Crystal-Chemical and Thermal Properties of Decorative Cement Composites
Abstract
1. Introduction
2. Raw Materials Composition and Samples Preparation
3. Experimental Methods
4. Results and Discussion
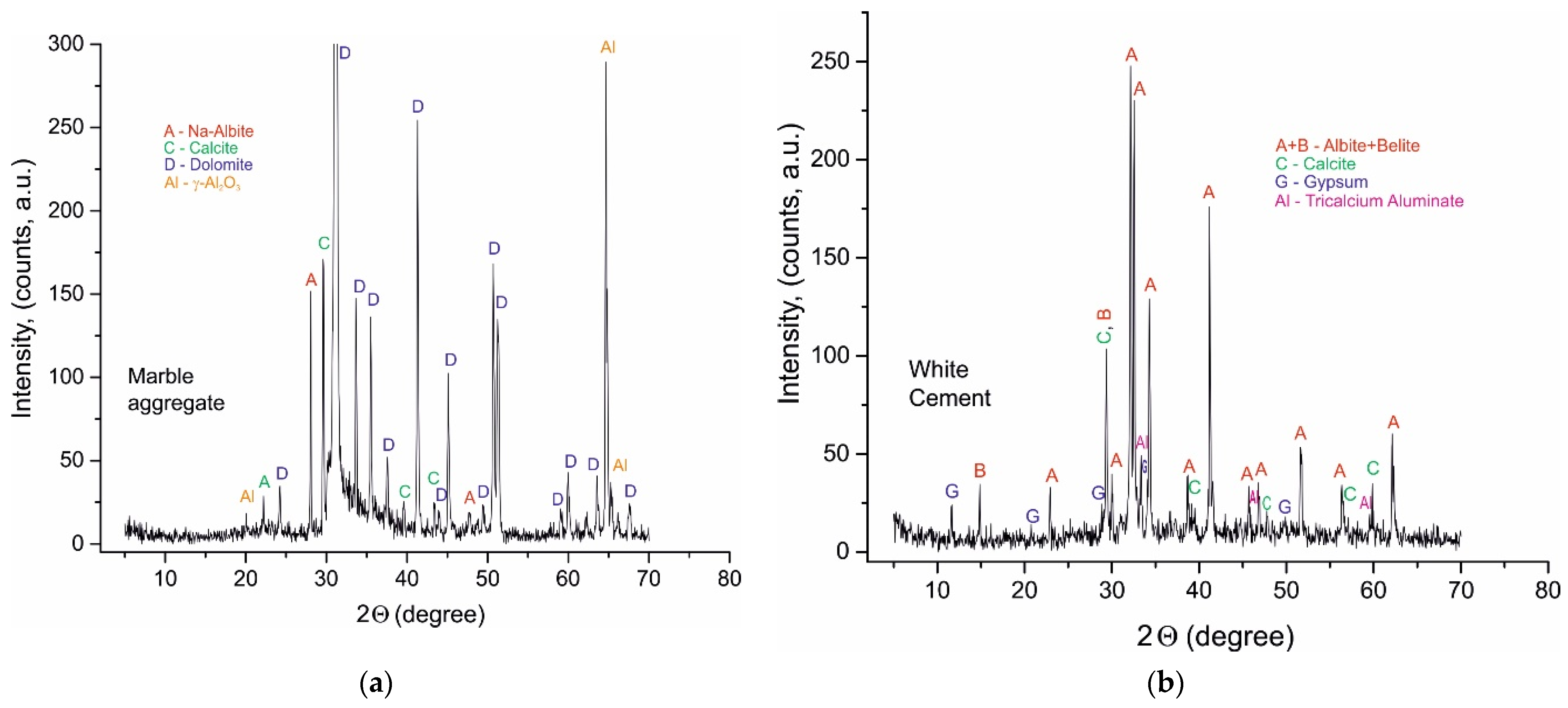
