Characterization and Sodium Cations Sorption Capacity of Chemically Modified Biochars Produced from Agricultural and Forestry Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Preparation and Activation
2.2. Biochar Analysis
2.3. Sodium Adsorption on Biochar Experiment
2.4. Calculations and Statistical Analysis
- qe—the equilibrium (instantaneous) amount of adsorbed Na ions on units of biochar mass, mg/g,
- qm—the maximum amount of adsorbed Na ions on units of biochar mass, mg/g, to form a complete monolayer on the surface,
- Ce—equilibrium concentration of Na ions, mg/L,
- B—Langmuir adsorption constant related to the energy of adsorption, L/mg.
- qe—the equilibrium (instantaneous) amount of adsorbed Na ions on units of biochar mass, mg/g,
- Kf—Freundlich adsorption constant, mg∙g–1∙(L∙mg–1)^(1/n),
- 1/n—empirical constant: heterogenicity coefficient.
- AIC—value of Akaike analysis,
- n—the number of measurements,
- e—the value of the rest of the model for a particular measurement point,
- K—the number of regression coefficients, including an intercept in the model.
3. Results and Discussion
3.1. Biochar Sorption Properties
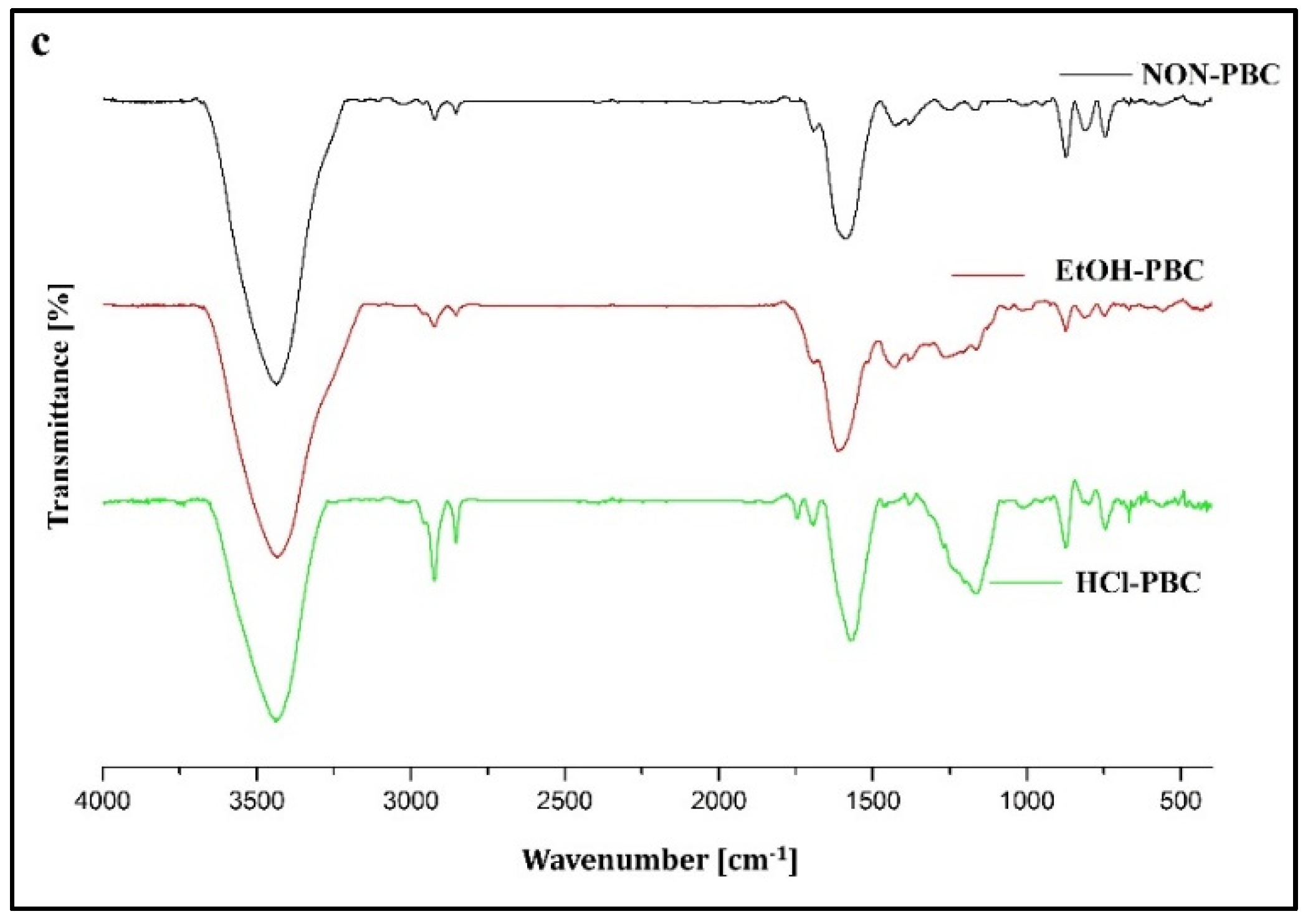
3.2. Surface and Structural Changes of Biochar after Modifications
3.3. Sodium Sorption Experiment
3.3.1. The Mechanism of the Adsorption
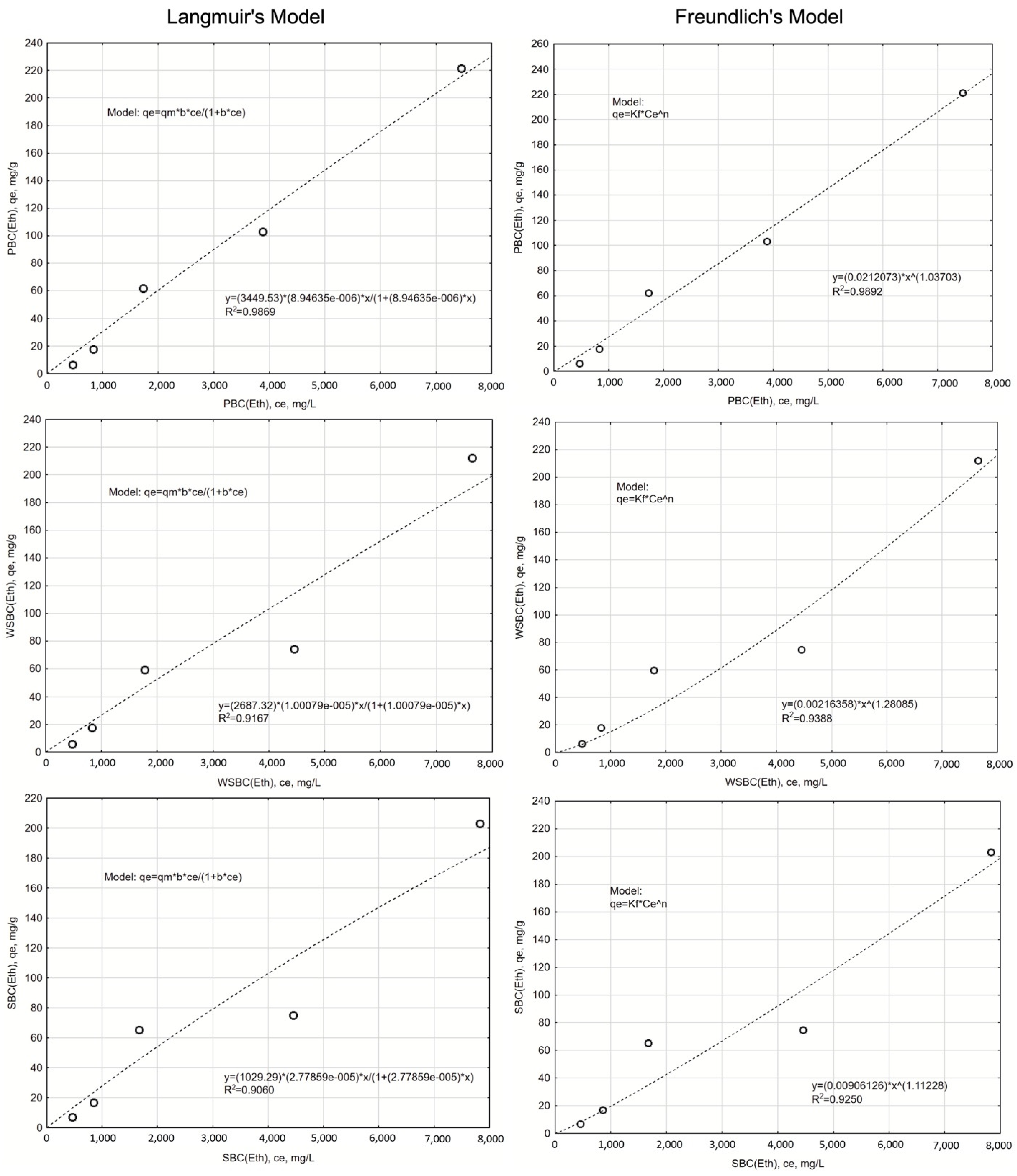
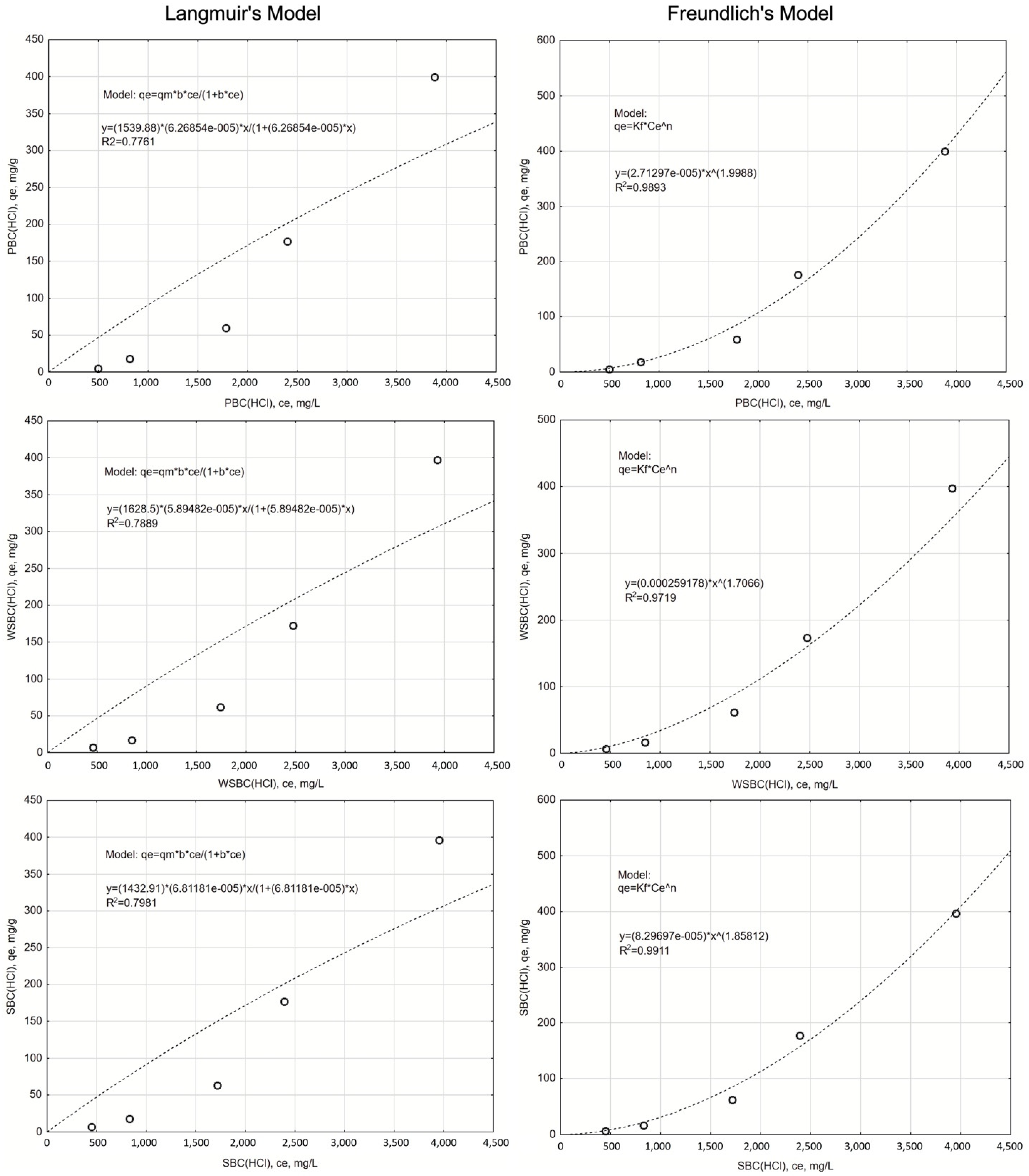
3.3.2. The Isotherms of the Na Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Biochar | NaCl (%) | |||||
|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.5 | 1.0 | 2.0 | ||
| Non-activated | Non-WSBC | Non-WSBC + 0.1 | Non-WSBC + 0.2 | Non-WSBC + 0.5 | Non-WSBC + 1.0 | Non-WSBC + 2.0 |
| Non-SBC | Non-SBC + 0.1 | Non-SBC + 0.2 | Non-SBC + 0.5 | Non-SBC + 1.0 | Non-SBC + 2.0 | |
| Non-PBC | Non-PBC + 0.1 | Non-PBC + 0.2 | Non-PBC + 0.5 | Non-PBC + 1.0 | Non-PBC + 2.0 | |
| Activated with 0.1 M HCl | HCl-WSBC | HCl-WSBC + 0.1 | HCl-WSBC + 0.2 | HCl-WSBC + 0.5 | HCl-WSBC + 1.0 | HCl-WSBC + 2.0 |
| HCl-SBC | HCl-SBC + 0.1 | HCl-SBC + 0.2 | HCl-SBC + 0.5 | HCl-SBC + 1.0 | HCl-SBC + 2.0 | |
| HCl-PBC | HCl-PBC + 0.1 | HCl-PBC + 0.2 | HCl-PBC + 0.5 | HCl-PBC + 1.0 | HCl-PBC + 2.0 | |
| Activated with 0.1 M C2H5OH | EtOH-WSBC | EtOH-WSBC + 0.1 | EtOH-WSBC + 0.2 | EtOH-WSBC + 0.5 | EtOH-WSBC + 1.0 | EtOH-WSBC + 2.0 |
