Sustainable Surface Modification of Polyetheretherketone (PEEK) Implants by Hydroxyapatite/Silica Coating—An In Vivo Animal Study
Abstract
1. Introduction
2. Materials and Methods
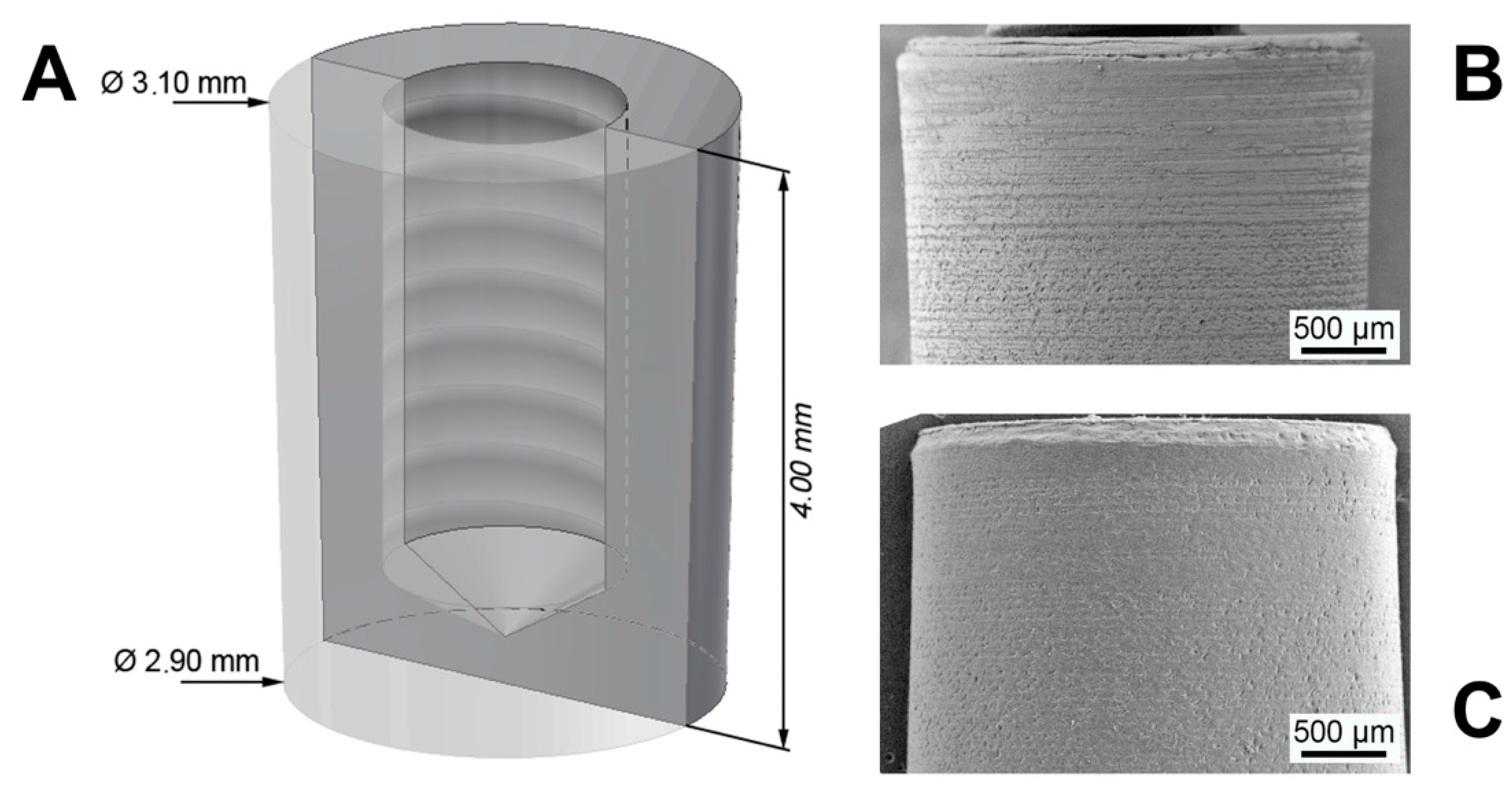
2.1. Study Materials
2.2. Material Characterization
2.3. Animal Model and Procedures
2.4. Pull-Out Tests
2.5. Histological Procedures and Histomorphometrical Measurement
2.6. Statistics
3. Results
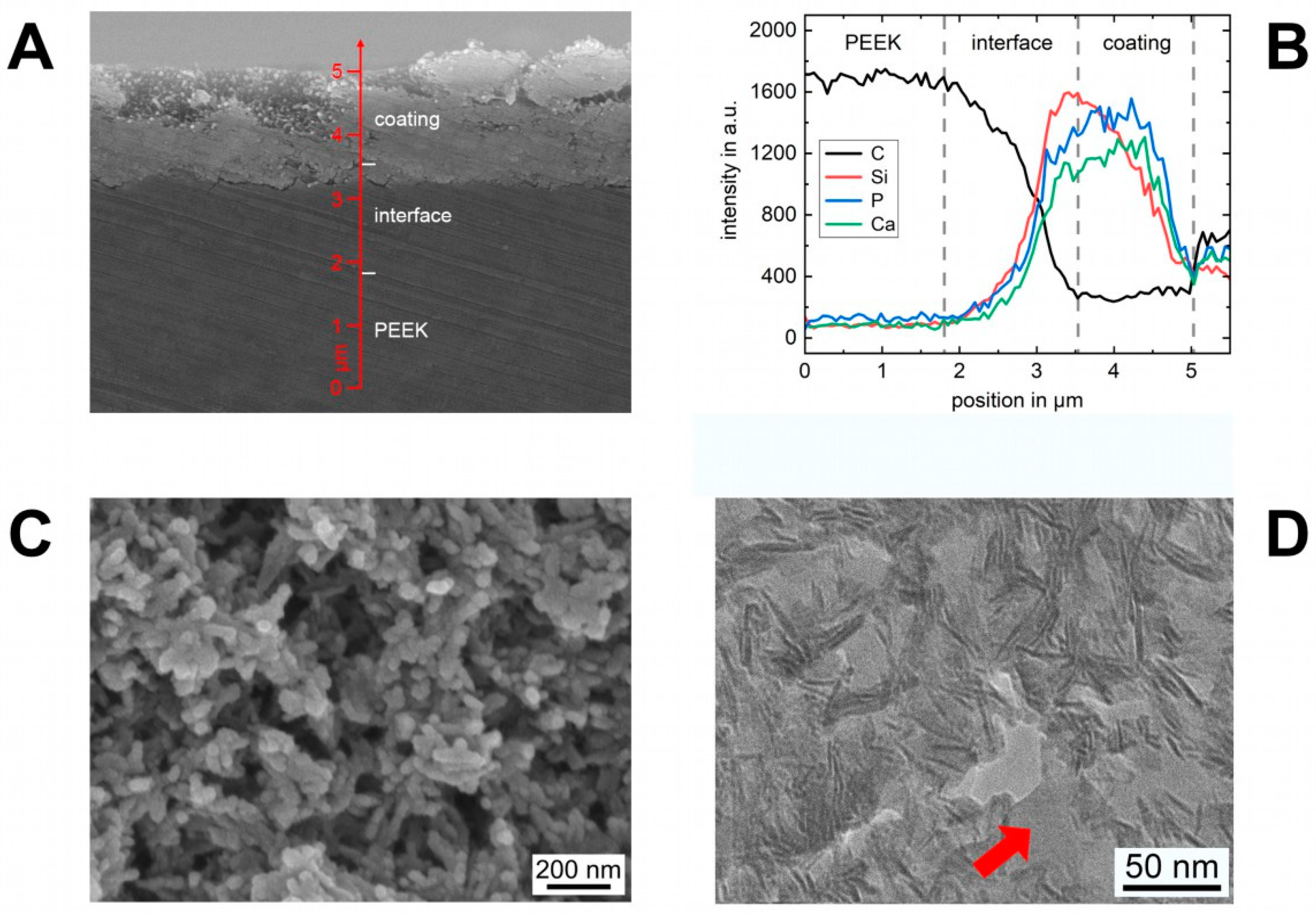
3.1. Coating Characterization
3.2. Interface Characterization
3.3. In Vivo Experiments
3.4. Pull-Out Tests
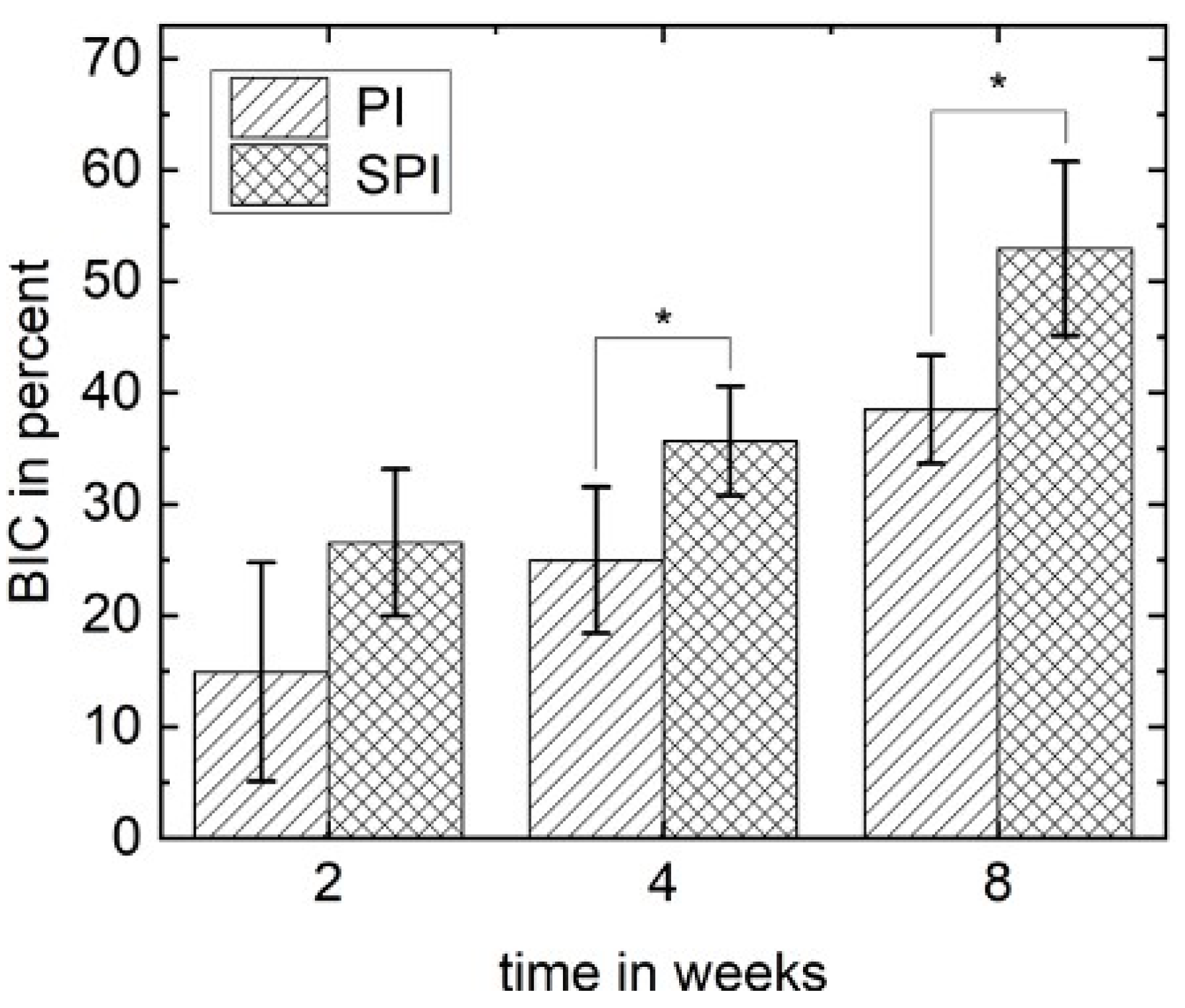
3.5. Histomorphometrical Analysis
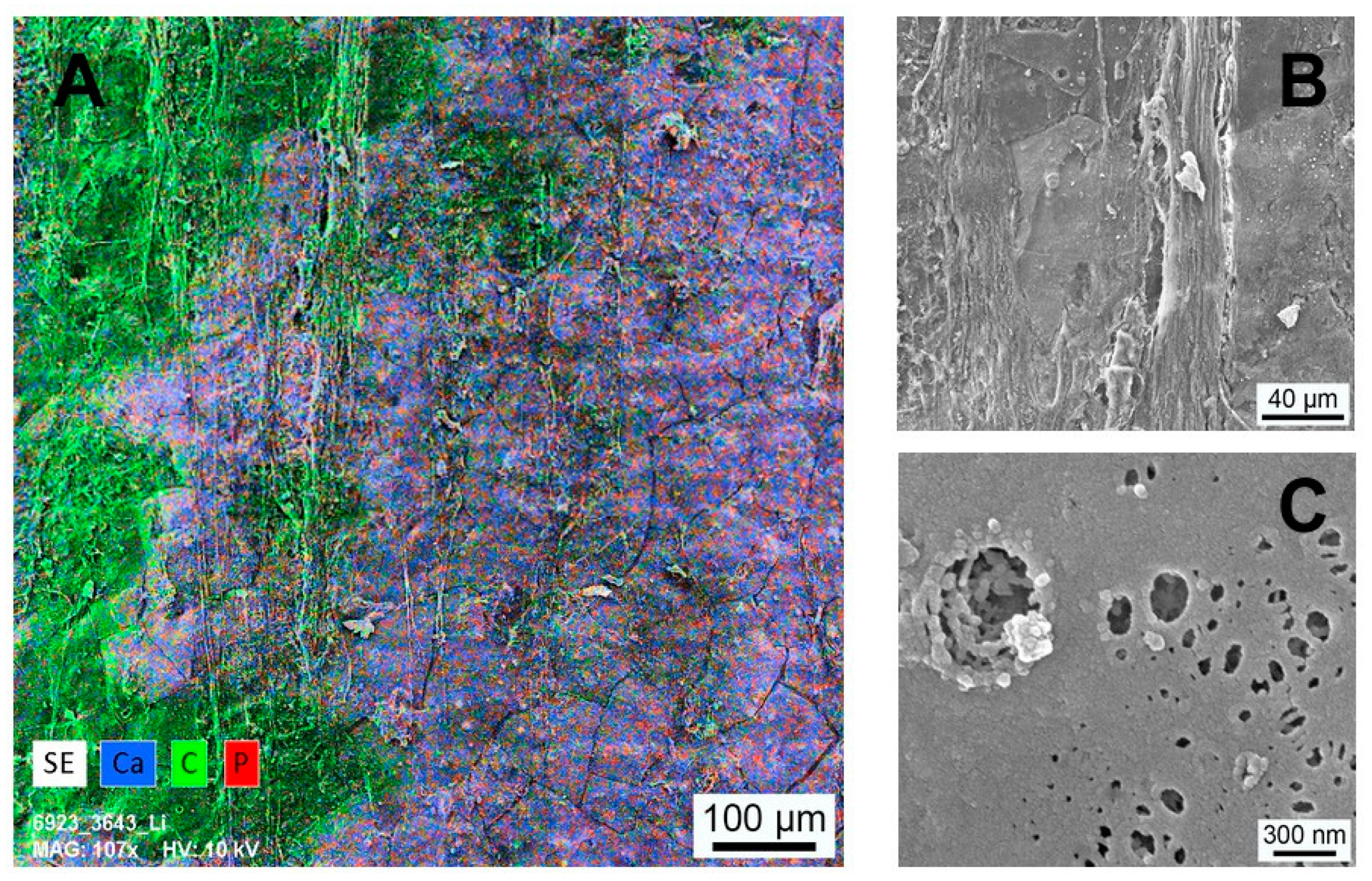
3.6. Histological Analysis
3.7. Implant Surface Characterization
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, M.H.; Zhang, F.; Yin, J.; Xu, H.C.; Lyu, F.Z. Titanium cages versus autogenous iliac crest bone grafts in anterior cervical discectomy and fusion treatment of patients with cervical degenerative diseases: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2017, 33, 803–811. [Google Scholar] [CrossRef]
- Svehla, M.; Morberg, P.; Zicat, B.; Bruce, W.; Sonnabend, D.; Walsh, W.R. Morphometric and mechanical evaluation of titanium implant integration: Comparison of five surface structures. J. Biomed. Mater. Res. 2000, 51, 15–22. [Google Scholar] [CrossRef]
- Rao, P.J.; Pelletier, M.H.; Walsh, W.R.; Mobbs, R.J. Spine interbody implants: Material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop. Surg. 2014, 6, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Ganz, C.; Xu, W.G.; Sarajian, H.R.; Frerich, B.; Gerber, T. How to enhance osseointegration—roughness, hydrophilicity or bioactive coating. Key Eng. Mater. 2012, 493–494, 467–472. [Google Scholar] [CrossRef]
