An Overview on Thermosensitive Oral Gel Based on Poloxamer 407
Abstract
1. Introduction
2. Subjects
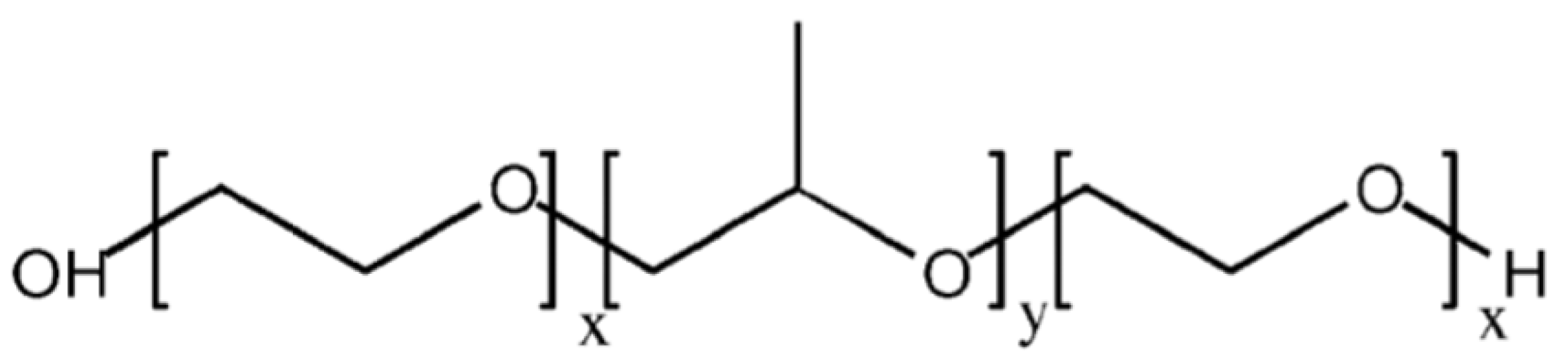
2.1. Poloxamers
2.1.1. Characteristics
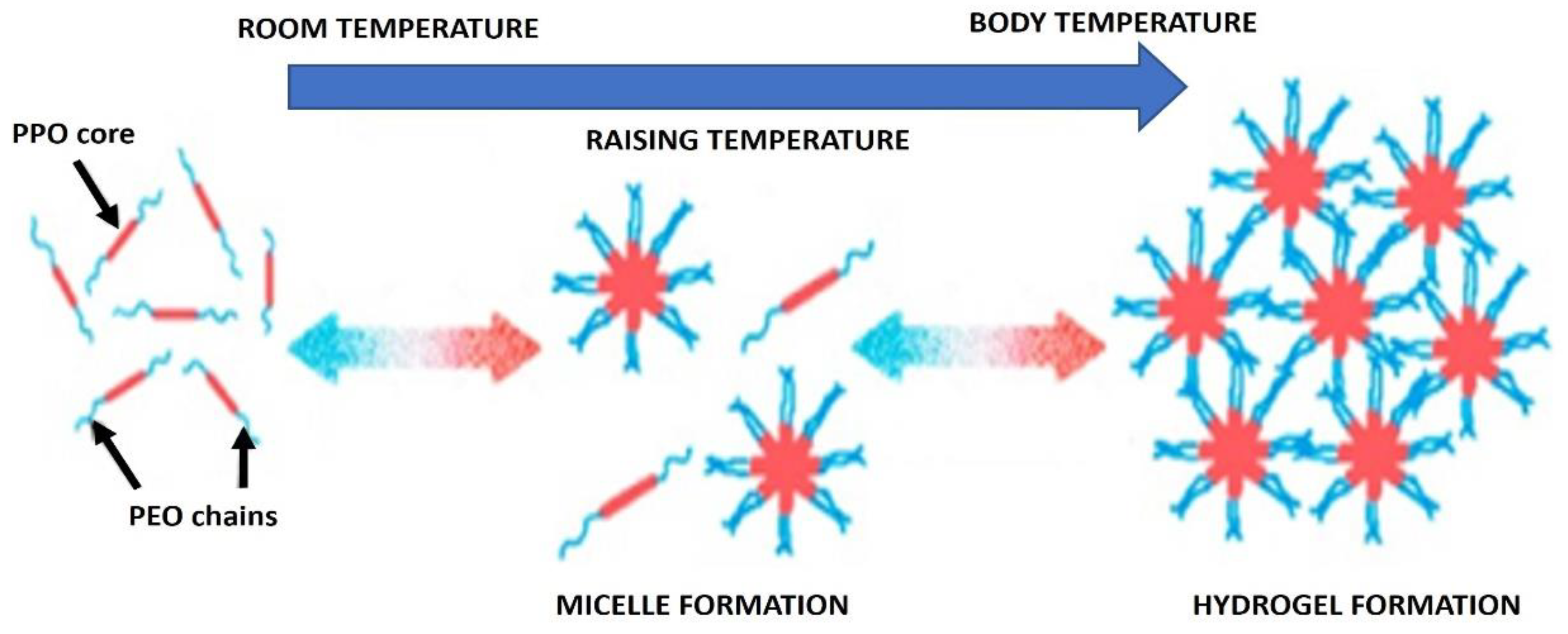
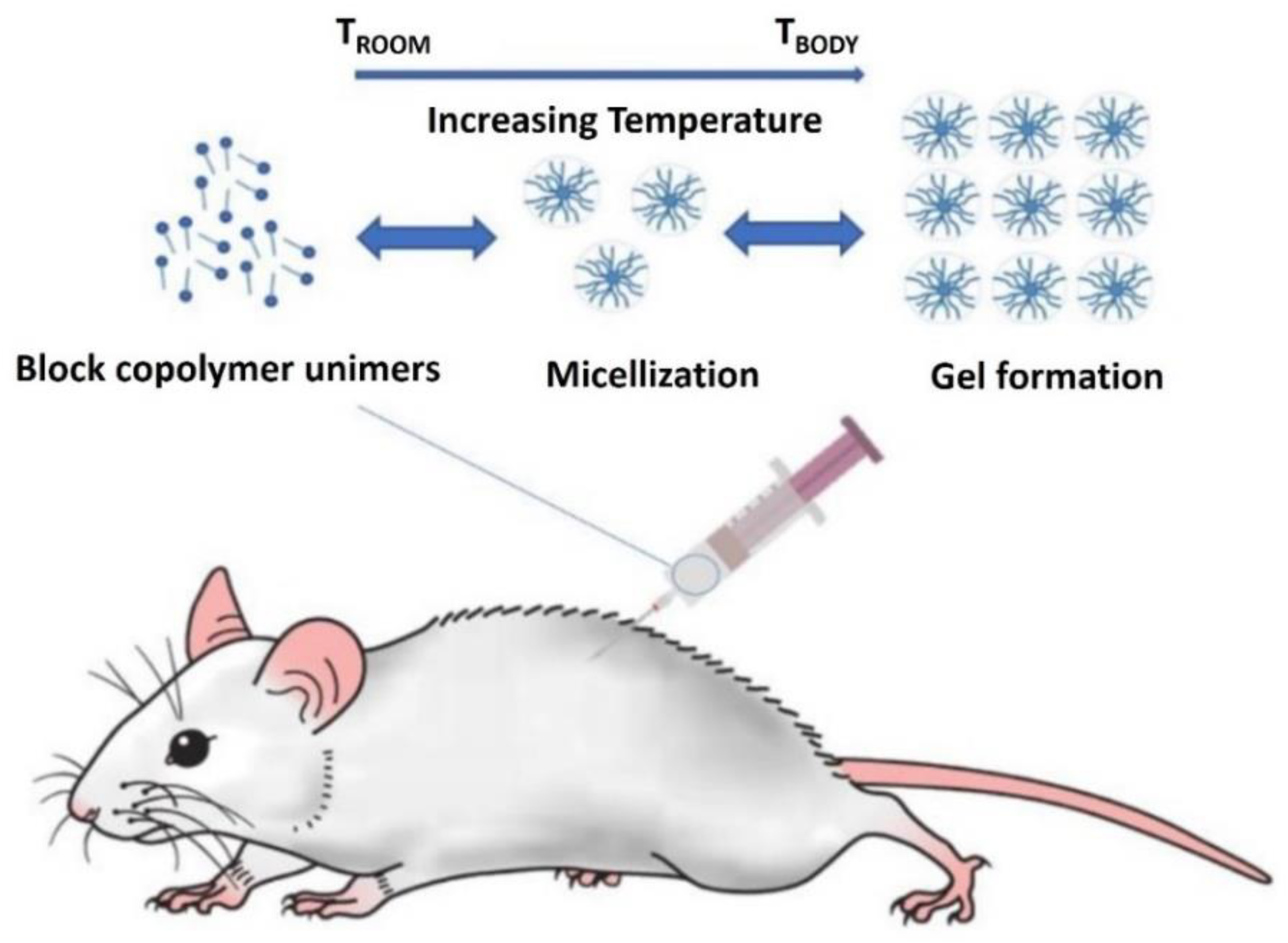
2.1.2. Micellization of Poloxamers
2.1.3. Rheological Properties of Poloxamers
2.1.4. Poloxamer 407
2.2. Research on Thermosensitive Gel
2.2.1. Preparation of Thermosensitive Gel
2.2.2. Formulation of Temperature-Sensitive Gel Based on Poloxamer 407
Formulation of Poloxamer 407 Temperature-Sensitive Gel
Carbomers
Hyaluronic Acid
Chitosan
2.2.3. Measurement of Sol-Gel Transition Temperature and Gelation Time
2.3. Drug Release of Poloxamer Temperature-Sensitive Gel
2.3.1. Diffusion of Drugs Using Thermosensitive Gels Based on Poloxamers
2.3.2. Hydrate Decomposition of Temperature-Sensitive Gel Based on Poloxamers
2.3.3. Additives Promote Drug Release
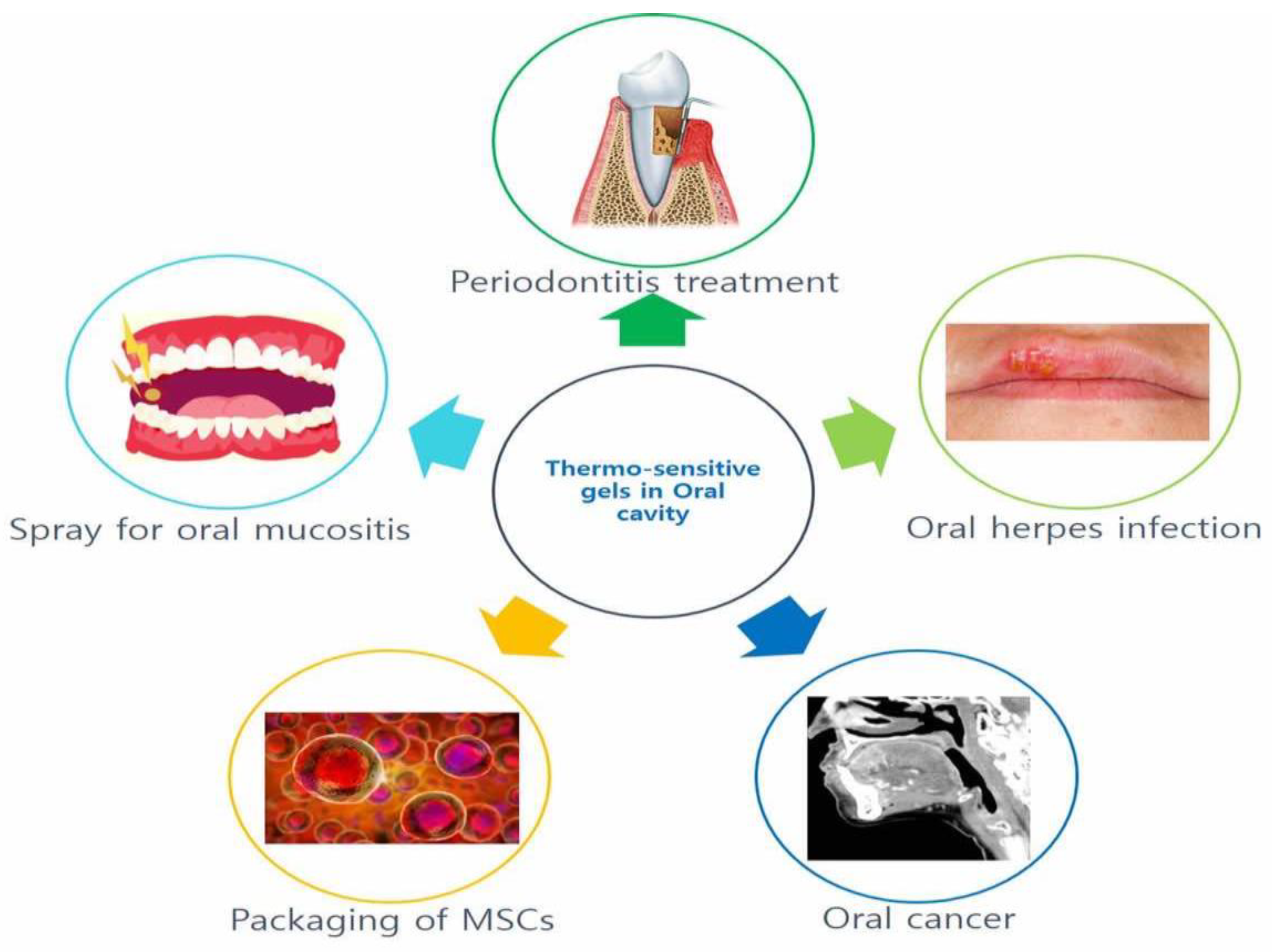
2.4. Application of Thermosensitive Gels in the Oral Cavity
2.4.1. Thermosensitive Gel for Periodontitis
2.4.2. Other Applications of Thermosensitive Gels in the Oral Cavity
Thermosensitive Gel for Oral Herpes Infection
Application of Thermosensitive Gels for Oral Cancer
Temperature-Sensitive Gel Used as the Packaging System of the Bracket
Temperature-Sensitive Gel as a Spray for Oral Mucositis
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Trends Biotechnol. 2000, 18, 412–420. [Google Scholar] [CrossRef]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N.; Sangfai, T.; Suknuntha, K. Thermosensitive Poloxamer 407/Poly(Acrylic Acid) Hydrogels with Potential Application as Injectable Drug Delivery System. AAPS PharmSciTech 2018, 19, 2103–2117. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef]
- Pagano, C.; Giovagnoli, S.; Perioli, L.; Tiralti, M.C.; Ricci, M. Development and characterization of mucoadhesive-thermoresponsive gels for the treatment of oral mucosa diseases. Eur. J. Pharm. Sci. 2020, 142, 105125. [Google Scholar] [CrossRef]
- Rajendran, S.; Kumar, K.S.; Ramesh, S.; Rao, S.R. Thermoreversible in situ gel for subgingival delivery of simvastatin for treatment of periodontal disease. Int. J. Pharm. Investig. 2017, 7, 101–106. [Google Scholar]
- Sheshala, R.; Quah, S.Y.; Tan, G.C.; Meka, V.S.; Jnanendrappa, N.; Sahu, P.S. Investigation on solution-to-gel characteristic of thermosensitive and mucoadhesive biopolymers for the development of moxifloxacin-loaded sustained release periodontal in situ gels. Drug Deliv. Transl. Res. 2019, 9, 434–443. [Google Scholar] [CrossRef]
- Swain, G.P.; Patel, S.; Gandhi, J.; Shah, P. Development of Moxifloxacin Hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J. Oral Biol. Craniofac. Res. 2019, 9, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Upadhayay, P.; Shankar, R.; Joshi, M.; Bhatt, S.; Malik, A. Chlorpheniramine maleate containing chitosan-based nanoparticle-loaded thermosensitive in situ gel for management in allergic rhinitis. Drug Deliv. Transl. Res. 2019, 9, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.L.; Ping, Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control. Release 2007, 120, 186–194. [Google Scholar] [CrossRef]