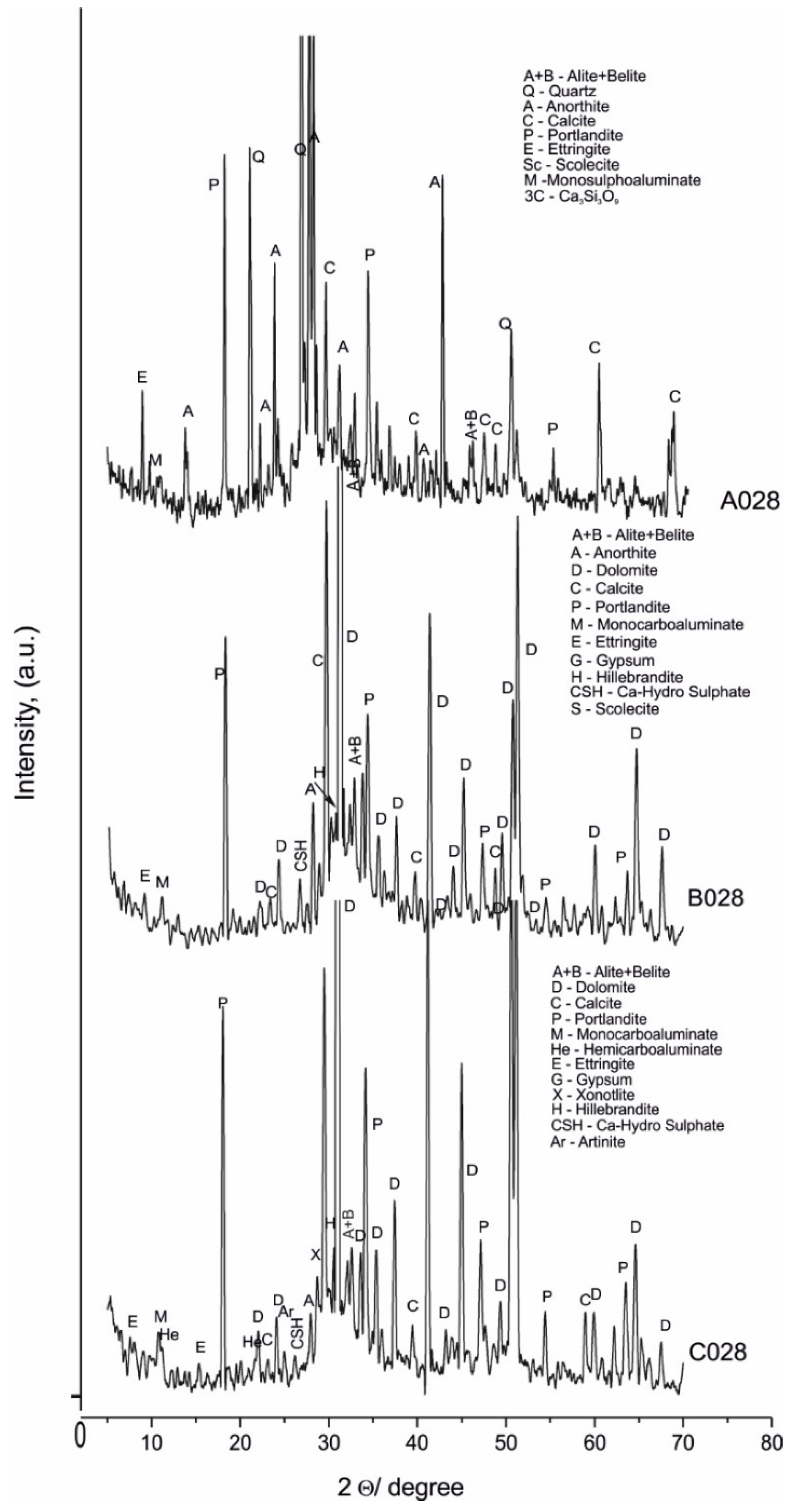
4.1. PXRD Phase Analysis
- -
- containing OH− and HCO3−/CO32−;
- -
- containing OH− and HSO4−/SO42−;
- -
- containing OH− in hydrate phase and OH− in hydrosilicates formed of main oxides CaO, Al2O3, SiO2.
