Kinetic Monte Carlo Simulation of Clustering in an Al-Mg-Si-Cu Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
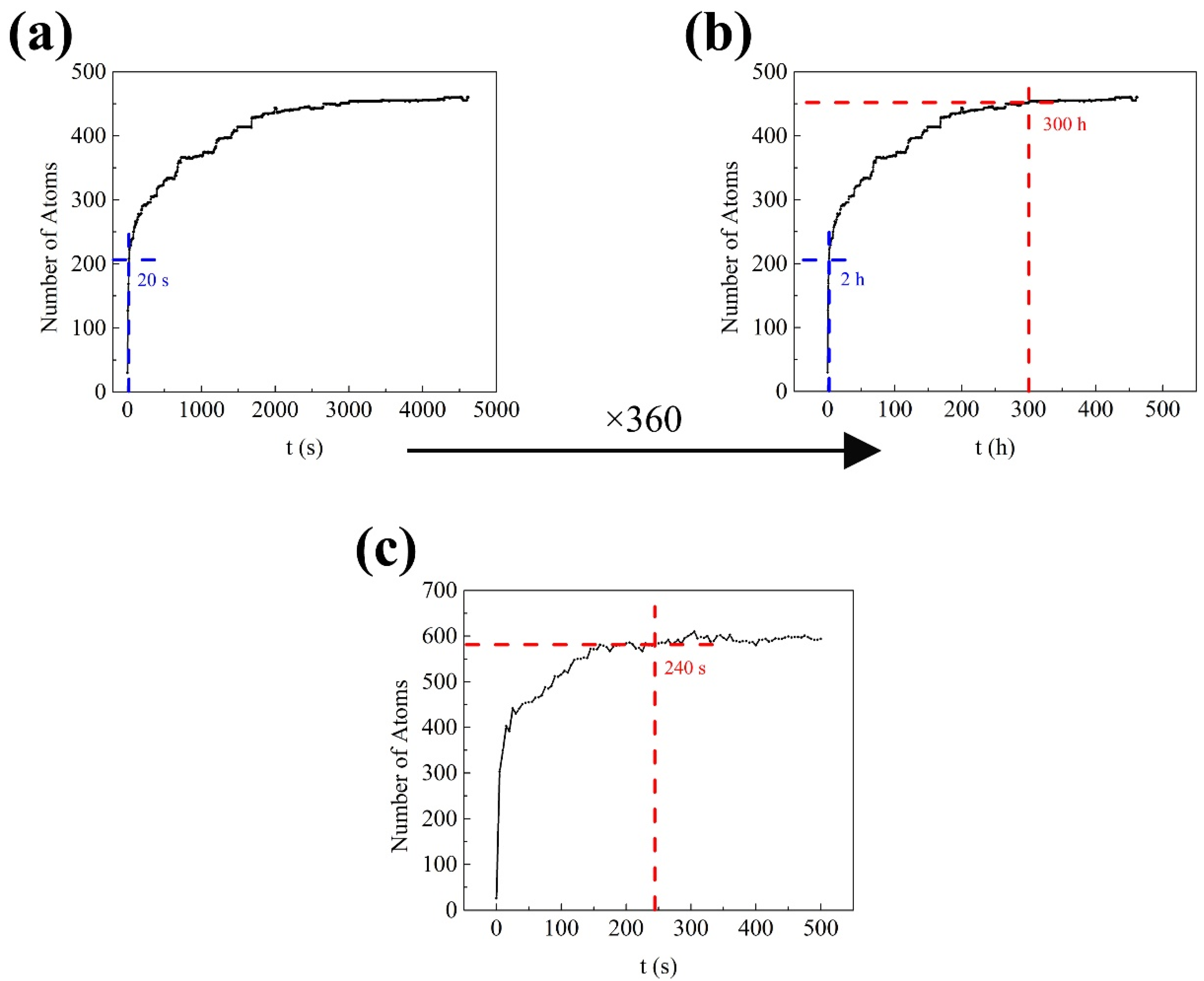
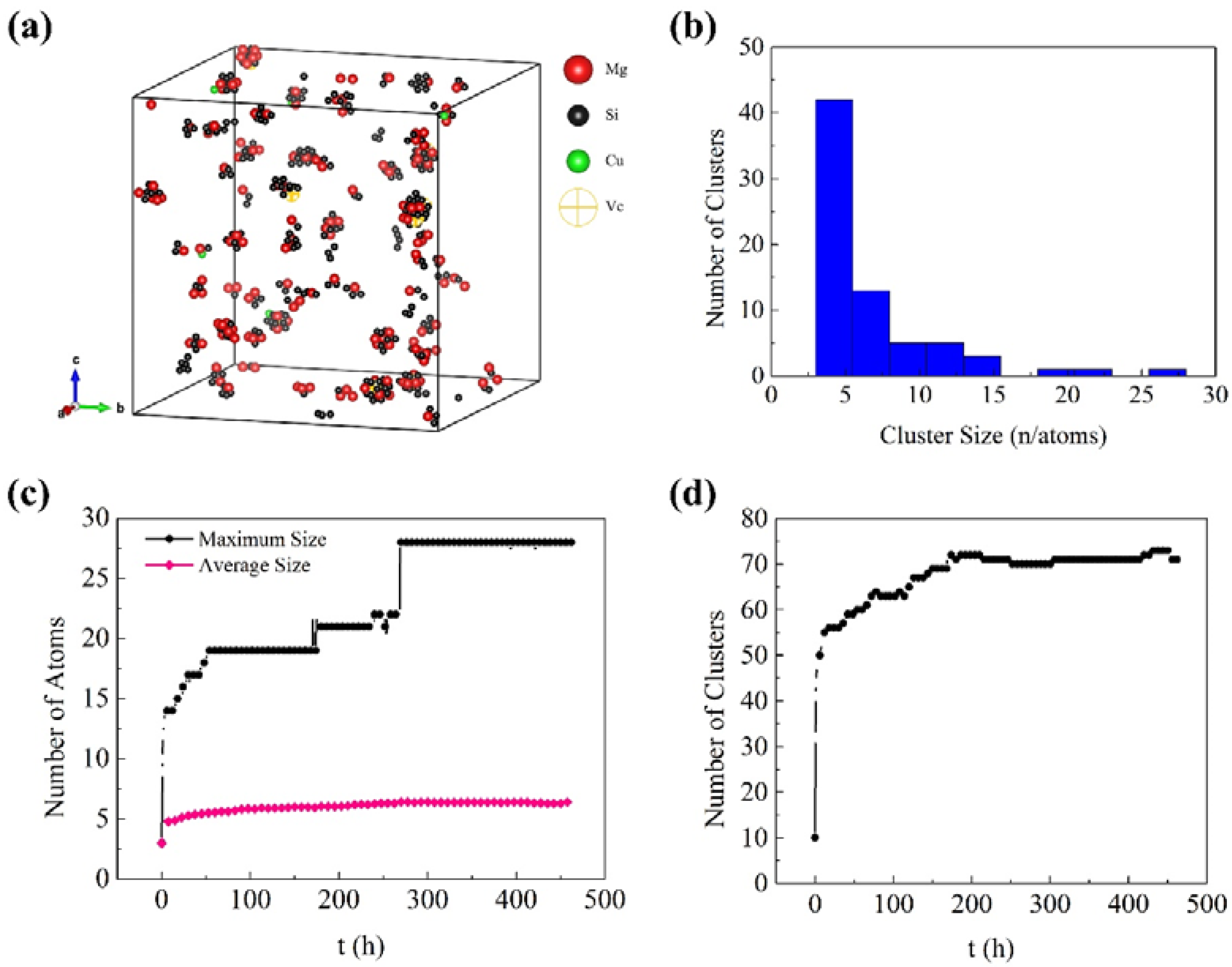
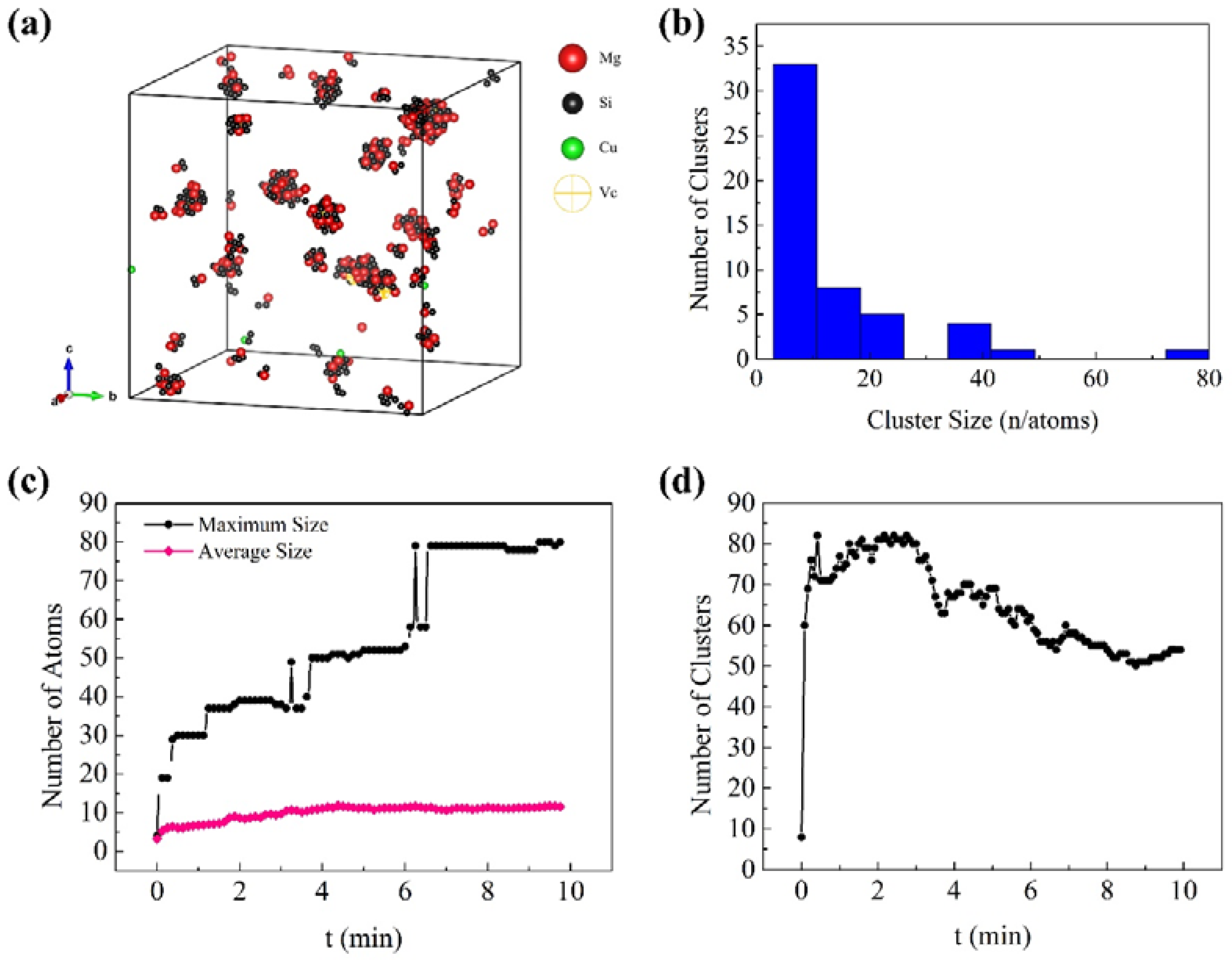
