Microstructural Design of Ba0.5La0.5Co0.5Fe0.5O3 Perovskite Ceramics
Abstract
:1. Introduction
2. Experimental Route
2.1. Synthesis of Material
2.2. Material Characterisation
3. Results and Discussion
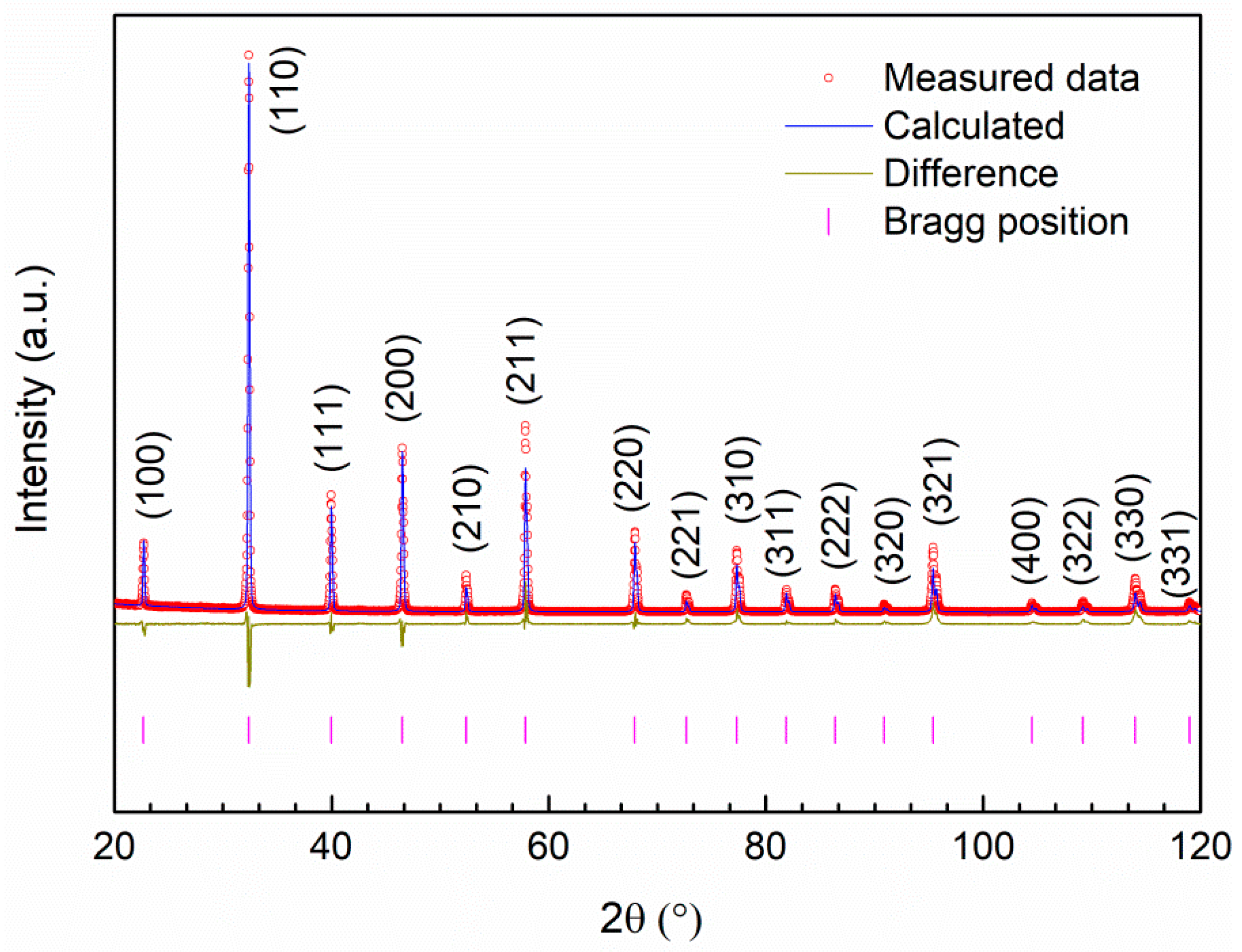
3.1. Structural Analysis
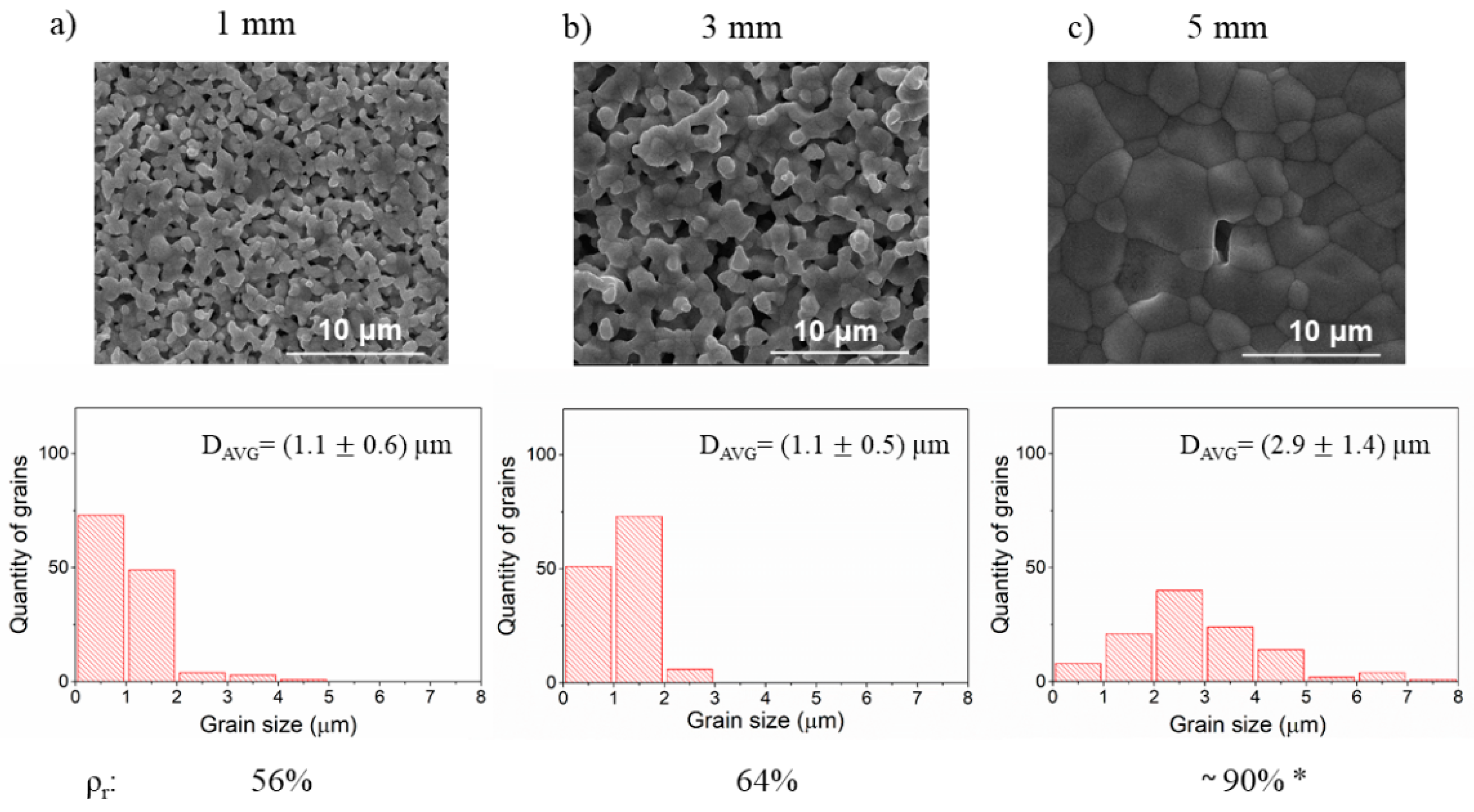
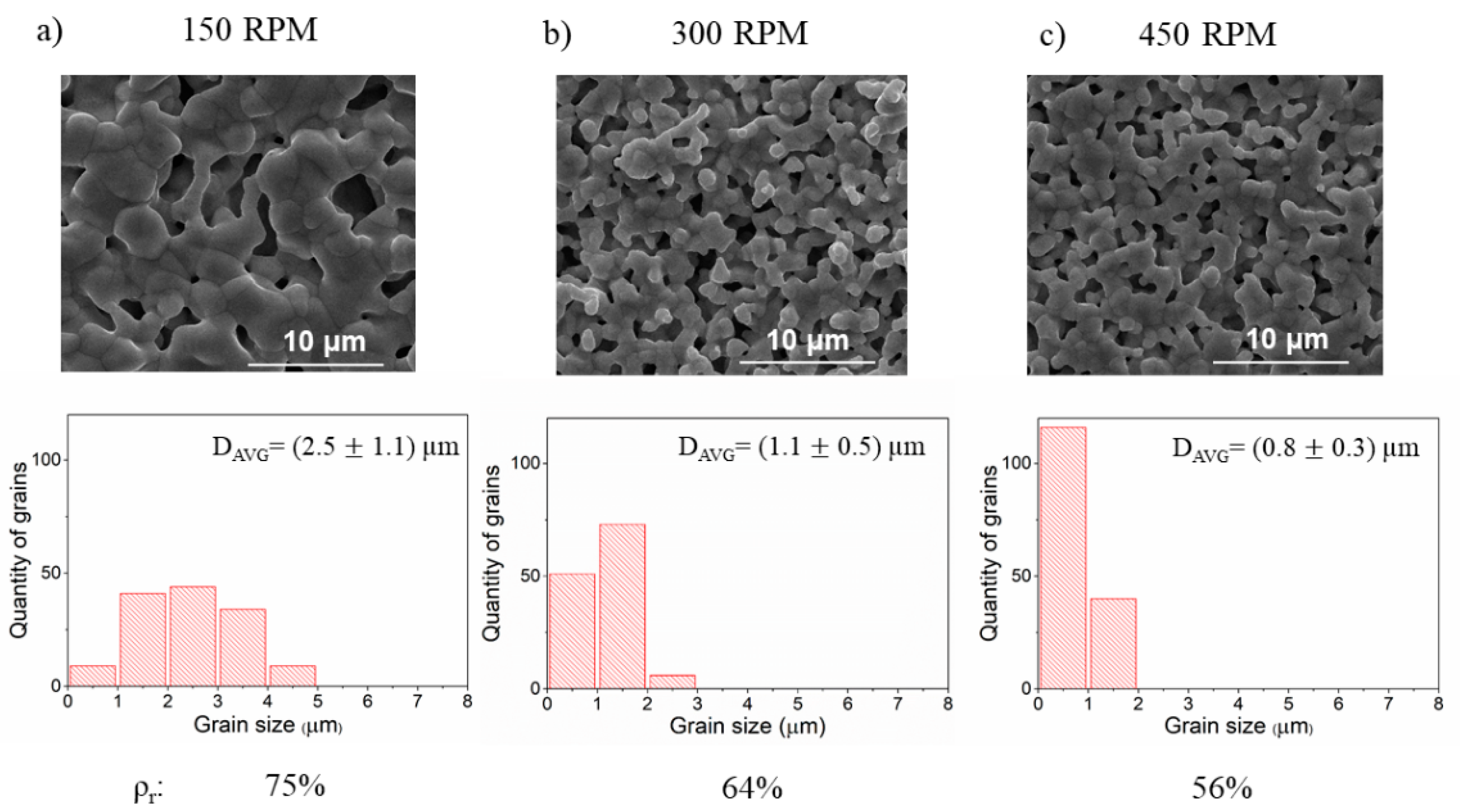
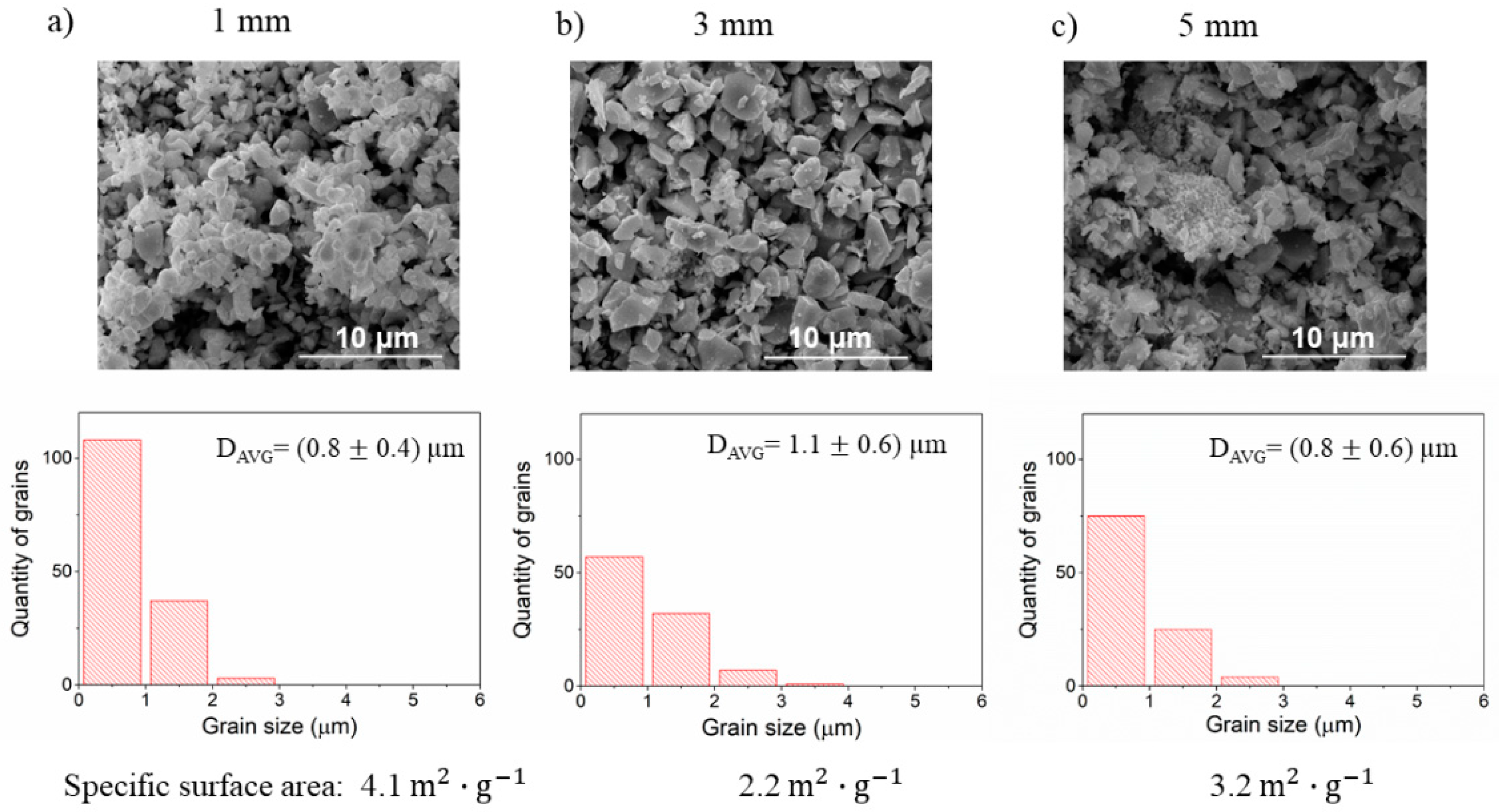
3.2. The Effect of Ball Milling of Precursors on the Microstructure of BLCF Ceramics
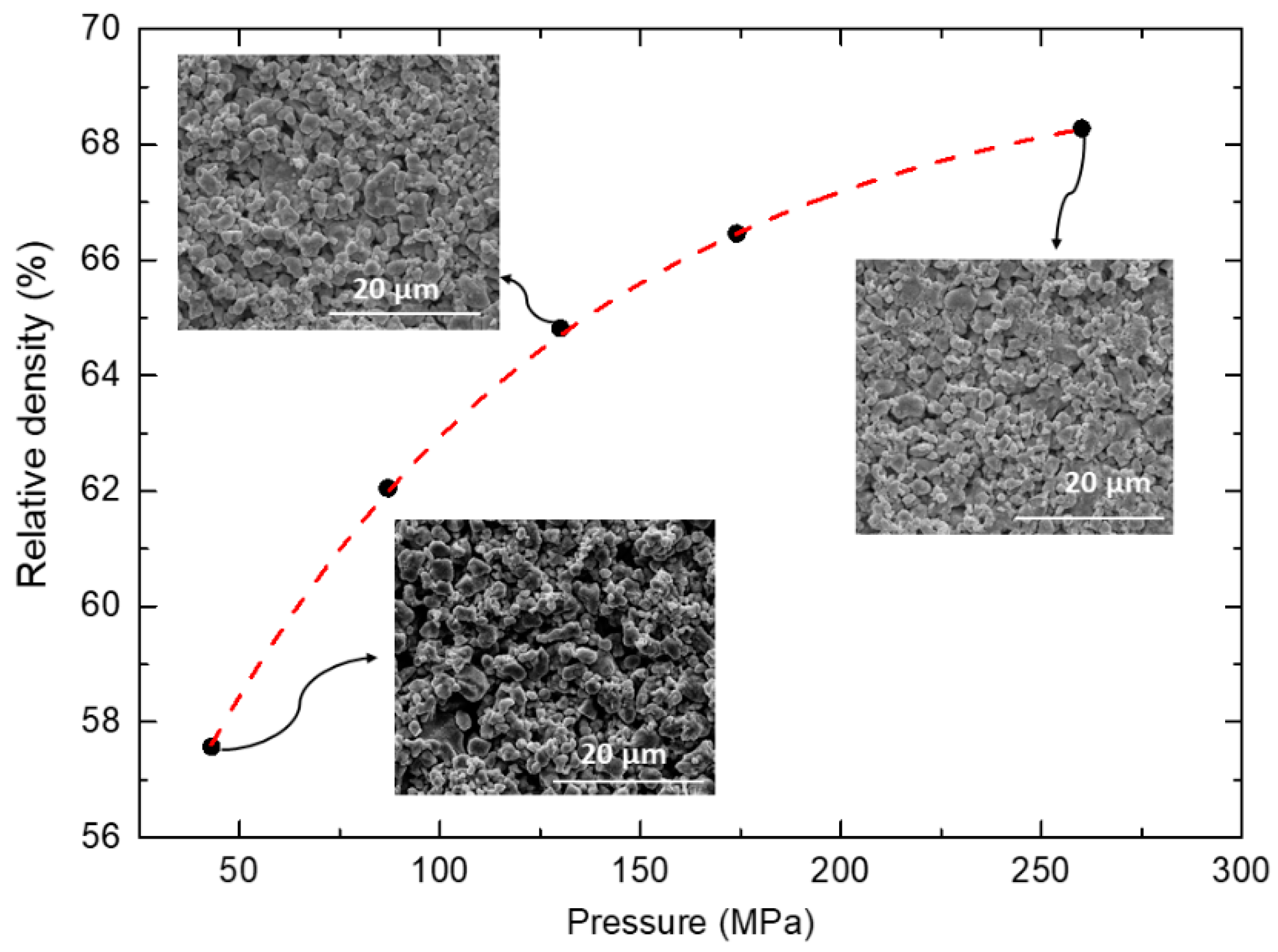
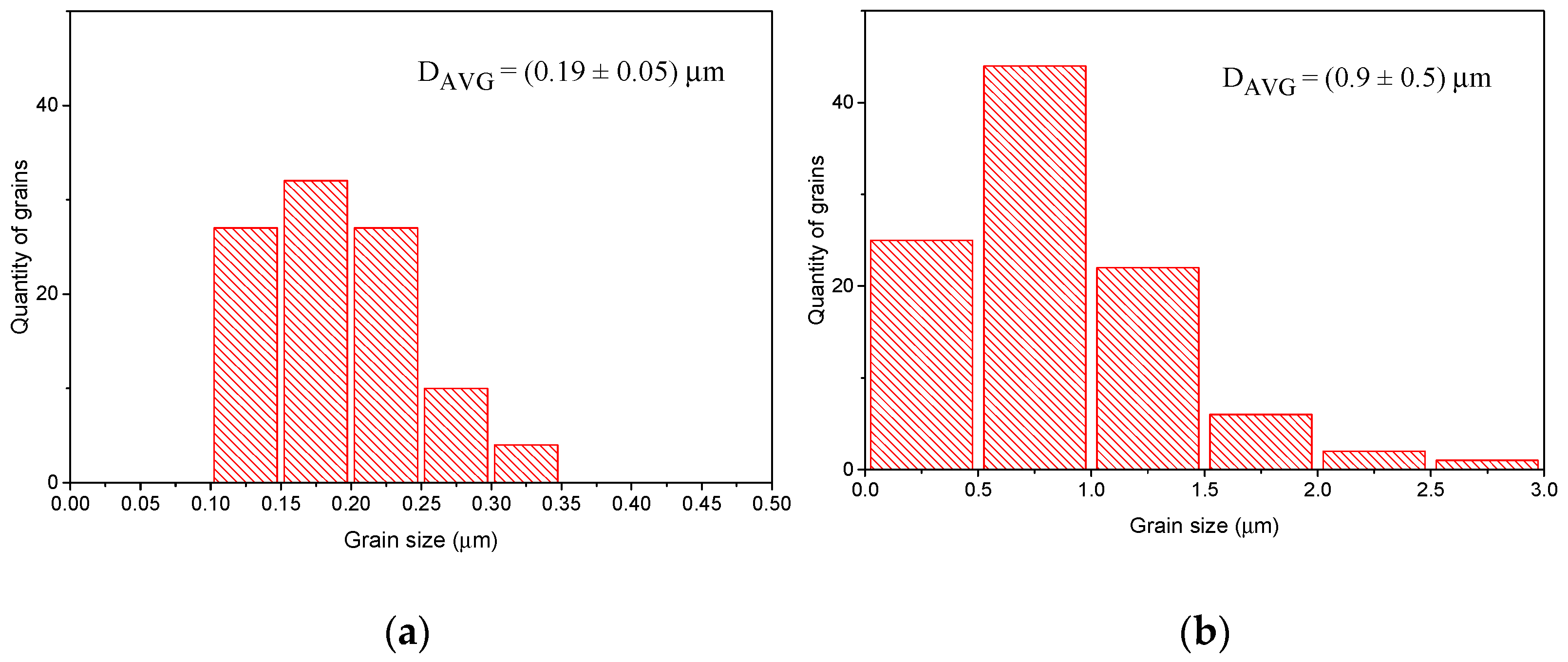
3.3. Influence of BLCF Pelletizing Pressure on the Ceramic Microstructure
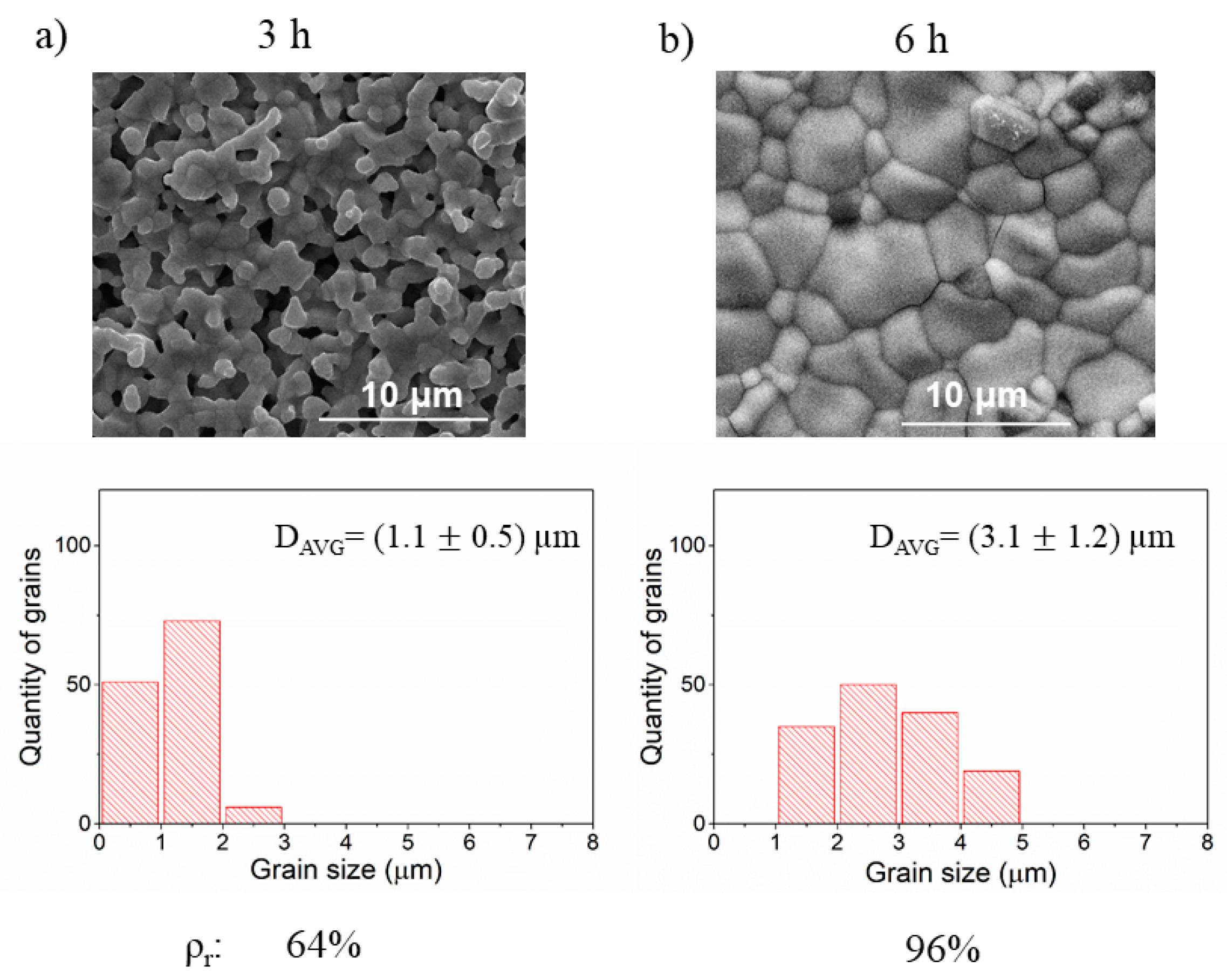
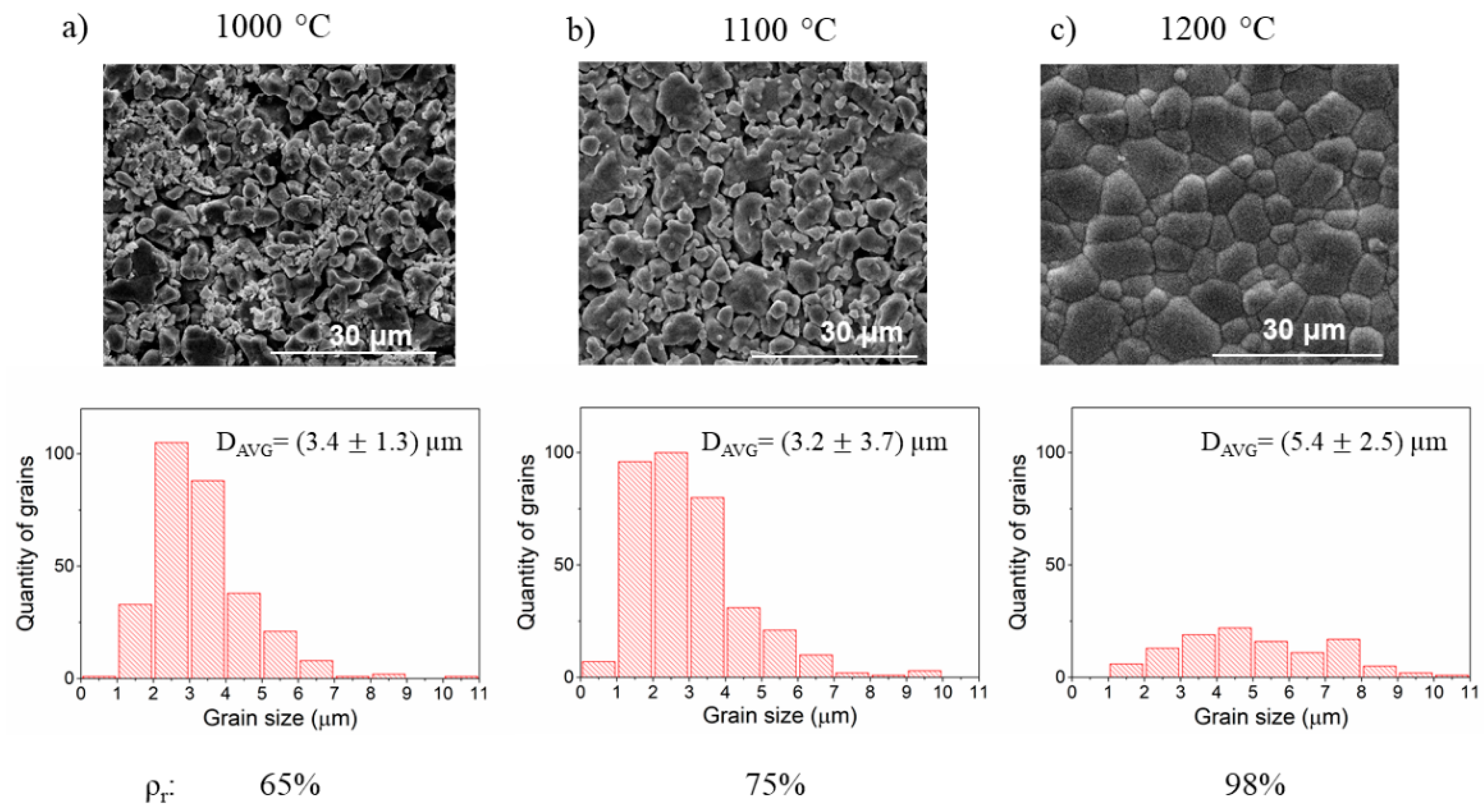
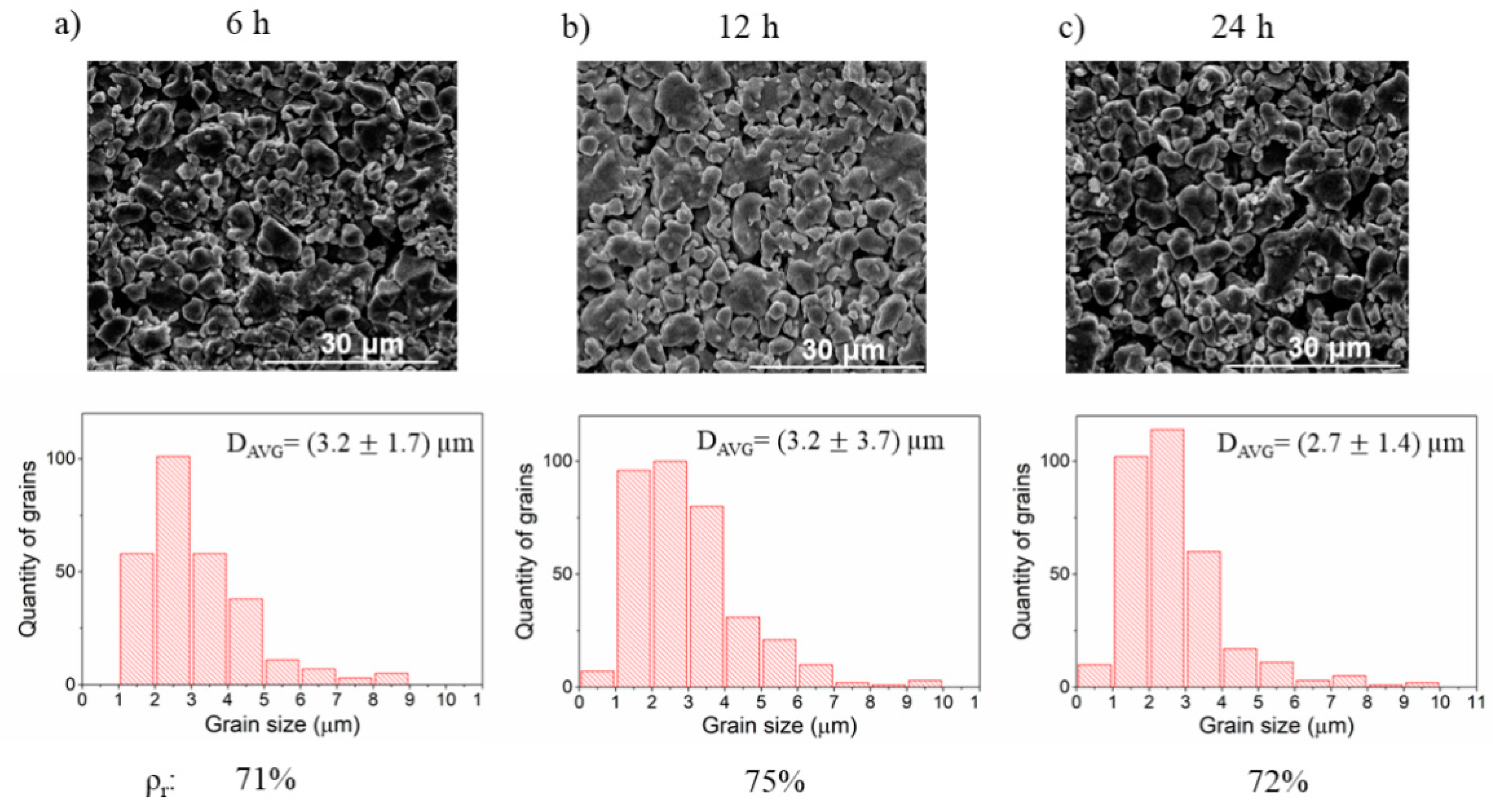
3.4. Influence of Time and Temperature of Annealing on Sintering of BLCF
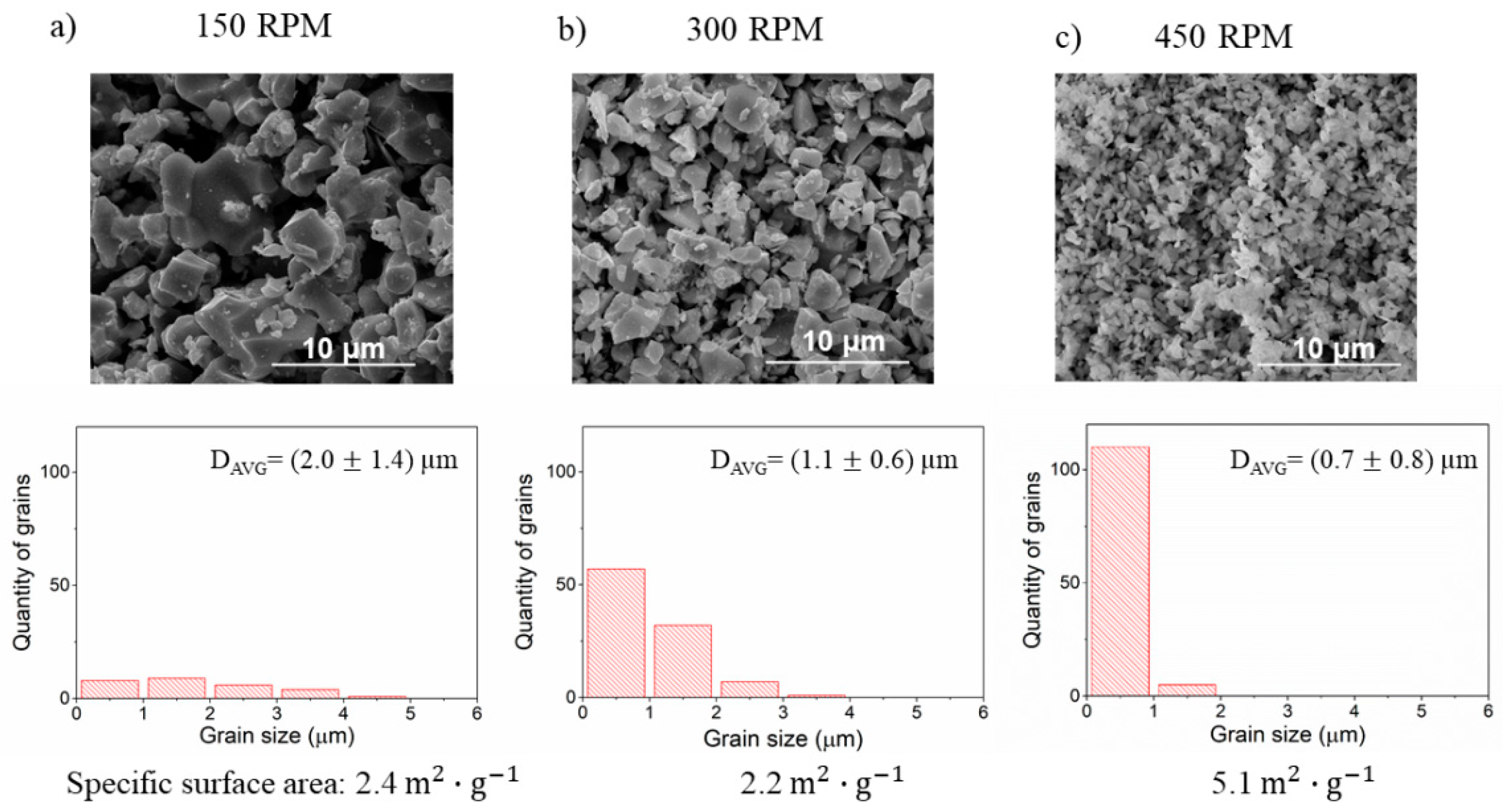
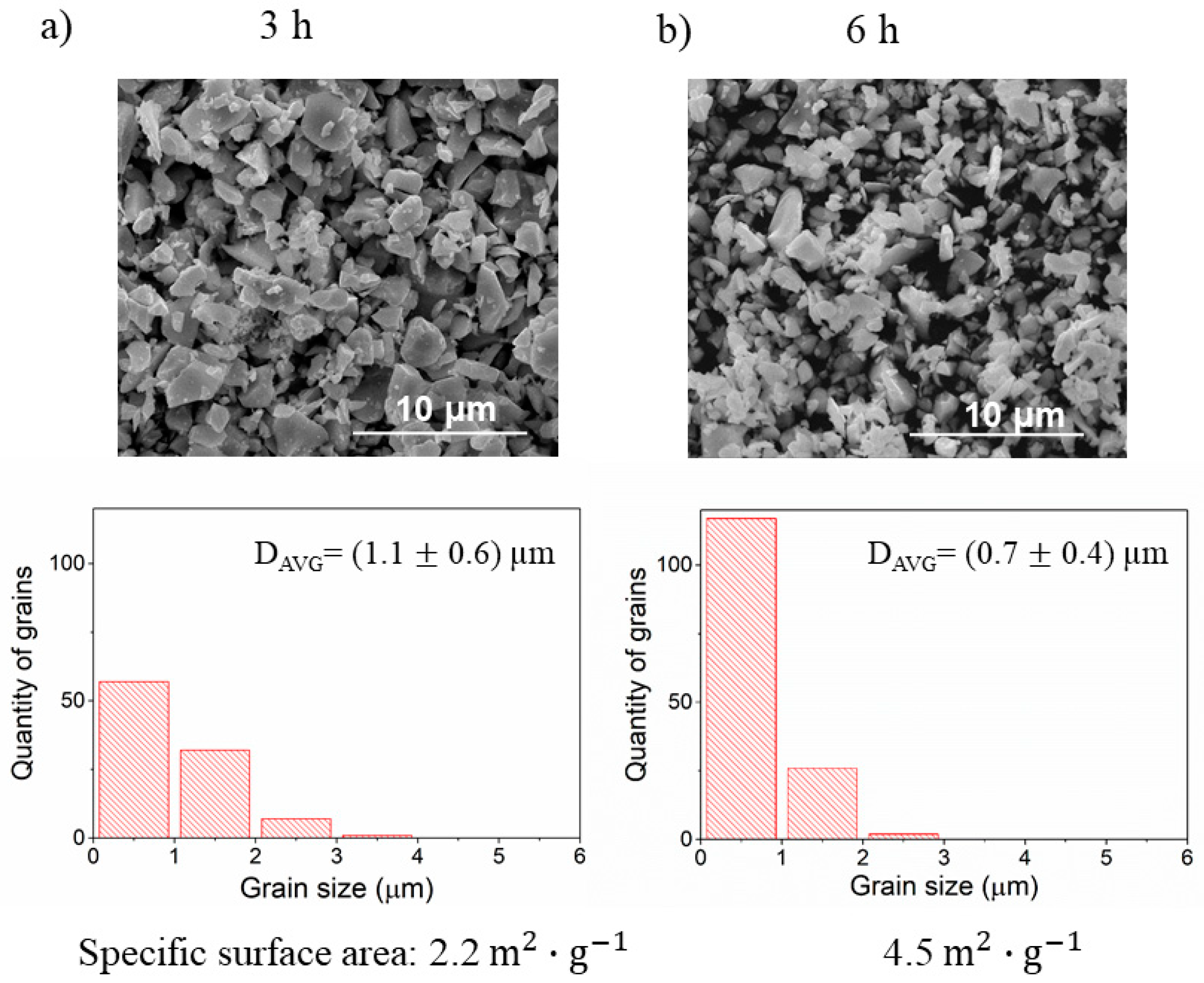
3.5. The Effect of Ball Milling of BLCF Powders on the Grain Size Distribution and Specific Surface Area
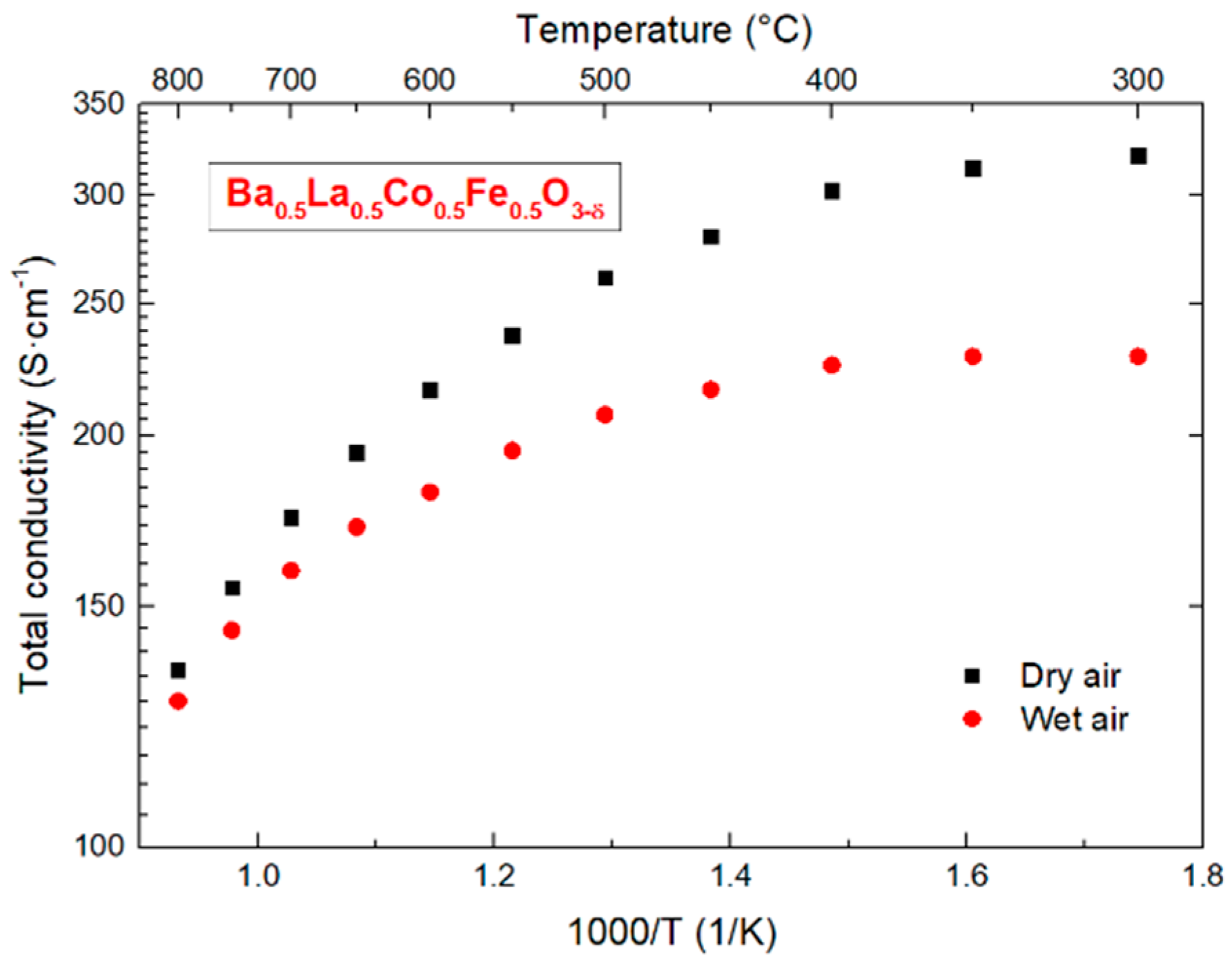
3.6. Conductivity of BLCF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grandjean, S.; Absi, J.; Smith, D.S. Numerical calculations of the thermal conductivity of porous ceramics based on micrographs. J. Eur. Ceram. Soc. 2006, 26, 2669–2676. [Google Scholar] [CrossRef]
- Pabst, W.; Gregorová, E. Conductivity of porous materials with spheroidal pores. J. Eur. Ceram. Soc. 2014, 34, 2757–2766. [Google Scholar] [CrossRef]
- Isobe, T.; Kameshima, Y.; Nakajima, A.; Okada, K.; Hotta, Y. Gas permeability and mechanical properties of porous alumina ceramics with unidirectionally aligned pores. J. Eur. Ceram. Soc. 2007, 27, 53–59. [Google Scholar] [CrossRef]
