The Impact of Chloride and Sulphate Aggressiveness on the Microstructure and Phase Composition of Fly Ash-Slag Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. CFBC Fly Ash
2.2. Ground Granulated Blast Furnace Slag
2.3. Specimen Preparation
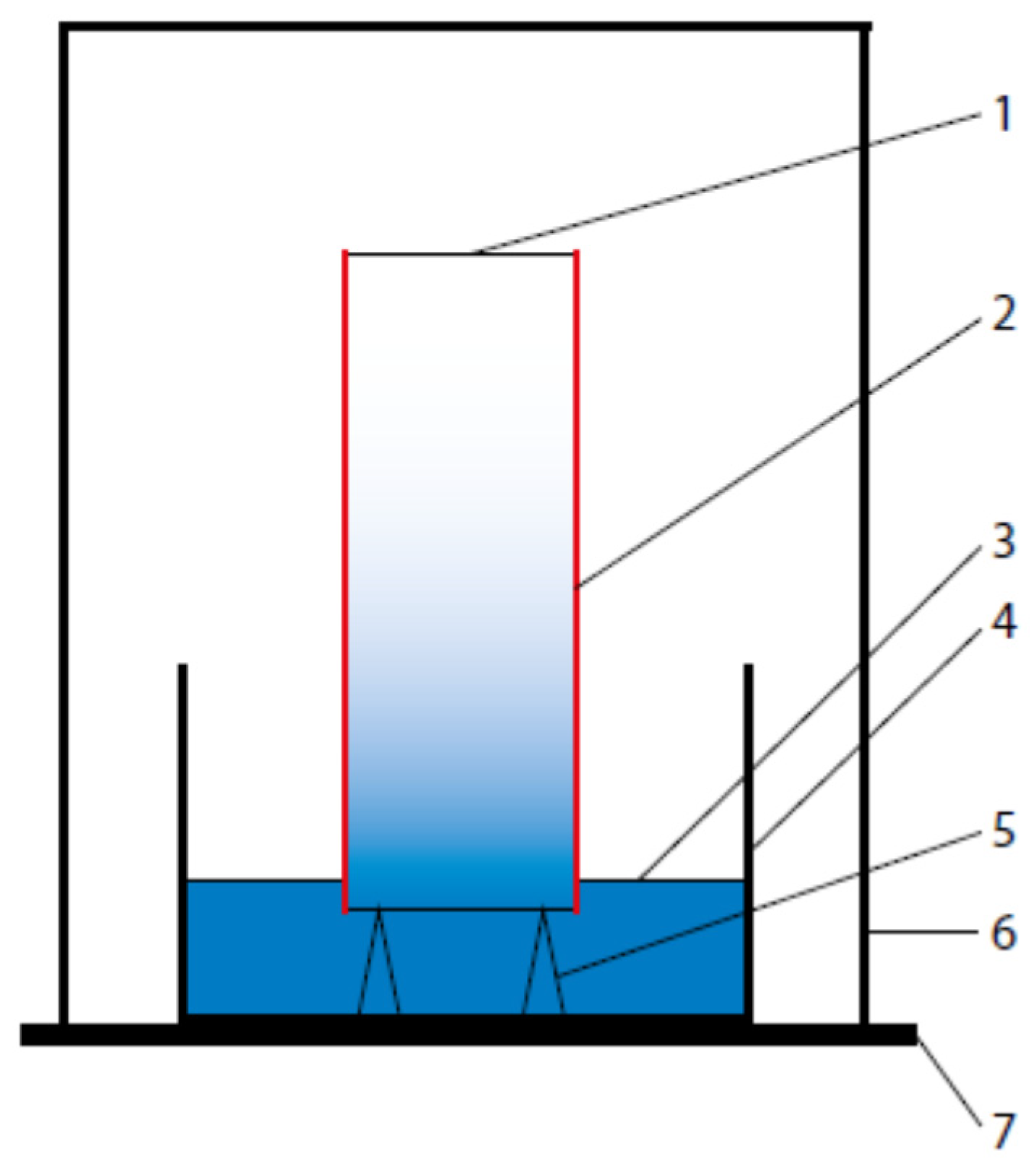
2.4. Test Methods
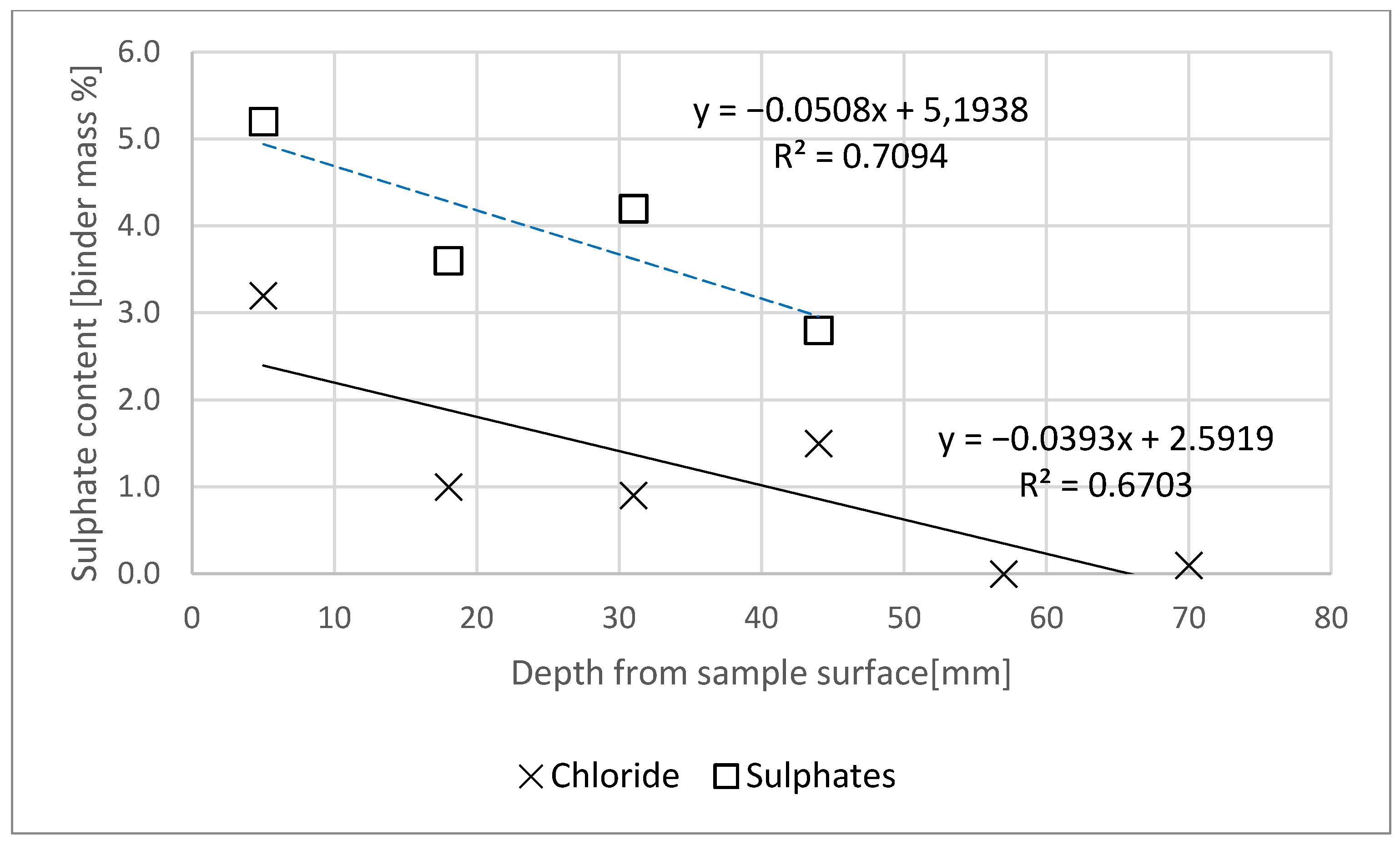
- The chloride and sulphate ion contents were assessed as per standard [23]. The test procedure set out in the standard defines transient state diffusion, since the stream of diffusing aggressive ions changes over time. As the changes can be induced by the binding of ions in the material, fragments of previously cut mortar slices were ground separately in a porcelain mortar, and the wet chemical analysis was applied [23]. Then the content of chloride ions (for 6 slices) and sulphate ions (for 4 slices) for each of the mortar slices exposed to the action of NaCl and Na2SO4 was determined. It was noted that the analysis results were related to the binder content in the mortars, taking into account the natural moisture of the specimens.
