Effects of Isothermal Temperature and Soaking Time on Water Quenched Microstructure of Nickel-Based Superalloy GH3536 Semi-Solid Billets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Semi-Solid Billets
2.3. Observation of Microstructure
3. Results
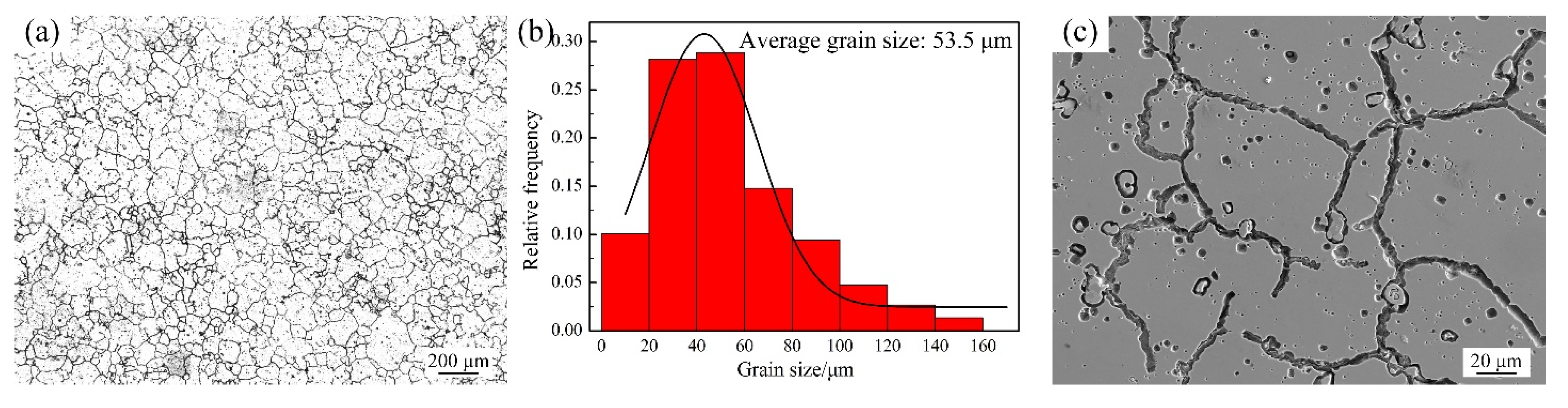
3.1. As-Received GH3536 Alloy
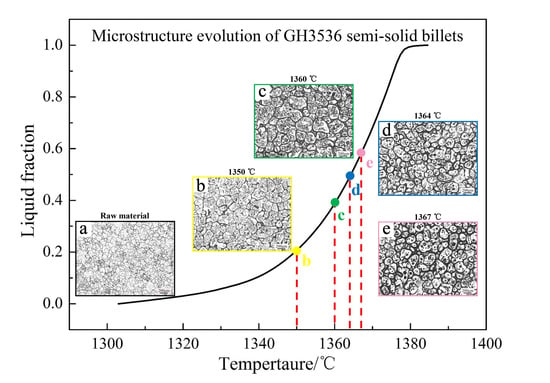