- Coronas, J.; Santamaría, J. Catalytic reactors based on porous ceramic membranes. Catal. Today 1999, 51, 377–389. [Google Scholar] [CrossRef]
- Martinelli, H.; Lamas, D.G.; Leyva, A.G.; Sacanell, J. Influence of particle size and agglomeration in solid oxide fuel cell cathodes using manganite nanoparticles. Mater. Res. Express 2018, 5, 075013. [Google Scholar] [CrossRef] [Green Version]
- Laohana, P.; Tanapongpisit, N.; Kim, S.; Eknapakul, T.; Fongkaew, I.; Supruangnet, R.; Nakajima, H.; Meevasana, W.; Bark, C.W.; Saenrang, W. Particle size dependence of the electrochemical properties of SrMnO3 supercapacitor electrodes. J. Solid State Electrochem. 2021, 25, 1121–1129. [Google Scholar] [CrossRef]
- Norby, T. Solid-state protonic conductors: Principles, properties, progress and prospects. Solid State Ion. 1999, 125, 1–11. [Google Scholar] [CrossRef]
- Marrony, M. (Ed.) Proton- Conducting Ceramics: From Fundamentals to Applied Research; Jenny Stanford Publishing: Boca Raton, FL, USA, 2016; ISBN 9789814613859. [Google Scholar]
- Nikodemski, S.; Tong, J.; O’Hayre, R. Solid-state reactive sintering mechanism for proton conducting ceramics. Solid State Ion. 2013, 253, 201–210. [Google Scholar] [CrossRef]
- Garcés, D.; Mogni, L. The soft chemical route improving IT-SOFC cathode performance: The lanthanum barium cobaltite case. ECS Trans. 2013, 58, 191–198. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Ma, J.; Yin, Y.; Fronzi, M.; Wang, X.; Bi, L. Tailoring electronic structure of perovskite cathode for proton-conducting solid oxide fuel cells with high performance. J. Power Sources 2021, 489, 1–9. [Google Scholar] [CrossRef]