3.1. Early Aging Behavior Simulation
3.2. Reversion Ageing Treatment
4. Conclusions
- (1)
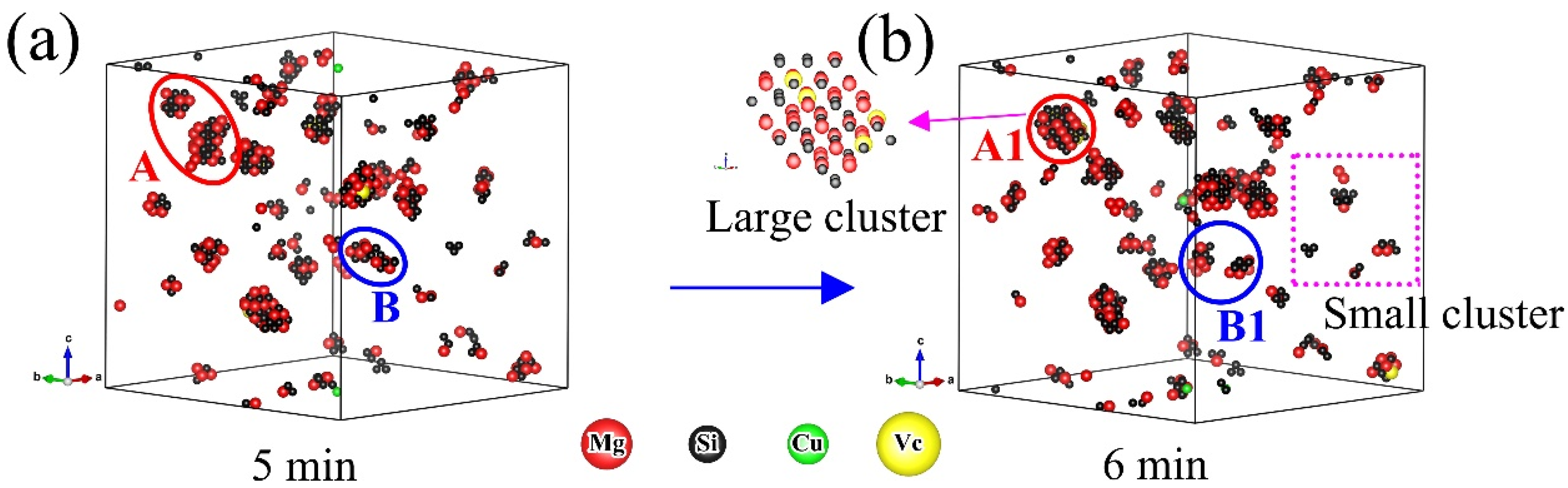
- The formation of many small clusters at the beginning of natural aging and artificial aging is mainly caused by the initial vacancies (quenching vacancies). The clusters containing capturing vacancies can merge with surrounding solute atoms and other clusters, and decompose before reaching a stable size.