- Zambanini, T.; Borges, R.; de Souza, A.C.S.; Justo, G.Z.; Machado, J., Jr.; de Araujo, D.R.; Marchi, J. Holmium-Containing Bioactive Glasses Dispersed in Poloxamer 407 Hydrogel as a Theragenerative Composite for Bone Cancer Treatment. Materials 2021, 14, 1459. [Google Scholar] [CrossRef]
- Jiang, Y.; Meng, X.; Wu, Z.; Qi, X. Modified chitosan thermosensitive hydrogel enables sustained and efficient anti-tumor therapy via intratumoral injection. Carbohydr. Polym. 2016, 144, 245–253. [Google Scholar] [CrossRef]
- Ferreira, S.B.S.; Braga, G.; Oliveira, É.L.; da Silva, J.B.; Rosseto, H.C.; de Castro Hoshino, L.V.; Baesso, M.L.; Caetano, W.; Murdoch, C.; Colley, H.E.; et al. Design of a nanostructured mucoadhesive system containing curcumin for buccal application: From physicochemical to biological aspects. Beilstein J. Nanotechnol. 2019, 10, 2304–2328. [Google Scholar] [CrossRef]
- Diniz, I.M.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Osman, D.A.; Abd El-Fattah, A.I. Formulation and in vitro evaluation of a thermoreversible mucoadhesive nasal gel of itopride hydrochloride. Drug Dev. Ind. Pharm. 2018, 44, 1857–1867. [Google Scholar] [CrossRef]
- Bonacucina, G.; Spina, M.; Misici-Falzi, M.; Cespi, M.; Pucciarelli, S.; Angeletti, M.; Palmieri, G.F. Effect of hydroxypropyl beta-cyclodextrin on the self-assembling and thermogelation properties of Poloxamer 407. Eur. J. Pharm. Sci. 2007, 32, 115–122. [Google Scholar] [CrossRef]
- Baghban, A.; Sasanipour, J.; Sarafbidabad, M.; Piri, A.; Razavi, R. On the prediction of critical micelle concentration for sugar-based non-ionic surfactants. Chem. Phys. Lipids 2018, 214, 46–57. [Google Scholar] [CrossRef]
- Khoshnood, A.; Lukanov, B.; Firoozabadi, A. Temperature Effect on Micelle Formation: Molecular Thermodynamic Model Revisited. Langmuir 2016, 32, 2175–2183. [Google Scholar] [CrossRef]
- Ahmed, S.; Gull, A.; Aqil, M.; Danish Ansari, M.; Sultana, Y. Poloxamer-407 thickened lipid colloidal system of agomelatine for brain targeting: Characterization, brain pharmacokinetic study and behavioral study on Wistar rats. Colloids Surf. B Biointerfaces 2019, 181, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Xuan, J.J.; Balakrishnan, P.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.G. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.; Rehman, K.; Sun, H.; Chen, S. Assessment of release kinetics, stability and polymer interaction of poloxamer 407-based thermosensitive gel of interleukin-1 receptor antagonist. Pharm. Dev. Technol. 2014, 19, 278–284. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; De Gaetano, F.; Ventura, C.A.; Fresta, M.; Paolino, D. The Rheolaser Master™ and Kinexus Rotational Rheometer(®) to Evaluate the Influence of Topical Drug Delivery Systems on Rheological Features of Topical Poloxamer Gel. Molecules 2020, 25, 1979. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Sosa, L.; Calpena, A.C.; Silva-Abreu, M.; Espinoza, L.C.; Rincón, M.; Bozal, N.; Domenech, O.; Rodríguez-Lagunas, M.J.; Clares, B. Thermoreversible Gel-Loaded Amphotericin B for the Treatment of Dermal and Vaginal Candidiasis. Pharmaceutics 2019, 11, 312. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef]