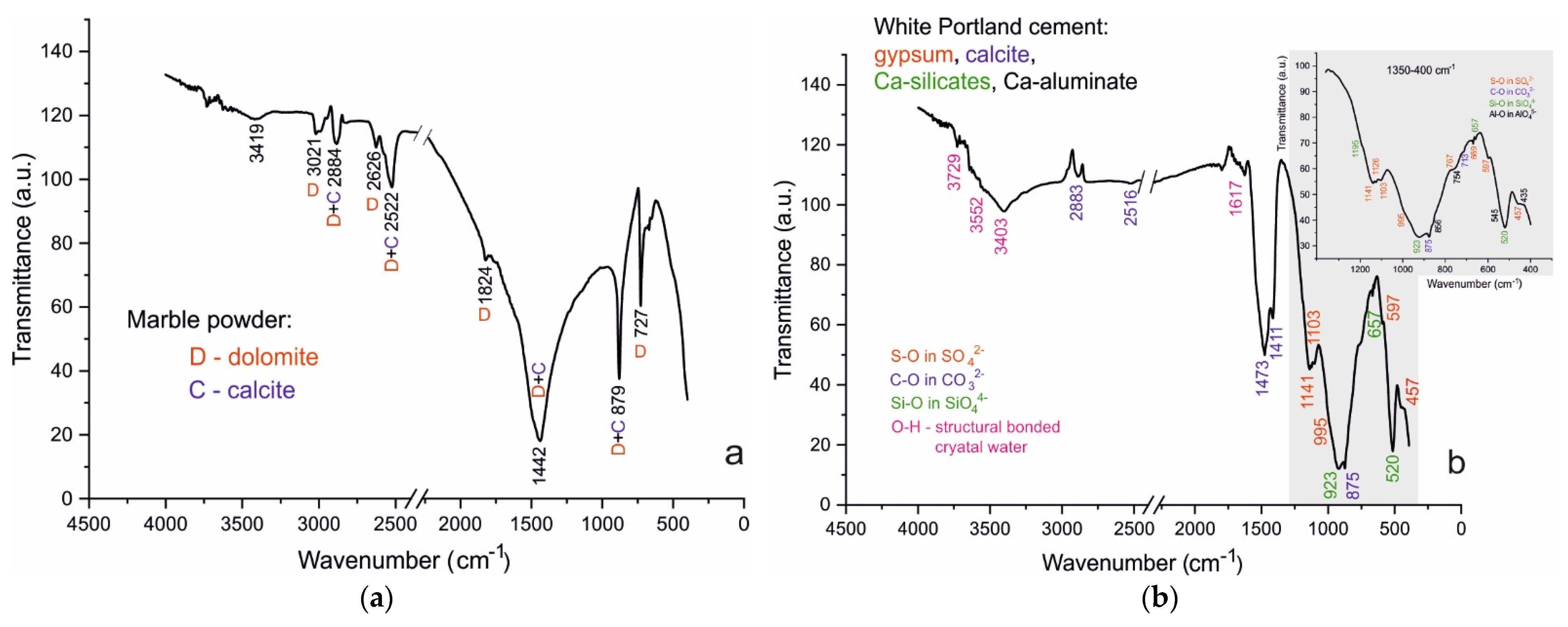