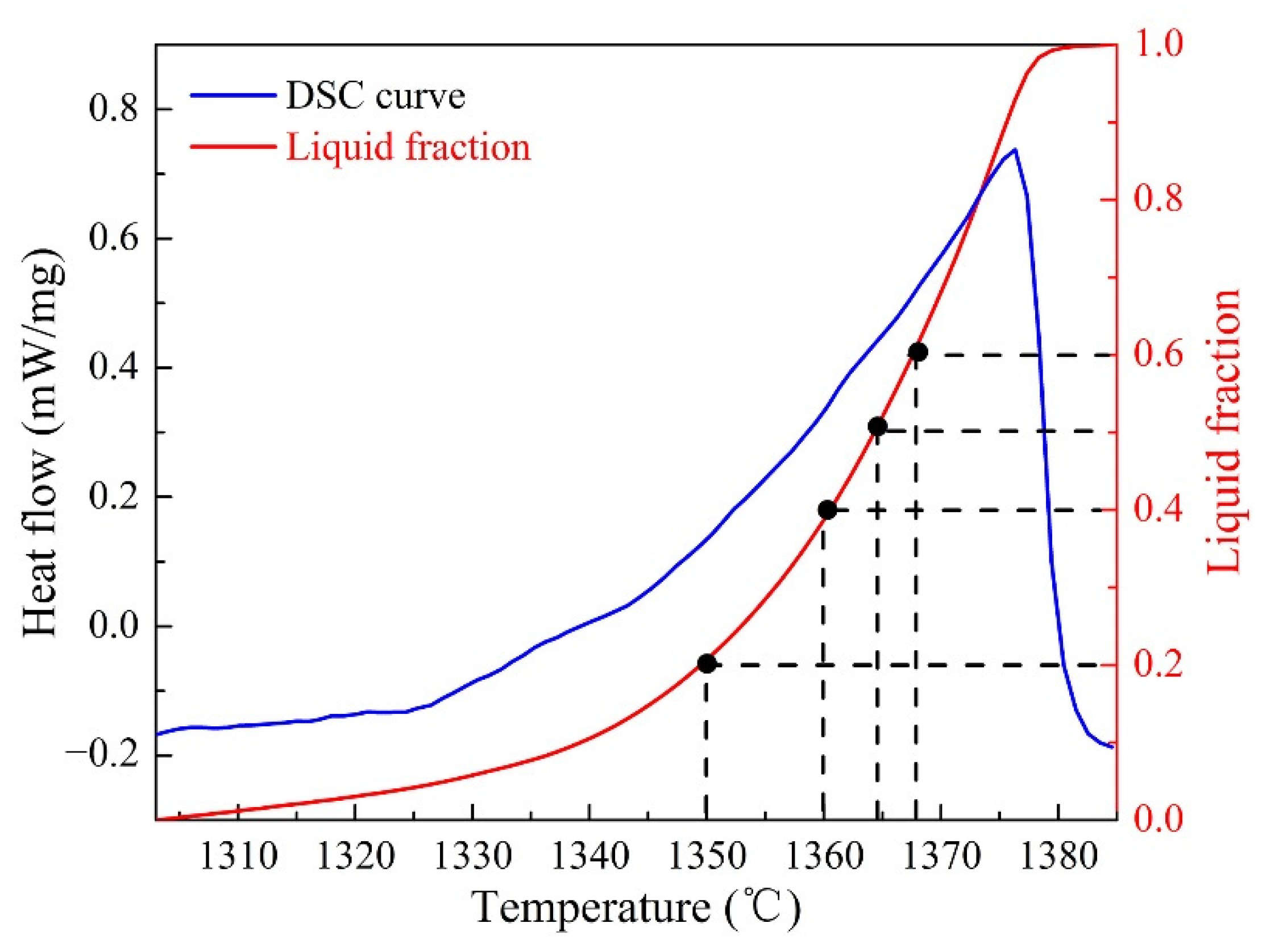
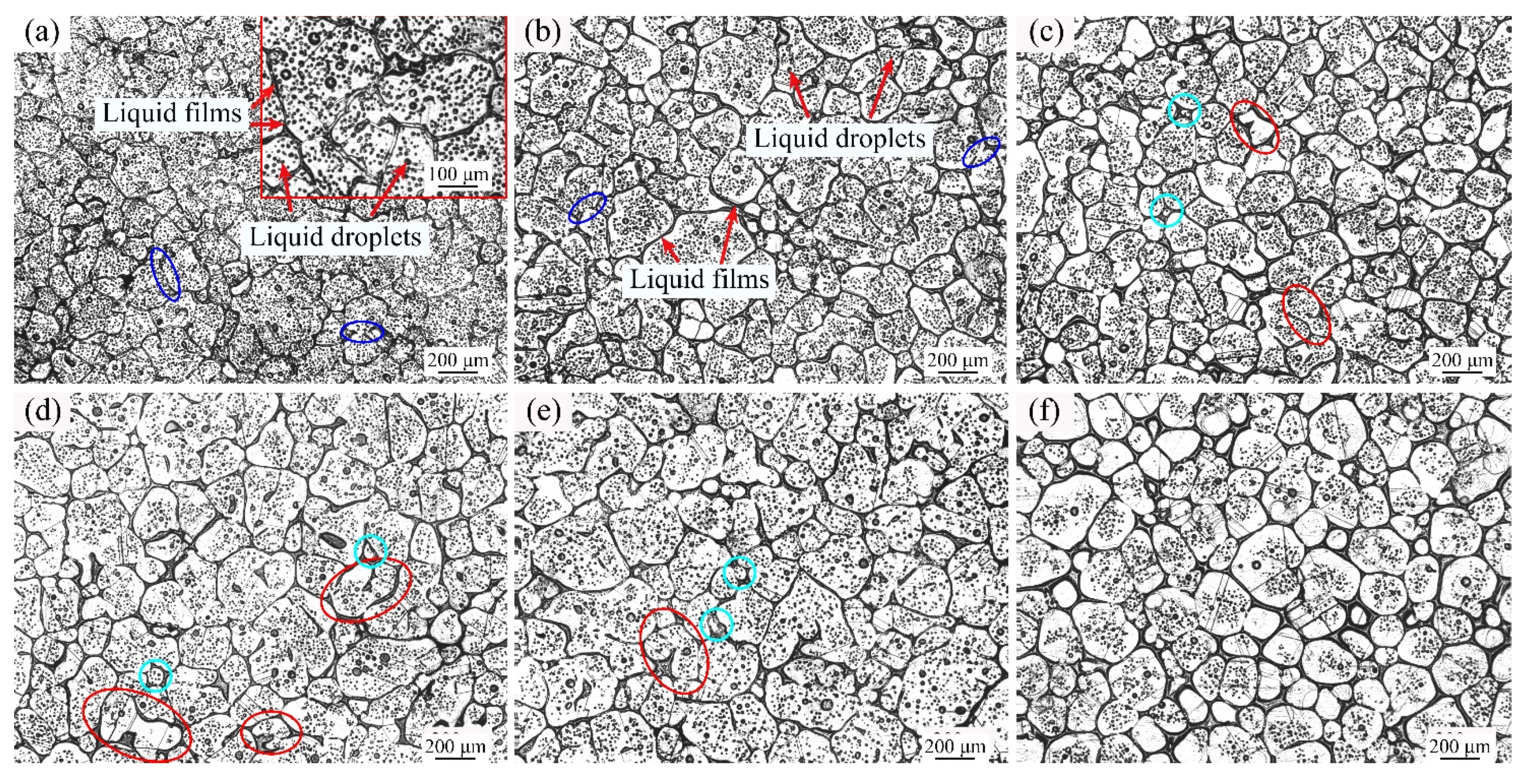
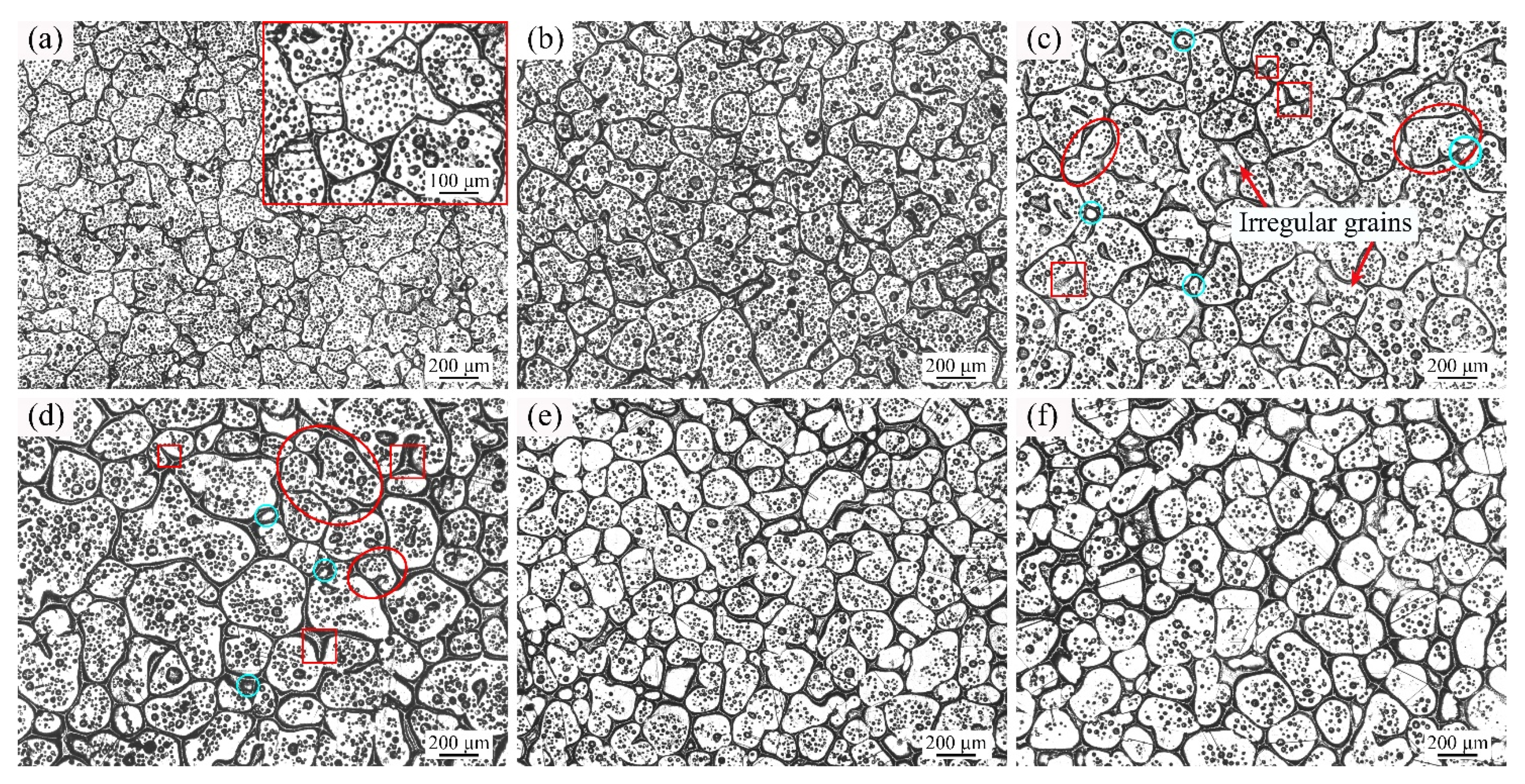
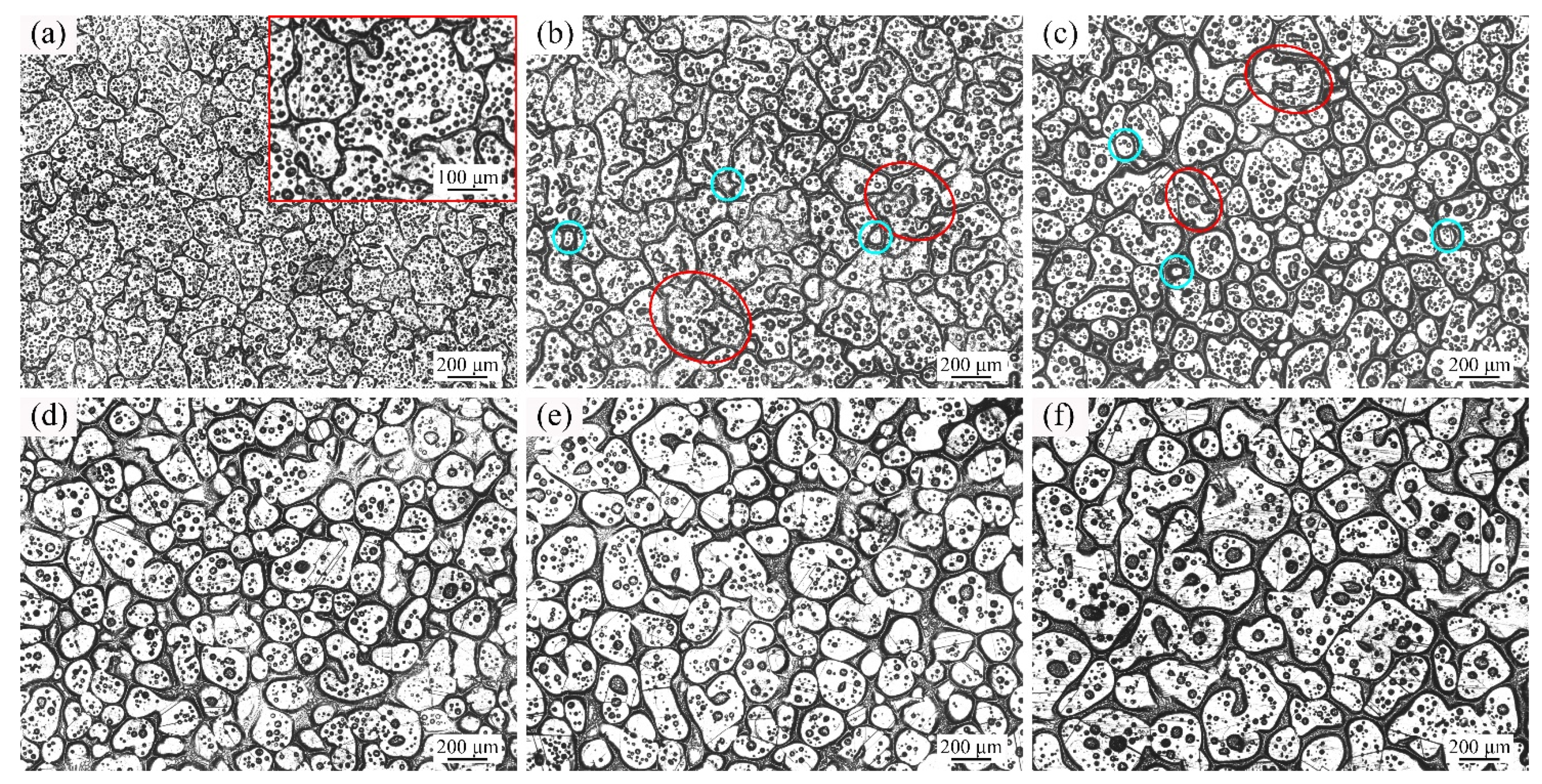
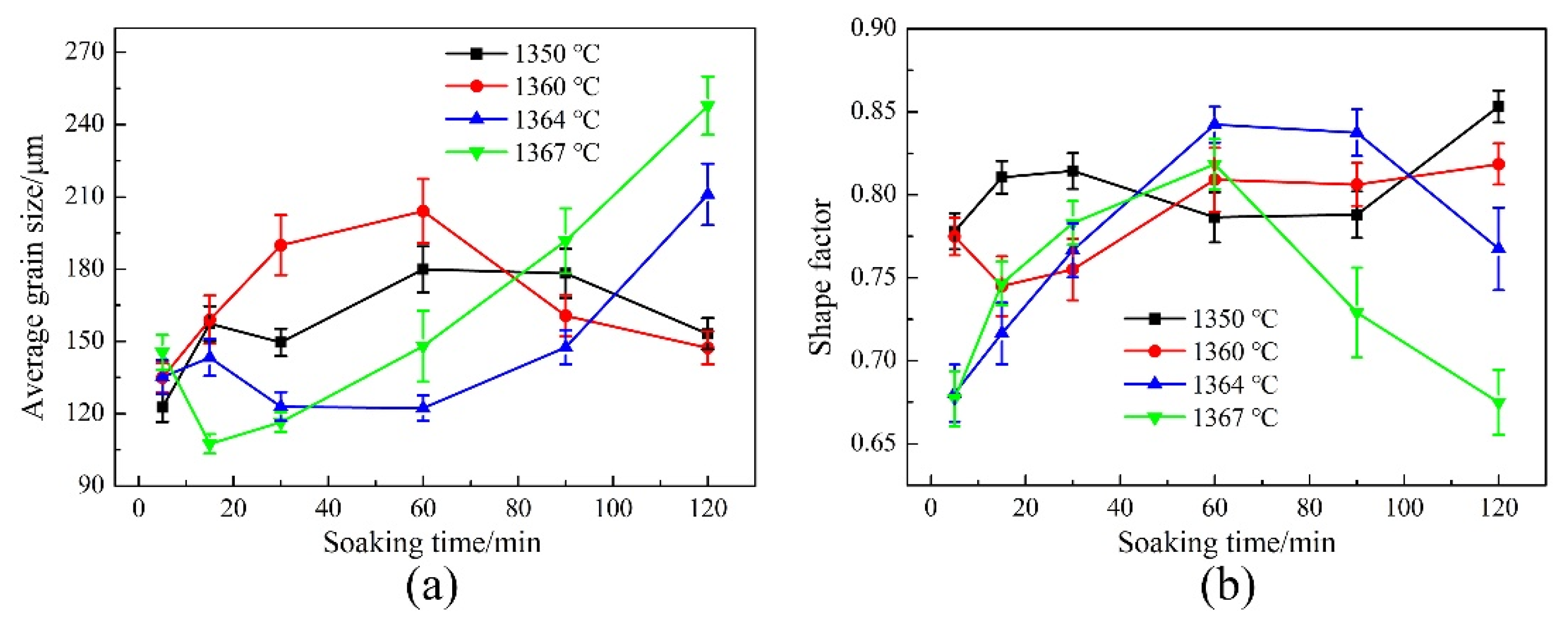
