1. Introduction
Radiation protective materials (sometime called shielding materials) are used widely in diagnostic radiology, nuclear facilities and hospitals to decrease the exposure of the ionizing radiations to medical physicist, patients and workers on the nuclear facilities. The main goal of the radiation shielding is to protect the persons who are using the X-ray equipment from the harmful effects of the photons that are emitted from these equipment. It is worth determining the type, thickness and composition of the shielding materials that can be used practically in real applications [
1,
2]. Glasses are utilized in the windows of the X-ray rooms and other constructions due to the amazing features of the glasses, such as the transparency of the light, low fabrication cost, the simplicity of the preparation method, and most importantly, the glasses show better radiation protection characteristics in comparison with other materials such as polymers, ceramics and concretes [
3,
4,
5,
6]. Many recent research has shown that the radiation shielding attitudes for glassy medium can be enhanced by changing the chemical composition and adding a certain amount of heavy metal oxides (HMO) and this is a very easy task during the preparation of the glasses [
7,
8,
9]. Moreover, the recent works proved that the glasses with HMO are preferable in practical applications than using thick concrete for many reasons such as the concrete is an opaque and this limited the utilizations of the concrete in some applications [
10,
11]. Among several types of glasses, B
2O
3-based glasses have a wide range of commercial applications thanks to the good physical features like low melting temperature, high transparency and high thermal stability. Moreover, B
2O
3 glasses can be prepared easily with HMO and these HMO are mainly added to the borate glasses for increasing the density of the prepared glass system and thus to enhance the radiation shielding attitudes [
12,
13,
14]. In the lanthanide series, La (Z = 57) is the first element in this series. Lanthanum oxide is an inorganic compound which has a very high dielectric constant and was utilized in fabricating certain types of optical glasses like telescope lenses and cameras. Moreover, it is used in some ferroelectric materials. The beneficial properties of La are of a high atomic number and density (the density of La
2O
3 is around 6.5 g/cm
3). In addition to this, La
2O
3 glasses are colorless, which allows these glasses to be used in typical applications as radiation shielding windows or eyeglasses [
15]. For these reason, we aim in this work to examine the influence of the addition of La
2O
3 in borosilicate glasses on radiation shielding properties.
2. Materials and Methods
The glass systems selected in this investigation have the composition of La
2O
3-CaO-B
2O
3-SiO
2 and were fabricated by Kaewjaeng et al. [
16] Five glass samples were labelled according to the content of La
2O
3 as follows:
BLa10: 10 La2O3-10 CaO-70 B2O3-10 SiO2, ρ = 3.01 g/cm3
BLa15: 15 La2O3-10 CaO-65 B2O3-10 SiO2, ρ = 3.16 g/cm3
BLa20: 20 La2O3-10 CaO-60B2O3-10 SiO2, ρ = 3.40 g/cm3
BLa25: 25 La2O3-10 CaO-55B2O3-10 SiO2, ρ = 3.60 g/cm3
BLa30: 30 La2O3-10 CaO-50B2O3-10 SiO2, ρ = 3.76 g/cm3
These compositions are in mol%. As we can see, the density of the BLaX glasses increases from 3.01 g/cm
3 to 3.76 g/cm
3 due to the increase in the La
2O
3 from 10 to 30 mol%. This is expected increment in the density, since the low molecular weight compound (i.e., B
2O
3) is replaced by the heavier one (i.e., La
2O
3). Therefore, the glass becomes denser with the addition of La
2O
3. It is worth mentioning that the density of the glass plays a very vital role in the shielding ability for these BLaX glasses. In the current investigation, we will examine the photons attenuation factors of the selected BLa5-BLa25 glasses by Phy-X software [
17]. This is a very useful and quick software that can evaluate several radiation shielding factors for any compounds, mixtures, glasses, etc., at different energy values. The calculations of the attenuation factors using the software can be summarized in 3 steps as follows: (a) definition of the materials (by adding the composition and the density); (b) selection the energy; and (c) chosen the factors to be evaluated. It is important to mention that in the first step, the use has two choices for the definition of the materials, namely either in mol% or in w.t %. Moreover, the density must be defined in the unit of g/cm
3. As an example for the first step, the BLa10 glass is defined in the main screen interface of this program (available on
https://phy-x.net/PSD by: 10 La
2O
3 + 10 CaO + 70 B
2O
3 + 10 SiO
2. In the current work, we chose 13 energies (ranging from 0.122 to 1.458 MeV).
We determined the basic radiation shielding parameters for the La
2O
3-CaO-B
2O
3-SiO
2 glasses. One of these parameters is the mass attenuation coefficient (
MAC) [
18,
19,
20]. For a certain medium, the
MAC simply describes how easily it can be penetrated by a beam of photons. For a medium composed of different elements (such as our samples in this work), the mixture rule is useful in determining the
MAC and can be written as:
where the symbol w denotes the weight fraction, and the (
MAC)
i is the mass attenuation coefficient of the
ith constituent element.