- Vøllestad, E.; Strandbakke, R.; Tarach, M.; Catalán-Martínez, D.; Fontaine, M.-L.; Beeaff, D.; Clark, D.R.; Serra, J.M.; Norby, T. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 2019, 18, 752–759. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Szpunar, I.; Wachowski, S.; Miruszewski, T.; Dzierzgowski, K.; Górnicka, K.; Klimczuk, T.; Sørby, M.H.; Balaguer, M.; Serra, J.M.; Strandbakke, R.; et al. Electric and magnetic properties of lanthanum barium cobaltite. J. Am. Ceram. Soc. 2020, 103, 1809–1818. [Google Scholar] [CrossRef]
- Szpunar, I.; Strandbakke, R.; Sørby, M.H.; Wachowski, S.L.; Balaguer, M.; Tarach, M.; Serra, J.M.; Witkowska, A.; Dzik, E.; Norby, T.; et al. High-temperature structural and electrical properties of BaLnCo2O6 positrodes. Materials 2020, 13, 4044. [Google Scholar] [CrossRef] [PubMed]
- Wachowski, S.L.; Szpunar, I.; Sørby, M.H.; Mielewczyk–Gryń, A.; Balaguer, M.; Ghica, C.; Istrate, M.C.; Gazda, M.; Gunnæs, A.E.; Serra, J.M.; et al. Structure and water uptake in BaLnCo2O6−δ (Ln = La, Pr, Nd, Sm, Gd, Tb and Dy). Acta Mater. 2020, 199, 297–310. [Google Scholar] [CrossRef]
- Goupil, G.; Delahaye, T.; Gauthier, G.; Sala, B.; Joud, F.L. Stability study of possible air electrode materials for proton conducting electrochemical cells. Solid State Ion. 2012, 209–210, 36–42. [Google Scholar] [CrossRef]
- Raimondi, G.; Giannici, F.; Longo, A.; Merkle, R.; Chiara, A.; Hoedl, M.F.; Martorana, A.; Maier, J. X-ray spectroscopy of (Ba,Sr,La)(Fe,Zn,Y)O3−δ identifies structural and electronic features favoring proton uptake. Chem. Mater. 2020, 32, 8502–8511. [Google Scholar] [CrossRef]