3.2. Microstructure Evolution of GH3536 Semi-Solid Billets
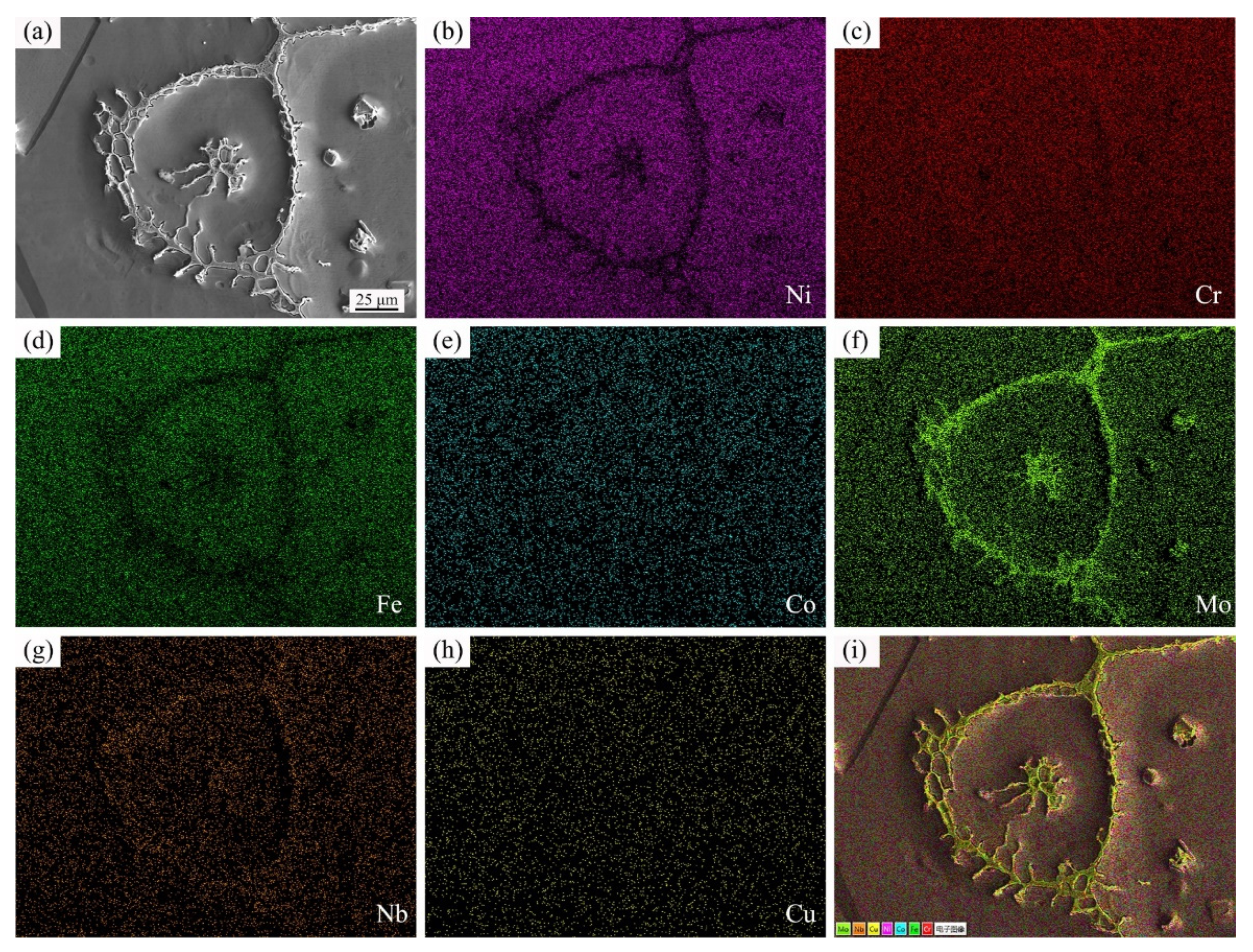
3.3. Distribution of Elements in the Microstructure
4. Discussion
5. Conclusions
- The microstructure of GH3536 semi-solid billets consisted of solid particles and liquid phases. The liquid phases were composed of intragranular liquid droplets and grain boundary liquid films.