If we take the density of the medium into the account, then we need to determine the linear attenuation coefficient (
LAC), which is given by:
The last parameter is helpful in evaluating the half value layer (
HVL) and the mean free path (
MFP). The
HVL gives information about the thickness of the medium which can attenuate half of the incoming photons, while, the
MFP gives information about the average distance traveled by the photon between two collisions. Both parameters are related to the
LAC by Equations (3) and (4) [
21]:
The effective atomic number (
Zeff) can be calculated using the following formula [
22]:
where
fi is the fraction by mole of the each constituent element and
Ai is the atomic weight.
3. Results
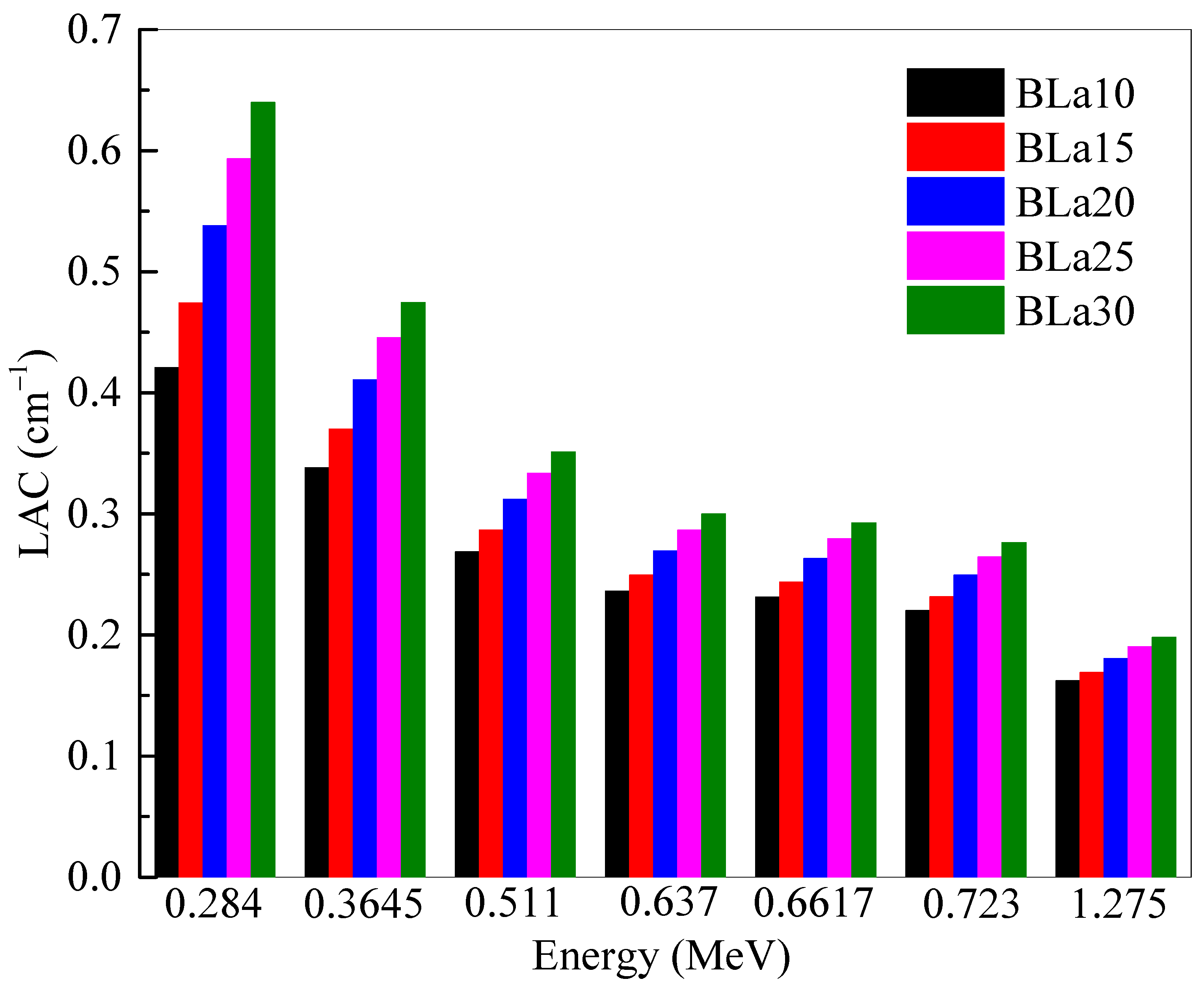
The
LAC of the BLa-X glasses was determined and plotted at selected energies in
Figure 1. The
LAC of a sample describes the attenuation ability of the material, with a greater value meaning more attenuation. The energies in this investigation were selected as: 0.284, 0.3645, 0.511, 0.637, 0.6617, 0.723 and 1.275 MeV. Two general trends can be observed in the figure. First, the