- Khoury, J.; Maxwell, M.; Cherian, R.E.; Bachand, J.; Kurz, A.C.; Walsh, M.; Assad, M.; Svrluga, R.C. Enhanced bioactivity and osseointegration of PEEK with accelerated neutral atom beam technology. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 531–543. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Phan, K.; Assem, Y.; Pelletier, M.; Walsh, W.R. Combination Ti/PEEK ALIF cage for anterior lumbar interbody fusion: Early clinical and radiological results. J. Clin. Neurosci. 2016, 34, 94–99. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Attin, T.; Schmidlin, P.R. In vitro effect of modified polyetheretherketone (PEEK) implant abutments on human gingival epithelial keratinocytes migration and proliferation. Materials 2019, 12, 1401. [Google Scholar] [CrossRef]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef]
- Walsh, W.R.; Bertollo, N.; Christou, C.; Schaffner, D.; Mobbs, R.J. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. 2015, 15, 1041–1049. [Google Scholar] [CrossRef]
- Henriques, B.; Fabris, D.; Mesquita-Guimaraes, J.; Sousa, A.C.; Hammes, N.; Souza, J.C.M.; Silva, F.S.; Fredel, M.C. Influence of laser structuring of PEEK, PEEK-GF30 and PEEK-CF30 surfaces on the shear bond strength to a resin cement. J. Mech. Behav. Biomed. Mater. 