- The heating temperature and soaking time had significant effects on the microstructure of GH3536 billets. The optimum parameters for fabricating GH3536 semi-solid billets were temperature of 1364–1367 °C and soaking time of 60–90 min. At these conditions, the solid grains had spherical shapes and suitable grain sizes.
- The microstructure evolution of GH3536 alloy was affected by the coalescence mechanism, Ostwald ripening mechanism and breaking up mechanism. The coalescence mechanism was dominant at short soaking times and low liquid fractions, while the Ostwald ripening mechanism worked at long heating times and high liquid fractions. When the intragranular liquid droplets were large enough, the breaking up mechanism occurred, resulting in the formation of vermiculate grains.
- The SEM observations and EDS results indicated that the eutectic components rich in Cr, Mo, and Nb were transformed from the grain boundary liquid films and intragranular liquid droplets after rapid quenching.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. Developments in investment casting process—A review. J. Mater. Process. Technol. 2012, 212, 2332–2348. [Google Scholar] [CrossRef]
- Deb, D.; Iyer, S.R.; Radhakrishnan, V.M. A comparative study of oxidation and hot corrosion of a cast nickel base superalloy in different corrosive environments. Mater. Lett. 1996, 29, 19–23. [Google Scholar] [CrossRef]
- Wen, D.X.; Lin, Y.C.; Li, H.B.; Chen, X.M.; Deng, J.; Li, L.T. Hot deformation behavior and processing map of a typical Ni-based superalloy. Mater. Sci. Eng. A 2014, 591, 183–192. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Z.; Ning, Y.; Yang, N. Effect of deformation temperature and strain rate on dynamic recrystallized grain size of a powder metallurgical nickel-based superalloy. J. Alloy. Compd. 2016, 691, 554–563. [Google Scholar] [CrossRef]
- Zheng, L.; Xiao, C.; Zhang, G.; Han, B.; Tang, D. Primary α phase and its effect on the impact ductility of a high Cr content cast Ni-base superalloy. J. Alloy. Compd. 2012, 527, 176–183. [Google Scholar] [CrossRef]
- D’Souza, N.; Roebuck, B.; Collins, D.M.; West, G.D.; Panwisawas, C. Relating micro-segregation to site specific high temperature deformation in single crystal nickel-base superalloy castings. Mater. Sci. Eng. A 2020, 773, 138862. [Google Scholar] [CrossRef] [Green Version]
- Flemings, M.C. Behavior of metal alloys in the semisolid state. Metall. Trans. 1991, 22, 269–293. [Google Scholar] [CrossRef]
- Gao, J.; Hu, X.; Zhu, Q.; Li, D.; Kang, Y. Cooling behavior and microstructure of semisolid A201 aluminum alloy prepared by the SEED process. Metals 2019, 9, 922. [Google Scholar] [CrossRef] [Green Version]
- Kapranos, P.; Ward, P.J.; Atkinson, H.V.; Kirkwood, D.H. Near net shaping by semi-solid metal processing. Mater. Des. 2000, 21, 387–394. [Google Scholar] [CrossRef]
- Li, K.N.; Zhang, Y.B.; Zeng, Q.; Huang, G.H.; Ji, B.; Yin, D.D. Effects of semisolid treatment and ECAP on the microstructure and mechanical properties of Mg-6.52Zn-0.95Y alloy with icosahedral phase. Mater. Sci. Eng. A 2019, 751, 283–291. [Google Scholar] [CrossRef]
- Guan, R.G.; Zhao, Z.Y.; Zhang, H.; Lian, C.; Lee, C.S.; Liu, C.M. Microstructure evolution and properties of Mg-3Sn-1Mn (wt%) alloy strip processed by semisolid rheo-rolling. J. Mater. Process. Technol. 2012, 212, 1430–1436. [Google Scholar] [CrossRef]