- (2)
- After repeated merging and decomposition, the clusters reach stability. Small clusters are generally rich in Si or Mg, while large clusters have similar contents of Mg and Si. The decrease in the number of clusters in the alloy is attributed to the merging and growth of small clusters. The fluctuations and irregularities of the number of clusters are caused by the repeated merging and decomposition of clusters during aging.
- (3)
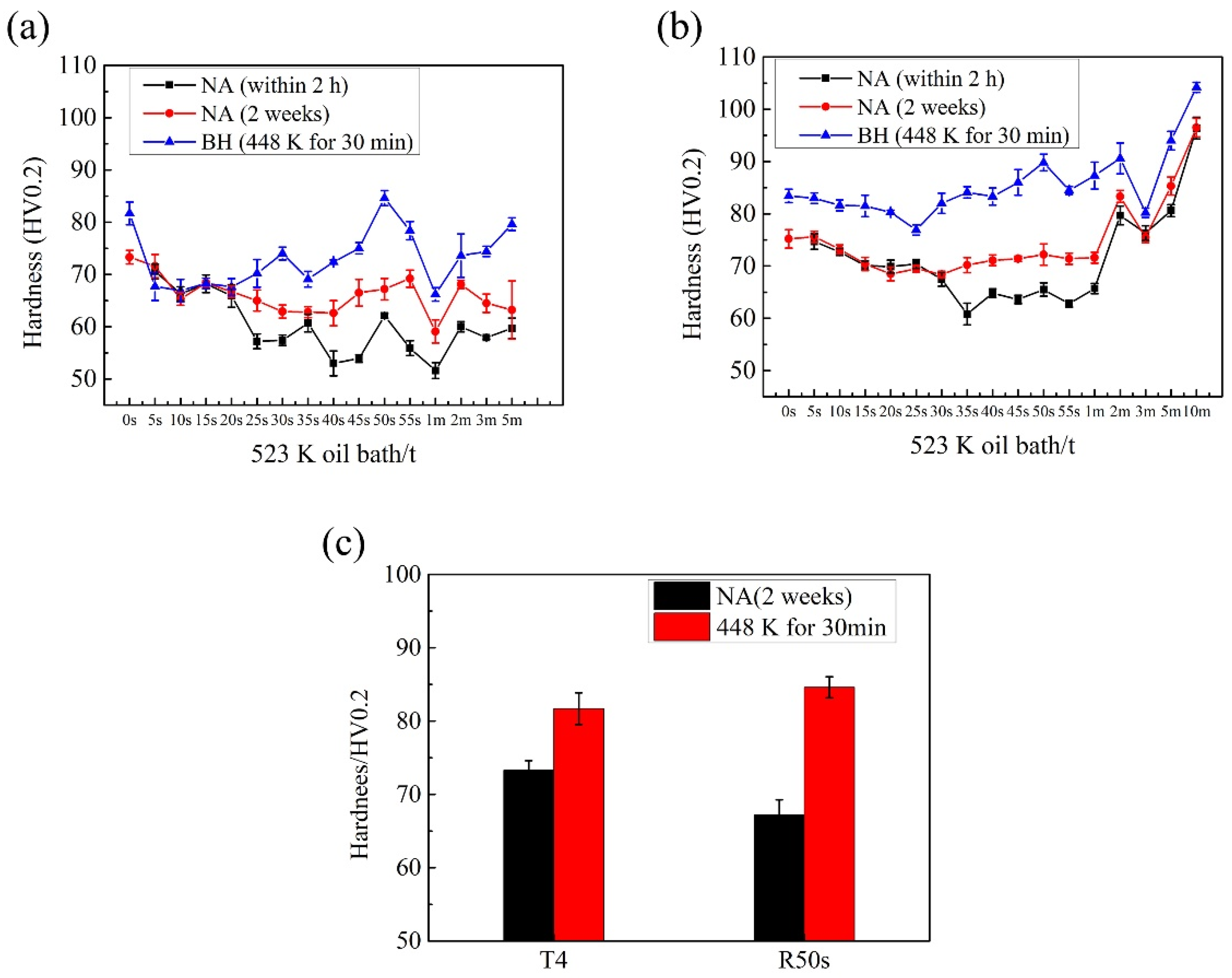
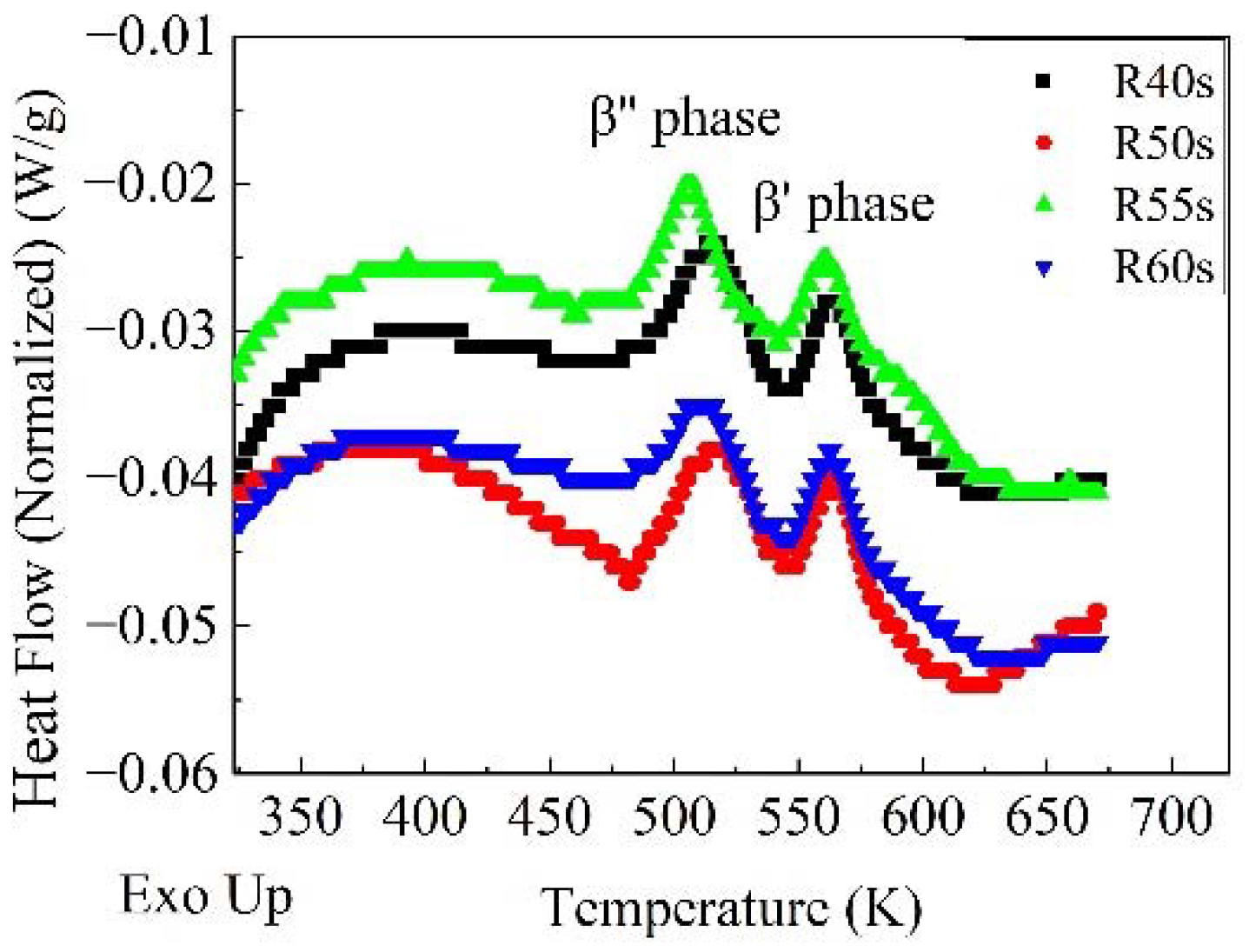
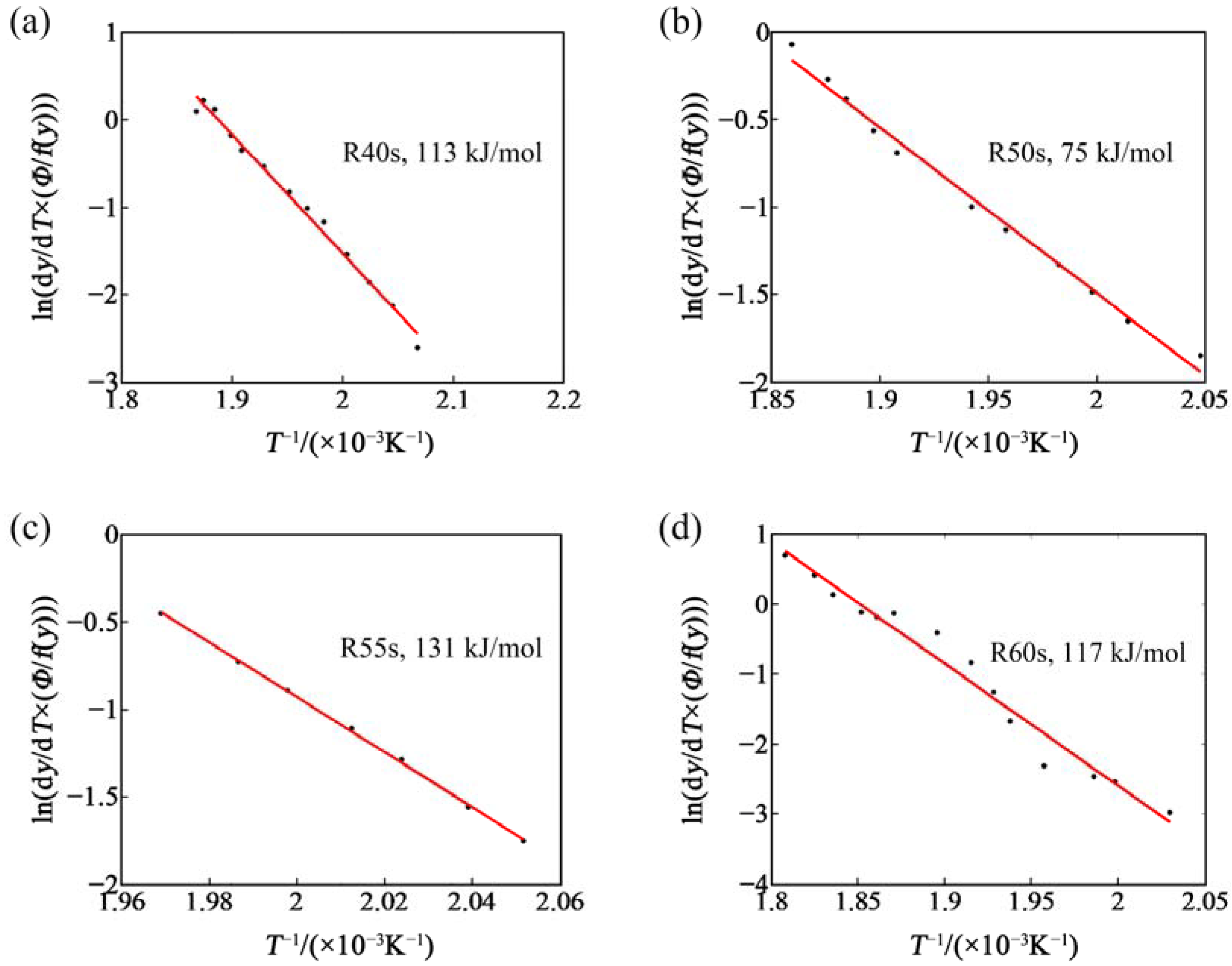
- In the process of reversion aging treatment, the baking hardening increases at first and then decreases, and the change of activation energy of β’’ phase is the opposite. There are some partial irregularities in the change of the decrease of in hardness and the activation energy of β″ phase with the extension of reversion aging time due to the interaction of decomposition and aggregation of clusters. The bake hardening of the sample after reversion aging in an oil bath at 523 K for 50 s is the best.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, W.; Zhuang, L.; Bottema, J.; Wittebrood, A.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Stene, T.N.; Hasting, H.; Walmsley, J.; Van Helvoort, A.T.J.; Holmestad, R. The effect of Cu on precipitation in Al–Mg–Si alloys. Philos. Mag. 2007, 87, 3385–3413. [Google Scholar] [CrossRef]
- Ding, L.; Jia, Z.; Nie, J.-F.; Weng, Y.; Cao, L.; Chen, H.; Wu, X.; Liu, Q. The structural and compositional evolution of precipitates in Al-Mg-Si-Cu alloy. Acta Mater. 2018, 145, 437–450. [Google Scholar] [CrossRef]
- Van Huis, M.A.; Chen, J.H.; Sluiter, M.H.F.; Zandbergen, H.W. Phase stability and structural features of matrix-embedded hardening precipitates in Al–Mg–Si alloys in the early stages of evolution. Acta Mater. 2007, 55, 2183–2199. [Google Scholar] [CrossRef]