2018, 84, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Akkan, C.K.; Hammadeh, M.E.; May, A.; Park, H.W.; Abdul-Khaliq, H.; Strunskus, T.; Aktas, O.C. Surface topography and wetting modifications of PEEK for implant applications. Lasers Med. Sci. 2014, 29, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.R.; Pelletier, M.H.; Bertollo, N.; Christou, C.; Tan, C. Does PEEK/HA enhance bone formation compared with Peek in a sheep cervical fusion model? Clin. Orthop. Relat. Res. 2016, 474, 2364–2372. [Google Scholar] [CrossRef]
- Willems, K.; Lauweryns, P.; Verleye, G.; Van Goethem, J. Randomized controlled trial of posterior lumbar interbody fusion with Ti- and CaP-nanocoated polyetheretherketone cages: Comparative study of the 1-year radiological and clinical outcome. Int. J. Spine Surg. 2019, 13, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Buck, E.; Li, H.; Cerruti, M. Surface modification strategies to improve the osseointegration of Poly(etheretherketone) and its composites. Macromol. Biosci. 2020, 20, e1900271. [Google Scholar] [CrossRef]
- Poulsson, A.H.; Eglin, D.; Zeiter, S.; Camenisch, K.; Sprecher, C.; Agarwal, Y.; Nehrbass, D.; Wilson, J.; Richards, R.G. Osseointegration of machined, injection moulded and oxygen plasma modified PEEK implants in a sheep model. Biomaterials 2014, 35, 3717–3728. [Google Scholar] [CrossRef]
- Sunarso; Tsuchiya, A.; Fukuda, N.; Toita, R.; Tsuru, K.; Ishikawa, K. Effect of micro-roughening of poly(ether ether ketone) on bone marrow derived stem cell and macrophage responses, and osseointegration. J. Biomater. Sci. Polym. Ed. 2018, 29, 1375–1388. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, M.; Wang, D.; Lu, T.; Wang, H.; Meng, F.; Yang, Y.; Ma, J.; Yeung, K.W.K.; Liu, X. Nano textured PEEK surface for enhanced osseointegration. ACS Biomater. Sci. Eng. 2019, 5, 1279–1289. [Google Scholar] [CrossRef]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Zhdanov, V.P.; Besenbacher, F. Enhancement of protein adsorption induced by surface roughness. Langmuir 2006, 22, 10885–10888. [Google Scholar] [CrossRef]