| EtOH-SBC | EtOH-SBC + 0.1 | EtOH-SBC + 0.2 | EtOH-SBC + 0.5 | EtOH-SBC + 1.0 | EtOH-SBC + 2.0 | |
| EtOH-PBC | EtOH-PBC + 0.1 | EtOH-PBC + 0.2 | EtOH-PBC + 0.5 | EtOH-PBC + 1.0 | EtOH-PBC + 2.0 | |
| Solution | Na | Ca | Mg | K |
|---|---|---|---|---|
| mg/L | ||||
| 0.1% NaCl | 594.3 | 0.07 | 0.02 | 0.09 |
| 0.2% NaCl | 1188.6 | 0.12 | 0.03 | 0.18 |
| 0.5% NaCl | 2971.5 | 0.38 | 0.04 | 0.45 |
| 1.0% NaCl | 5943.2 | 0.8 | 0.05 | 0.9 |
| 2.0% NaCl | 11,886.5 | 1.7 | 0.06 | 1.8 |
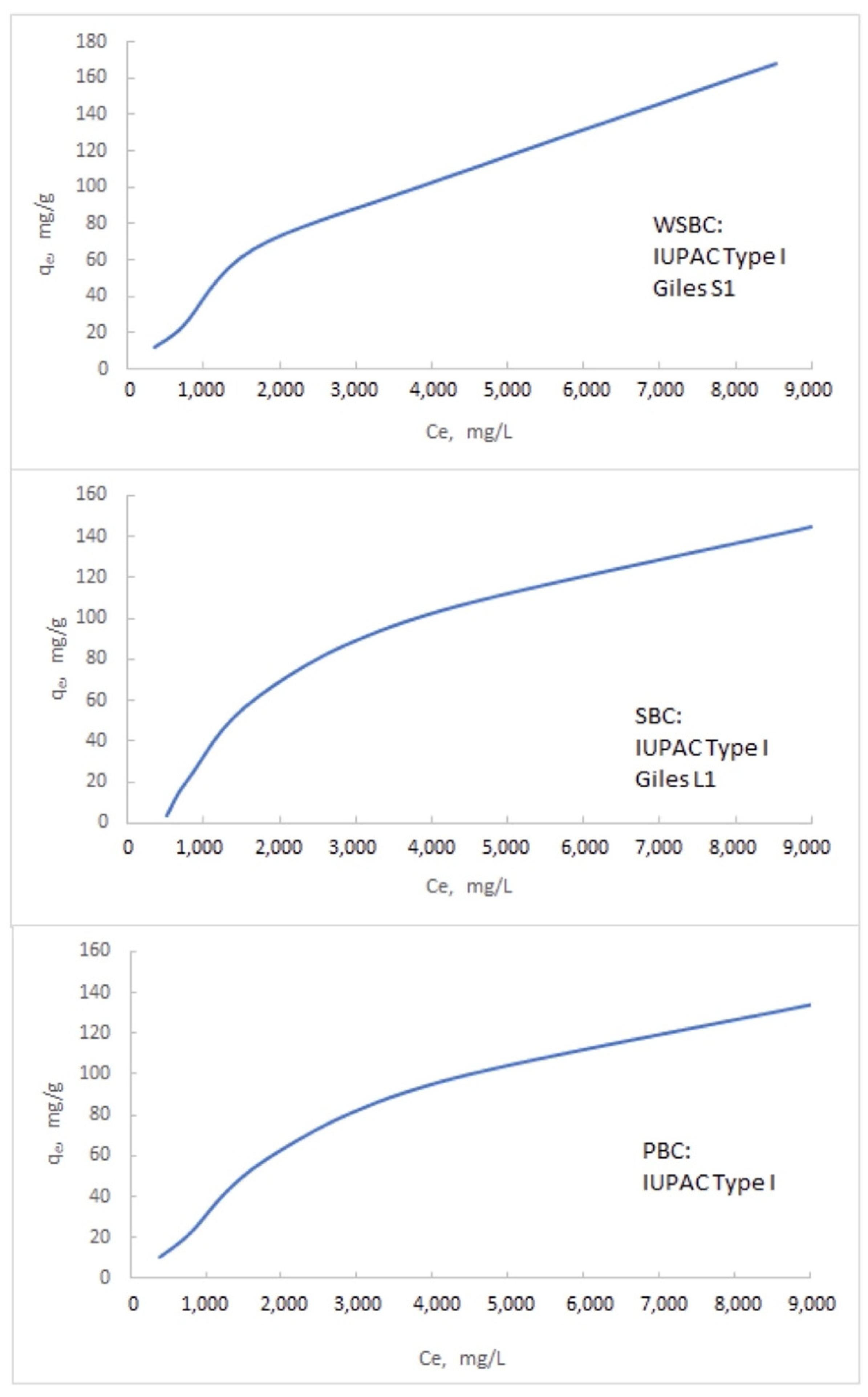
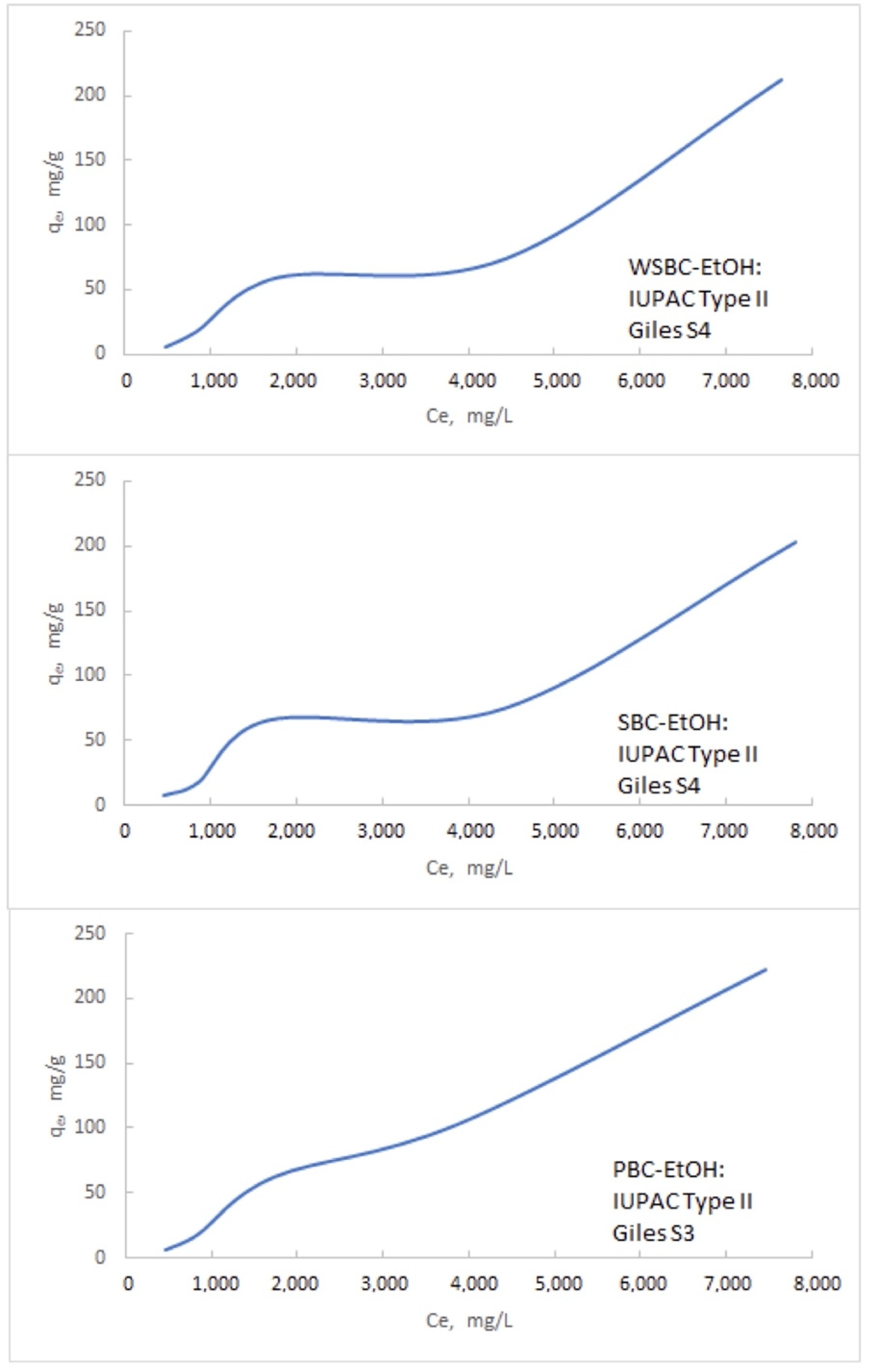
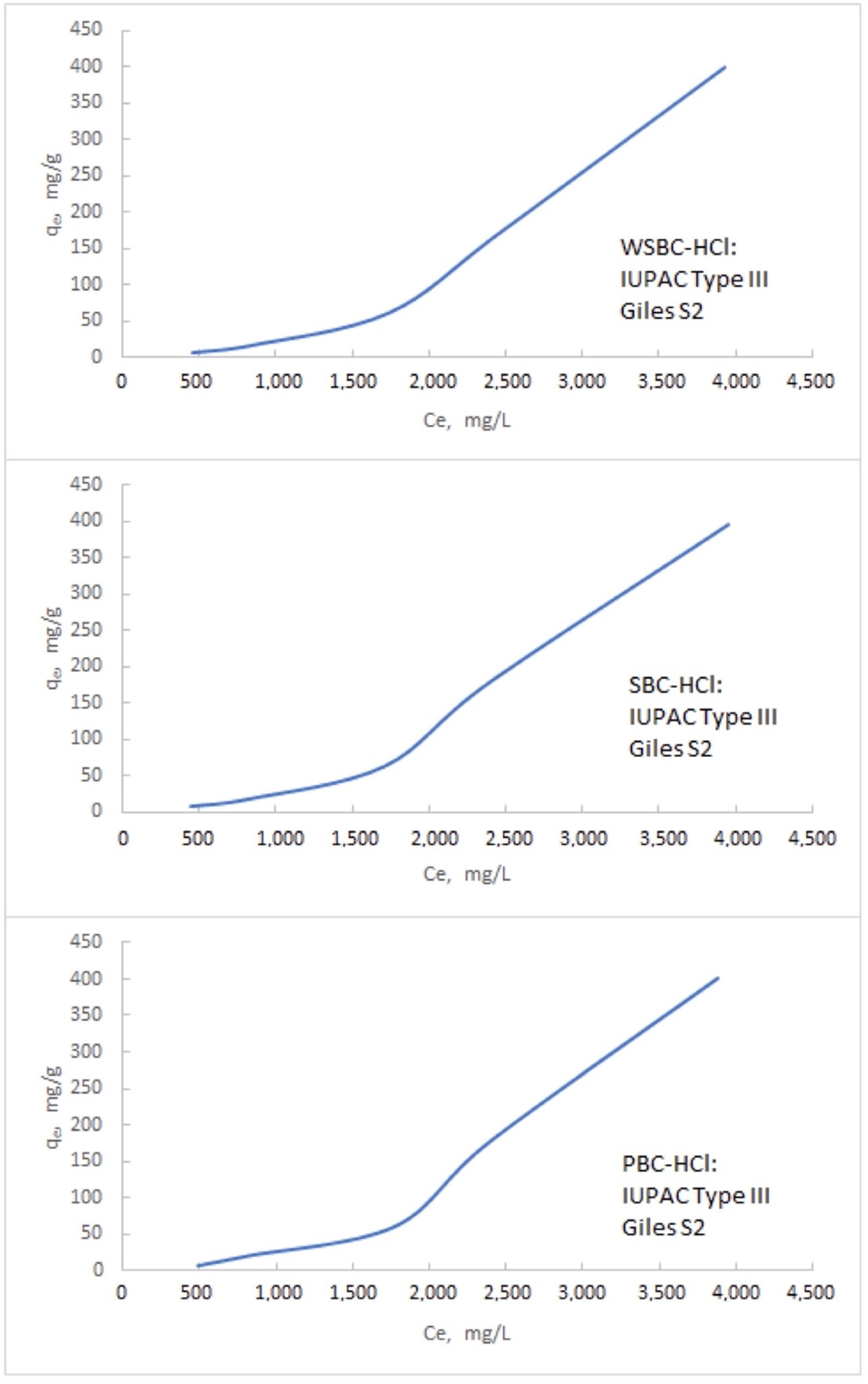
Appendix B
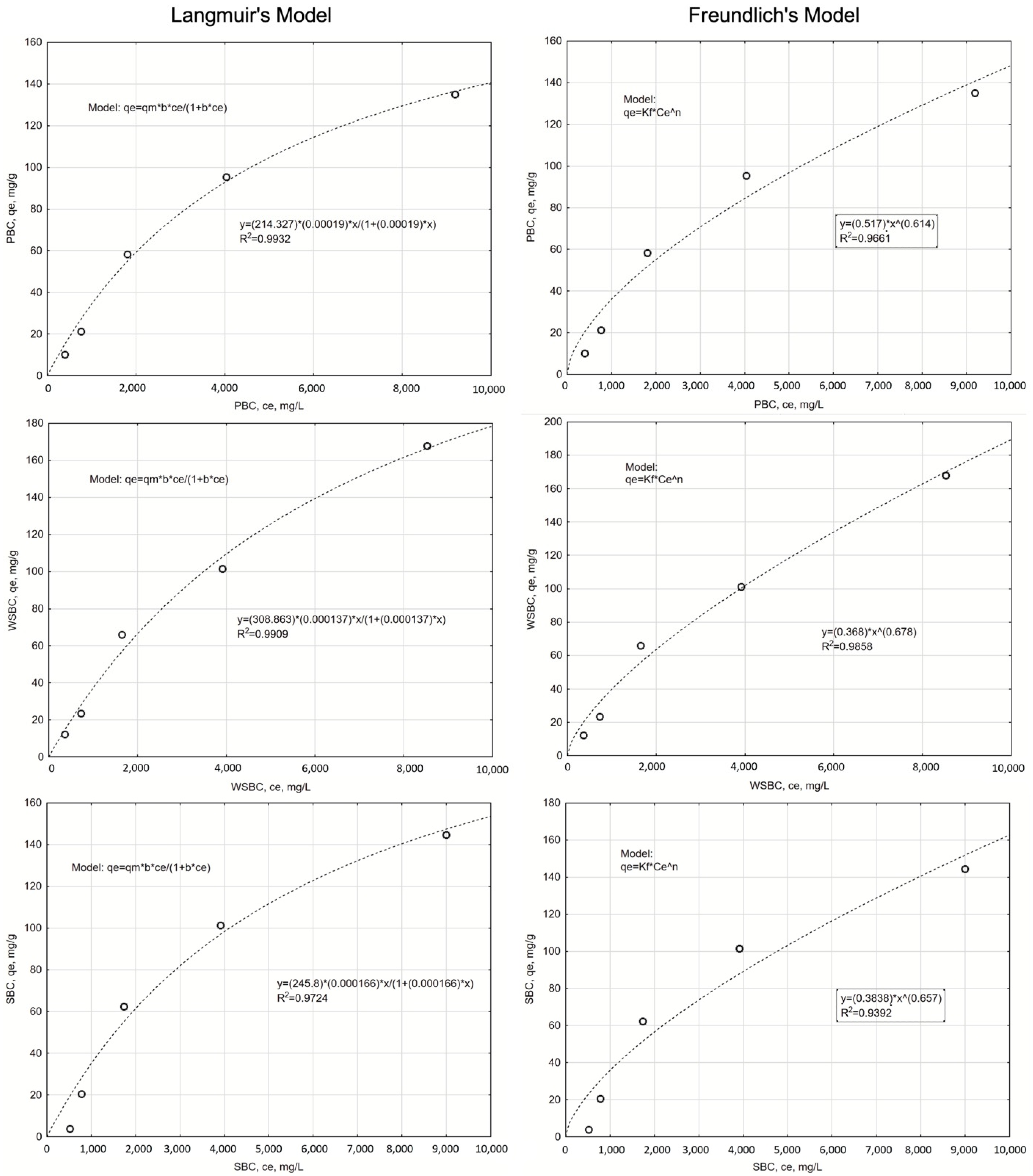
References
- Singh, R.; Mavi, M.S.; Choudhary, O.P. Saline Soils Can Be Ameliorated by Adding Biochar Generated from Rice-Residue Waste. CLEAN Soil Air Water 2018, 47, 1700656. [Google Scholar] [CrossRef]
- Amini, S.; Ghadiri, H.; Chen, C.; Marschner, P. Salt-affected soils, reclamation, carbon dynamics, and biochar: A review. J. Soils Sediments 2015, 16, 939–953. [Google Scholar] [CrossRef]