- Alexandridis, P.; Alan Hatton, T. Poly(ethylene oxide)-poly(propylene oxide )-poly (ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017, 302, 1600266. [Google Scholar] [CrossRef]
- Wang, B.; Shao, J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. A Novel Thermoresponsive Gel as a Potential Delivery System for Lipoxin. J. Dent. Res. 2019, 98, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Wu, Y.; Zhao, Y.; Chen, H.; Yuan, Y.; Xu, K.; Zhang, H.; Lu, Y.; Wang, J.; et al. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials 2018, 168, 24–37. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Di, X. Thermosensitive In Situ Gel Based on Solid Dispersion for Rectal Delivery of Ibuprofen. AAPS PharmSciTech 2018, 19, 338–347. [Google Scholar] [CrossRef]
- Bansal, M.; Mittal, N.; Yadav, S.K.; Khan, G.; Gupta, P.; Mishra, B.; Nath, G. Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: Preparation, in-vitro characterization and antimicrobial study. J. Oral Biol. Craniofac. Res. 2018, 8, 126–133. [Google Scholar] [CrossRef]
- Hemelryck, S.V.; Dewulf, J.; Niekus, H.; van Heerden, M.; Ingelse, B.; Holm, R.; Mannaert, E.; Langguth, P. In vitro evaluation of poloxamer in situ forming gels for bedaquiline fumarate salt and pharmacokinetics following intramuscular injection in rats. Int. J. Pharm. X 2019, 1, 100016. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan-loaded nanoethosomes: Formulation, optimization, evaluation and permeation studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-G.; Jung, J.-H.; Ryu, J.-M.; Yoon, S.-J.; Oh, Y.-K.; Kim, C.-K. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int. J. Pharm. 1998, 165, 33–44. [Google Scholar] [CrossRef]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Guneri, T. Rheological and mechanical properties of poloxamer mixtures as a mucoadhesive gel base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef]
- Radivojša, M.; Grabnar, I.; Ahlin Grabnar, P. Thermoreversible in situ gelling poloxamer-based systems with chitosan nanocomplexes for prolonged subcutaneous delivery of heparin: Design and in vitro evaluation. Eur. J. Pharm. Sci. 2013, 50, 93–101. [Google Scholar] [CrossRef]
- Caretti, L.; La Gloria Valerio, A.; Piermarocchi, R.; Badin, G.; Verzola, G.; Masarà, F.; Scalora, T.; Monterosso, C. Efficacy of carbomer sodium hyaluronate trehalose vs hyaluronic acid to improve tear film instability and ocular surface discomfort after cataract surgery. Clin. Ophthalmol. 2019, 13, 1157–1163. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Salomão, D.R.; Iezzi, R.; Barkmeier, A.J. Subconjunctival Exposure to Carbopol Causes Chronic Histiocytic Inflammatory Response in Rabbits. Transl. Vis. Sci. Technol. 