- Banhart, J.; Chang, C.S.T.; Liang, Z.; Wanderka, N.; Lay, M.D.H.; Hill, A.J. Natural Aging in Al-Mg-Si Alloys—A Process of Unexpected Complexity. Adv. Eng. Mater. 2010, 12, 559–571. [Google Scholar] [CrossRef]
- Buha, J.; Lumley, R.N.; Crosky, A.G.; Hono, K. Secondary precipitation in an Al–Mg–Si–Cu alloy. Acta Mater. 2007, 55, 3015–3024. [Google Scholar] [CrossRef]
- Pogatscher, S. Phase Transitions in Quenched Nonferrous Metallic Systems; Montanuniversität Leoben: Leoben, Austria, 2017. [Google Scholar]
- Aruga, Y.; Kozuka, M.; Sato, T. Formulation of initial artificial age-hardening response in an Al-Mg-Si alloy based on the cluster classification using a high-detection-efficiency atom probe. J. Alloys Compd. 2018, 739, 1115–1123. [Google Scholar] [CrossRef]
- Dumitraschkewitz, P.; Gerstl, S.S.A.; Stephenson, L.T.; Uggowitzer, P.J.; Pogatscher, S. Clustering in Age-Hardenable Aluminum Alloys. Adv. Eng. Mater. 2018, 1800255. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, L.T.; Moody, M.P.; Gault, B.; Ringer, S.P. Estimating the physical cluster-size distribution within materials using atom-probe. Microsc. Res. Tech. 2011, 74, 799–803. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Kinetic Monte Carlo simulation of clustering in an Al–Zn–Mg–Cu alloy (7050). Acta Mater. 2005, 53, 907–917. [Google Scholar] [CrossRef]
- Soisson, F.; Barbu, A.; Martin, G. Monte Carlo simulations of copper precipitation in dilute iron-copper alloys during thermal ageing and under electron irradiation. Acta Mater. 1996, 44, 3789–3800. [Google Scholar] [CrossRef]
- Abinandanan, T.A.; Haider, F.; Martin, G. Computer simulations of diffusional phase transformations: Monte Carlo algorithm and application to precipitation of ordered phases. Acta Mater. 1998, 46, 4243–4255. [Google Scholar] [CrossRef]