- Løken, A.; Ricote, S.; Wachowski, S. Thermal and chemical expansion in proton ceramic electrolytes and compatible electrodes. Crystals 2018, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Zohourian, R.; Merkle, R.; Raimondi, G.; Maier, J. Mixed-conducting perovskites as cathode materials for protonic ceramic fuel cells: Understanding the trends in proton uptake. Adv. Funct. Mater. 2018, 1801241, 1–10. [Google Scholar] [CrossRef]
- Burgio, N.; Iasonna, A.; Magini, M.; Martelli, S.; Padella, F. Mechanical alloying of the Fe−Zr system. Correlation between input energy and end products. Il Nuovo Cimento D 1991, 13, 459–476. [Google Scholar] [CrossRef]
- Kho, H.X.; Bae, S.; Bae, S.; Kim, B.-W.; Kim, J.S. Planetary ball mill process in aspect of milling energy. J. Korean Powder Metall. Inst. 2014, 21, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Graulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography open database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Ivanova, N.B.; Ovchinnikov, S.G.; Korshunov, M.M.; Eremin, I.M.; Kazak, N.V. Specific features of spin, charge, and orbital ordering in cobaltites. Physics-Uspekhi 2009, 52, 789–810. [Google Scholar] [CrossRef]
- Minet, Y.; Lefranc, V.; Nguyen, N.; Domengès, B.; Maignan, A.; Raveau, B. Magnetic properties of the oxygen deficient perovskites La0.5Ba0.5Co1−xFexO3−δ. J. Solid State Chem. 1996, 121, 158–166. [Google Scholar] [CrossRef]
- Kundu, A.K.; Yu Mychinko, M.; Caignaert, V.; Lebedev, O.I.; Volkova, N.E.; Deryabina, K.M.; Cherepanov, V.A.; Raveau, B. Coherent intergrowth of simple cubic and quintuple tetragonal perovskites in the system Nd2−εBa3+ε(Fe,Co)5O15−δ. J. Solid State Chem. 2015, 231, 36–41. [Google Scholar] [CrossRef]