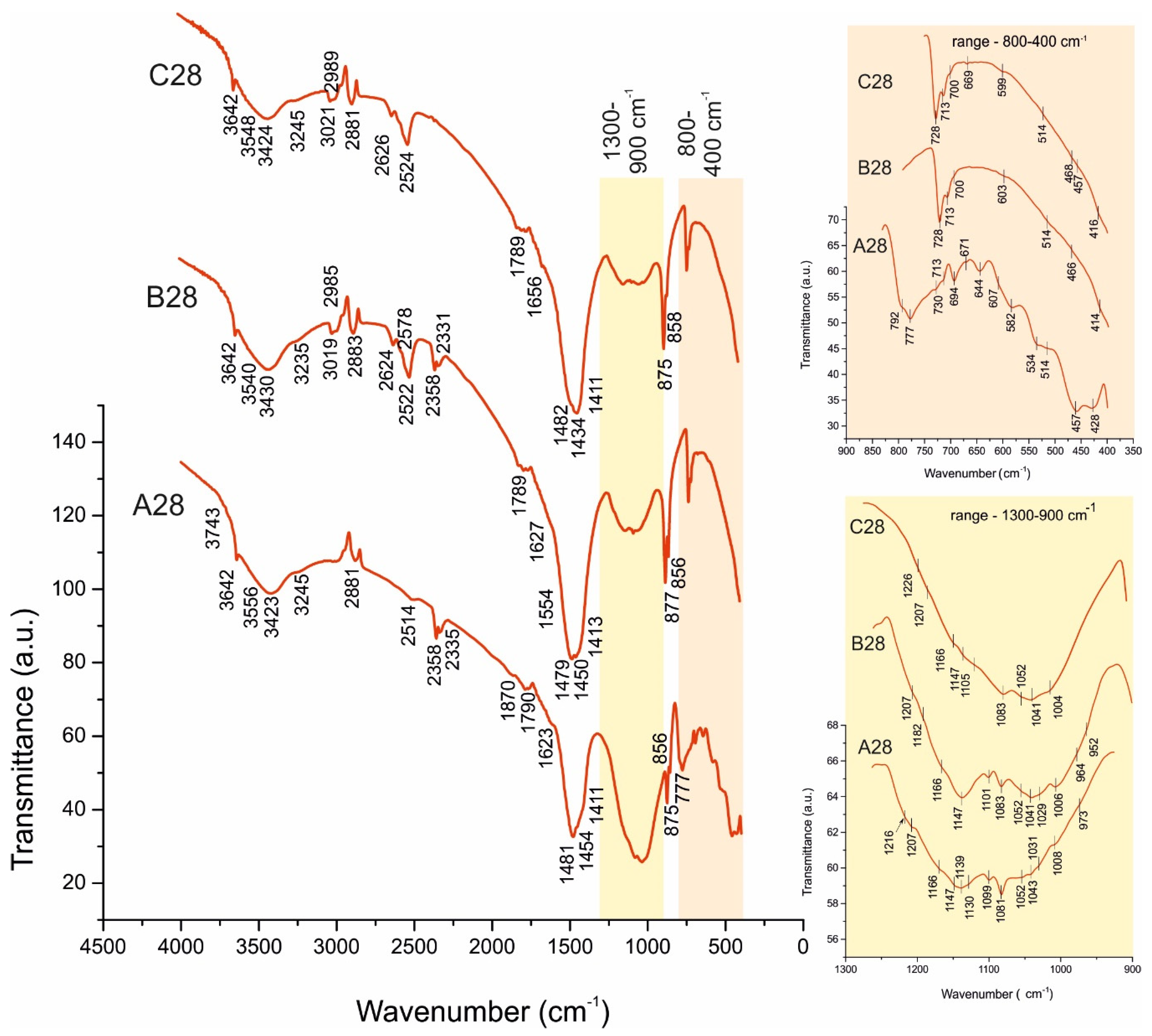
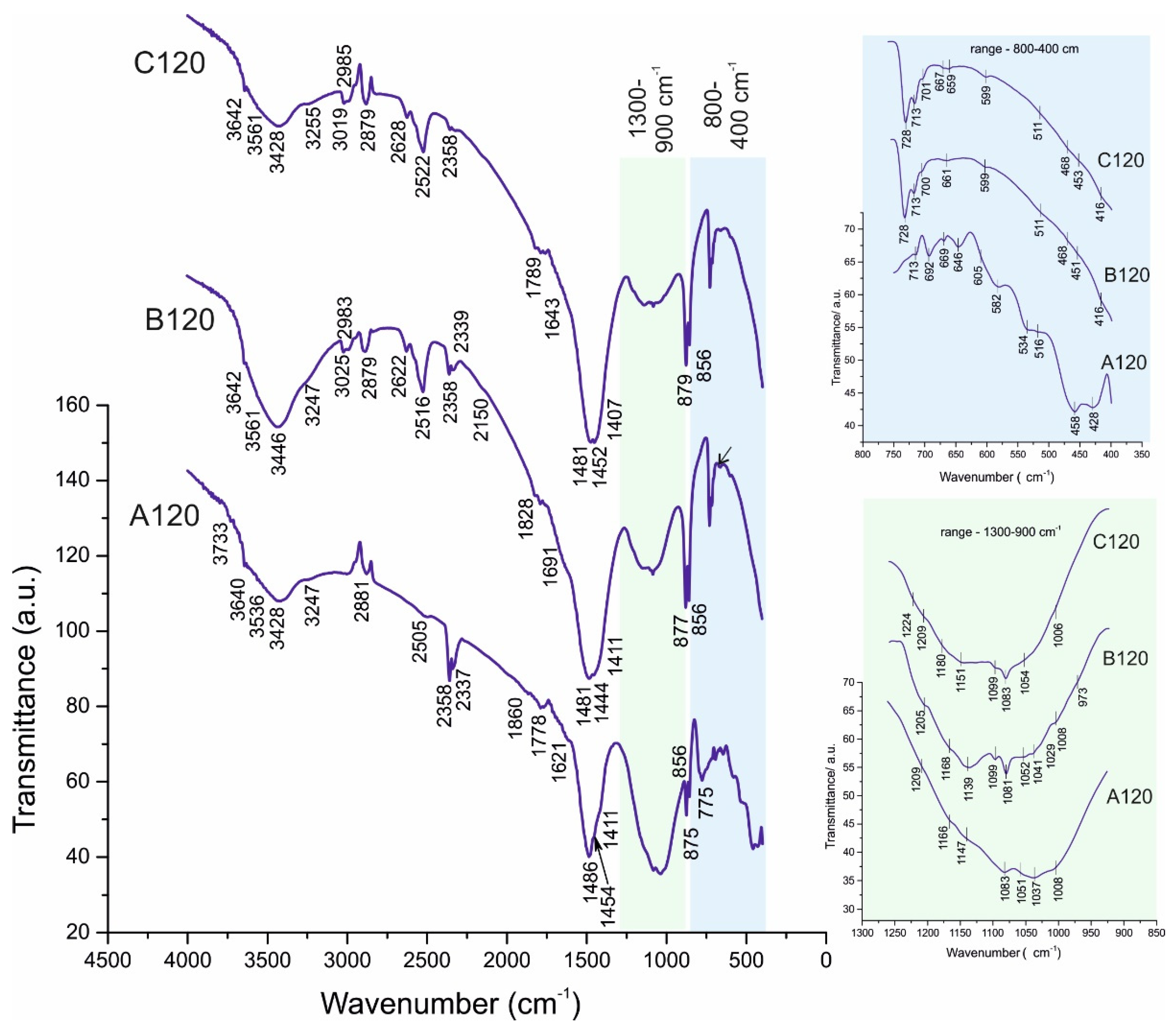