- Trenouth, W.R.; Gharabaghi, B.; Perera, N. Road salt application planning tool for winter de-icing operations. J. Hydrol. 2015, 524, 401–410. [Google Scholar] [CrossRef]
- Gunarathne, V.; Senadeera, A.; Gunarathne, U.; Biswas, J.K.; Almaroai, Y.A.; Vithanage, M. Potential of biochar and organic amendments for reclamation of coastal acidic-salt affected soil. Biochar 2020, 2, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Tana, H.; Onga, P.Y.; Klemešb, J.J.; Bonga, C.P.C.; Lic, C.; Gaoc, Y.; Leea, C.T. Mitigation of Soil Salinity Using Biochar Derived from Lignocellulosic Biomass. Chem. Eng. Trans. 2021, 83, 235–240. [Google Scholar] [CrossRef]
- Sadegh-Zadeh, F.; Parichehreh, M.; Jalili, B.; Bahmanyar, M.A. Rehabilitation of calcareous saline-sodic soil by means of biochars and acidified biochars. Land Degrad. Dev. 2018, 29, 3262–3271. [Google Scholar] [CrossRef]
- Qadir, M.; Schubert, S.; Ghafoor, A.; Murtaza, G. Amelioration strategies for sodic soils: A review. Land Degrad. Dev. 2001, 12, 357–386. [Google Scholar] [CrossRef]
- Rostamian, R.; Heidarpour, M.; Mousavi, S.F.; Afyuni, M. Characterization and Sodium Sorption Capacity of Biochar and Activated Carbon Prepared from Rice Husk. J. Agric. Sci. Technol. 2015, 17, 1057–1069. [Google Scholar]