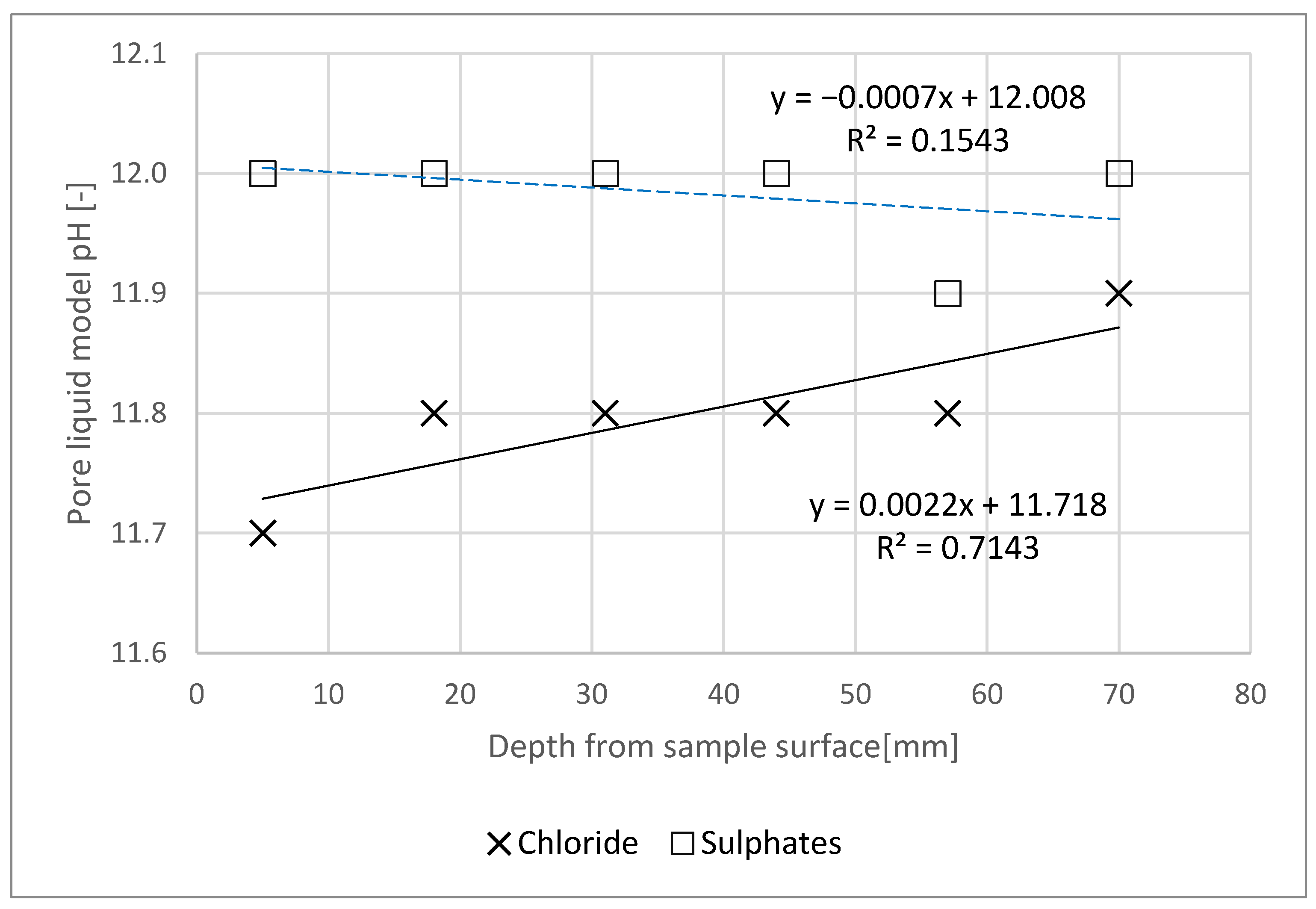
- The sulphate content results were then converted into SO42− content. Furthermore, water extraction as per [24] was executed for each of the slices (6) of each specimen, after previously grinding and sieving the material through a 1.0 mm mesh-sized filter. Water extracts were used as a mortar pore liquid model, where the pH was determined.