LAC values for all five BLa-X glasses decrease with increasing energy [
18]. This decrease in value is due to the increased penetration power of higher energy photons, reducing the attenuation of the samples, and decreasing
LAC. The second trend of the figure can be seen by analyzing the values at a single energy. As can be observed, BLa10 has the least
LAC at all the tested energies, while BLa30 has the greatest. This indicates that the shielding ability for the BLa-X glasses is enhanced when the La
2O
3 changes from 10 to 30 mol%. This is because the LA has much higher atomic number than that of B. Therefore, increasing the amount of La
2O
3 at the expense of B
2O
3 causes an enhancement in the density value, and this explained the increase in the
LAC with more La
2O
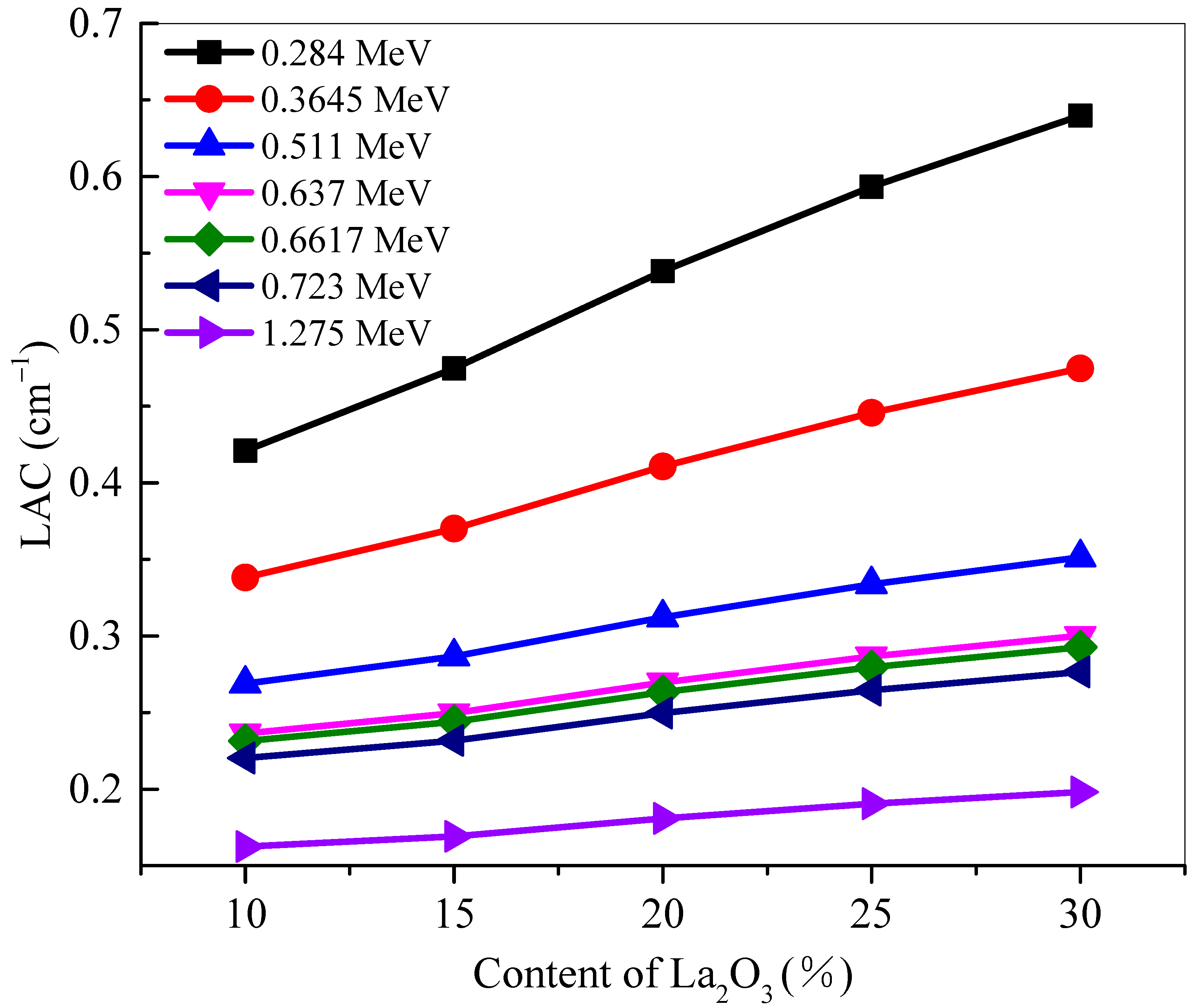
3 added to the glasses. This observation can be confirmed by looking at the positive correlation between La
2O
3 mol% and the
LAC of the glasses (see
Figure 2).