- Kubasiewicz-Ross, P.; Hadzik, J.; Seeliger, J.; Kozak, K.; Jurczyszyn, K.; Gerber, H.; Dominiak, M.; Kunert-Keil, C. New nano-hydroxyapatite in bone defect regeneration: A histological study in rats. Ann. Anat. 2017, 213, 83–90. [Google Scholar] [CrossRef]
- Lukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hedzelek, W.; Dorocka-Bobkowska, B. Effects of a hydroxyapatite coating on the stability of endosseous implants in rabbit tibiae. Dent. Med. Probl. 2019, 56, 123–129. [Google Scholar] [CrossRef]
- Khaled, H.; Atef, M.; Hakam, M. Maxillary sinus floor elevation using hydroxyapatite nano particles vs tenting technique with simultaneous implant placement: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2019, 21, 1241–1252. [Google Scholar] [CrossRef]
- Almasi, D.; Izman, S.; Assadian, M.; Ghanbari, M.; Abdul Kadir, M.R. Crystalline ha coating on peek via chemical deposition. Appl. Surf. Sci. 2014, 314, 1034–1040. [Google Scholar] [CrossRef]
- Adam, M.; Ganz, C.; Xu, W.; Sarajian, H.R.; Gotz, W.; Gerber, T. In vivo and in vitro investigations of a nanostructured coating material—A preclinical study. Int. J. Nanomed. 2014, 9, 975–984. [Google Scholar] [CrossRef][Green Version]
- Rogers, W.J. Steam and Dry Heat Sterilization of Biomaterials and Medical Devices. In Sterilisation of Biomaterials and Medical Devices; Lerouge, S., Simmons, A., Eds.; Woodhead Publishing: Shaston, UK, 2012; p. 352. [Google Scholar]
