The Curing Kinetics of E-Glass Fiber/Epoxy Resin Prepreg and the Bending Properties of Its Products
Abstract
:1. Introduction
2. Materials and Methods
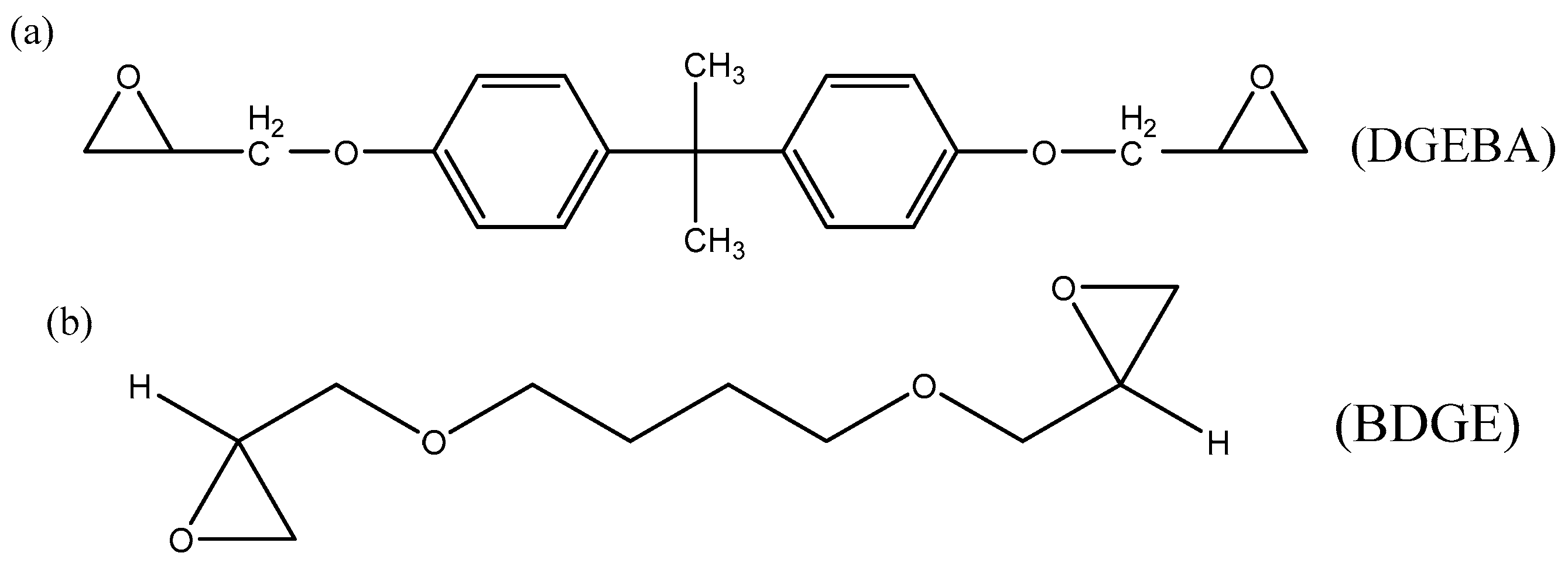
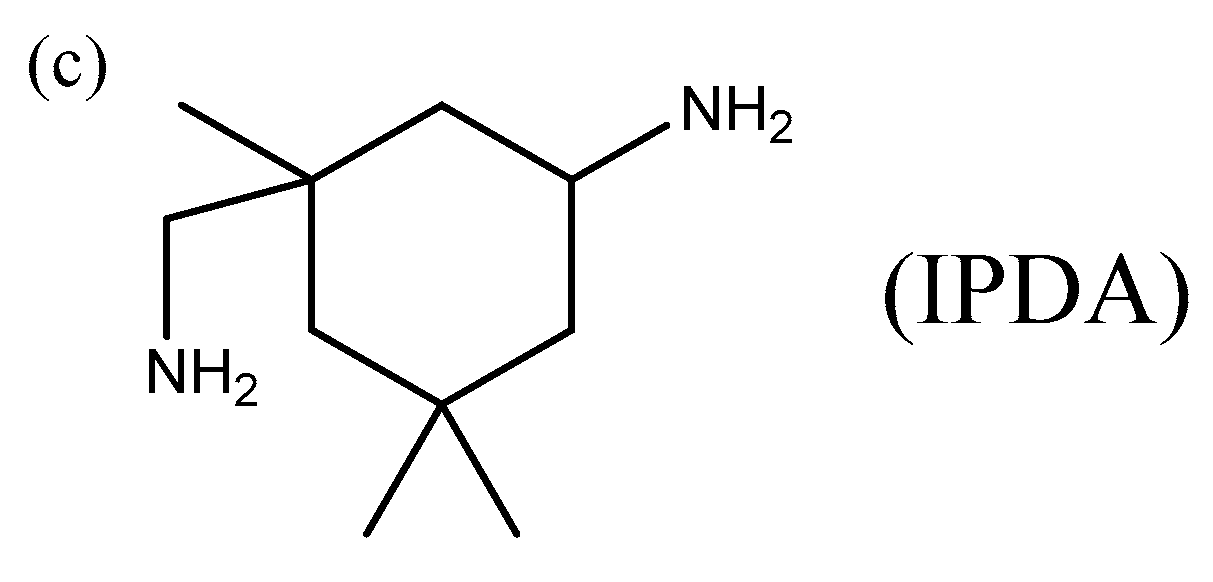
2.1. Materials
2.2. Manufacturing of Composite Laminates
2.3. Three-Point Bending Test
2.4. Characterization
3. Results and Discussion
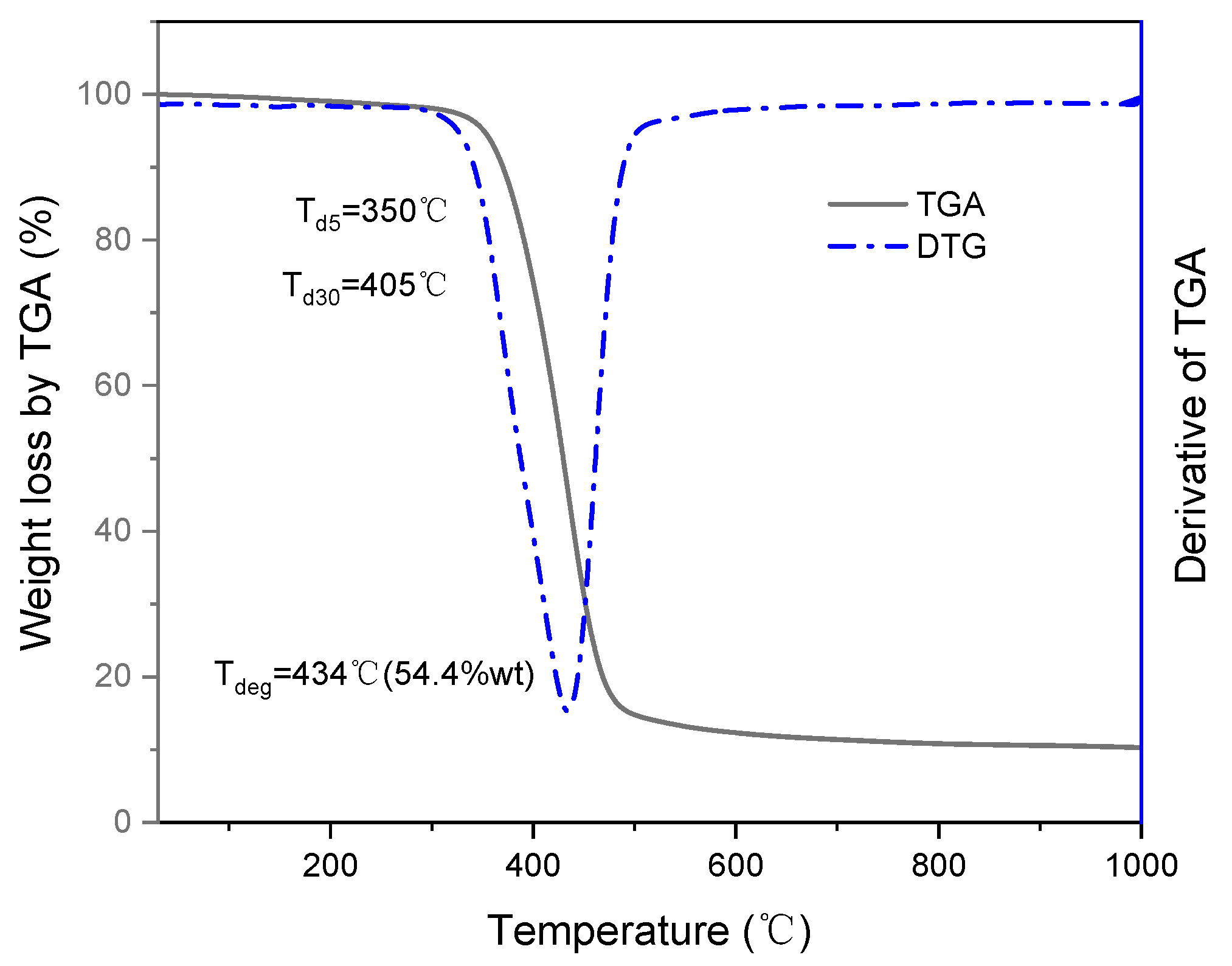
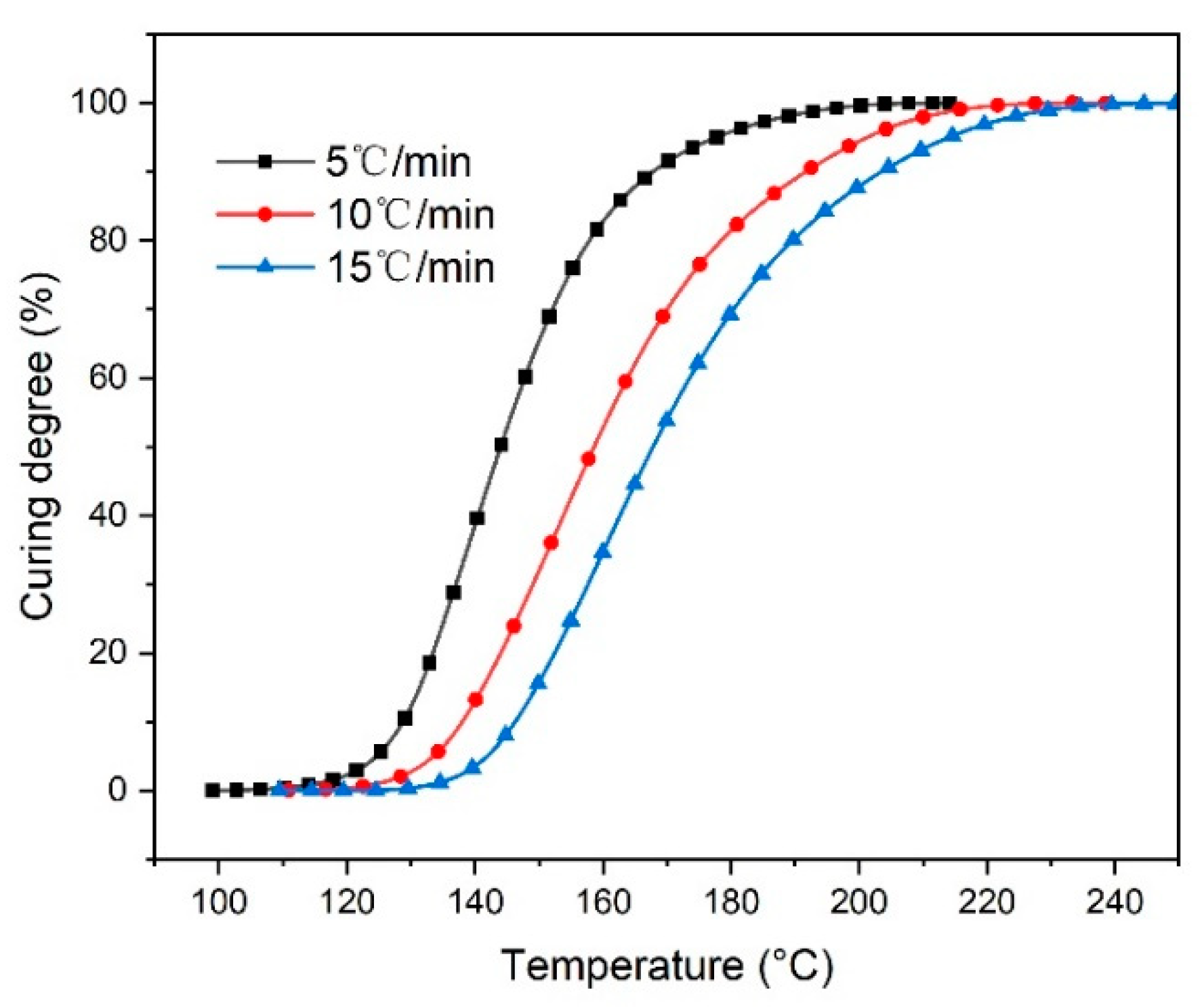
3.1. Thermogravimetric Analysis
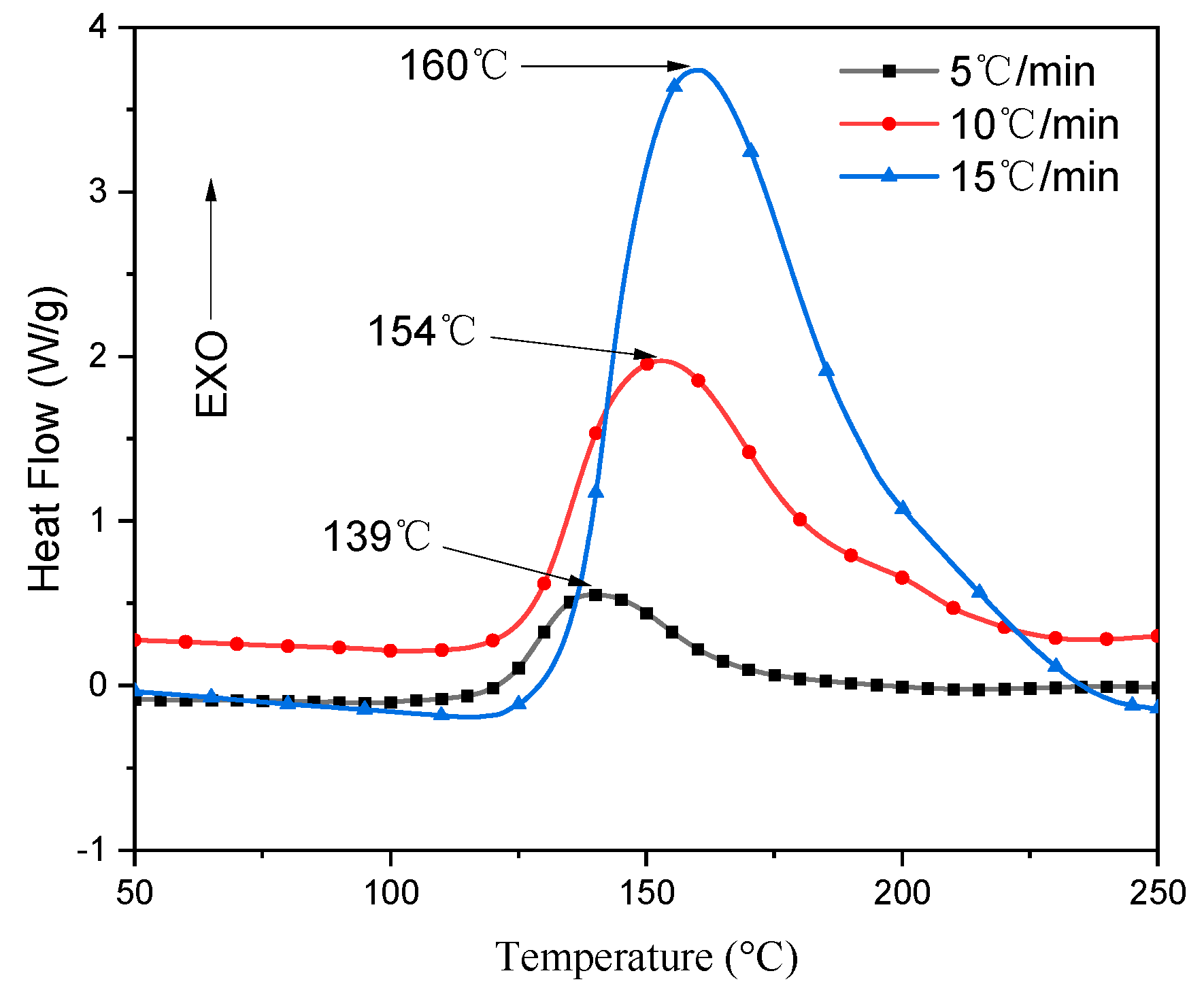
3.2. DSC Curve Analysis
3.3. Curing Kinetics
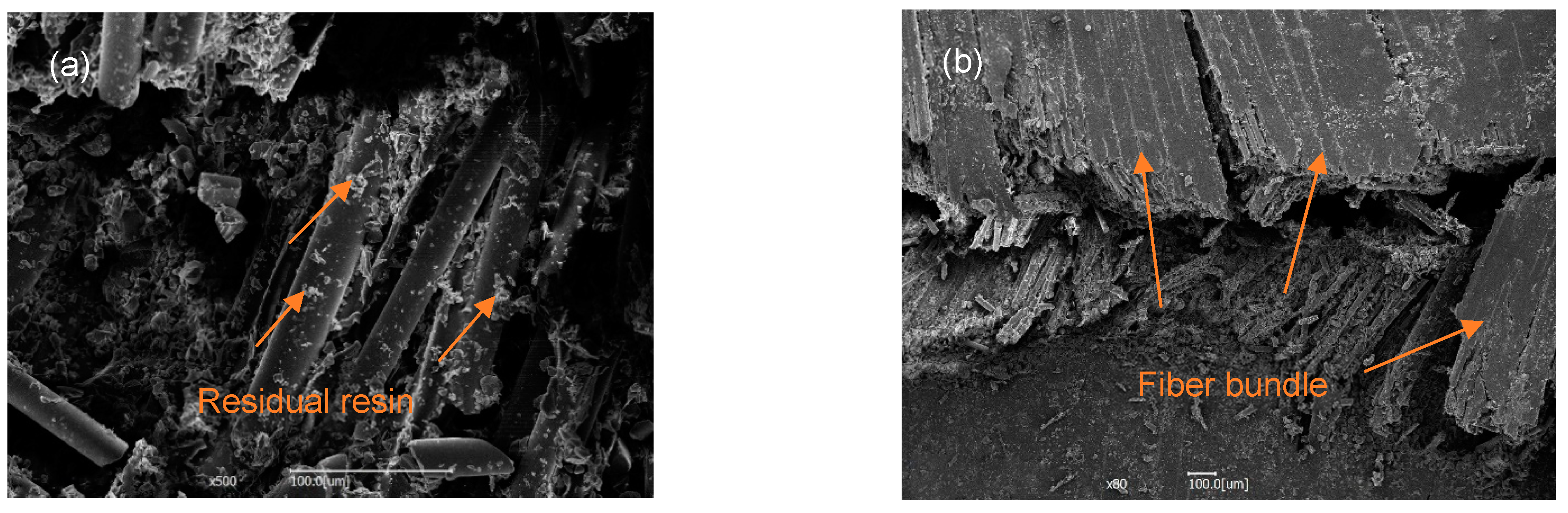
3.4. Bending Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stickel, J.M.; Nagarajan, M. Glass Fiber-Reinforced Composites: From Formulation to Application. Int. J. Appl. Glass Sci. 2012, 3, 122–136. [Google Scholar] [CrossRef]
- Yıldız, S.; Karaağaç, B.; Güzeliş, S.G. Utilization of Glass Fiber Reinforced Polymer Wastes. Polym. Compos. 2021, 42, 412–423. [Google Scholar] [CrossRef]
- Islam, M.; Ar-Rashid, H.; Islam, F.; Karmaker, N.; Koly, F.A.; Mahmud, J.; Keya, K.N.; Khan, R.A. Fabrication and Characterization of E-Glass Fiber Reinforced Unsaturated Polyester Resin Based Composite Materials. In Nano Hybrids and Composites; Trans Tech Publications Ltd.: Pfaffikon, Switzerland, 2019; Volume 24, pp. 1–7. [Google Scholar]