- Lee, J.E.; Park, Y.-K. Applications of Modified Biochar-Based Materials for the Removal of Environment Pollutants: A Mini Review. Sustainability 2020, 12, 6112. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Ćwieląg-Piasecka, I.; Jerzykiewicz, M.; Trynda, J. Wheat Straw Biochar as a Specific Sorbent of Cobalt in Soil. Materials 2020, 13, 2462. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Awan, S.; Ippolito, J.A.; Ullman, J.L.; Ansari, K.; Cui, L.; Siyal, A.A. Biochars reduce irrigation water sodium adsorption ratio. Biochar 2021, 3, 77–87. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Li, J.-H.; Lv, G.-H.; Bai, W.-B.; Liu, Q.; Zhang, Y.-C.; Song, J.-Q. Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water. DESALINATION Water Treat. 2014, 1–13. [Google Scholar] [CrossRef]
- Du, J.; Kim, S.H.; Hassan, M.A.; Irshad, S.; Bao, J. Application of biochar in advanced oxidation processes: Supportive, adsorptive, and catalytic role. Environ. Sci. Pollut. Res. 2020, 27, 1–27. [Google Scholar] [CrossRef]
- Sarpong, K.A.; Amiri, A.; Ellis, S.; Idowu, O.J.; Brewer, C.E. Short-term leachability of salts from Atriplex-derived biochars. Sci. Total Environ. 2019, 688, 701–707. [Google Scholar] [CrossRef]
- Wu, H.; Yip, K.; Kong, Z.; Li, C.-Z.; Liu, D.; Yu, Y.; Gao, X. Removal and Recycling of Inherent Inorganic Nutrient Species in Mallee Biomass and Derived Biochars by Water Leaching. Ind. Eng. Chem. Res. 2011, 50, 12143–12151. [Google Scholar] [CrossRef]
- Dietrich, C.C.; Rahaman, A.; Robles-Aguilar, A.A.; Latif, S.; Intani, K.; Müller, J.; Jablonowski, N.D. Nutrient Loaded Biochar Doubled Biomass Production in Juvenile Maize Plants (Zea mays L.). Agronomy 2020, 10, 567. [Google Scholar] [CrossRef] [Green Version]
- Dou, G.; Jiang, Z. Preparation of Sodium Humate-Modified Biochar Absorbents for Water Treatment. ACS Omega 2019, 4, 16536–16542. [Google Scholar] [CrossRef] [Green Version]
- Munera-Echeverri, J.; Martinsen, V.; Strand, L.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Standard Reference Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues ASTM D7348—13. Available online: https://www.astm.org/Standards/D7348.htm (accessed on 16 August 2021).