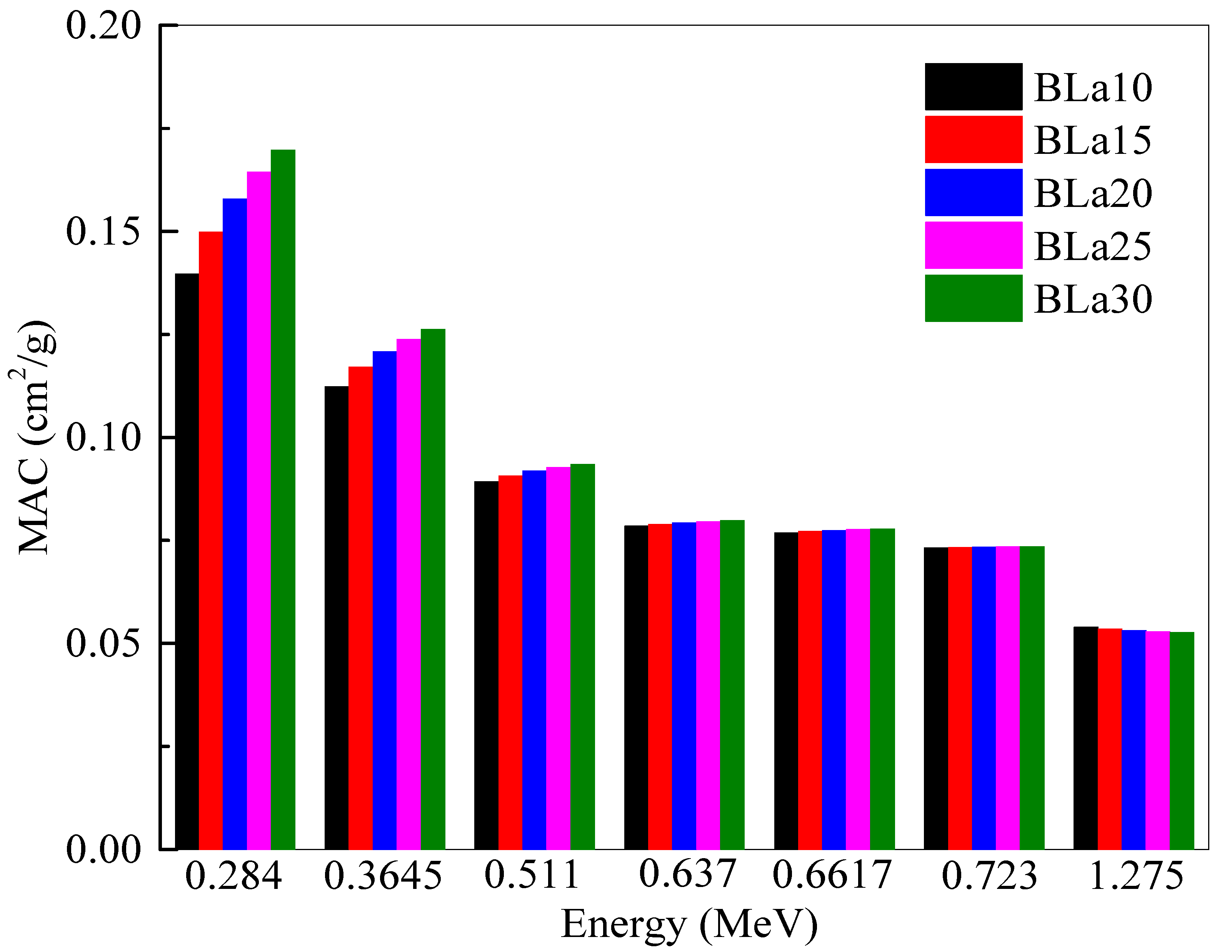
Figure 3 shows the
MAC of the BLa-X glasses against energy. Like
LAC, a greater
MAC represents a more radiation shielding efficient shield [
19]. Unlike
LAC, however,
MAC factors out density to compare the glasses. The same energies were chosen as the previous figures. Even though the two parameters are alike,
Figure 3 demonstrates an interesting trend between
MAC and energy.