- Karvanis, K.; Rusnáková, S.; Žaludek, M.; Čapka, A. Preparation and Dynamic Mechanical Analysis of Glass or Carbon Fiber/Polymer Composites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 362, p. 012005. [Google Scholar]
- Budelmann, D.; Schmidt, C.; Meiners, D. Prepreg Tack: A Review of Mechanisms, Measurement, and Manufacturing Implication. Polym. Compos. 2020, 41, 3440–3458. [Google Scholar] [CrossRef]
- Hou, J.P.; Cherruault, J.Y.; Nairne, I.; Jeronimidis, G.; Mayer, R.M. Evolution of the Eye-End Design of a Composite Leaf Spring for Heavy Axle Loads. Compos. Struct. 2007, 78, 351–358. [Google Scholar] [CrossRef]
- Kim, H.G. Dielectric Cure Monitoring for Glass/Polyester Prepreg Composites. Compos. Struct. 2002, 57, 91–99. [Google Scholar] [CrossRef]
- Du, S.; Guo, Z.-S.; Zhang, B.; Wu, Z. Cure Kinetics of Epoxy Resin Used for Advanced Composites. Polym. Int. 2004, 53, 1343–1347. [Google Scholar] [CrossRef]
- Hardis, R.; Jessop, J.L.; Peters, F.E.; Kessler, M.R. Cure Kinetics Characterization and Monitoring of an Epoxy Resin Using DSC, Raman Spectroscopy, and DEA. Compos. Part A Appl. Sci. Manuf. 2013, 49, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Zhang, X.; Ju, F.; Tsai, S.-B. A Study on Curing Kinetics of Nano-Phase Modified Epoxy Resin. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Cai, H.; Li, P.; Sui, G.; Yu, Y.; Li, G.; Yang, X.; Ryu, S. Curing Kinetics Study of Epoxy Resin/Flexible Amine Toughness Systems by Dynamic and Isothermal DSC. Thermochim. Acta 2008, 473, 101–105. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, J.; Bai, S.; Zhang, W. Study on Phenomenological Curing Model of Epoxy Resin for Prediction of Degree of Cure. J. Therm. Anal. Calorim. 2012, 109, 1555–1561. [Google Scholar] [CrossRef]
- Mphahlele, K.; Ray, S.S.; Kolesnikov, A. Cure Kinetics, Morphology Development, and Rheology of a High-Performance Carbon-Fiber-Reinforced Epoxy Composite. Compos. Part B Eng. 2019, 176, 107300. [Google Scholar] [CrossRef]
- Azzam, A.; Li, W. An Experimental Investigation on the Three-Point Bending Behavior of Composite Laminate. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2014; Volume 62, p. 012016. [Google Scholar]
- Mouzakis, D.E.; Zoga, H.; Galiotis, C. Accelerated Environmental Ageing Study of Polyester/Glass Fiber Reinforced Composites (GFRPCs). Compos. Part B Eng. 2008, 39, 467–475. [Google Scholar] [CrossRef]
- Ren, D.; Li, K.; Chen, L.; Chen, S.; Han, M.; Xu, M.; Liu, X. Modification on Glass Fiber Surface and Their Improved Properties of Fiber-Reinforced Composites via Enhanced Interfacial Properties. Compos. Part B Eng. 2019, 177, 107419. [Google Scholar] [CrossRef]