3. Results and Analysis
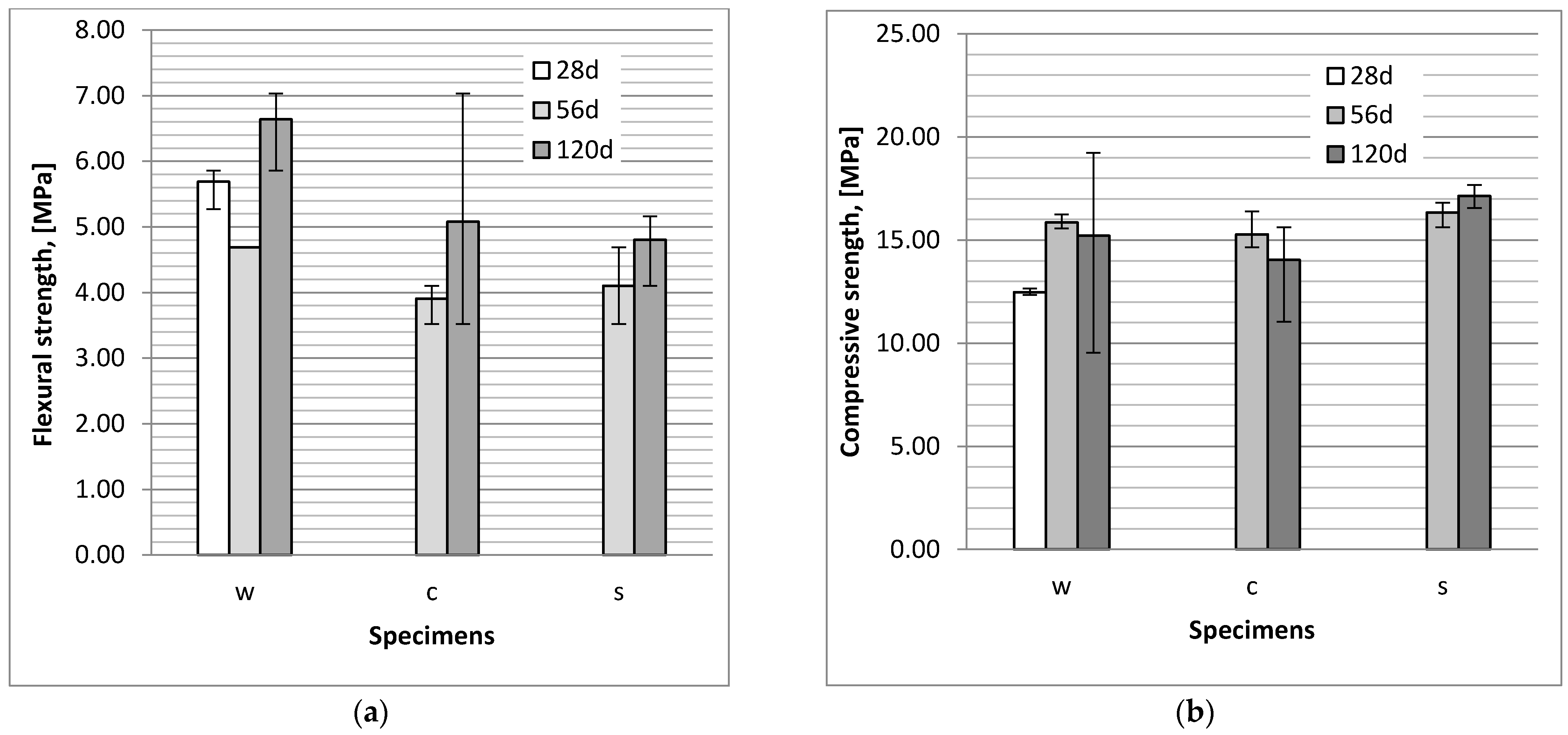
3.1. Flexural and Compressive Strength
3.2. Chloride and Sulphate Ion Content
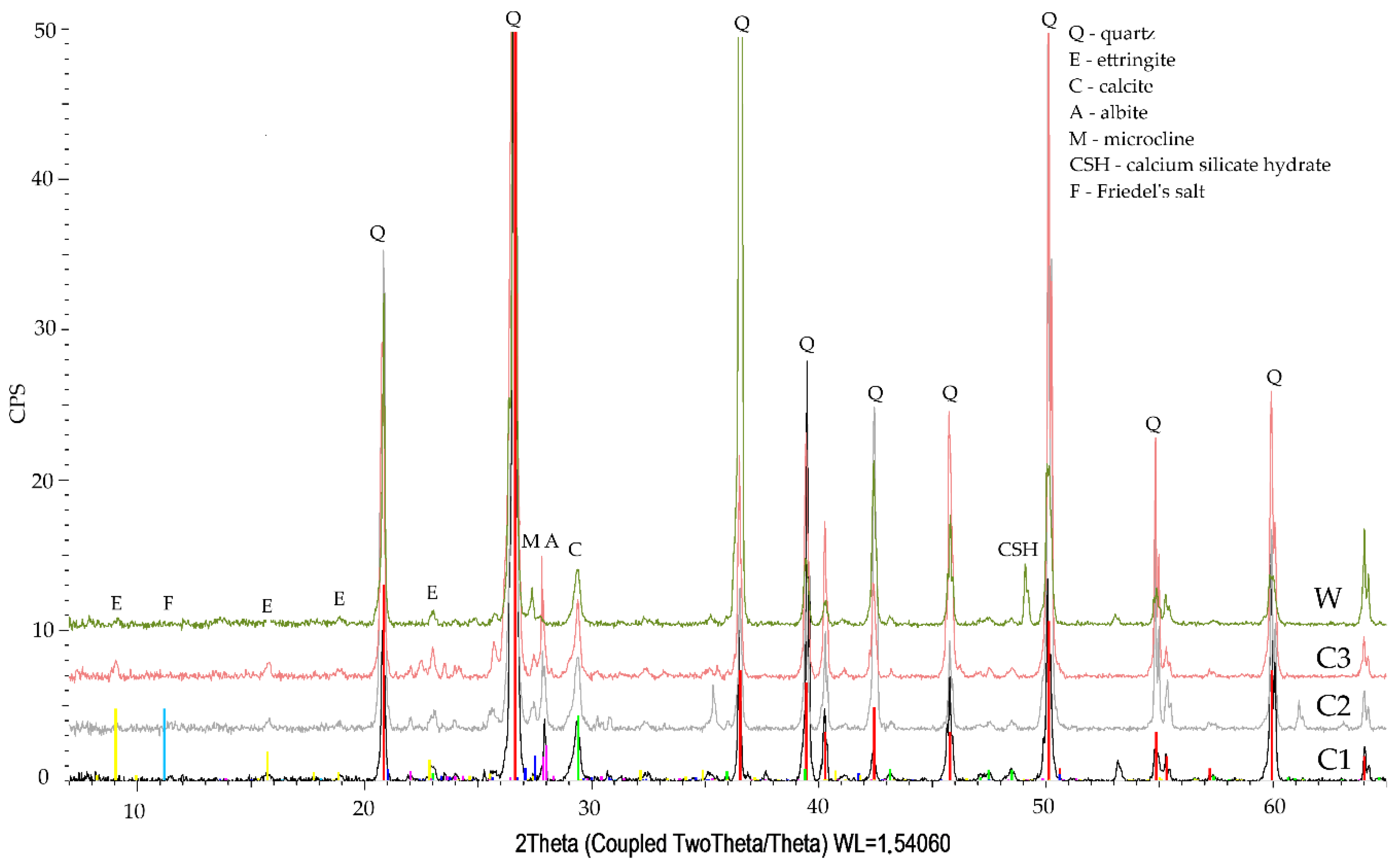
