Y.; Zhu, Y.; Wang, Y.; Jiang, H. Synthesis and characterization of hydroxyapatite–bacterial cellulose nanocomposites. Compos. Sci. Technol. 2006, 66, 1825–1832. [Google Scholar] [CrossRef]
- Na, Y.; Chen, S.-Y.; Ouyang, Y.; Lian, T.; Yang, J.-X.; Wang, H.-P. Biomimetic mineralization synthesis of hydroxyapatite bacterial cellulose nanocomposites. Prog. Nat. Sci. Mater. Int. 2011, 21, 472–477. [Google Scholar]
- Vielreicher, M.; Kralisch, D.; Völkl, S.; Sternal, F.; Arkudas, A.; Friedrich, O. Bacterial nanocellulose stimulates mesenchymal stem cell expansion and formation of stable collagen-I networks as a novel biomaterial in tissue engineering. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman, M.; Haring, A.P.; Bertucio, T.J. The growing merits and dwindling limitations of bacterial cellulose-based tissue engineering scaffolds. Curr. Opin. Chem. Eng. 2019, 24, 98–106. [Google Scholar] [CrossRef]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: A review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Gutierrez, E.; Burdiles, P.A.; Quero, F.; Palma, P.; Olate-Moya, F.; Palza, H. 3D Printing of antimicrobial alginate/bacterial-cellulose composite hydrogels by incorporating copper nanostructures. ACS Biomater. Sci. Eng. 2019, 5, 6290–6299. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Ullah, M.W.; Booth, P.; Pei, E.; Yang, G. The use of bacterial polysaccharides in bioprinting. Biotechnol. Adv. 2019, 37, 107448. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Yu, T.; Wang, N.; Wang, C.; Wang, H. Preparation of polylactic acid/TEMPO-oxidized bacterial cellulose nanocomposites for 3D printing via Pickering emulsion approach. Compos. Commun. 2019, 16, 162–167. [Google Scholar] [CrossRef]
- Apelgren, P.; Karabulut, E.; Amoroso, M.; Mantas, A.; Martínez Ávila, H.C.; Kölby, L.; Kondo, T.; Toriz, G.; Gatenholm, P. In vivo human cartilage formation in three-dimensional bioprinted constructs with a novel bacterial nanocellulose bioink. ACS Biomater. Sci. Eng. 2019, 5, 2482–2490. [Google Scholar] [CrossRef]


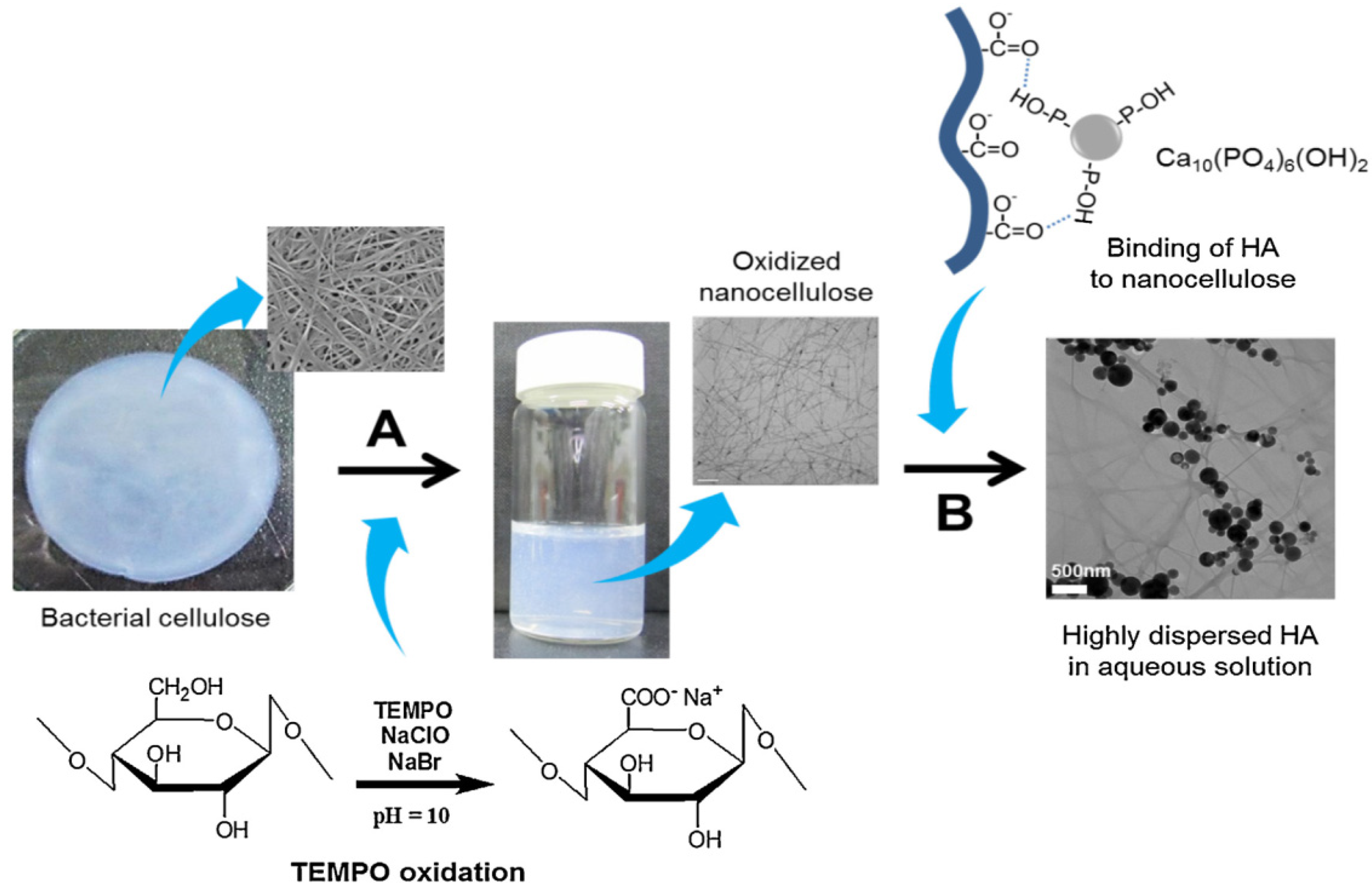
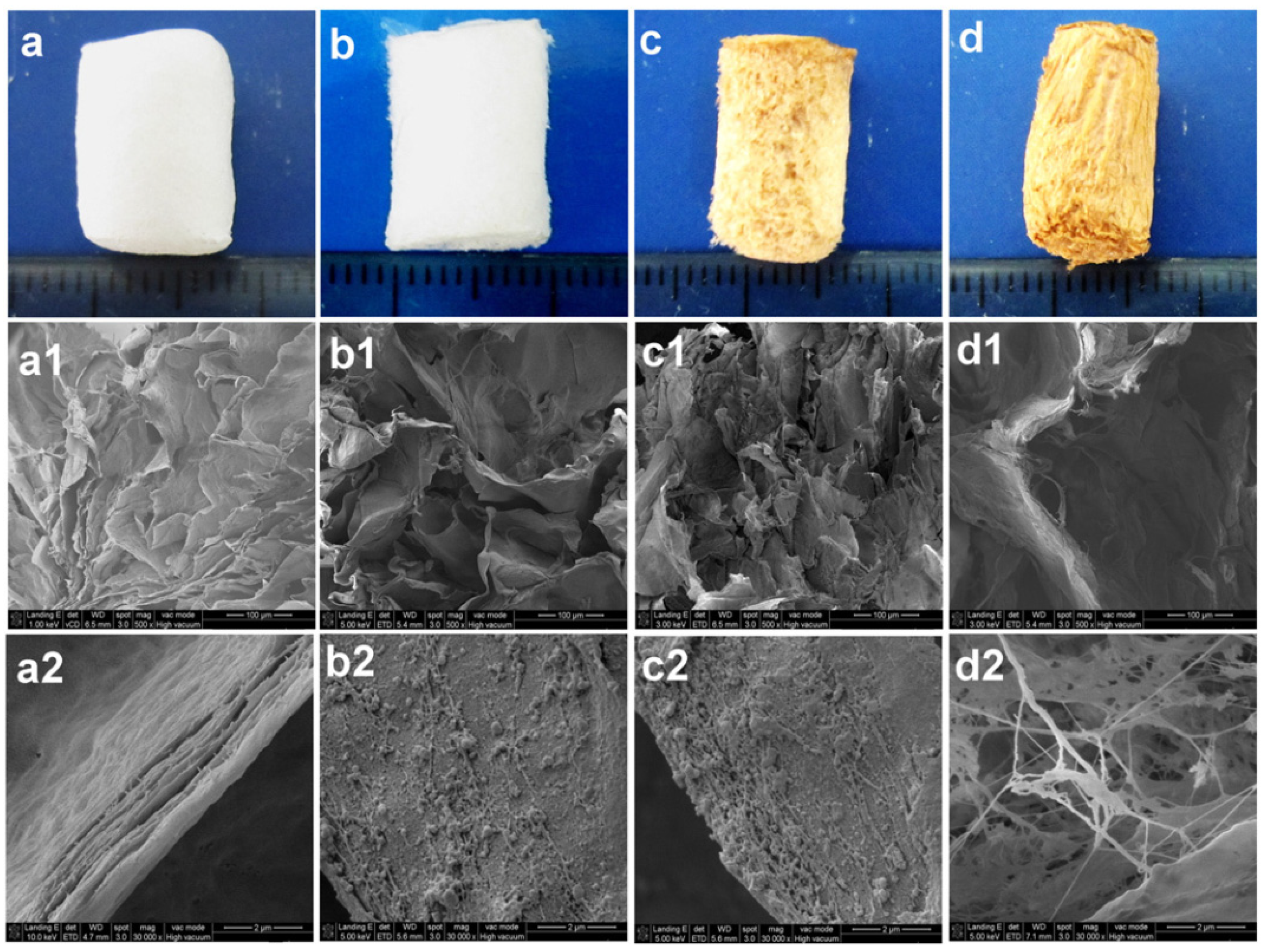
| Composition | Scaffold Form | Cell/Drug/Biomolecule | Features | Ref. |
|---|---|---|---|---|
| BNC | Membrane | NIH-3T3 fibroblast cells | Suitable biocompatibility and enhanced cell viability, remarkably formation of large new bone area | [99] |