- Jariwala, H.; Jain, P.; Maisuriya, V. Experimental and Statistical Analysis of Strength of Glass Fiber Reinforced Polymer Composite for Different Fiber Architecture. Polym. Compos. 2021, 42, 1407–1419. [Google Scholar] [CrossRef]
- Shin, P.-S.; Wang, Z.-J.; Kwon, D.-J.; Choi, J.-Y.; Sung, I.; Jin, D.-S.; Kang, S.-W.; Kim, J.-C.; DeVries, K.L.; Park, J.-M. Optimum Mixing Ratio of Epoxy for Glass Fiber Reinforced Composites with High Thermal Stability. Compos. Part B Eng. 2015, 79, 132–137. [Google Scholar] [CrossRef]
- Memon, H.; Wei, Y. Welding and Reprocessing of Disulfide-Containing Thermoset Epoxy Resin Exhibiting Behavior Reminiscent of a Thermoplastic. J. Appl. Polym. Sci. 2020, 137, 49541. [Google Scholar] [CrossRef]
- Xu, W.-B.; Bao, S.-P.; Shen, S.-J.; Hang, G.-P.; He, P.-S. Curing Kinetics of Epoxy Resin–Imidazole–Organic Montmorillonite Nanocomposites Determined by Differential Scanning Calorimetry. J. Appl. Polym. Sci. 2003, 88, 2932–2941. [Google Scholar] [CrossRef]
- Yousefi, A.; Lafleur, P.G.; Gauvin, R. Kinetic Studies of Thermoset Cure Reactions: A Review. Polym. Compos. 1997, 18, 157–168. [Google Scholar] [CrossRef]
- Garschke, C.; Parlevliet, P.P.; Weimer, C.; Fox, B.L. Cure Kinetics and Viscosity Modelling of a High-Performance Epoxy Resin Film. Polym. Test. 2013, 32, 150–157. [Google Scholar] [CrossRef]