- Madanat, M.; Liu, M.; Banhart, J. Reversion of natural ageing in Al-Mg-Si alloys. Acta Mater. 2018, 159, 163–172. [Google Scholar] [CrossRef]
- Hirosawa, S.; Sato, T.; Kamio, A.; Flower, H.M. Classification of the role of microalloying elements in phase decomposition of Al based alloys. Acta Mater. 2000, 48, 1797–1806. [Google Scholar] [CrossRef]
- Slabanja, M.; Wahnström, G. Kinetic Monte Carlo study of Al–Mg precipitation. Acta Mater. 2005, 53, 3721–3728. [Google Scholar] [CrossRef]
- Clouet, E.; Hin, C.; Gendt, D.; Nastar, M.; Soisson, F. Kinetic Monte Carlo Simulations of Precipitation. Adv. Eng. Mater. 2006, 8, 1210–1214. [Google Scholar] [CrossRef]
- Grimvall, G. Thermophysical Properties of Materials; North-Holland Publishing Company: North-Holland, The Netherlands, 1986. [Google Scholar]
- Carling, K.M.; Wahnström, G.; Mattsson, T.R.; Sandberg, N.; Grimvall, G. Vacancy concentration in Al from combined first-principles and model potential calculations. Phys. Rev. B 2003, 67, 054101. [Google Scholar] [CrossRef]
- Doyama, M. Vacancy-solute interactions in metals. J. Nucl. Mater. 1978, 69, 350–361. [Google Scholar] [CrossRef]
- Doyama, M.; Koehler, J.S. The relation between the formation energy of a vacancy and the nearest neighbor interactions in pure metals and liquid metals. Acta Metall. 1976, 24, 871–879. [Google Scholar] [CrossRef]
- Brandes, E.A.; Brook, G.B. Smithells Metals Reference Book; Butterworth-Heinemann Ltd.: Oxford, UK, 1992. [Google Scholar]
- Du, Y.; Chang, Y.; Huang, B.; Gong, W.; Jin, Z.; Xu, H.; Yuan, Z.; Liu, Y.; He, Y.; Xie, F.-Y. Diffusion coefficients of some solutes in fcc and liquid Al: Critical evaluation and correlation. Mater. Sci. Eng. A 2003, 363, 140–151. [Google Scholar] [CrossRef]
- Pogatscher, S.; Antrekowitsch, H.; Leitner, H.; Ebner, T.; Uggowitzer, P.J. Mechanisms controlling the artificial aging of Al–Mg–Si Alloys. Acta Mater. 2011, 59, 3352–3363. [Google Scholar] [CrossRef]