MAC also decreases with increasing energy for the same reason as previously stated. The
MAC of BLa15 decreases from 0.150 to 0.054 cm
2/g at energies of 0.284 and 1.275 MeV, respectively, while the
MAC of BLa25 decreases from 0.164 to 0.053 cm
2/g for the previously mentioned energies, respectively. Clearly, at higher energies, the difference between the
MAC is almost negligible, and this is explained according to the Compton scattering as stated in [
23]. At 1.275 MeV, BLa10 has an
MAC equal to 0.054 cm
2/g, while BLa30 has a
MAC equal to 0.053 cm
2/g. These results contrast with the trend observed in
Figure 1, where BLa10 had the least
MAC at all energies. The trend in this figure demonstrates that at low energies, BLa30 remains the most desirable shield, but at greater energies, there is almost no difference between the attenuation abilities of the glasses, with BLa10 having a slightly greater advantage due to the slightly higher
MAC of BLa10 than the other glasses. The reason for the very slight difference in the
MAC for the selected glasses between 0.637 and 1.275 MeV is related directly to the Compton scattering, as it is known that this process has a small dependence on the atomic number of the medium.
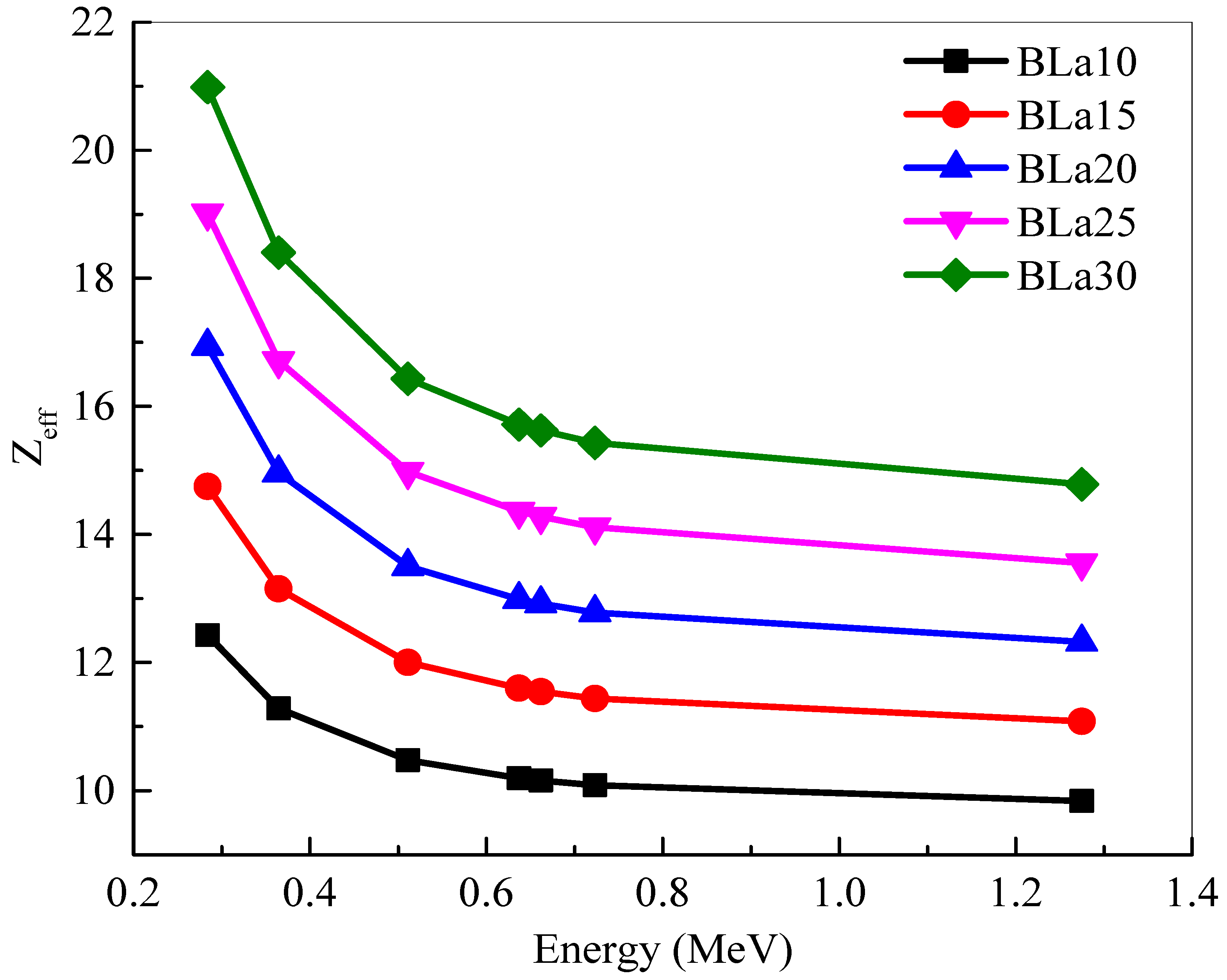
The
Zeff of the BLa-X glasses are plotted in
Figure 4. The
Zeff values follow the trend BLa10 < BLa15 < BLa20 < BLa25 < BLa30. In this figure, BLa30 clearly has the greater
Zeff at all energies. This trend is due to BLa30 having the greatest La
2O
3 content, which has a greater atomic number than B
2O
3, which it is being substituted by. This conclusion also explains why BLa10 has the least
Zeff at all energies. Nevertheless, all the
Zeff values decrease with the increasing energy. At 0.284 and 0.365 MeV, the
Zeff is sharply decreased. This sharp trend is thanks to the dominance of the photoelectric effect at these two energies. As energy further increases, the Compton scattering becomes the most important process. At this medium energy range, the
Zeff can be observed to only slightly decrease. Despite this decrease, BLa30 maintains the highest
Zeff at all chosen energies.