- Yeh, T.-S.; Sacks, M.D. Effect of particle size distribution on the sintering of alumina. J. Am. Ceram. Soc. 1988, 71, C-484–C-487. [Google Scholar] [CrossRef]
- Hasan, Z.; Pandey, R.K.; Arif Siddiqui, M. Effect of compaction pressure on ceramic compacts. Interceram Int. Ceram. Rev. 2004, 53, 90–95. [Google Scholar]
- Özkan, N. Compaction and Sintering of Ceramic Powders. Ph.D. Thesis, Imperial College London, London, UK, 1994. [Google Scholar]
- Gallas, M.R.; Rosa, A.R.; Costa, T.H.; Da Jornada, J.A.H. High pressure compaction of nanosize ceramic powders. J. Mater. Res. 1997, 12, 764–768. [Google Scholar] [CrossRef]
- Helle, A.S.; Easterling, K.E.; Ashby, M.F. Hot-isostatic pressing diagrams: New developments. Acta Metall. 1985, 33, 2163–2174. [Google Scholar] [CrossRef]
- Vøllestad, E.; Schrade, M.; Segalini, J.; Strandbakke, R.; Norby, T. Relating defect chemistry and electronic transport in the double perovskite Ba1−xGd0.8La0.2+xCo2O6−δ (BGLC). J. Mater. Chem. A 2017, 5, 15743–15751. [Google Scholar] [CrossRef] [Green Version]
- Miruszewski, T.; Dzierzgowski, K.; Winiarz, P.; Wachowski, S.; Mielewczyk-Gryń, A.; Gazda, M. Structural properties and water uptake of STi1−xFexO3−x/2−δ. Materials 2020, 13, 965. [Google Scholar] [CrossRef] [Green Version]
- Zohourian, R.; Merkle, R.; Maier, J. Proton uptake into the protonic cathode material BaCo0.4Fe0.4Zr0.2O3−δ and comparison to protonic electrolyte materials. Solid State Ion. 2017, 299, 64–69. [Google Scholar] [CrossRef]
- Gdula-Kasica, K.; Mielewczyk-Gryn, A.; Molin, S.; Jasinski, P.; Krupa, A.; Kusz, B.; Gazda, M. Optimization of microstructure and properties of acceptor-doped barium cerate. Solid State Ion. 2012, 225, 245–249. [Google Scholar] [CrossRef]