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Dennie, L. Safety and Efficacy of 0.5% Carbomer 980 Gel for Treatment of Symptoms of Common Cold: Results of 2 Randomized Trials. Drugs R D 2019, 19, 191–200. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Li, L.; Li, B.; Zhang, X.; Su, J. Mechanical, rheological and release behaviors of a poloxamer 407/ poloxamer 188/carbopol 940 thermosensitive composite hydrogel. Molecules 2013, 18, 12415–12425. [Google Scholar] [CrossRef]
- Garala, K.; Joshi, P.; Shah, M.; Ramkishan, A.; Patel, J. Formulation and evaluation of periodontal in situ gel. Int. J. Pharm. Investig. 2013, 3, 29–41. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Dalmedico, M.M.; Meier, M.J.; Felix, J.V.; Pott, F.S.; Petz Fde, F.; Santos, M.C. Hyaluronic acid covers in burn treatment: A systematic review. Rev. Esc. Enferm. USP 2016, 50, 522–528. [Google Scholar] [CrossRef]
- López-Ruiz, E.; Jiménez, G.; Álvarez de Cienfuegos, L.; Antic, C.; Sabata, R.; Marchal, J.A.; Gálvez-Martín, P. Advances of hyaluronic acid in stem cell therapy and tissue engineering, including current clinical trials. Eur. Cell Mater. 2019, 37, 186–213. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar] [PubMed]
- Abdelraouf, S.A.; Dahab, O.A.; Elbarbary, A.; El-Din, A.M.; Mostafa, B. Assessment of Hyaluronic Acid Gel Injection in the Reconstruction of Interdental Papilla: A Randomized Clinical Trial. Open Access Maced. J. Med. Sci. 2019, 7, 1834–1840. [Google Scholar] [CrossRef]
- Brandt, F.S.; Cazzaniga, A. Hyaluronic acid gel fillers in the management of facial aging. Clin. Interv. Aging 2008, 3, 153–159. [Google Scholar]
- Alcântara, C.E.P.; Castro, M.A.A.; Noronha, M.S.; Martins-Junior, P.A.; Mendes, R.M.; Caliari, M.V.; Mesquita, R.A.; Ferreira, A.J. Hyaluronic acid accelerates bone repair in human dental sockets: A randomized triple-blind clinical trial. Braz. Oral Res. 2018, 32, 13. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Lin, W.Y.; Lee, A.L.; Li, Y.C.; Chen, Y.J.; Chen, K.C.; Young, T.H. Hyaluronic acid on the urokinase sustained release with a hydrogel system composed of poloxamer 407: HA/P407 hydrogel system for drug delivery. PLoS ONE 2020, 15, e0227784. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, H.; Choi, E.S.; Park, M.H.; Ryu, J.H. Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy. Pharmaceutics 2019, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef]
- Li, X.; Li, A.; Feng, F.; Jiang, Q.; Sun, H.; Chai, Y.; Yang, R.; Wang, Z.; Hou, J.; Li, R. Effect of the hyaluronic acid-poloxamer hydrogel on skin-wound healing: In vitro and in vivo studies. Anim. Model. Exp. Med. 2019, 2, 107–113. [Google Scholar] [CrossRef]
- Lima, L.C.B.; Coelho, C.C.; Silva, F.C.; Meneguin, A.B.; Barud, H.S.; Bezerra, R.D.S.; Viseras, C.; Osajima, J.A.; Silva-Filho, E.C. Hybrid Systems Based on Talc and Chitosan for Controlled Drug Release. Materials 2019, 12, 3634. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.Y.; Lu, S.T.; Li, P.W.; Li, S.D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef]