4.2. FTIR Spectroscopy
4.2.1. Sample A
4.2.2. Samples B and C:
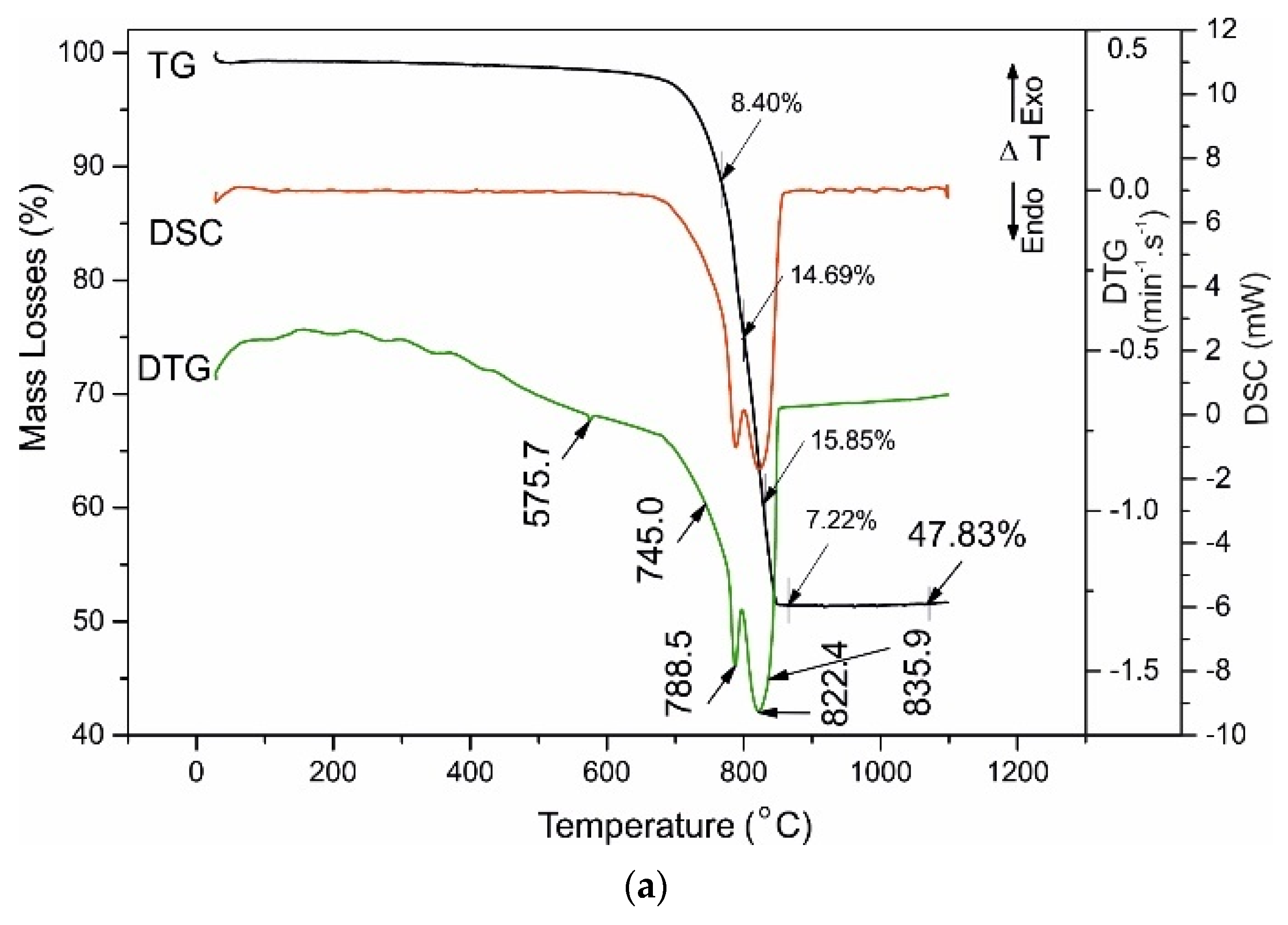