- Kratz, J.; Hsiao, K.; Fernlund, G.; Hubert, P. Thermal Models for MTM45-1 and Cycom 5320 out-of-Autoclave Prepreg Resins. J. Compos. Mater. 2013, 47, 341–352. [Google Scholar] [CrossRef]
- Ferdosian, F.; Zhang, Y.; Yuan, Z.; Anderson, M.; Xu, C.C. Curing Kinetics and Mechanical Properties of Bio-Based Epoxy Composites Comprising Lignin-Based Epoxy Resins. Eur. Polym. J. 2016, 82, 153–165. [Google Scholar] [CrossRef]
- Gonis, J.I.M.; Simon, G.P.; Cook, W.D. Cure Properties of Epoxies with Varying Chain Length as Studied by DSC. J. Appl. Polym. Sci. 1999, 72, 1479–1488. [Google Scholar] [CrossRef]
- Yang, L.F.; Yao, K.D.; Koh, W. Kinetics Analysis of the Curing Reaction of Fast Cure Epoxy Prepregs. J. Appl. Polym. Sci. 1999, 73, 1501–1508. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Tudorachi, N.; Mustata, F. Curing and Thermal Degradation of Diglycidyl Ether of Bisphenol A Epoxy Resin Crosslinked with Natural Hydroxy Acids as Environmentally Friendly Hardeners. Arab. J. Chem. 2020, 13, 671–682. [Google Scholar] [CrossRef]
- Li, L.; Zou, H.; Liang, M.; Chen, Y. Study on the Effect of Poly (Oxypropylene) Diamine Modified Organic Montmorillonite on Curing Kinetics of Epoxy Nanocomposties. Thermochim. Acta 2014, 597, 93–100. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Xu, S. Synthesis and Curing Kinetics of Cardanol-Based Curing Agents for Epoxy Resin by in Situ Depolymerization of Paraformaldehyde. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 472–480. [Google Scholar] [CrossRef]