- Kong, X.; Xu, W.; Zhang, C.; Kong, W. Chitosan temperature-sensitive gel loaded with drug microspheres has excellent effectiveness, biocompatibility and safety as an ophthalmic drug delivery system. Exp. Ther. Med. 2018, 15, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Madrazo-Jiménez, M.; Rodríguez-Caballero, Á.; Serrera-Figallo, M.; Garrido-Serrano, R.; Gutiérrez-Corrales, A.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. The effects of a topical gel containing chitosan, 0,2% chlorhexidine, allantoin and despanthenol on the wound healing process subsequent to impacted lower third molar extraction. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e696–e702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: Mechanical, rheological and sol-gel transition analysis. Carbohydr. Polym. 2020, 240, 116268. [Google Scholar] [CrossRef]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.P.; Guo, Y.S.; Zhong, B.; Hu, X.; Wang, X.H.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef]
- Dumortier, G.; El Kateb, N.; Sahli, M.; Kedjar, S.; Boulliat, A.; Chaumeil, J.C. Development of a thermogelling ophthalmic formulation of cysteine. Drug Dev. Ind. Pharm. 2006, 32, 63–72. [Google Scholar] [CrossRef]
- Abdeltawab, H.; Svirskis, D.; Sharma, M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin. Drug Deliv. 2020, 17, 495–509. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Akkari, A.C.S.; Papini, J.Z.B.; Garcia, G.K.; Franco, M.; Cavalcanti, L.P.; Gasperini, A.; Alkschbirs, M.I.; Yokaichyia, F.; de Paula, E.; Tófoli, G.R.; et al. Poloxamer 407/188 binary thermosensitive hydrogels as delivery systems for infiltrative local anesthesia: Physico-chemical characterization and pharmacological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 299–307. [Google Scholar] [CrossRef]
- Nascimento, M.H.M.; Franco, M.; Yokaichyia, F.; de Paula, E.; Lombello, C.B.; de Araujo, D.R. Hyaluronic acid in Pluronic F-127/F-108 hydrogels for postoperative pain in arthroplasties: Influence on physico-chemical properties and structural requirements for sustained drug-release. Int. J. Biol. Macromol. 2018, 111, 1245–1254. [Google Scholar] [CrossRef]
- Fathalla, Z.M.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef]
- Nazar, H.; Fatouros, D.G.; van der Merwe, S.M.; Bouropoulos, N.; Avgouropoulos, G.; Tsibouklis, J.; Roldo, M. Thermosensitive hydrogels for nasal drug delivery: The formulation and characterisation of systems based on N-trimethyl chitosan chloride. Eur. J. Pharm. Biopharm. 2011, 77, 225–232. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.Y.; Wei, G.; Lu, W.Y. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: Improved in vitro and in vivo sustained-release properties. Eur. J. Pharm. Sci. 2009, 37, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, X.; Lin, X.; Yao, C.; Shen, L.; Feng, Y. Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats. Drug Deliv. 2015, 22, 375–382. [Google Scholar] [CrossRef]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef]