| BNC | Membrane | Low biocompatibility and large amount of mature connective tissue in filling the defect (adult male rat) | [100] | |
| BNC | Nanofibrous | BMP-2, C2C12 cells | Suitable biocompatibility and osteogenic differentiation of fibroblast-like cells, and BNC scaffold with BMP-2 exhibited more bone formation and higher calcium content than that of BNC only | [92] |
| BNC | Micro-/nanofibrous | Osteoblasts and fibroblasts, BMP-2 | Promoted optimal bone formation and sustained release of BMP-2 | [28] |
| BNC | Macro-/micro-/nanofibrous | C3H10T1/2 cells, BMP-2 | Low dose of BMP-2 exhibited excellent cell adhesion and growth, remarkably improved bone matrix secretion and maturation, and facilitated the mineralization of cells to some extent | [98] |
| BNC | Nanofibrous | HASCs | Successful osteogenic differentiation of HASCs on BNC and tissue-repairing ability | [97] |
| BNC | Nanofibrous | L929 fibroblasts, doxycycline | Suitable biocompatibility and antibiotic efficiency against pathogenic oral bacteria | [101] |
| BNC/β-CD-CHX | Membrane | CHX | Ten-fold increase in release rate of CHX, all CHX-loaded membranes showed antibacterial activity, but BNC/β-CD-CHX exhibited greater inhibition zone | [104] |
| BNC/collagen | Fibrous | UCB-MSCs and NIH3T3 cells, BMP-2, dexamethasone | Favorable cell adhesion and growth, more up-regulated osteogenic markers and remarkably uplifted proteins and calcium deposition, and positive signals (α-smooth muscle actin) for neovascularization | [85] |
| BNC/collagen | 3D mesoporous microspheres | MC3T3-E1 cellsBMP-2 | High surface area, suitable biocompatibility, effective promotion of cell adhesion, proliferation, and osteogenic differentiation | [128] |
| BNC/Gel | Nanofibrous | NIH-3T3 fibroblast cells | Decreased crystallinity and improved thermal stability, Enhanced Young’s modulus and decreased tensile strength, and excellent biocompatibility | [75] |
| BNC/MWCNTs | Nanofibrous | Osteoblastic cells (human inferior maxillary bone) | Excellent adhesion and proliferation of osteoblastic cells | [83] |
| BNC/fisetin | Nanofibrous | BMSCs | Suitable cytocompatibility with enhanced cell viability, differentiation of BMSCs to osteoblasts and promoted the expression of osteocalcin and osteopontin genes | [88] |