- Liu, H.Q.; Wang, J.G.; Wang, H.Y.; Jiang, Q.C. Effect of predeformation on the globular grains in AZ91D alloy during strain induced melt activation (SIMA) process. J. Alloy. Compd. 2007, 431, 141–147. [Google Scholar]
- Atkinson, H.V. Semisolid processing of metallic materials. Mater. Sci. Technol. 2010, 26, 1401–1413. [Google Scholar] [CrossRef] [Green Version]
- Yan, G.; Zhao, S.; Ma, S.; Shou, H. Microstructural evolution of A356.2 alloy prepared by the SIMA process. Mater. Charact. 2012, 69, 45–51. [Google Scholar] [CrossRef]
- Parshizfard, E.; Shabestari, S.G. An investigation on the microstructural evolution and mechanical properties of A380 aluminum alloy during SIMA process. J. Alloy. Compd. 2011, 509, 9654–9658. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, Q.; Zhang, Y. Effects of plastic energy on thixotropic microstructure of C5191 alloys during SIMA process. J. Alloy. Compd. 2017, 721, 220–228. [Google Scholar] [CrossRef]
- Fu, J.; Yang, D.; Wang, K. Correlation between the liquid fraction, microstructure and tensile behaviors of 7075 aluminum alloy processed by recrystallization and partial remelting (RAP). Metals 2018, 8, 508. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Sugiyama, S.; Yanagimoto, J. Microstructural evolution during RAP process and deformation behavior of semi-solid SKD61 tool steel. J. Mater. Process. Technol. 2012, 212, 1731–1741. [Google Scholar] [CrossRef]
- Zhang, L.J.; Fan, J.T.; Liu, D.J.; Zhang, M.D.; Yu, P.F.; Jing, Q.; Ma, M.Z.; Liaw, P.K.; Li, G.; Liu, R.P. The microstructural evolution and hardness of the equiatomic CoCrCuFeNi high-entropy alloy in the semi-solid state. J. Alloy. Compd. 2018, 745, 75–83. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, G.; Wang, Y.; Qi, Y. Microstructure evolution of wrought nickel based superalloy GH4037 in the semi-solid state. Mater. Charact. 2018, 141, 229–237. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Bi, G.; Huang, X.; Chen, T.; Ma, Y. Effects of melt treatment temperature and isothermal holding parameter on water-quenched microstructures of A356 aluminum alloy semisolid slurry. Trans. Nonferrous Met. Soc. 2018, 28, 393–403. [Google Scholar] [CrossRef]
- Xiao, G.; Jiang, J.; Liu, Y.; Wang, Y.; Guo, B. Recrystallization and microstructure evolution of hot extruded 7075 aluminum alloy during semi-solid isothermal treatment. Mater. Charact. 2019, 156, 109874. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, Y.; Liu, W.; Ding, W. Microstructure evolution of semi-solid Mg-10Gd-3Y-0.5Zr alloy during isothermal heat treatment. J. Magnes. Alloy. 2013, 1, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Pan, F.; Chen, R.; Shen, J. Effects of holding temperature and time on semi-solid isothermal heat-treated microstructure of ZA84 magnesium alloy. Trans. Nonferrous Met. Soc. 2008, 18, 566–572. [Google Scholar] [CrossRef]
- Meng, Y.; Sugiyama, S.; Yanagimoto, J. Microstructural evolution during partial melting and semisolid forming behaviors of two hot-rolled Cr-V-Mo tool steels. J. Mater. Process. Technol. 2015, 225, 203–212. [Google Scholar] [CrossRef]
- Meng, Y.; Sugiyama, S.; Soltanpour, M.; Yanagimoto, J. Effects of predeformation and semi-solid processing on microstructure and mechanical properties of Cr-V-Mo steel. J. Mater. Process. Technol. 2013, 213, 426–433. [Google Scholar] [CrossRef]