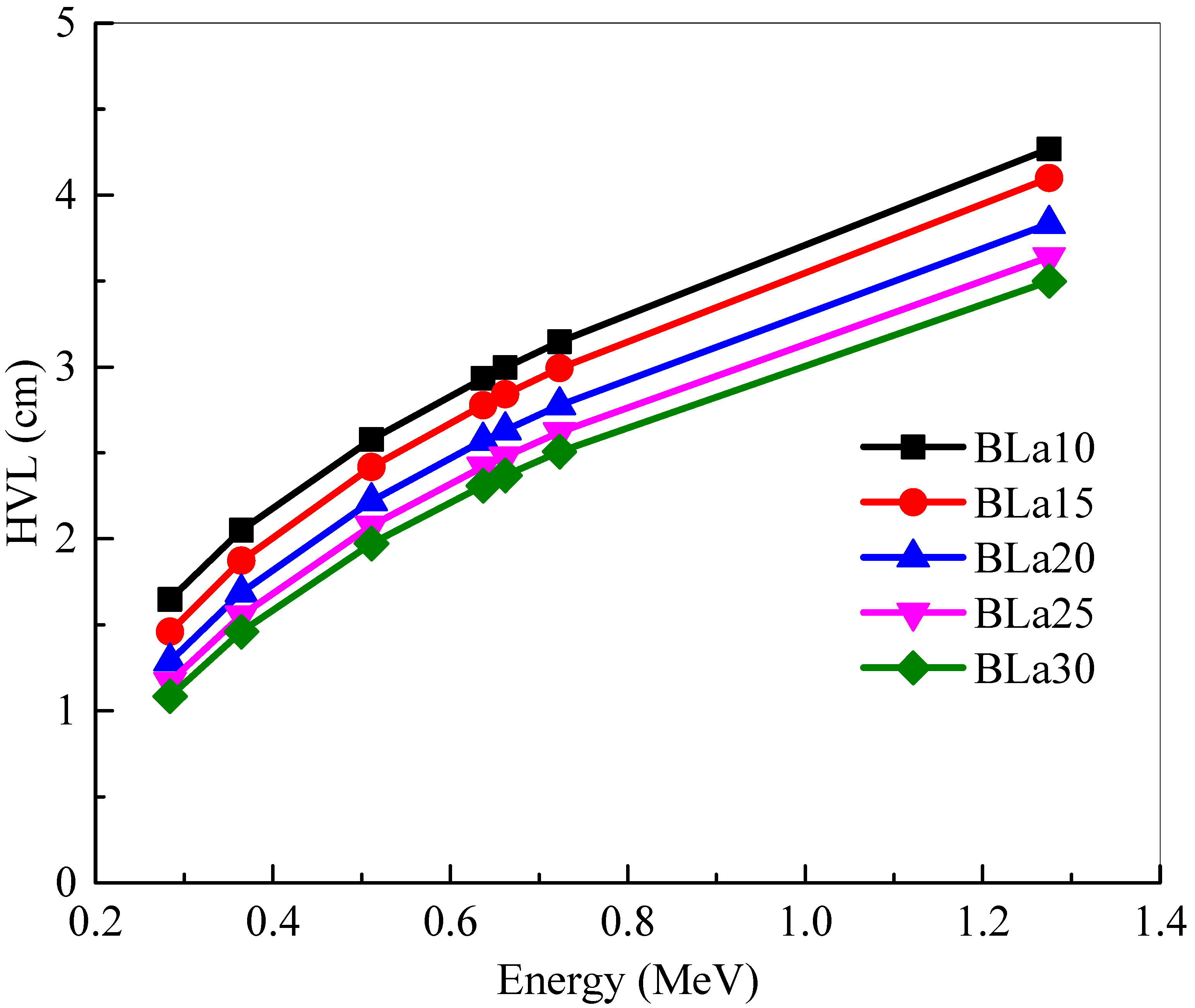
The
HVL of the BLa-X glass shields were investigated at selected energies and in
Figure 5. One must remember that a lower
HVL signifies a more space-efficient shield. The minimum
HVL values are found at 0.284 MeV, which is as expected.