- Ćwieląg-Piasecka, I.; Medyńska-Juraszek, A.; Jerzykiewicz, M.; Debicka, M.; Bekier, J.; Jamroz, E.; Kawałko, D. Humic acid and biochar as specific sorbents of pesticides. J. Soils Sediments 2018, 18, 2692–2702. [Google Scholar] [CrossRef] [Green Version]
- Fathy, M.; Mousa, M.A.; Moghny, T.A.; Awadallah, A.E. Characterization and evaluation of amorphous carbon thin film (ACTF) for sodium ion adsorption. Appl. Water Sci. 2017, 7, 4427–4435. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Alam, S.; Konhauser, K.O.; Alessi, D.; Xu, S.; Tian, W.; Liu, Y. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater. J. Clean. Prod. 2018, 209, 927–936. [Google Scholar] [CrossRef]
- Heymann, K.; Lehmann, J.; Solomon, D.; Schmidt, M.W.; Regier, T. C 1s K-edge near edge X-ray absorption fine structure (NEXAFS) spectroscopy for characterizing functional group chemistry of black carbon. Org. Geochem. 2011, 42, 1055–1064. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Tomczak, E.; Sulikowski, R. Opis równowagi i kinetyki sorpcji jonów metali ciężkich na klinoptylocie. Inżynieria Apar. Chem. 2010, 49, 113–114. [Google Scholar]
- El-Banna, M.F.; Mosa, A.; Gao, B.; Yin, X.; Ahmad, Z.; Wang, H. Sorption of lead ions onto oxidized bagasse-biochar mitigates Pb-induced oxidative stress on hydroponically grown chicory: Experimental observations and mechanisms. Chemosphere 2018, 208, 887–898. [Google Scholar] [CrossRef] [PubMed]

| Biochar | pH | Ash | CEC | Na+ | K+ | Ca2+ | Mg2+ |
|---|---|---|---|---|---|---|---|
| - | % DM | cmol(+)/kg | |||||
| Non-WSBC | 9.98 1 ± 0.03 2 | 36.4 ± 0.6 | 30.72 | 27.01 ± 0.2 | 1.42 ± 0.02 | 1.43 ± 0.04 | 0.19 ± 0.02 |
| EtOH-WSBC | 9.43 1 ± 0.03 2 | 19.5 | 19.67 | 16.02 ± 0.02 | 1.11 ± 0.02 | 1.98 ± 0.02 | 0.56 ± 0.02 |
| HCl-WSBC | 8.89 ± 0.02 | 33.8 | 27.71 | 23.24 ± 0.02 | 1.15 ± 0.02 | 2.34 ± 0.02 | 0.98 ± 0.02 |
| Non-SBC | 8.83 ± 0.02 | 20.3 ± 0.3 | 3.08 | 1.13 ± 0.1 | 1.01 ± 0.01 | 0.93 ± 0.02 | 0.11 ± 0.03 |
| EtOH-SBC | 8.67 ± 0.02 | 19.7 | 3.69 | 0.98 ± 0.02 | 1.03 ± 0.02 | 1.34 ± 0.02 | 0.34 ± 0.02 |
| HCl-SBC | 8.02 ± 0.02 | 21.0 | 4.96 | 1.11 ± 0.02 | 0.98 ± 0.02 | 1.98 ± 0.02 | 0.89 ± 0.02 |
| Non-PBC | 6.81 ± 0.02 | 10.5 ± 0.9 | 6.64 | 1.99 ± 0.7 | 1.45 ± 0.03 | 2.93 ± 0.01 | 0.26 ± 0.02 |
| EtOH-PBC | 6.63 ± 0.02 | 7.6 | 7.31 | 1.48 ± 0.02 | 1.94 ± 0.02 | 3.65 ± 0.02 | 0.24 ± 0.02 |
| HCl-PBC | 6.43 ± 0.02 | 4.08 | 8.13 | 1.78 ± 0.02 | 1.34 ± 0.02 | 3.89 ± 0.02 | 1.12 ± 0.02 |
| Biochar | SSA (g/m2) |
|---|---|
| Non-WSBC | 265 1 ± 2.1 2a |
| HCl-WSBC | 278 ± 3.1 b |
| EtOH-WSBC | 387 ± 4.5 b |
| Non-SBC | 80.5 ± 2.1 a |
| HCl-SBC | 85.4 ± 1.4 a |
| EtOH-SBC | 93.2 ± 1.9 b |
| Non-PBC | 16.5 ± 1.8 a |
| HCl-PBC | 14.5 ± 1.3 a |
| EtOH-PBC | 17.8 ± 0.9 a |
| Biochar | C | N | H | O | S | C/N | H/C | O/C |
|---|---|---|---|---|---|---|---|---|
| (% w/dw) | (molar) | |||||||
| Non-WSBC | 63.61 1 ± 0.2 2a | 0.74 ± 0.1 a | 2.2 ± 0.01 a | 33.42 ± 0.9 a | 0.001 ± 0.0 a | 85.98 | 0.03 | 0.52 |
| HCl-WSBC | 66.12 ± 0.1 a | 0.64 ± 0.05 b | 1.98 ± 0.02 a | 30.92 ± 1.1 a | 0.0355 ± 0.01 b | 104.09 | 0.02 | 0.46 |
| EtOH-WSBC | 89.42 ± 3.2 b | 0.63 ± 0.04 b | 1.81 ± 0.01 b | 8.11 ± 0.4 b | 0.0425 ± 0.01 b | 143.07 | 0.02 | 0.09 |
| Non-SBC | 79.74 ± 2.1 a | 0.95 ± 0.1 a | 2.57 ± 0.03 a | 16.73 ± 0.3 a | 0.001 ± 0.03 a | 83.49 | 0.03 | 0.20 |
| HCl-SBC | 78.95 ± 1.2 a | 0.92 ± 0.11 a | 1.80 ± 0.03 b | 18.28 ± 1.1 a | 0.047 ± 0.04 b | 85.81 | 0.02 | 0.23 |
| EtOH-SBC | 80.33 ± 1.3 a | 0.87 ± 0.12 b | 2.82 ± 0.04 b | 15.92 ± 0.9 a | 0.048 ± 0.01 b | 91.80 | 0.03 | 0.19 |
| Non-PBC | 89.55 ± 2.7 a | 0.42 ± 0.03 a | 2.44 ± 0.01 a | 7.58 ± 0.3 a | 0.003 ± 0.01 a | 213.21 | 0.03 | 0.08 |
| HCl-PBC | 75.92 ± 2.1 b | 0.53 ± 0.04 b | 3.10 ± 0.05 b | 20.43 ± 0.7 b | 0.013 ± 0.02 b | 143.24 | 0.04 | 0.26 |
| EtOH-PBC | 72.36 ± 3.1 b | 0.69 ± 0.06 b | 3.21 ± 0.03 b | 23.69 ± 0.9 b | 0.034 ± 0.03 b | 104.11 | 0.04 | 0.32 |
| Biochar | 3188–3720 | 2800–2989 | 1480–1660 |