| BNC/otoliths | Stimulation of the formation of mineralized tissue barrier and reparative pulp reaction | [107] | ||
| BNC/goat bone apatite | 3D porous | L929 fibroblasts | Suitable bioactivity and stimulation of cell proliferation and differentiation | [108] |
| BNC/HAp | Nanofibrous | 3D porous network with homogenous precipitation of carbonated-HAp crystals on BC fibers | [129] | |
| BNC/HAp | Nanofibrous | 3D porous network with homogenous precipitation of carbonated-HAp crystals on BC fibers | [130] | |
| BNC/HAp | Nanofibrous | Oxidized-BNC/HAp is more bioactive and degradable than BNC/HAp and high glucose levels in BNC degradation | [79] | |
| BNC/HAp | Nanofibrous | Surface-treated carbon nanofibres (CNFs) (from BNC) showed enhanced biomineralization and changed morphology from needle-like to rod-like HAp formed on CNFs | [106] | |
| BNC/HAp-CNCs | Nanofibrous | CNCs-assisted dispersibility of HAp exhibited promising results | [115] | |
| BNC/HAp-CNCs | Nanofibrous | L929 fibroblasts | Suitable dispersibility and had less effect of HAp/CNCs on crystallinity, whereas slight increase in thermal stability, and suitable cytocompatibility | [116] |
| BNC/MNPs/HAp | Nanofibrous | MC3T3-E1 cells | Enhanced mechanical and physiochemical properties, superparamagnetic and remarkable thermal stability, and significant cell adhesion and proliferation | [113] |
| BNC/HAp/Sr and BNC/SrAp | Porous membrane | L929 fibroblasts | Oxidized-BNC/SrAp exhibited improved degradation under physiological conditions with suitable cytocompatibility, low inflammatory reaction, and enhanced connective tissue repair, including degradation (in vivo) | [80] |
| BNC/HAp/Sr and BNC/SrAp | Porous membrane | L929 fibroblasts | Oxidized-BNC/SrAp exhibited improved degradation under physiological conditions with suitable cytocompatibility, low inflammatory reaction, and enhanced connective tissue repair, including degradation (in vivo) | [80] |
| BNC-PVP/HAp (in situ using SBF) | Nanofibrous | Improved apatite formation ability of BNC with higher HAp deposition | [131] | |
| BNC-PA-Gel/HAp | Nanofibrous | MSCs | Excellent cellular compatibility and bone-like properties | [122] |