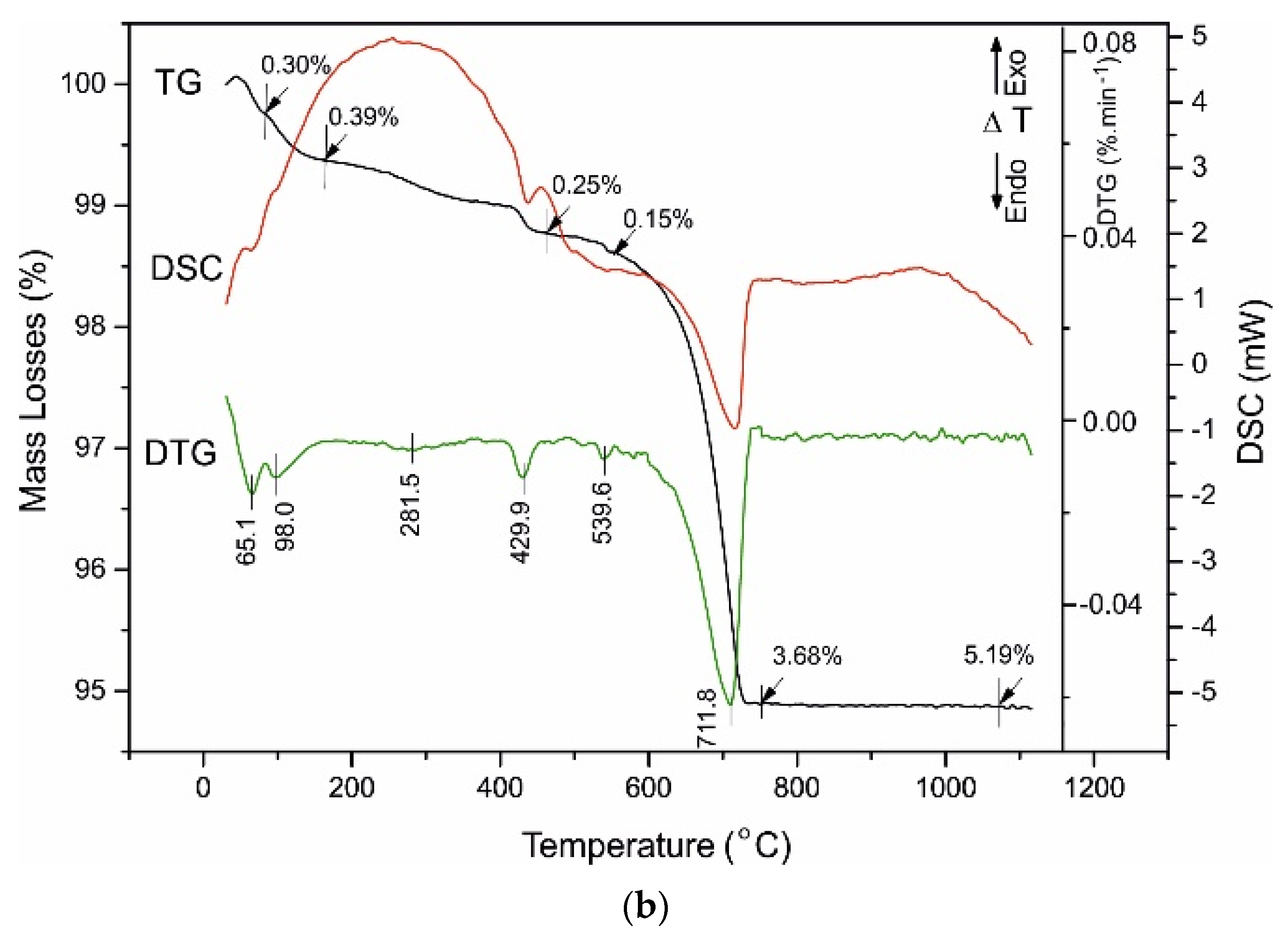
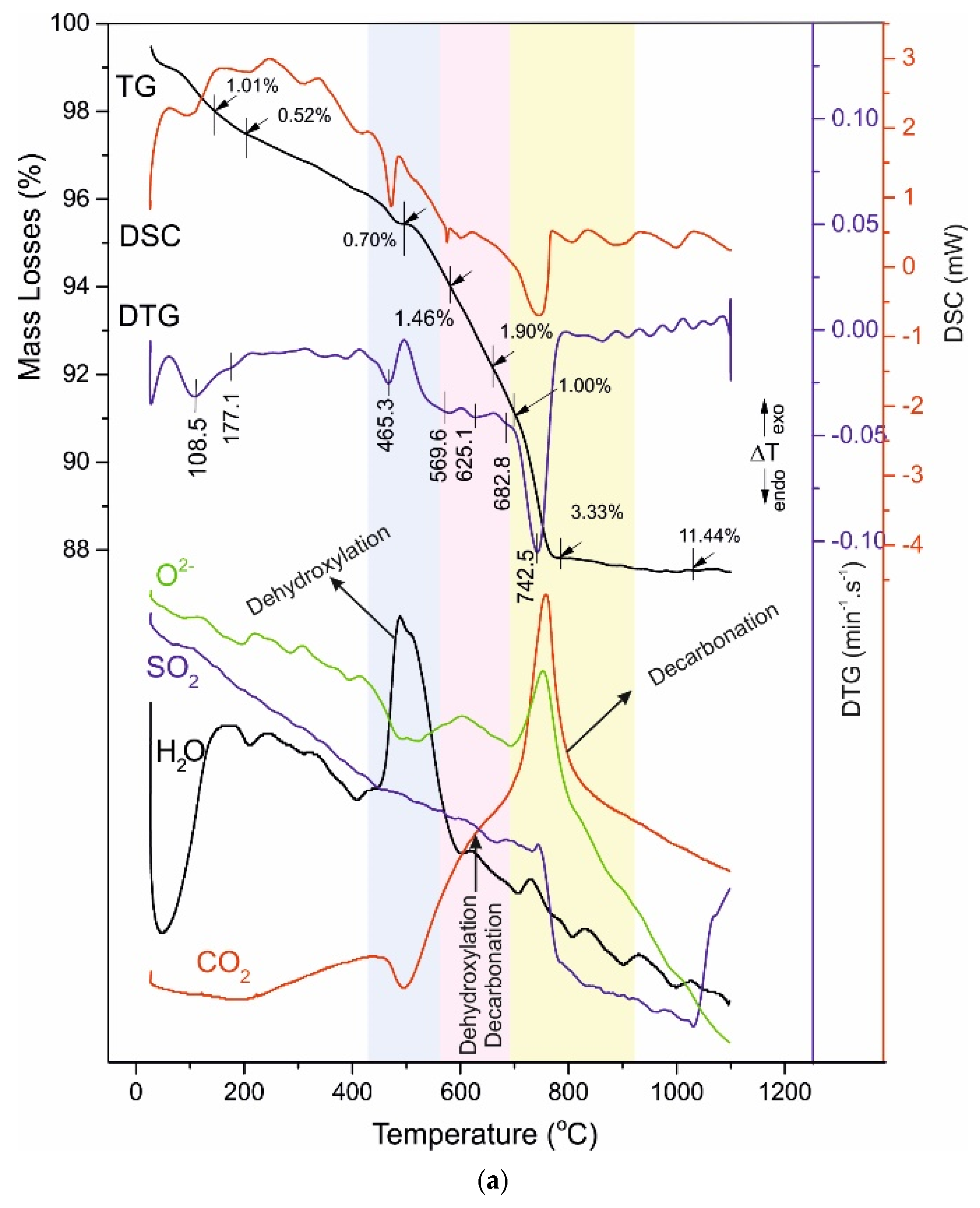