Figure 5 demonstrated that BLa30 has the least
HVL and BLa10 has the greatest, which means that BLa30 is the most space-efficient shield. As energy increases, the
HVLs of the glasses increase as well. The
HVL of BLa10 increases from 1.646 (for E = 0.284 MeV) to 4.268 cm (for E = 1.275 MeV), but it is increased for BLa30 from 1.083 to 3.499 cm for the same respective energies. Since energy and
HVL are directly correlated, the maximum
HVL can be found at the highest selected energy, 1.275 MeV. This positive trend occurs since, as the photon energy increases, the dimension of the medium required to shield 50% of the radiation increases, increasing the
HVL. Even with the increase in values, however, BLa30 has the least
HVL.
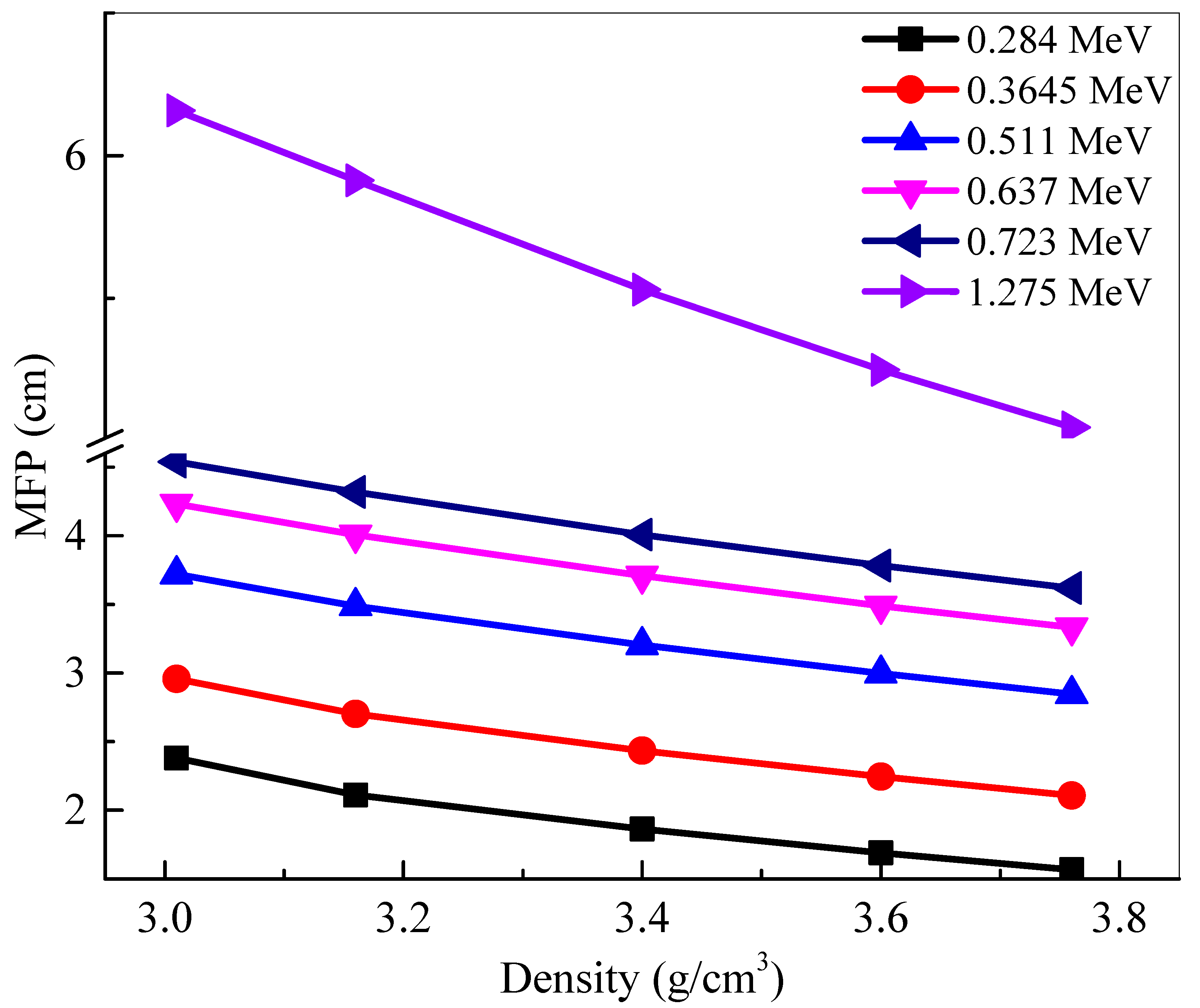
Mean free path represents the distance between subsequent collisions between the incoming photons and the atoms within the sample. A lower
MFP signifies that the distance between collisions is short, increasing the number of total collisions, and increasing the amount of attenuation. Therefore, a smaller
MFP is more desirable.