- Wachowski, S.; Mielewczyk-Gryń, A.; Zagórski, K.; Li, C.; Jasiński, P.; Skinner, S.J.; Haugsrud, R.; Gazda, M. Influence of Sb-substitution on ionic transport in lanthanum orthoniobates. J. Mater. Chem. A 2016, 4, 11696–11707. [Google Scholar] [CrossRef] [Green Version]
- Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G. Cobalt based layered perovskites as cathode material for intermediate temperature Solid Oxide Fuel Cells: A brief review. J. Power Sources 2015, 298, 46–67. [Google Scholar] [CrossRef]
- Zuev, A.Y.; Petrov, A.N.; Vylkov, A.I.; Tsvetkov, D.S. Oxygen nonstoichiometry and defect structure of undoped and doped lanthanum cobaltites. J. Mater. Sci. 2007, 42, 1901–1908. [Google Scholar] [CrossRef]
- Patrakeev, M.; Leonidov, I.; Kozhevnikov, V.; Kharton, V. Ion–electron transport in strontium ferrites: Relationships with structural features and stability. Solid State Sci. 2004, 6, 907–913. [Google Scholar] [CrossRef]
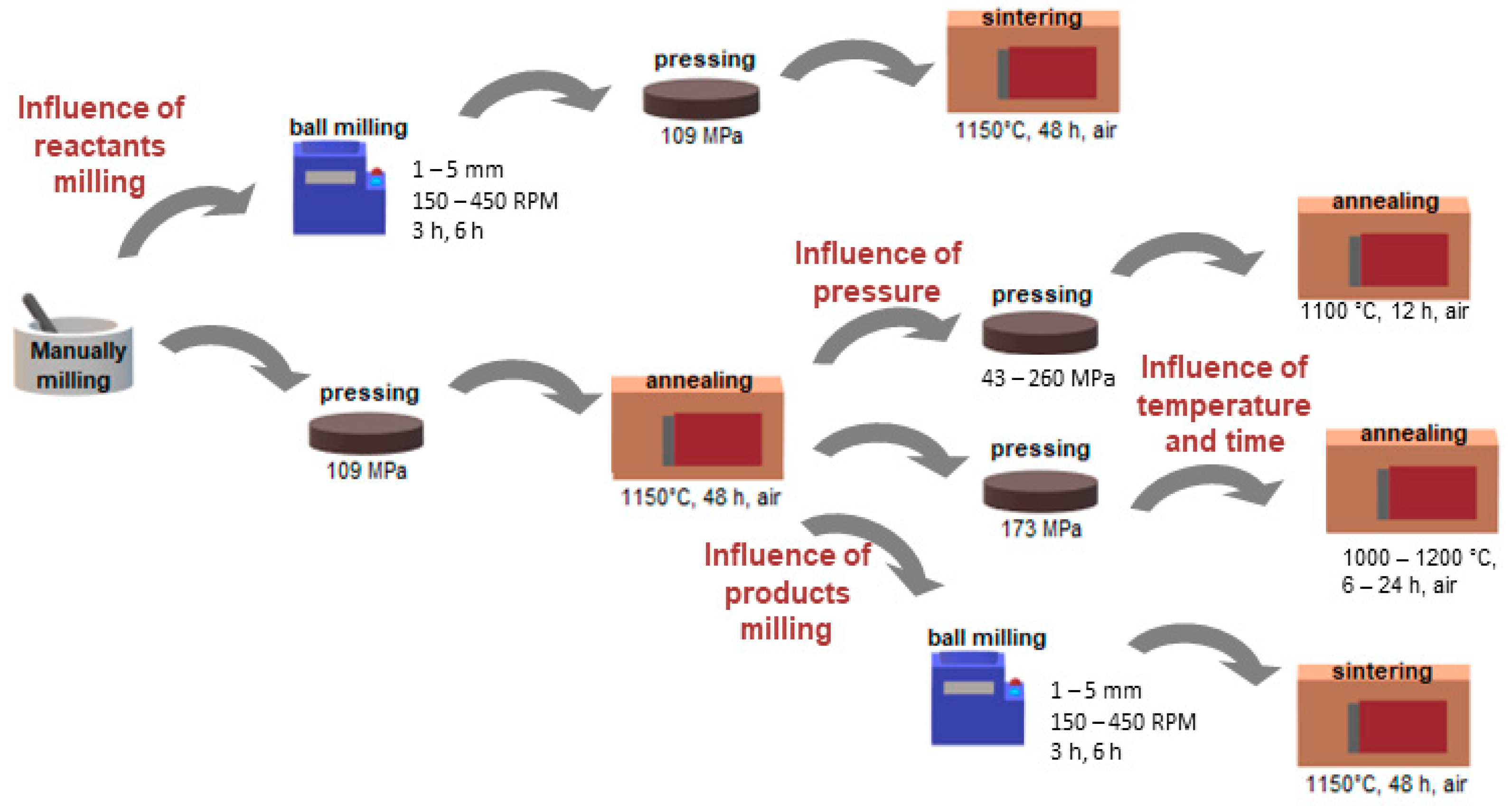
| Proces | Sample ID | Changed Parameters | Additional Information | ||
|---|---|---|---|---|---|
| Size of Media (mm) | Velocity (RPM) | Time (h) | |||
| Precursors milling | PM1 | 1 | constant 300 | constant 3 | In each preparation, the mass of media was constant and equal to 8.5 g. The mass of precursors was about 2 g. Isopropanol was used as the solution during ball milling. |
| PM2 | 3 | ||||
| PM3 | 5 | ||||
| PM4 | constant 3 | 150 | constant 3 | ||
| PM2 | 300 | ||||
| PM5 | 450 | ||||
| PM2 | constant 3 | constant 300 | 3 | ||
| PM6 | 6 | ||||
| Pelletizing pressure | Pressure (MPa) | All samples during preparation were manually ground, pressed under 109 MPa into pellets, and sintered at 1150 °C for 48 h in the air before examination of how pelletizing pressure affects the microstructure. | |||