- Püttgen, W.; Hallsted, B.; Blecka, W.; Uggowitzer, P.J. On the microstructure formation in chromium steels rapidly cooled from the semi-solid state. Acta Mater. 2007, 55, 1033–1042. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Samuel, F.H.; Al kahtani, S. Influence of Mg and solution heat treatment on the occurrence of incipient melting in Al–Si–Cu–Mg cast alloys. Mater. Sci. Eng. A 2012, 543, 22–34. [Google Scholar] [CrossRef]
- Liu, D.; Atkinson, H.V. Microstructural coarsening of semi-solid aluminium alloys. Mater. Sci. Eng. A 2008, 496, 439–446. [Google Scholar]
- Jiang, J.; Wang, Y.; Xiao, G.; Nie, X. Comparison of microstructural evolution of 7075 aluminum alloy fabricated by SIMA and RAP. J. Mater. Process. Technol. 2016, 238, 361–372. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Li, M.; Li, F. Texture evolution in semi-solid partial remelting and its effect on the microstructure of magnesium alloy. Mater. Lett. 2019, 237, 141–144. [Google Scholar] [CrossRef]
- Hardy, S.C.; Voorhees, P.W. Ostwald ripening in a system with a high volume fraction of coarsening phase. Metall. Trans. A 1988, 19, 2713–2721. [Google Scholar] [CrossRef]
- Li, J.; Sugiyama, S.; Yanagimoto, J. Microstructural evolution and flow stress of semi-solid type 304 stainless steel. J. Mater. Process. Technol. 2005, 161, 396–406. [Google Scholar] [CrossRef]
- Yi, Y.; Meng, Y.; Li, D.; Sugiyama, S.; Yanagimoto, J. Partial melting behavior and thixoforming properties of extruded magnesium alloy AZ91 with and without addition of SiC particles with a volume fraction of 15%. J. Mater. Sci. Technol. 2018, 34, 1149–1161. [Google Scholar] [CrossRef]

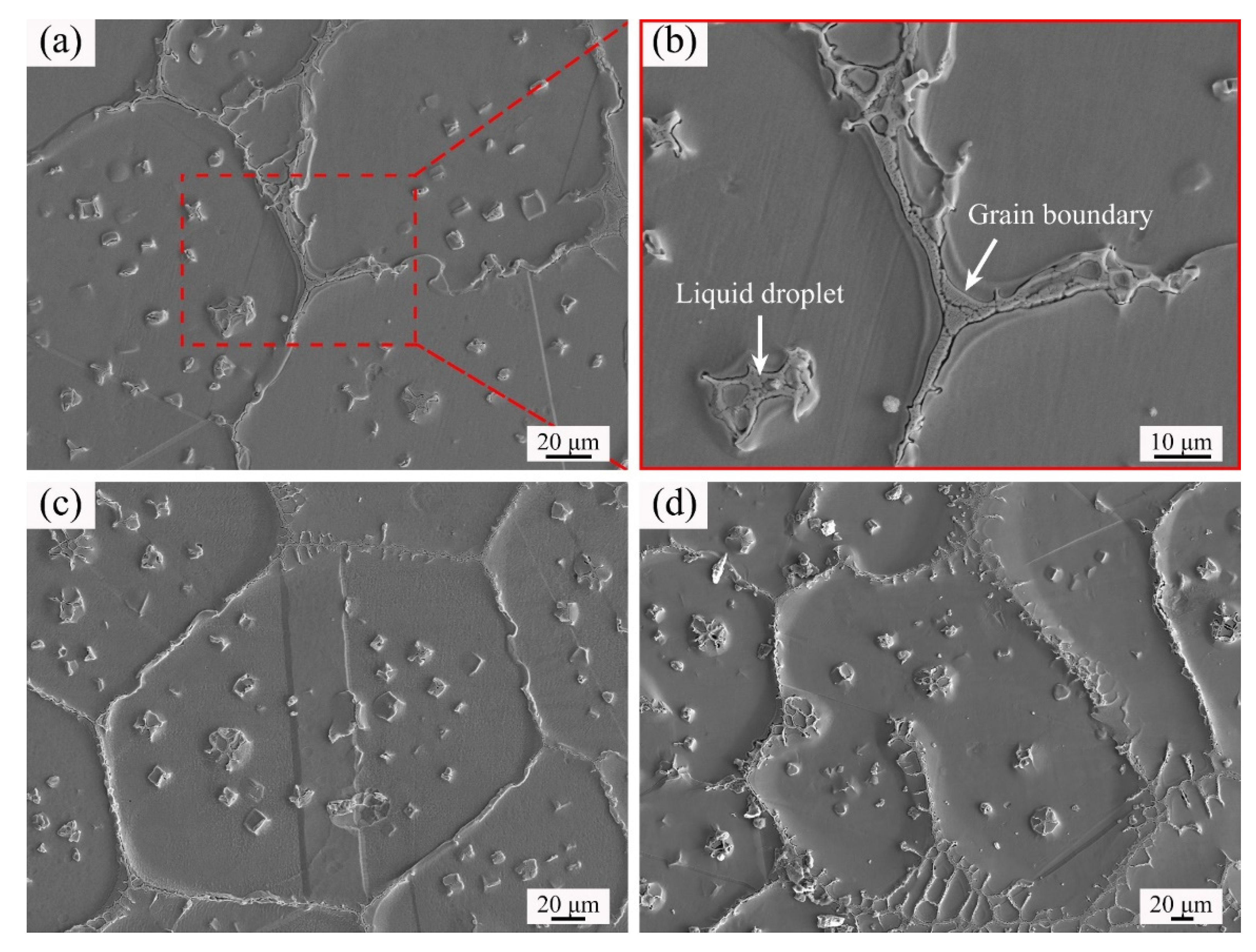

| Location | - | Cr | Fe | Co | Ni | Cu | Nb | Mo |
|---|---|---|---|---|---|---|---|---|
| 1 | wt % | 30.35 | 10.59 | 0.70 | 20.37 | 0.25 | 0.26 | 37.48 |
| at % | 38.16 | 12.39 | 0.78 | 22.69 | 0.26 | 0.18 | 25.54 | |
| 2 | wt % | 32.35 | 10.24 | 0.57 | 18.22 | 0.11 | 0.84 | 37.67 |
| at % | 40.70 | 12.00 | 0.63 | 20.28 | 0.12 | 0.59 | 25.68 | |
| 3 | wt % | 21.68 | 20.13 | 0.75 | 49.56 | 0.00 | 0.11 | 7.77 |
| at % | 24.29 | 20.99 | 0.74 | 49.18 | 0.00 | 0.07 | 4.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, G.; Jiang, J.; Wang, Y.; Liu, Y.; Zhang, Y.; Tian, Y. Effects of Isothermal Temperature and Soaking Time on Water Quenched Microstructure of Nickel-Based Superalloy GH3536 Semi-Solid Billets. Materials 2021, 14, 4668. https://doi.org/10.3390/ma14164668
Xiao G, Jiang J, Wang Y, Liu Y, Zhang Y, Tian Y. Effects of Isothermal Temperature and Soaking Time on Water Quenched Microstructure of Nickel-Based Superalloy GH3536 Semi-Solid Billets. Materials. 2021; 14(16):4668. https://doi.org/10.3390/ma14164668
Chicago/Turabian StyleXiao, Guanfei, Jufu Jiang, Ying Wang, Yingze Liu, Ying Zhang, and Yinfeng Tian. 2021. "Effects of Isothermal Temperature and Soaking Time on Water Quenched Microstructure of Nickel-Based Superalloy GH3536 Semi-Solid Billets" Materials 14, no. 16: 4668. https://doi.org/10.3390/ma14164668
APA StyleXiao, G., Jiang, J., Wang, Y., Liu, Y., Zhang, Y., & Tian, Y. (2021). Effects of Isothermal Temperature and Soaking Time on Water Quenched Microstructure of Nickel-Based Superalloy GH3536 Semi-Solid Billets. Materials, 14(16), 4668. https://doi.org/10.3390/ma14164668