| BNC-PA-Gel/HAp | Fibrous structure | hBMSCs and rBMSCs | Excellent mechanical properties and cytocompatiblity (adhesion, proliferation, and osteogenic differentiation), and high new bone formation | [123] |
| BNC-HAp/BC-GAG | Bilayer | Osteosarcoma cells, hADMSCs, and human articular chondrocytes | Suitable tissue ingrowth and no adverse immunological responses, progressive regeneration of cartilage tissue, ECM deposition, and subchondral bone regeneration, and remarkably higher mineral density and volume ratio of bone to tissue | [118] |
| BNC-Gel/HAp | Nanofibrous | Oxidation of BNC and increased content of Gel induced the formation of tiny HAp crystallites and Gel (0.3 wt%)-incorporated composite system exhibited promising effects | [119] | |
| BNC-Gel/HAp | Nanofibrous | Calvarial osteoblasts | Excellent mechanical properties and improved cell proliferation and differentiation | [76] |
| BNC-Gel/HAp | Nanofibrous | rBMSCs | Rough surface morphology, enhanced mechanical properties, better adhesion, and higher proliferation and differentiation of cells | [120] |
| BNC-boron-doped HAp/Gel | 3D porous | Saos-2 cells | Suitable degradation rate and in vitro bioactivity, excellent cytocompatibility, and intracellular calcium deposition | [124] |
| BNC-CMC/HAp (in situ using SBF) | Nanofibrous | Osteoprogenitor cells (MC3T3-E1) | Calcium-deficient HAp enhanced BNC fibril density and improved cell attachment and growth | [74] |
| BNC/Alg-CS-Gel/HAp | MC3T3-E1 cells, RGD | Suitable 3D structure with well-defined porous network, enhanced compressive properties, and remarkable biocompatibility | [77] | |
| BNC-β-glucan/HAp-GO | 3D porous | MC3T3-E1 cells | Suitable mechanical and antibacterial properties, significant cell adhesion and proliferation | [84] |
| BNC/CPs | Nanofibrous | AFSCs | BNC was used as template and calcinated to prepare 3D calcium phosphate-based scaffold as bioactive filler or bone tissue regeneration with suitable biocompatibility and bioactivity | [93] |
| BNC/CPs | Membrane | CHO-K1 cells | Suitable deposition of calcium phosphate and wettability, and suitable cytocompatibility | [117] |
| BNC/CPs | 3D fibrous | Suitable intrinsic magnetic properties for effective cell adhesion and growth | [95] | |
| BNC/cerium-doped-CPs | Nanofibrous | GM07492 human fibroblasts | Achieved trabecular morphology with interconnected pores and suitable cell viability | [111] |