- Esmaeili, S.; Lloyd, D.J.; Poole, W.J. Modeling of precipitation hardening for the naturally aged Al-Mg-Si-Cu alloy AA6111. Acta Mater. 2003, 51, 3467–3481. [Google Scholar] [CrossRef]
- Aruga, Y.; Kozuka, M.; Takaki, Y.; Sato, T. Formation and reversion of clusters during natural aging and subsequent artificial aging in an Al–Mg–Si alloy. Mater. Sci. Eng. A 2015, 631, 86–96. [Google Scholar] [CrossRef]
- Cao, L.; Rometsch, P.A.; Couper, M.J. Clustering behaviour in an Al–Mg–Si–Cu alloy during natural ageing and subsequent under-ageing. Mater. Sci. Eng. A 2013, 559, 257–261. [Google Scholar] [CrossRef]
- Guo, M.X.; Zhang, Y.D.; Li, G.J.; Jin, S.B.; Sha, G.; Zhang, J.S.; Zhuang, L.Z.; Lavernia, E.J. Solute clustering in Al-Mg-Si-Cu-(Zn) alloys during aging. J. Alloys Compd. 2019, 774, 347–363. [Google Scholar] [CrossRef]
- Buha, J.; Lumley, R.N.; Crosky, A.G. Precipitation and solute distribution in an interrupted-aged Al–Mg–Si–Cu alloy. Philos. Mag. 2008, 88, 373–390. [Google Scholar] [CrossRef]
- Marceau, R.K.W.; de Vaucorbeil, A.; Sha, G.; Ringer, S.P.; Poole, W.J. Analysis of strengthening in AA6111 during the early stages of aging: Atom probe tomography and yield stress modelling. Acta Mater. 2013, 61, 7285–7303. [Google Scholar] [CrossRef]
- Esmaeili, S.; Vaumousse, D.; Zandbergen, M.W.; Poole, W.J.; Cerezo, A.; Lloyd, D.J. A study on the early-stage decomposition in the Al–Mg–Si–Cu alloy AA6111 by electrical resistivity and three-dimensional atom probe. Philos. Mag. 2007, 87, 3797–3816. [Google Scholar] [CrossRef]
- Uchida, H.; Yoshida, H. Improvement in paint bake hardenability of an Al-Mg-Si alloy by reversion. J. Jpn. Inst. Light Met. 1996, 46, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.J.; Li, Y.; Wang, Z.D.; Misra, R.D.K.; Li, J.D.; Xu, G.M. Study of retrogression response in naturally and multi-step aged Al-Mg-Si automotive sheets. J. Alloys Compd. 2018, 753, 457–464. [Google Scholar] [CrossRef]