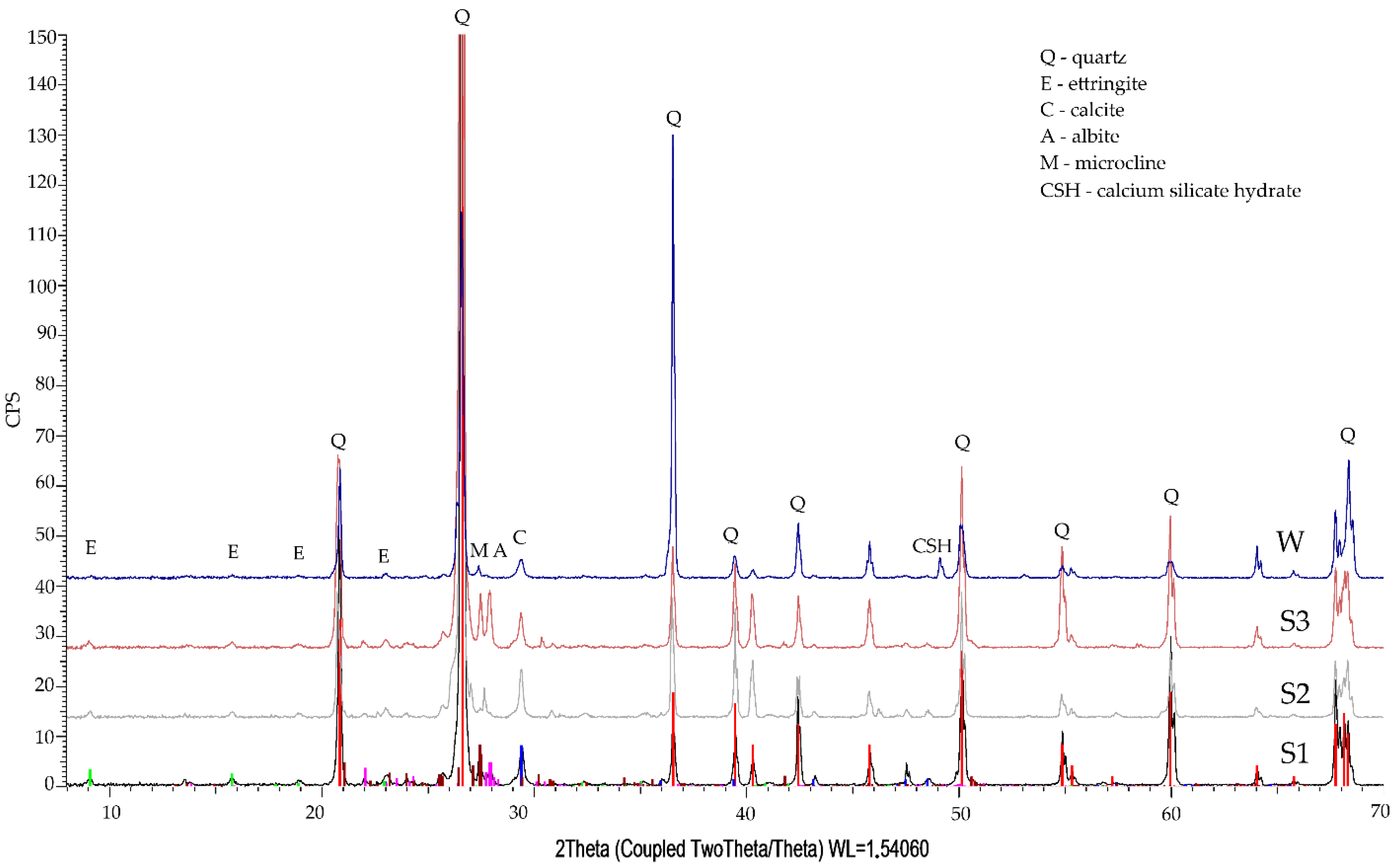
3.3. Phase Composition Qualitative Tests
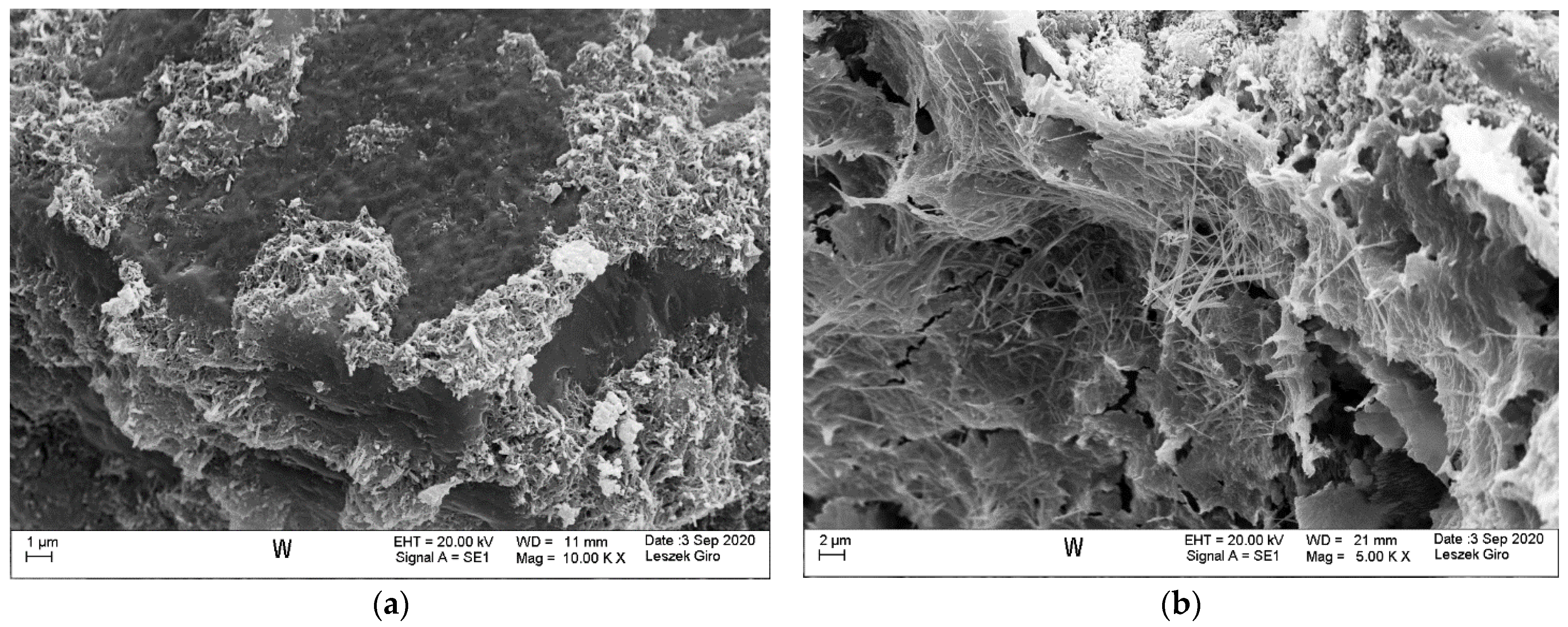
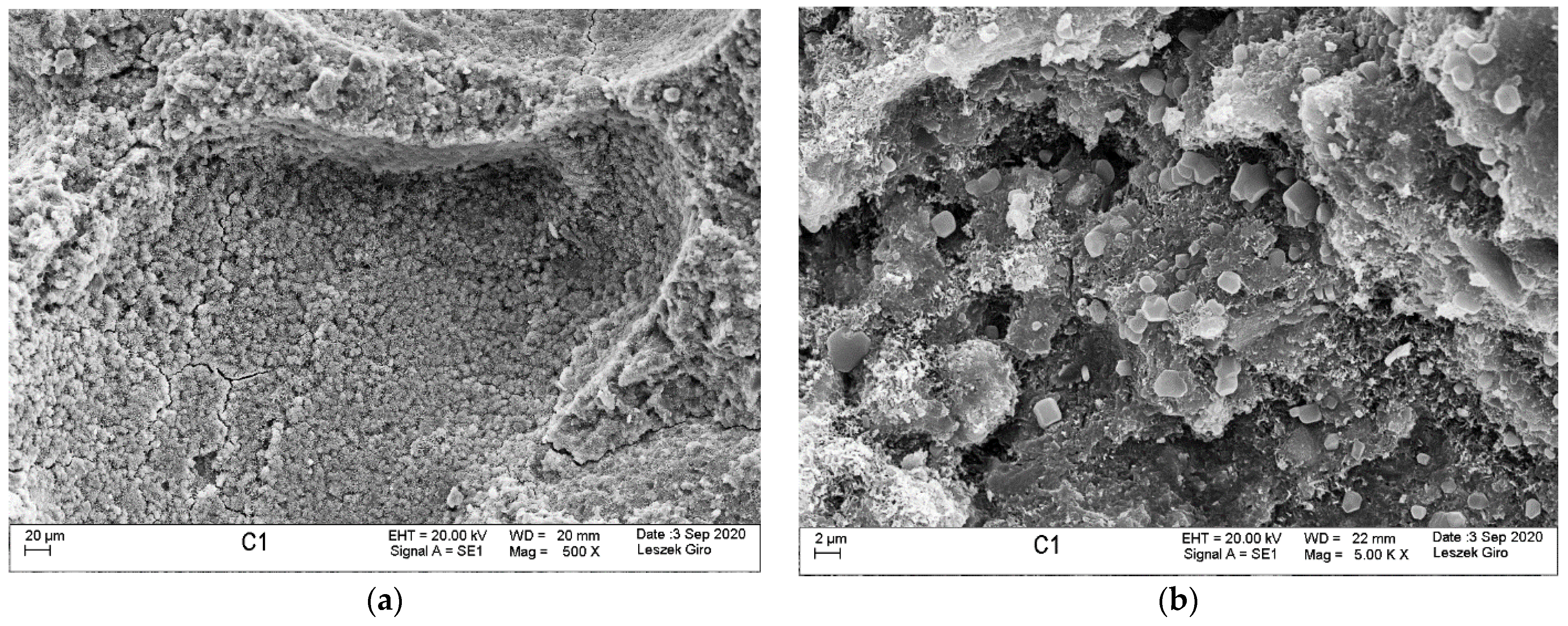