|---|---|---|---|
| –OH | C–H Aliphatic | C–C Aromatic | |
| % of Area | |||
| Non-WSBC | 77.72 | 0.71 | 13.90 |
| HCl-WSBC | 72.35 | 1.47 | 15.04 |
| EtOH-WSBC | 71.62 | 0.96 | 31.50 |
| Non-SBC | 66.91 | 2.21 | 13.02 |
| HCl-SBC | 82.00 | 0.56 | 17.70 |
| EtOH-SBC | 85.55 | 0.87 | 13.65 |
| Non-PBC | 73.83 | 0.74 | 15.60 |
| HCl-PBC | 56.16 | 3.93 | 16.65 |
| EtOH-PBC | 63.31 | 0.97 | 17.16 |
| Biochar | Radical Concentration ×10−19 (spin/gram) | g-Parameter |
|---|---|---|
| Non-WSBC | 0.80 | 2.0028 |
| HCl-WSBC | 0.71 | 2.0028 |
| EtOH-WSBC | 0.42 | 2.0028 |
| Non-SBC | 1.85 | 2.0028 |
| HCl-SBC | 1.88 | 2.0027 |
| EtOH-SBC | 1.20 | 2.0029 |
| Non-PBC | 1.58 | 2.0029 |
| HCl-PBC | 1.63 | 2.0030 |
| EtOH-PBC | 0.46 | 2.0030 |
| Biochar Type | Langmuir’s Model | Freundlich’s Model | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax, Maximum Adsorption Capacity, mg/g | b, the Ratio of Adsorption Constant Rate to Desorption Constant Rate (k/k’) | R2—Determination Coefficient | AIC, Akaike Criterion Coefficient | Kf, mg∙g−1∙(L∙mg−1)^(1/n) | (1/n), Heterogenicity Coefficient | R2—Determination Coefficient | AIC, Akaike Criterion Coefficient | |
| WSBC | 308.86 | 0.00014 | 0.9909 | 29.09 | 0.368 | 0.678 | 0.9858 | 31.13 |
| SBC | 245.80 | 0.00017 | 0.9724 | 33.60 | 0.384 | 0.657 | 0.9392 | 37.50 |
| PBC | 214.33 | 0.00019 | 0.9932 | 25.46 | 0.517 | 0.614 | 0.9661 | 33.51 |
| Biochar Type | Langmuir’s Model | Freundlich’s Model | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax, Maximum Adsorption Capacity, mg/g | b, the Ratio of Adsorption Constant Rate to Desorption Constant Rate (k/k’) | R2—Determination Coefficient | AIC, Akaike Criterion Coefficient | Kf, mg∙g−1∙(L∙mg−1)^(1/n) | (1/n), Heterogenicity Coefficient | R2—Determination Coefficient | AIC, Akaike criterion Coefficient | |
| EtOH-WSBC | 2687.32 | 0.00001 | 0.9167 | 42.60 | 0.002164 | 1.281 | 0.9388 | 41.06 |
| EtOH-SBC | 1029.30 | 0.000028 | 0.906 | 42.73 | 0.009061 | 1.112 | 0.925 | 41.58 |
| EtOH-PBC | 3449.54 | 0.000009 | 0.9869 | 33.91 | 0.021207 | 1.037 | 0.9892 | 32.92 |
| Biochar Type | Langmuir’s Model | Freundlich’s Model | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax, Maximum Adsorption Capacity, mg/g | b, the Ratio of Adsorption Constant Rate to Desorption Constant Rate (k/k’) | R2—Determination Coefficient | AIC, Akaike Criterion Coefficient | Kf, mg∙g−1∙(L∙mg−1)^(1/n) | (1/n), Heterogenicity Coefficient | R2—Determination Coefficient | AIC, Akaike Criterion Coefficient | |
| HCl-WSBC | 1628.50 | 0.000059 | 0.7889 | 54.09 | 0.000259 | 1.707 | 0.9719 | 44.04 |
| HCl-SBC | 1432.91 | 0.000068 | 0.7981 | 53.83 | 0.000083 | 1.858 | 0.9911 | 38.21 |
| HCl-PBC | 1539.88 | 0.000063 | 0.7761 | 54.48 | 0.000027 | 1.999 | 0.9893 | 39.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medyńska-Juraszek, A.; Álvarez, M.L.; Białowiec, A.; Jerzykiewicz, M. Characterization and Sodium Cations Sorption Capacity of Chemically Modified Biochars Produced from Agricultural and Forestry Wastes. Materials 2021, 14, 4714. https://doi.org/10.3390/ma14164714
Medyńska-Juraszek A, Álvarez ML, Białowiec A, Jerzykiewicz M. Characterization and Sodium Cations Sorption Capacity of Chemically Modified Biochars Produced from Agricultural and Forestry Wastes. Materials. 2021; 14(16):4714. https://doi.org/10.3390/ma14164714
Chicago/Turabian StyleMedyńska-Juraszek, Agnieszka, María Luisa Álvarez, Andrzej Białowiec, and Maria Jerzykiewicz. 2021. "Characterization and Sodium Cations Sorption Capacity of Chemically Modified Biochars Produced from Agricultural and Forestry Wastes" Materials 14, no. 16: 4714. https://doi.org/10.3390/ma14164714
APA StyleMedyńska-Juraszek, A., Álvarez, M. L., Białowiec, A., & Jerzykiewicz, M. (2021). Characterization and Sodium Cations Sorption Capacity of Chemically Modified Biochars Produced from Agricultural and Forestry Wastes. Materials, 14(16), 4714. https://doi.org/10.3390/ma14164714