| BNC/CPs/barium titanate (CaO-BaO-P2O5/TiO2) | 3D porous | hMSCs | Only crystalline phase emerged as TiO2 in 3D structure and exhibited no cytotoxic effect | [61] |
| BNC/CPs/BaTiO3 | 3D fibrous | MSCs | BNC-acted as sacrificial template and scaffold exhibited suitable biocompatibility | [96] |
| BNC/BG | Vero cells | Improved BNC yield with enhanced biocompatibility and antimicrobial properties | [81] | |
| BNC/BG | Nanofibrous | BNC was used as template and calcinated to prepare highly bioactive 3D nanofibrous BG-based scaffold with high bioactivity | [93] | |
| BNC/BG | Nanofibrous | Eeffective absorption of deposited CaO and SiO2 precursors on the surface of BNC, 3D porous interconnected-NBG nanofibrous scaffolds, and higher bioactivity | [94] | |
| BNC/mesoporous BG | Nanofibrous | hBMSCs, rhBMP-2 | A sustained release of rhBMP-2 for 28 days and enhanced cell proliferation and osteogenic-related gene expression | [82] |
| BNC/silicate glass | 3D structure | MSCs | The behavior of BNC with silicate glasses (cements) exhibited enhanced features, especially in terms of setting time (i.e., faster) and biological properties as cell survival and accelerated cell proliferation | [120] |
| BNC-PVA/hexagonal boron nitride | Microporous (printed) | human osteoblast cells | Well-defined porous structure with significantly enhanced cell viability and mechanical properties | [73] |
| BNC-Alg/LAP | Microporous (printed) | L929 fibroblast cells, BSA | Excellent printability, improved stability of printed hydrogel with sustained and long-term protein delivery due to nanoclay | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Han, S.-S. Efficacy of Bacterial Nanocellulose in Hard Tissue Regeneration: A Review. Materials 2021, 14, 4777. https://doi.org/10.3390/ma14174777
Kumar A, Han S-S. Efficacy of Bacterial Nanocellulose in Hard Tissue Regeneration: A Review. Materials. 2021; 14(17):4777. https://doi.org/10.3390/ma14174777
Chicago/Turabian StyleKumar, Anuj, and Sung-Soo Han. 2021. "Efficacy of Bacterial Nanocellulose in Hard Tissue Regeneration: A Review" Materials 14, no. 17: 4777. https://doi.org/10.3390/ma14174777
APA StyleKumar, A., & Han, S.-S. (2021). Efficacy of Bacterial Nanocellulose in Hard Tissue Regeneration: A Review. Materials, 14(17), 4777. https://doi.org/10.3390/ma14174777