- Birol, Y. Restoration of the bake hardening response in a naturally aged twin-roll cast AlMgSi automotive sheet. Scr. Mater. 2006, 54, 2003–2008. [Google Scholar] [CrossRef]
- Mittemeijer, E.J. Analysis of the kinetics of phase transformations. J. Mater. Sci. 1992, 27, 3977–3987. [Google Scholar] [CrossRef]



| Element | Cohesive Energy (kJ/mol) | Diffusion Constant D0 (m2/s) | Activation Energy Q (kJ/mol) | Vacancy Formation Energy ΔHvf (kJ/mol) | Solute-Vacancy Binding Energy Ebi-v (eV) |
|---|---|---|---|---|---|
| Al | 327.279 | 1.76 × 10−5 | 126.4 | 67.54 | - |
| Mg | 145.643 | 6.23 × 10−6 | 115.0 | 55.96 | 3.86 |
| Si | 445.783 | 2.48 × 10−4 | 137.0 | 385.94 | 2.89 |
| Cu | 336.236 | 6.54 × 10−5 | 136.0 | 119.64 | 4.34 |
| Al | Mg | Cu | Si | Vacancy | |
|---|---|---|---|---|---|
| Al | −54.5 | −34.5 | −49.3 | −50.8 | −21.9 |
| Mg | - | −16.8 | −32.2 | −38.1 | −10.5 |
| Cu | - | - | −48.5 | −45.3 | −15.8 |
| Si | - | - | - | −52.2 | −25.9 |
| Vacancy | - | - | - | - | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Wu, J.; Zhao, J.; Yang, G.; Yang, B. Kinetic Monte Carlo Simulation of Clustering in an Al-Mg-Si-Cu Alloy. Materials 2021, 14, 4523. https://doi.org/10.3390/ma14164523
Ye Q, Wu J, Zhao J, Yang G, Yang B. Kinetic Monte Carlo Simulation of Clustering in an Al-Mg-Si-Cu Alloy. Materials. 2021; 14(16):4523. https://doi.org/10.3390/ma14164523
Chicago/Turabian StyleYe, Qilu, Jianxin Wu, Jiqing Zhao, Gang Yang, and Bin Yang. 2021. "Kinetic Monte Carlo Simulation of Clustering in an Al-Mg-Si-Cu Alloy" Materials 14, no. 16: 4523. https://doi.org/10.3390/ma14164523
APA StyleYe, Q., Wu, J., Zhao, J., Yang, G., & Yang, B. (2021). Kinetic Monte Carlo Simulation of Clustering in an Al-Mg-Si-Cu Alloy. Materials, 14(16), 4523. https://doi.org/10.3390/ma14164523