- Rangabhatla, A.S.L.; Tantishaiyakul, V.; Oungbho, K.; Boonrat, O. Fabrication of pluronic and methylcellulose for etidronate delivery and their application for osteogenesis. Int. J. Pharm. 2016, 499, 110–118. [Google Scholar] [CrossRef]
- Sridhar, V.; Wairkar, S.; Gaud, R.; Bajaj, A.; Meshram, P. Brain targeted delivery of mucoadhesive thermosensitive nasal gel of selegiline hydrochloride for treatment of Parkinson’s disease. J. Drug Target. 2018, 26, 150–161. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Mahrous, G.M.; Alanazi, F.K. Novel in-situ gel for intravesical administration of ketorolac. Saudi Pharm. J. 2018, 26, 845–851. [Google Scholar] [CrossRef]
- Lu, C.; Liu, M.; Fu, H.; Zhang, W.; Peng, G.; Zhang, Y.; Cao, H.; Luo, L. Novel thermosensitive in situ gel based on poloxamer for uterus delivery. Eur. J. Pharm. Sci. 2015, 77, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, M.; Kim, M.; Kim, C. Effect of additives on the physicochemical properties of liquid suppository bases. Int. J. Pharm. 1999, 190, 13–19. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, M.; Miglani, G.; Mali, A.; Paradkar, A.; Yamamura, S.; Kadam, S. Development of tetracycline-serratiopeptidase-containing periodontal gel: Formulation and preliminary clinical study. AAPS PharmSciTech 2006, 7, E162–E171. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kim, K.R.; Noh, Y.; Chung, W.H.; Cho, Y.S.; Chung, S.K.; Dhong, H.J.; Kim, H.Y. Intranasal distribution and clearance of thermoreversible gel in an animal model. Int. Forum Allergy Rhinol. 2017, 7, 705–711. [Google Scholar] [CrossRef]
- Nasra, M.M.; Khiri, H.M.; Hazzah, H.A.; Abdallah, O.Y. Formulation, in-vitro characterization and clinical evaluation of curcumin in-situ gel for treatment of periodontitis. Drug Deliv. 2017, 24, 133–142. [Google Scholar] [CrossRef]
- Kumari, N.; Pathak, K. Dual controlled release, in situ gelling periodontal sol of metronidazole benzoate and serratiopeptidase: Statistical optimization and mechanistic evaluation. Curr. Drug Deliv. 2012, 9, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Patil, D.; Hegde, S.; Potdar, R.; Metgud, R.; Jalalpure, S.; Roy, S.; Jadhav, K.; et al. Formulation of thermoreversible gel of cranberry juice concentrate: Evaluation, biocompatibility studies and its antimicrobial activity against periodontal pathogens. Mater. Sci Eng. C Mater. Biol. Appl. 2017, 75, 1506–1514. [Google Scholar]
- Kukhanova, M.K.; Korovina, A.N.; Kochetkov, S.N. Human herpes simplex virus: Life cycle and development of inhibitors. Biochemistry 2014, 79, 1635–1652. [Google Scholar] [CrossRef]
- Worrall, G. Herpes labialis. BMJ Clin. Evid. 2009, 23, 1704. [Google Scholar]
- Bader, C.; Crumpacker, C.S.; Schnipper, L.E.; Ransil, B.; Clark, J.E.; Arndt, K.; Freedberg, I.M. The natural history of recurrent facial-oral infection with herpes simplex virus. J. Infect. Dis. 1978, 138, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Verma, S. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. Sci. World J. 2014, 24, 280928. [Google Scholar]