| PP1 | 43 | ||||
| PP2 | 87 | ||||
| PP3 | 130 | ||||
| PP4 | 174 | ||||
| PP5 | 260 | ||||
| Annealing | Temperature (°C) | Time (h) | Each sample was manually ground, pressed under 109 MPa, sintered, and reground before pressing under 173 MPa. During further annealing, different temperatures and time values were applied. | ||
| A1 | 1000 | constant 12 | |||
| A2 | 1100 | ||||
| A3 | 1200 | ||||
| A4 | constant 1100 | 6 | |||
| A2 | 12 | ||||
| A5 | 24 | ||||
| BLCF powders milling | Size of media (mm) | Velocity (RPM) | Time (h) | In each preparation, the mass of media equaled 8.5 g. The mass of precursors was about 2 g. Isopropanol was used as the solution during ball milling. | |
| BM1 | 1 | constant 300 | constant 3 | ||
| BM2 | 3 | ||||
| BM3 | 5 | ||||
| BM4 | constant 3 | 150 | constant 3 | ||
| BM2 | 300 | ||||
| BM5 | 450 | ||||
| BM2 | constant 3 | constant 300 | 3 | ||
| BM6 | 6 | ||||
| Balls Diameter (mm) | |||||
|---|---|---|---|---|---|
| 1 | 1 | 1.06 | 1700 | 9863 | 0.99 |
| 3 | 1 | 1.01 | 100 | 543 | 0.99 |
| 5 | 1 | 0.98 | 22 | 115 | 0.99 |
| Atom | a (Å) | Wyckoff Position | Position | Occupancy | BAVG | δ | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Ba | 3.9033(1) | 1a | 0 | 0 | 0 | 0.49 | 3.42 | 0.04 |
| La | 1a | 0 | 0 | 0 | 0.51 | |||
| Co | 1b | ½ | ½ | ½ | 0.5 | |||
| Fe | 1b | ½ | ½ | ½ | 0.5 | |||
| O | 1c | ½ | ½ | 0 | 0.96 | |||
| Change | D (mm) | m (g) | N | Ω (RPM) | Eimp | f | P* (10−2 W) | ttot (min) | Etot* (J) |
|---|---|---|---|---|---|---|---|---|---|
| diameter | 1 | 0.005 | 1700 | 300 | 0.1 | 4785 | 6.4 | 150 | 580 |