- Li, X.; Xue, W.; Cao, Y.; Long, Y.; Xie, M. Effect of lycopene on titanium implant osseointegration in ovariectomized rats. J. Orthop. Surg. Res. 2018, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- NemToi, A.; Trandafir, V.; Pasca, A.S.; Sindilar, E.V.; Dragan, E.; Odri, G.A.; NemToi, A.; Haba, D.; Sapte, E. Osseointegration of chemically modified sandblasted and acid-etched titanium implant surface in diabetic rats: A histological and scanning electron microscopy study. Rom. J. Morphol. Embryol. 2017, 58, 881–886. [Google Scholar] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Park, P.J.; Lehman, R.A. Optimizing the spinal interbody implant: Current advances in material modification and surface treatment technologies. Curr. Rev. Musculoskelet. Med. 2020, 13, 688–695. [Google Scholar] [CrossRef]
- Gu, X.; Sun, X.; Sun, Y.; Wang, J.; Liu, Y.; Yu, K.; Wang, Y.; Zhou, Y. Bioinspired modifications of PEEK implants for bone tissue engineering. Front. Bioeng. Biotechnol. 2020, 8, 631616. [Google Scholar] [CrossRef]
- Torstrick, F.B.; Lin, A.S.P.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.J.; Lorman, B.; Fedder, I.L. Improved response of osteoprogenitor cells to titanium plasma-sprayed PEEK surfaces. Colloids Surf. B Biointerfaces 2019, 175, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.M.; Tan, K.K.; Phang, M.Y.; Aziyati, O.; Tan, G.H.; Isa, M.R.; Aminuddin, B.S.; Naseem, M.; Fauziah, O.; Ruszymah, B.H. Differential osteogenic activity of osteoprogenitor cells on HA and TCP/HA scaffold of tissue engineered bone. J. Biomed. Mater. Res. A 2008, 85, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Barkarmo, S.; Wennerberg, A.; Hoffman, M.; Kjellin, P.; Breding, K.; Handa, P.; Stenport, V. Nano-hydroxyapatite-coated PEEK implants: A pilot study in rabbit bone. J. Biomed. Mater. Res. A 2013, 101, 465–471. [Google Scholar] [CrossRef]
- Johansson, P.; Jimbo, R.; Naito, Y.; Kjellin, P.; Currie, F.; Wennerberg, A. Polyether ether ketone implants achieve increased bone fusion when coated with nano-sized hydroxyapatite: A histomorphometric study in rabbit bone. Int. J. Nanomed. 2016, 11, 1435–1442. [Google Scholar] [CrossRef]
- Suska, F.; Omar, O.; Emanuelsson, L.; Taylor, M.; Gruner, P.; Kinbrum, A.; Hunt, D.; Hunt, T.; Taylor, A.; Palmquist, A. Enhancement of CRF-PEEK osseointegration by plasma-sprayed hydroxyapatite: A rabbit model. J. Biomater. Appl. 2014, 29, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Gerber, T.; Lenz, S.; Holzhüter, G.; Götz, W.; Helms, K.; Harms, C.; Mittlmeier, T. Nanostructured bone grafting substitutes—A pathway to osteoinductivity. Key Eng. Mater. 2012, 493–494, 147–152. [Google Scholar] [CrossRef]
- Gotz, W.; Papageorgiou, S.N. Molecular, cellular and pharmaceutical aspects of synthetic hydroxyapatite bone substitutes for oral and maxillofacial grafting. Curr. Pharm. Biotechnol. 2017, 18, 95–106. [Google Scholar] [CrossRef]
- Punke, C.; Zehlicke, T.; Just, T.; Holzhuter, G.; Gerber, T.; Pau, H.W. Matrix change of bone grafting substitute after implantation into guinea pig bulla. Folia Morphol. 2012, 71, 109–114. [Google Scholar]
- Xu, W.; Holzhuter, G.; Sorg, H.; Wolter, D.; Lenz, S.; Gerber, T.; Vollmar, B. Early matrix change of a nanostructured bone grafting substitute in the rat. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 692–699. [Google Scholar] [CrossRef]