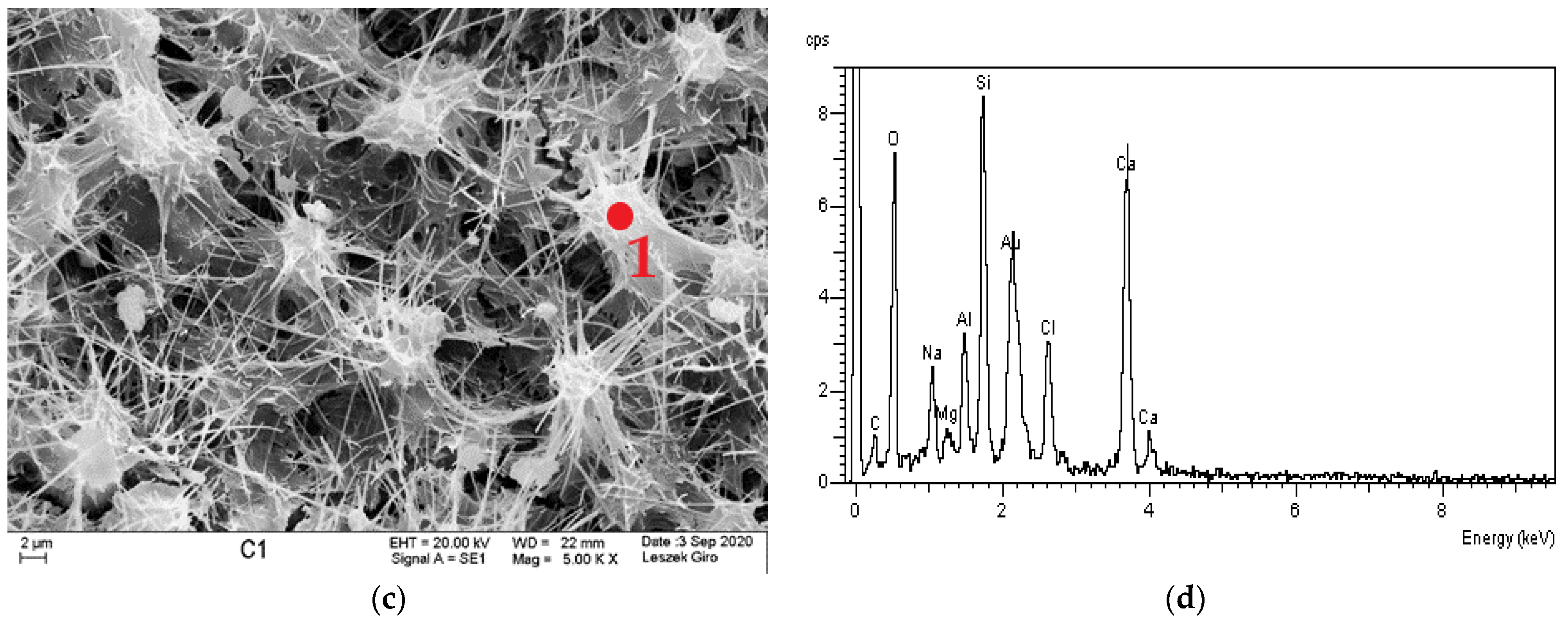
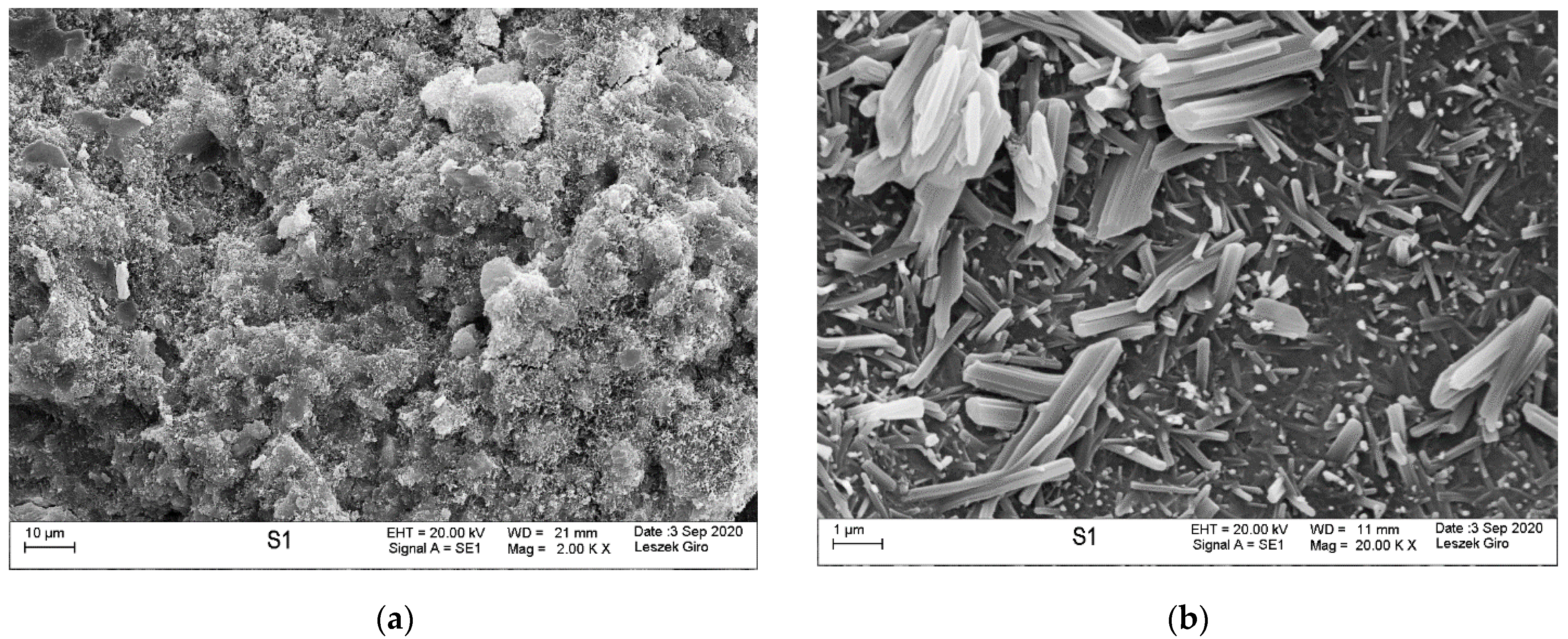
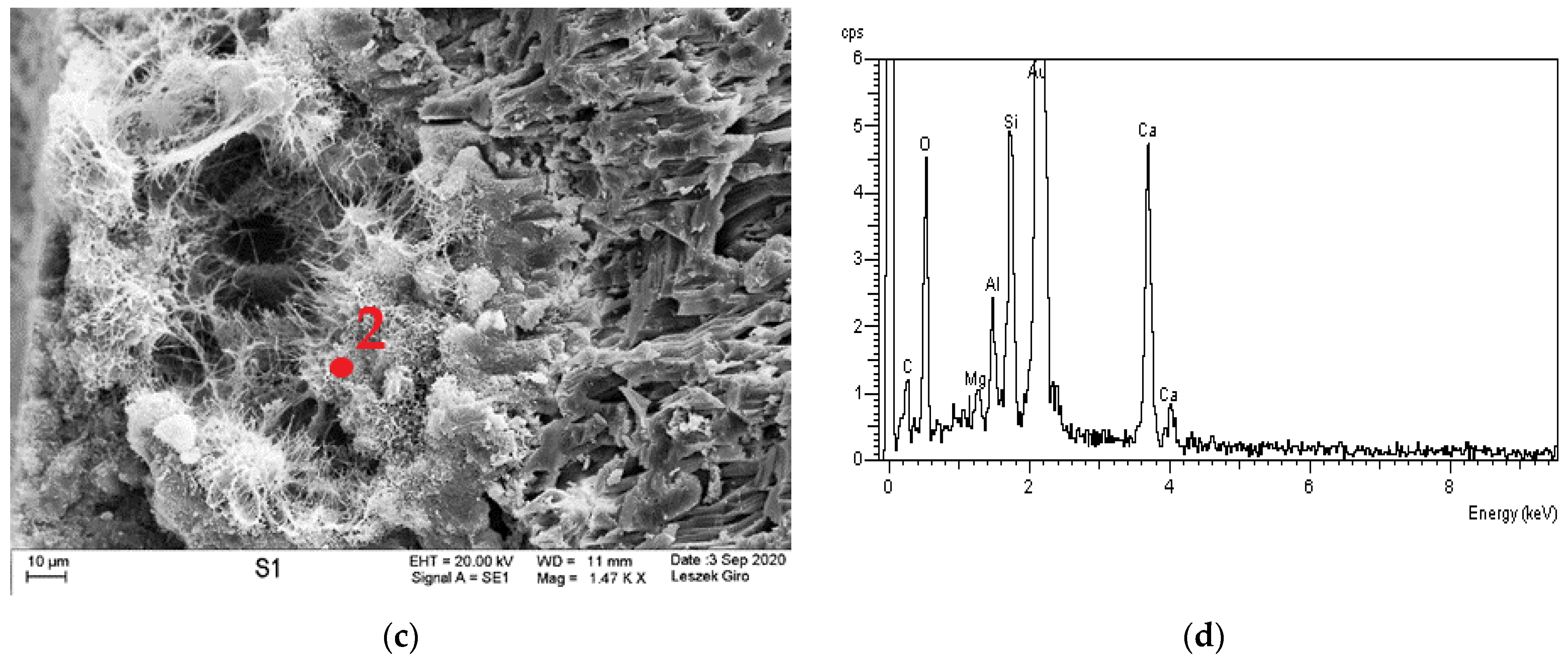
3.4. Microstructure
4. Conclusions
- The action of a NaCl and Na2SO4 solution caused a reduction of flexural strength of bars in comparison to the strength of specimens stored in tap water, probably due to the microcracks which were observed in the specimens.