| 3 | 0.087 | 100 | 300 | 2.2 | 269 | 5.7 | 150 | 516 | |
| 5 | 0.387 | 22 | 300 | 9.0 | 57 | 5.1 | 150 | 458 | |
| velocity | 3 | 0.087 | 100 | 150 | 0.5 | 134 | 0.7 | 150 | 65 |
| 3 | 0.087 | 100 | 300 | 2.2 | 269 | 5.7 | 150 | 516 | |
| 3 | 0.087 | 100 | 450 | 4.9 | 403 | 19.3 | 150 | 1740 | |
| time | 3 | 0.087 | 100 | 300 | 2.2 | 269 | 5.7 | 150 | 516 |
| 3 | 0.087 | 100 | 300 | 2.2 | 269 | 5.7 | 300 | 1030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gierszewska, D.; Szpunar, I.; Oseko, F.; Pośpiech, J.; Nadolska, M.; Pieragowska, M.; Reniecka, K.; Waniek, K.; Leszczyński, K.; Mielewczyk-Gryń, A.; et al. Microstructural Design of Ba0.5La0.5Co0.5Fe0.5O3 Perovskite Ceramics. Materials 2021, 14, 4656. https://doi.org/10.3390/ma14164656
Gierszewska D, Szpunar I, Oseko F, Pośpiech J, Nadolska M, Pieragowska M, Reniecka K, Waniek K, Leszczyński K, Mielewczyk-Gryń A, et al. Microstructural Design of Ba0.5La0.5Co0.5Fe0.5O3 Perovskite Ceramics. Materials. 2021; 14(16):4656. https://doi.org/10.3390/ma14164656
Chicago/Turabian StyleGierszewska, Daria, Iga Szpunar, Francis Oseko, Joanna Pośpiech, Małgorzata Nadolska, Martyna Pieragowska, Karolina Reniecka, Kinga Waniek, Karol Leszczyński, Aleksandra Mielewczyk-Gryń, and et al. 2021. "Microstructural Design of Ba0.5La0.5Co0.5Fe0.5O3 Perovskite Ceramics" Materials 14, no. 16: 4656. https://doi.org/10.3390/ma14164656
APA StyleGierszewska, D., Szpunar, I., Oseko, F., Pośpiech, J., Nadolska, M., Pieragowska, M., Reniecka, K., Waniek, K., Leszczyński, K., Mielewczyk-Gryń, A., Gazda, M., & Wachowski, S. (2021). Microstructural Design of Ba0.5La0.5Co0.5Fe0.5O3 Perovskite Ceramics. Materials, 14(16), 4656. https://doi.org/10.3390/ma14164656