Figure 6 graphs the
MFP of the samples against the density of the five glasses at selected energies. At any single energy, an inverse correlation can be observed between density and
MFP. This decrease in value demonstrates that increasing the density of the glasses, by increasing the amount of La
2O
3 content, lowers
MFP, and increases attenuation, which means that BLa30, the glass with the greatest density, absorbs the most amount of radiation. This conclusion can also be drawn by analyzing any of the other densities. By the same logic, the glass with the least density—BLa10—has the greatest
MFP at all energies. The
MFP values can also be seen to increase with the increasing energy. An especially large change in values occurs between 0.72 MeV and 1.28 MeV, which shows how the glasses decrease in efficiency as the photon energy increases. This is because the penetrating ability of the high energy photons is very high and thus we needed a relatively thick glass to attenuate these high energy photons. With this in mind, to maximize attenuation, the BLa-X glass with the greatest density should be used at low energies.
To put the shielding ability of the BLa-X glasses into perspective, the
HVL of the tested samples were compared against three commercially available glasses, RS-360, RS-520, and RS-253-G18, in
Figure 7. It should first be stated that the
HVL of all the glasses increases with increasing energy, even the commercially available glasses. The same trend can be observed at all three energies, following the order of RS-253-G18 > BLa10 > BLa15 > BLa20 > BLa25 > BLa20 > RS-360 > RS-520. The high
MFP for the RS-253-G18 is due to the low density of this glass compared to our selected glasses, where the density of RS-253-G18 is 2.53 g/cm
3, while the density of our glasses varied between 3.01 and 3.76 g/cm
3. This figure shows that all the BLa-X glasses are more effective than RS-253-G18, but they are all outperformed by RS-360 and RS-520 at low, medium, and high energies. It can be observed, however, that at 1.275 MeV BLa30 has comparable attenuation to RS-360, although RS-520 has a much smaller
MFP than all the other tested samples.
4. Conclusions
The linear attenuation coefficient (LAC) of the BLa-X glasses was reported to be between 0.284 and 1.275 MeV. The LAC values for all five BLa-X glasses decrease with the increasing energy, indicating the reduction in the attenuation of the samples. At 0.284 MeV, the LAC values varied between 0.421 cm−1 for BLa10 and 0.640 cm−1 for BLa30. Whereas, at 1.275 MeV, the LAC values varied between 0.162 and 0.198 cm−1 for BLa10 and BLa30, respectively. BLa10 has the least LAC as well as MAC at all the tested energies, while BLa30 has the greatest. At 0.511 MeV, the Zeff values varied between 10.48 and 16.43. The MAC of BLa15 decreases from 0.150 cm2/g to 0.054 cm2/g at energies of 0.284 MeV and 1.275 MeV, respectively, while the MAC of BLa25 decreases from 0.164 cm2/g to 0.053 cm2/g. The minimum HVL values are found at 0.284 MeV, and they are equal to 1.646, 1.460, 1.288, 1.168, and 1.083 for BLa10, BLa15, BLa20, BLa25, and BLa30, respectively. BLa30 has the least TVL and HVL, while BLa10 has the greatest, which emphasized the positive correlation between La2O3 content and shielding ability, as well as the conclusion that BLa30 has the most potential for radiation shielding applications. Moreover, an inverse correlation was observed between density and MFP and from the MFP results, we found that BLa30, the glass with the greatest density, absorbs the most amount of radiation. All the BLa-X glasses are more effective than RS-253-G18, but they are all outperformed by RS-360 and RS-520 at low, medium, and high energies. Finally, at 1.275 MeV, BLa30 has comparable attenuation to RS-360.