- Zheng, T.; Xi, H.; Wang, Z.; Zhang, X.; Wang, Y.; Qiao, Y.; Wang, P.; Li, Q.; Li, Z.; Ji, C. The Curing Kinetics and Mechanical Properties of Epoxy Resin Composites Reinforced by PEEK Microparticles. Polym. Test. 2020, 91, 106781. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, Q.; Wang, J.; Huo, Z.; Sun, H. Curing Kinetics and Properties of Epoxy Resin–Fluorenyl Diamine Systems. Polymer 2008, 49, 4399–4405. [Google Scholar] [CrossRef]
- Hu, J.; Shan, J.; Wen, D.; Liu, X.; Zhao, J.; Tong, Z. Flame Retardant, Mechanical Properties and Curing Kinetics of DOPO-Based Epoxy Resins. Polym. Degrad. Stab. 2014, 109, 218–225. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Geng, C.; Li, Z.; Dai, G.; Cui, W. Curing Kinetics and the Properties of KH560-SiO 2/Polyethersulfone/Bismaleimide-Phenolic Epoxy Resin Composite. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1735–1743. [Google Scholar] [CrossRef]
- Newcomb, B.A. Time-Temperature-Transformation (TTT) Diagram of a Carbon Fiber Epoxy Prepreg. Polym. Test. 2019, 77, 105859. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Y.; Wang, Y.; Tan, H. Curing Behavior of Epoxy Resins in Two-Stage Curing Process by Non-Isothermal Differential Scanning Calorimetry Kinetics Method. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Cheng, L.; Zhang, L. Indirect Microwave Curing Process Design for Manufacturing Thick Multidirectional Carbon Fiber Reinforced Thermoset Composite Materials. Appl. Compos. Mater. 2019, 26, 533–552. [Google Scholar] [CrossRef]



| Physical Properties | Unit | Value |
|---|---|---|
| The weight of prepreg per unit area | g/m2 | 2352 ± 5% |
| Fiber weight per unit area | g/m2 | 1600 ± 5% |
| Single-layer thickness | mm | 1.2 ± 0.1 |
| Resin content | % | 32.0 ± 3.0 |
| Volatile content | % | ≤1.0 |
| β/(°C·min−1) | Cure Temperature (°C) | Htot/(J·g−1) | ||
|---|---|---|---|---|
| Ti/°C | Tp/°C | Tf/°C | ||
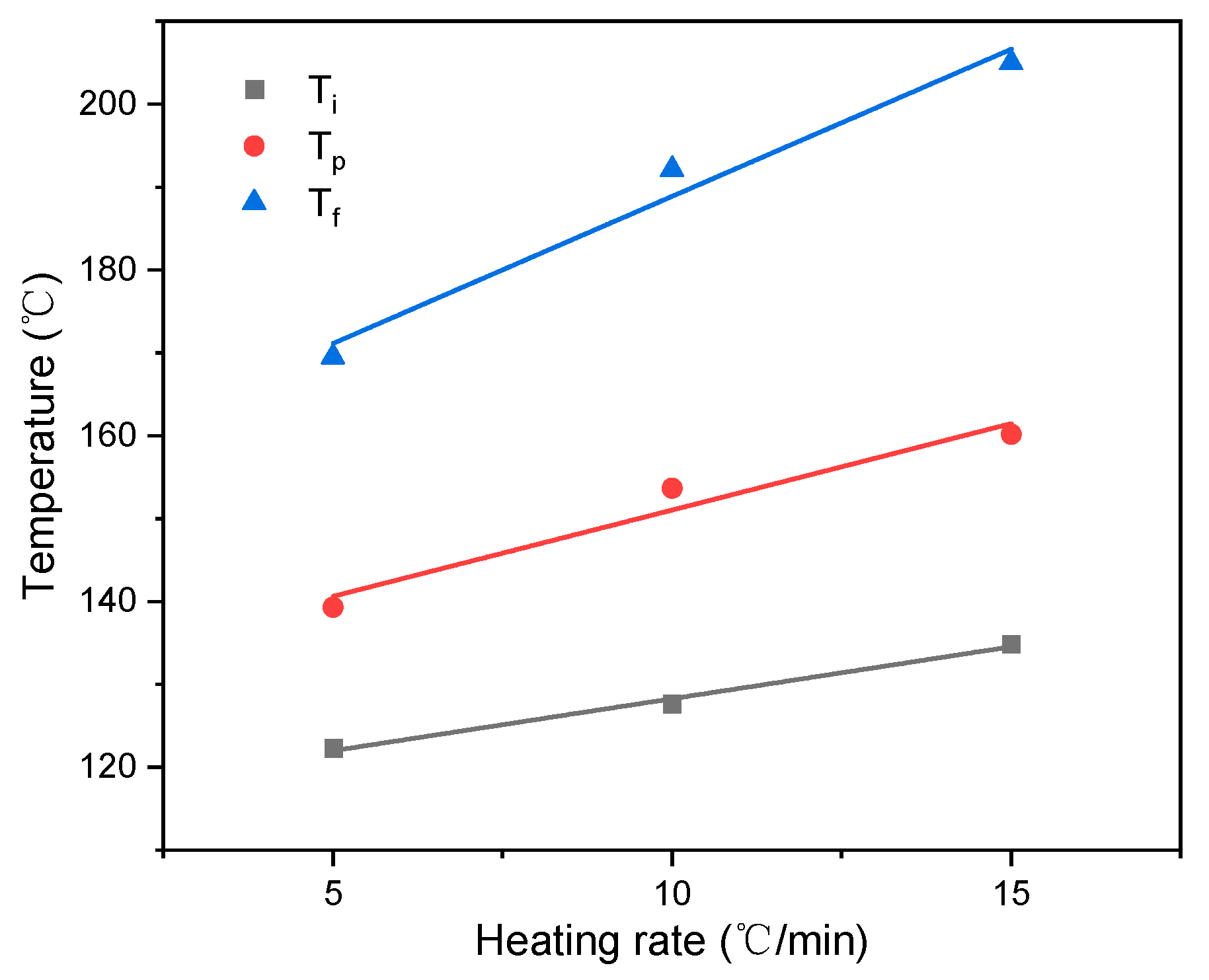
| 5 | 122 | 139 | 169 | 257 |
| 10 | 128 | 154 | 192 | 499 |
| 15 | 135 | 160 | 205 | 780 |
| β/(°C∙min−1) | Tp/K | 1/Tp(1000/K) | lnβ | |
|---|---|---|---|---|
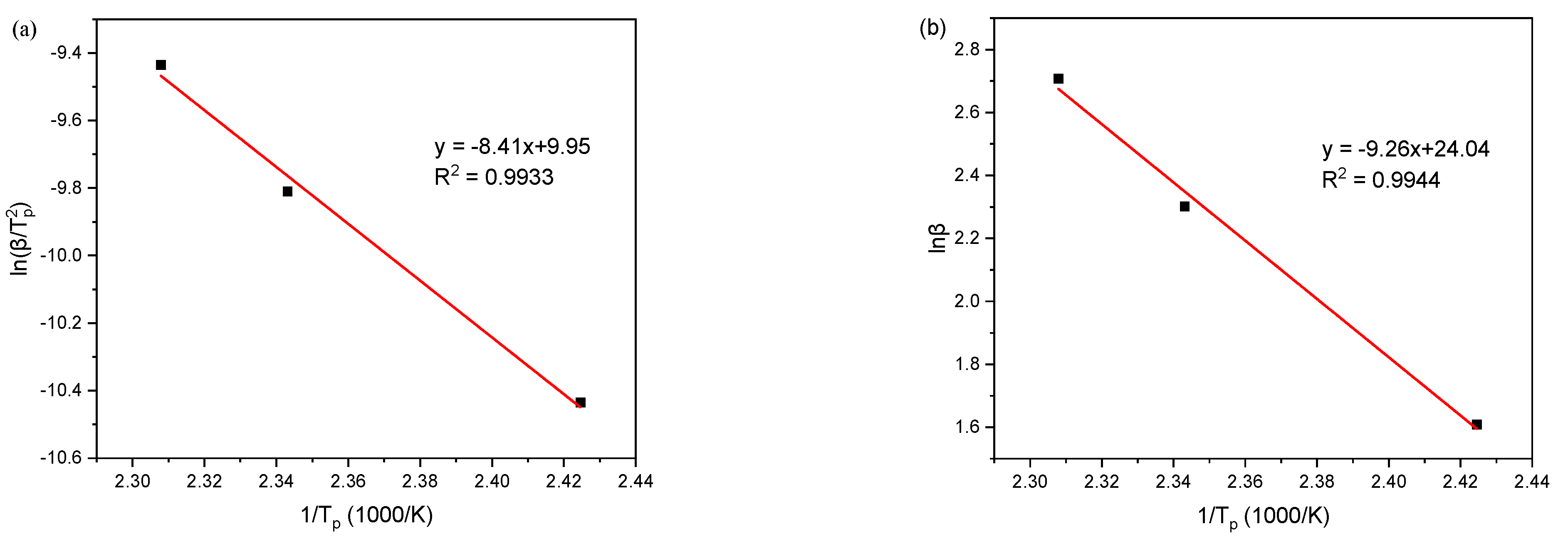
| 5 | 412 | 2.42 | 1.61 | −10.43 |
| 10 | 427 | 2.34 | 2.30 | −9.81 |
| 15 | 433 | 2.31 | 2.71 | −9.43 |
| Type of Bender | Ea (kJ/mol) | Reaction Order | Ref Number |
|---|---|---|---|
| Bisphenol-A | 71.42 | 1.3435 | [31] |
| TDE-85/BMAPF | 73.99 | 0.908 | [32] |
| Multifunctional P-containing | 72.96 | 3.21 | [33] |
| Phenolic | 96.03 | - | [34] |
| VTM264 | 96.4 | - | [35] |