- Ettinger, K.S.; Ganry, L.; Fernandes, R.P. Oral Cavity Cancer. Oral Maxillofac Surg. Clin. N. Am. 2019, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Colley, H.E.; Hearnden, V.; Jones, A.V.; Weinreb, P.H.; Violette, S.M.; Macneil, S.; Thornhill, M.H.; Murdoch, C. Development of tissue-engineered models of oral dysplasia and early invasive oral squamous cell carcinoma. Br. J. Cancer 2011, 105, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Du, L.; Liu, Y.; Li, X.; Li, M.; Jin, Y.; Qian, X. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-β-cyclodextrin for melanoma treatment. Int. J. Pharm. 2014, 469, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Du, J.; Duan, Y.; Zang, Y.; Zhang, H.; Yang, C.; Cao, F.; Zhai, G. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: Preparation, optimization and in vitro characterization. Colloids Surf. B Biointerfaces 2012, 97, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Borden, B.A.; Yockman, J.; Kim, S.W. Thermoresponsive hydrogel as a delivery scaffold for transfected rat mesenchymal stem cells. Mol. Pharm. 2010, 7, 963–968. [Google Scholar] [CrossRef]
- Huang, J.W.; Chen, W.J.; Liao, S.K.; Yang, C.Y.; Lin, S.S.; Wu, C.C. Osteoblastic differentiation of rabbit mesenchymal stem cells loaded in A carrier system of Pluronic F127 and Interpore. Chang Gung Med. J. 2006, 29, 363–372. [Google Scholar]
- Rustad, K.C.; Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Major, M.R.; Rajadas, J.; Longaker, M.T.; Gurtner, G.C. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2012, 33, 80–90. [Google Scholar] [CrossRef]


| Poloxamer | Pluronic | PEO% | Average Molecular Weight | Melting Point (℃) | Viscosity (Pa·s) | Surface Tension (dyn cm−1) | HLB * |
|---|---|---|---|---|---|---|---|
| P105 | L35 | 50 | 1900 | 7 | 0.375 | 49 | 18–23 |
| P108 | F38 | 80 | 4700 | 48 | 0.260 | 52 | >24 |
| P122 | L42 | 20 | 1630 | −26 | 0.280 | 46 | 7–12 |
| P123 | L43 | 30 | 1850 | −1 | 0.310 | 47 | 7–12 |
| P124 | L44 | 40 | 2200 | 16 | 0.440 | 45 | 12–18 |
| P182 | L62 | 20 | 2500 | −4 | 0.450 | 43 | 1–7 |
| P183 | L63 | 30 | 2650 | 10 | 0.490 | 43 | 7–12 |
| P184 | L64 | 40 | 2900 | 16 | 0.850 | 43 | 12–18 |
| P185 | P65 | 50 | 3400 | 27 | 0.180 | 46 | 12–18 |
| P188 | F68 | 80 | 8400 | 52 | 1.000 | 50 | >24 |
| P212 | L72 | 20 | 2750 | −7 | 0.510 | 39 | 1–7 |
| P215 | P75 | 50 | 4150 | 27 | 0.250 | 43 | 12–18 |
| P217 | F77 | 70 | 6600 | 48 | 0.480 | 47 | >24 |
| P234 | P84 | 40 | 4200 | 34 | 0.280 | 42 | 12–18 |
| P235 | P85 | 50 | 4600 | 34 | 0.310 | 42 | 12–18 |
| P237 | F87 | 70 | 7700 | 49 | 0.700 | 44 | >24 |
| P238 | F88 | 80 | 11,400 | 54 | 2.300 | 48 | >24 |
| P288 | F98 | 80 | 13,000 | 58 | 2.700 | 43 | >24 |
| P333 | P103 | 30 | 4950 | 30 | 0.285 | 34 | 7–12 |
| P334 | P104 | 40 | 5900 | 32 | 0.390 | 33 | 12–18 |
| P335 | P105 | 50 | 6500 | 35 | 0.750 | 39 | 12–18 |
| P338 | F108 | 80 | 14,600 | 57 | 2.800 | 41 | >24 |
| P402 | L122 | 20 | 5000 | 20 | 1.750 | 33 | 1–7 |
| P403 | P123 | 30 | 5750 | 31 | 0.350 | 34 | 7–12 |
| P407 | F127 | 70 | 12,600 | 56 | 3.100 | 41 | 18–23 |