- Rodella, L.F.; Bonazza, V.; Labanca, M.; Lonati, C.; Rezzani, R. A review of the effects of dietary silicon intake on bone homeostasis and regeneration. J. Nutr. Health Aging 2014, 18, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Milne, D.B. Growth-promoting effects of silicon in rats. Nature 1972, 239, 333–334. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frankenberger, T.; Graw, C.L.; Engel, N.; Gerber, T.; Frerich, B.; Dau, M. Sustainable Surface Modification of Polyetheretherketone (PEEK) Implants by Hydroxyapatite/Silica Coating—An In Vivo Animal Study. Materials 2021, 14, 4589. https://doi.org/10.3390/ma14164589
Frankenberger T, Graw CL, Engel N, Gerber T, Frerich B, Dau M. Sustainable Surface Modification of Polyetheretherketone (PEEK) Implants by Hydroxyapatite/Silica Coating—An In Vivo Animal Study. Materials. 2021; 14(16):4589. https://doi.org/10.3390/ma14164589
Chicago/Turabian StyleFrankenberger, Thomas, Constantin Leon Graw, Nadja Engel, Thomas Gerber, Bernhard Frerich, and Michael Dau. 2021. "Sustainable Surface Modification of Polyetheretherketone (PEEK) Implants by Hydroxyapatite/Silica Coating—An In Vivo Animal Study" Materials 14, no. 16: 4589. https://doi.org/10.3390/ma14164589
APA StyleFrankenberger, T., Graw, C. L., Engel, N., Gerber, T., Frerich, B., & Dau, M. (2021). Sustainable Surface Modification of Polyetheretherketone (PEEK) Implants by Hydroxyapatite/Silica Coating—An In Vivo Animal Study. Materials, 14(16), 4589. https://doi.org/10.3390/ma14164589