| E51 | 102.55 | 1.3064 | [36] |
| Epoxy | 88.549 | 1.323 | [37] |
| 7901 DNK | 69.95 | 0.91 | This work |
| Sample | Load Time (s) | Displacement (mm) | Load (N) | Flexural Modulus (MPa) |
|---|---|---|---|---|
| 1 | 96.93 | 16.82 | 6112 | 411 |
| 2 | 75.72 | 13.22 | 6106 | 408 |
| 3 | 72.95 | 12.76 | 6356 | 438 |
| 4 | 79.21 | 13.79 | 6315 | 421 |
| 5 | 74.34 | 12.99 | 6231 | 423 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Wang, Z.; Rahman, M.B.; Shen, W.; Zhu, C. The Curing Kinetics of E-Glass Fiber/Epoxy Resin Prepreg and the Bending Properties of Its Products. Materials 2021, 14, 4673. https://doi.org/10.3390/ma14164673
Zhu L, Wang Z, Rahman MB, Shen W, Zhu C. The Curing Kinetics of E-Glass Fiber/Epoxy Resin Prepreg and the Bending Properties of Its Products. Materials. 2021; 14(16):4673. https://doi.org/10.3390/ma14164673
Chicago/Turabian StyleZhu, Lvtao, Zhenxing Wang, Mahfuz Bin Rahman, Wei Shen, and Chengyan Zhu. 2021. "The Curing Kinetics of E-Glass Fiber/Epoxy Resin Prepreg and the Bending Properties of Its Products" Materials 14, no. 16: 4673. https://doi.org/10.3390/ma14164673
APA StyleZhu, L., Wang, Z., Rahman, M. B., Shen, W., & Zhu, C. (2021). The Curing Kinetics of E-Glass Fiber/Epoxy Resin Prepreg and the Bending Properties of Its Products. Materials, 14(16), 4673. https://doi.org/10.3390/ma14164673