| Publication | Components | Advantages |
|---|---|---|
| Ganesh P. Swain, Shivani Patel (2019) [11] | Moxifloxacin Hydrochloride, Methyl cellulose, Carbopol P934, poloxamer 407, Gellan gum, Sodium citrate, Triethanolamine, Deionized water. | The formulations extend their residence time in the infected cavity to improve the local effect of periodontitis. There is no indication of incompatibility between the drug and gel. |
| Ravi Sheshala (2018) [10] | Moxifloxacin HCl, poloxamer 407, poloxamer 188, HPMC E4M, Chitosan in 1.5% v/v lactic acid, Benzalkonium chloride, β-glycerophosphate. | All formulations have moderate viscosity and prolong the residence time in the affected area. The pH value has no irritating effect on the oral mucosa. |
| Maha M.A. Nasra(2017) [84] | Curcumin Cur, Pluronic 127, Carbopol P934, polyethylene glycol 400, potassium di-hydrogen phosphate and sodium lauryl sulfate, tri-ethanol amine, PEG 7-glyceryl-cocoate. | In this drug delivery system, the probe depth and bleeding index of curcumin can be reduced and can therefore be used as a new method for the treatment of periodontal disease. It also leads to better patient compliance. |
| Monika Bansal(2017) [37] | Levofloxacin (LVF), metronidazole (MZ), chitosan, poloxamer 407. | The duration of drug release could be extended up to 48 h, and the high viscosity makes it easier to store the gel in the periodontal pocket for a considerable period. In addition, compared with carrier gels, in situ gels exhibit stronger antibacterial effects. |
| Pathak, K. and N. Kumari(2012) [85] | Metronidazole benzoate, Metronidazole, serratiopeptidase, poloxamer 407. | The developed gel has good injectability and forms a gel that is more accessible to pathogenic bacteria deep in the periodontal pockets and can maintain an effective drug dose for considerable period. |
| Rajeshwari H. R(2017) [86] | Poloxamer 407, chlorhexidine gluconate, Carbopol P934. The original gel has good adhesive strength that can prolong its retention time in the periodontal pocket, which in turn can prolong drug release. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lee, J.-H.; Meng, M.; Cui, N.; Dai, C.-Y.; Jia, Q.; Lee, E.-S.; Jiang, H.-B. An Overview on Thermosensitive Oral Gel Based on Poloxamer 407. Materials 2021, 14, 4522. https://doi.org/10.3390/ma14164522
Chen Y, Lee J-H, Meng M, Cui N, Dai C-Y, Jia Q, Lee E-S, Jiang H-B. An Overview on Thermosensitive Oral Gel Based on Poloxamer 407. Materials. 2021; 14(16):4522. https://doi.org/10.3390/ma14164522
Chicago/Turabian StyleChen, Yabing, Jeong-Ho Lee, Mingyue Meng, Naiyu Cui, Chun-Yu Dai, Qi Jia, Eui-Seok Lee, and Heng-Bo Jiang. 2021. "An Overview on Thermosensitive Oral Gel Based on Poloxamer 407" Materials 14, no. 16: 4522. https://doi.org/10.3390/ma14164522
APA StyleChen, Y., Lee, J.-H., Meng, M., Cui, N., Dai, C.-Y., Jia, Q., Lee, E.-S., & Jiang, H.-B. (2021). An Overview on Thermosensitive Oral Gel Based on Poloxamer 407. Materials, 14(16), 4522. https://doi.org/10.3390/ma14164522