- The impact of chloride and sulphate ion diffusion on the compressive strength of mortars is minor and within measurement uncertainty limits. The compressive strength of the specimens exposed to chloride aggressiveness is lower than the strength of specimens exposed to sulphate aggressiveness.
- Chloride action had a slight (adverse) influence on the pH of the pore liquid. However, no impact of sulphate action of the pore liquid pH was noted—this outcome may be due to its formation in a NaOH solution.
- Chloride and sulphate concentration in the specimen decreased with increasing specimen depth.
- Based on the tests, it can be concluded that the content of chlorides and sulphates in the specimen was lower than the limit concentration at a distance of less than 60 mm from the specimen’s plane of contact with the aggressive medium.
- The qualitative tests of phase composition using the XRD method revealed minor amounts of Friedel’s salt in the specimen exposed to the action of chloride ions.
- The diffraction patterns for all studied specimens exhibit the clear presence of quartz, calcite, calcium aluminosilicates, and ettringite. The presence of a small amount of Friedel’s salt was recorded in specimens subjected to diffusion with chloride ions.
- SEM-EDS microstructure tests indicated that there were more microcracks on the surface of specimens subjected to ion diffusion than the surface of other specimens. Moreover, sodium chloride in the form of regular crystals was evident. In addition, chlorine ions were incorporated into the hydrated calcium aluminosilicates.
- The local presence of ettringite crystals in the form of pseudo-hexagonal columns were identified in the microstructure of specimens exposed to sulphate ion diffusion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ECOBA. Production and Utilisation of CCPs in 2016 in Europe. Available online: http://www.ecoba.com/ecobaccpprod.html (accessed on 15 February 2021).
- Bashandy, A.A. Self-curing concrete under sulphate attack. Arch. Civ. Eng. 2016, 62, 3–18. [Google Scholar] [CrossRef]
- Łukowski, P.; Dębska, D. Effect of polymer addition on performance of Portland cement mortar exposed to sulphate attack. Materials 2020, 13, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramlochan, T.; Zacarias, P.; Thomas, M.D.A.; Hooton, R.D. The effect of pozzolans and slag on the expansion of mortars cured at elevated temperature. Part 2. In Microstructural and Microchemical Investigations. Cem. Concr. Res. 2004, 34, 1341–1356. [Google Scholar] [CrossRef]
- Bensted, J.; Munn, J. Delayed ettringite formation—A concise view. Cem. Lime Concr. 2009, 5, 240–244. [Google Scholar]
- Kelham, S. Effects of Cement Parameters on Expansion Associated with DEF. In International RILEM Workshop on Internal Sulphate Attack and Delayed Ettrinigite Formation; RILEM Publications SARL: Paddyfield, HongKong, 2004; pp. 197–211. [Google Scholar] [CrossRef]
- Kurdowski, W. Korozja chlorkowa betonu. Cement Wapno Beton 2002, 2, 56–60. [Google Scholar]
- Hewlett, P.C.; Liska, M. Lea’s Chemistry of Cement and Concrete, 5th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Jaśniok, T.; Słomka-Słupik, B.; Zybura, A. Przebieg korozji chlorkowej zbrojenia w betonie, bezpośrednio po inicjacji. Cement Wapno Beton 2014, 3, 158–165. [Google Scholar]
- Machowska, A. Spoiwa Niskoemisyjne i o Niskim Cieple Hydratacji z Wybranych Ubocznych Produktów Przemysłowych Jako Potencjalny Składnik Betonów Masywnych i Hydrotechnicznych; Circular Economy-Technologie (monograph): OWPW, Warsaw, Poland, 2019. [Google Scholar]
- PN-EN 451-2:2017. Method of Testing Fly Ash. Determination of Fineness by Wet Sieving; Polish Committee for Standardization: Warsaw, Poland, 2017. [Google Scholar]
- PN-EN 196-6:2019. Methods of testing cement. Determination of Fineness; Polish Committee for Standardization: Warsaw, Poland, 2019. [Google Scholar]
- PN-EN 1015-3:2000. Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table); Polish Committee for Standardization: Warsaw, Poland, 2000. [Google Scholar]
- Kledyński, Z.; Machowska, A.; Pacewska, B.; Wilińska, I. Investigation of hydration products of fly ash-slag pastes. J. Therm. Anal. Calorim. 2017, 130, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Roszczynialski, W. The role of calcium sulphate (VI) in the processes of shaping the strength of road binders containing steel slag and fly ash. Ph.D. Thesis, AGH Cracow, Kraków, Poland, 2014. [Google Scholar]
- Zapotoczna-Sytek, G.; Łaskawiec, K.; Gębarowski, P.; Małolepszy, J.; Szymczak, J. Popioły Lotne Nowej Generacji do Produkcji Autoklawizowanego Betonu Komórkowego—Monografia ICiMB; Wydawnictwo Instytut Śląski: Opole, Poland, 2013. [Google Scholar]
- Mehta, P.K. Pozzolanic and Cementitious By-Products in Concrete—Another look. In Fly Ash, Silica Fume, Slag and Natural Pozzolans in Concrete, Proceedings of the 3rd International Conference, Bangkok, Thailand, 31 May–5 June 1989; American Concrete Institute (ACI): Trondheim, Norway, 1989; Volume 114, pp. 1–44. [Google Scholar]
- Neville, A.M. Properties of Concrete, 5th ed.; Polish Cement Association: Cracow, Poland, 2012. [Google Scholar]
- PN-EN 197-1:2012. Cement. Composition, Specifications and Conformity Criteria for Common Cements; Polish Committee for Standardization: Warsaw, Poland, 2012. [Google Scholar]
- Machowska, A.; Kledyński, Z.; Wilińska, I.; Pacewska, B. Study of the early hydration processes and properties of fly ash-slag binders. Bull. Mater. Sci. 2019, 42, 213. [Google Scholar] [CrossRef] [Green Version]
- PN-EN 196-1:2016. Methods of Testing Cement. Determination of Strength; Polish Committee for Standardization: Warsaw, Poland, 2016. [Google Scholar]
- PN-EN 12390-11:2015-09. Testing Hardened Concrete—Part 11: Determination of the Chloride Resistance of Concrete, Unidirectional Diffusion; Polish Committee for Standardization: Warsaw, Poland, 2015. [Google Scholar]
- PN-EN 196-2:2013-11. Methods of Testing Cement. Chemical Analysis of Cement; Polish Committee for Standardization: Warsaw, Poland, 2013. [Google Scholar]
- PN-EN 12457-2:2006. Characterisation of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges. One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size below 4 mm (without or with Size Reduction); Polish Committee for Standardization: Warsaw, Poland, 2006. [Google Scholar]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al (OH) 6 (Cl, OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Conc. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Reiterman, P.; Keppert, M. Effect of various de-icers containing chloride ions on scaling resistance and chloride penetration depth of highway concrete. Roads Bridges-Drog. I Mosty 2020, 19, 51–64. [Google Scholar]
- Kurdowski, W. Cement and Concrete Chemistry; Springer Science & Business: Berlin, Germany, 2014. [Google Scholar]
- Ściślewski, Z. Ochrona Konstrukcji Żelbetowych; Arkady: Warszawa, Polska, 1999. [Google Scholar]
- PN-EN 206+A1:2016-12. Concrete—Specification, Performance, Production and Conformity; Polish Committee for Standardization: Warsaw, Poland, 2016. [Google Scholar]
- Glass, G.K.; Buenfeld, N.R. The influence of chloride binding on the chloride induced corrosion risk in reinforced concrete. Corros. Sci. 2000, 42, 329–344. [Google Scholar] [CrossRef]
- Słomka-Słupik, B.; Zybura, A. Doświadczalne Badania Dekalcyfikacji Zaczynu Cementowego. In Konferencja Dni Betonu; Polish Cement Association: Wisła, Poland, 2010; pp. 787–796. [Google Scholar]
- Glasser, F.P.; Marchand, J.; Samson, E. Durability of concrete—Degradation phenomena involving detrimental chemical reactions. Cem. Concr. Res. 2008, 38, 226–246. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, D.; De Schutter, G.; Yu, Z. Chemical sulfate attack performance of partially exposed cement and cement + fly ash paste. Constr. Build. Mat. 2012, 28, 230–237. [Google Scholar] [CrossRef]
- Marchand, J.; Samson, E.; Maltais, Y. The Role of Ettringite in External Sulfate Attack. In Material Science of Concrete: Sulfate Attack Mechanisms; Marchand, J., Skalny, J., Eds.; The American Ceramic Society: Westerville, OH, USA, 1999. [Google Scholar]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Owsiak, Z. Ocena stanu betonu w elementach wiaduktu drogowego. Przegląd Bud. 2007, 78, 24–27. [Google Scholar]
- Pawluk, J. Korozja siarczanowa betonu, analiza problemu. Cem. Wapno Beton 2017, 22, 230–238. [Google Scholar]
- Irbe, L.; Beddoe, R.E.; Heinz, D. The role of aluminium in CASH during sulfate attack on concrete. Cem. Concr. Res. 2019, 116, 71–80. [Google Scholar] [CrossRef]
- Bellmann, F.; Möser, B.; Stark, J. Influence of sulfate solution concentration on the formation of gypsum in sulfate resistance test specimen. Cem. Concr. Res. 2006, 36, 358–363. [Google Scholar] [CrossRef]
- Luo, Y.; Niu, D.; Su, L. Chloride Diffusion Property of Hybrid Basalt–Polypropylene Fibre-Reinforced Concrete in a Chloride–Sulphate Composite Environment under Drying—Wetting Cycles. Materials 2021, 14, 1138. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, S.; Wang, C.; Fang, Z. Effects of cations in sulfate on the thaumasite form of sulfate attack of cementitious materials. Constr. Build. Mater. 2019, 229, 116865. [Google Scholar] [CrossRef]
- Wang, P.; Mo, R.; Li, S.; Xu, J.; Jin, Z.; Zhao, T.; Wang, D. A chemo-damage-transport model for chloride ions diffusion in cement-based materials: Combined effects of sulfate attack and temperature. Constr. Build. Mater. 2021, 288, 123121. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Academic Press: London, UK, 1990. [Google Scholar]
- Beaudin, J.J.; Ramachandran, V.S.; Feldman, R.F. Interaction of chloride and C-S-H. Cem. Concr. Res. 1990, 20, 875. [Google Scholar] [CrossRef] [Green Version]
- Divet, L.; Randriambololona, R.; Leger, D. Contribution à l’études des mécanismes de sorption des ions Cl− et SO4-2 sur les silicates de calcium hydrates (CSH). Silic. Ind. 2005, 25–31. [Google Scholar]
- Mohammed, T.U.; Hamada, H. Relationship between free and total chloride contents in concrete. Cem. Concr. Res. 2003, 33, 1487–1490. [Google Scholar] [CrossRef]
| No. | Ingredient | Ingredient Content [g] |
|---|---|---|
| 1 | Water | 225 |
| 2 | GGBFS | 315 |
| 3 | CFBC fly ash | 135 |
| 4 | Standard sand | 1350 |
| 5 | w/b ratio | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falaciński, P.; Machowska, A.; Szarek, Ł. The Impact of Chloride and Sulphate Aggressiveness on the Microstructure and Phase Composition of Fly Ash-Slag Mortar. Materials 2021, 14, 4430. https://doi.org/10.3390/ma14164430
Falaciński P, Machowska A, Szarek Ł. The Impact of Chloride and Sulphate Aggressiveness on the Microstructure and Phase Composition of Fly Ash-Slag Mortar. Materials. 2021; 14(16):4430. https://doi.org/10.3390/ma14164430
Chicago/Turabian StyleFalaciński, Paweł, Agnieszka Machowska, and Łukasz Szarek. 2021. "The Impact of Chloride and Sulphate Aggressiveness on the Microstructure and Phase Composition of Fly Ash-Slag Mortar" Materials 14, no. 16: 4430. https://doi.org/10.3390/ma14164430
APA StyleFalaciński, P., Machowska, A., & Szarek, Ł. (2021). The Impact of Chloride and Sulphate Aggressiveness on the Microstructure and Phase Composition of Fly Ash-Slag Mortar. Materials, 14(16), 4430. https://doi.org/10.3390/ma14164430