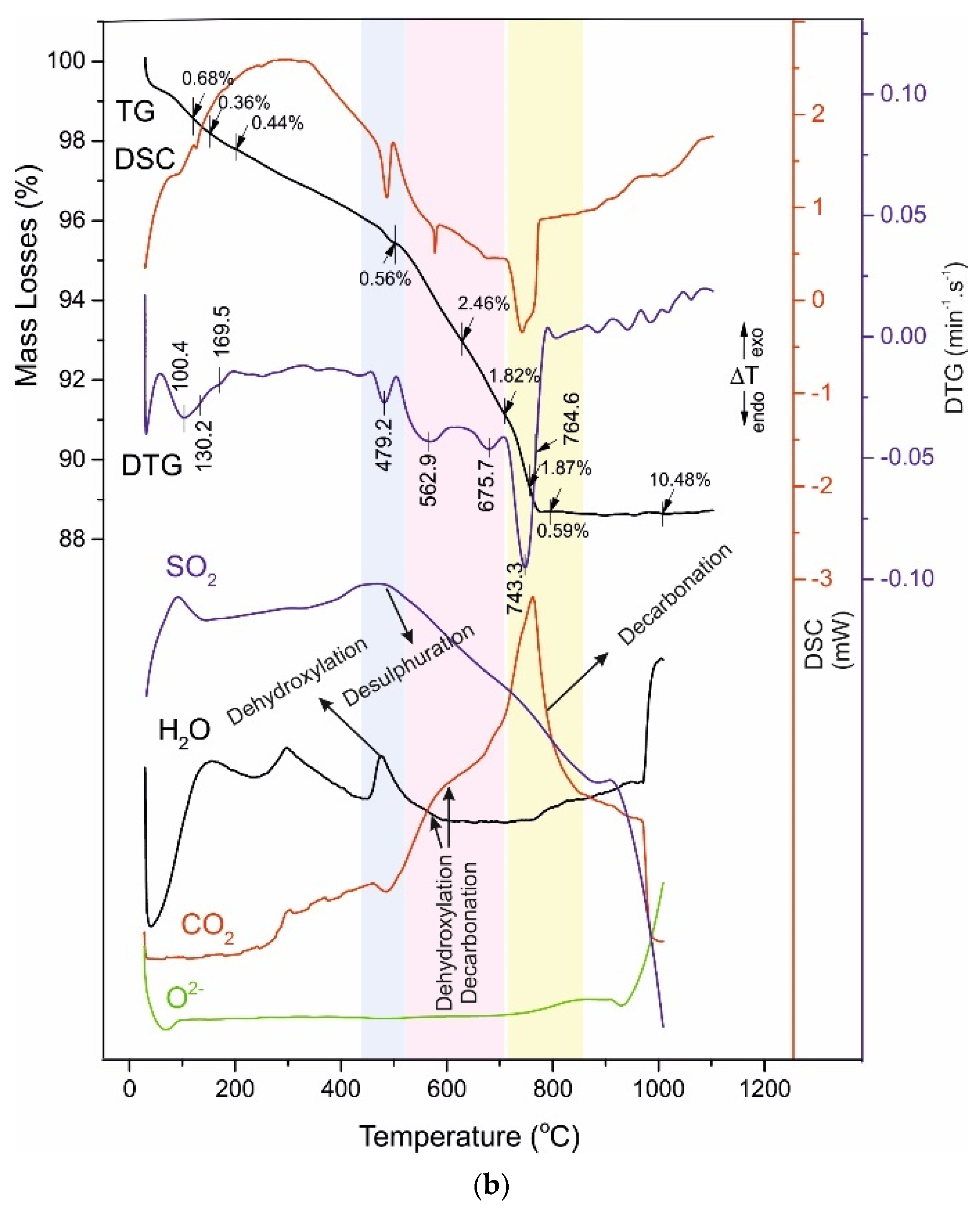
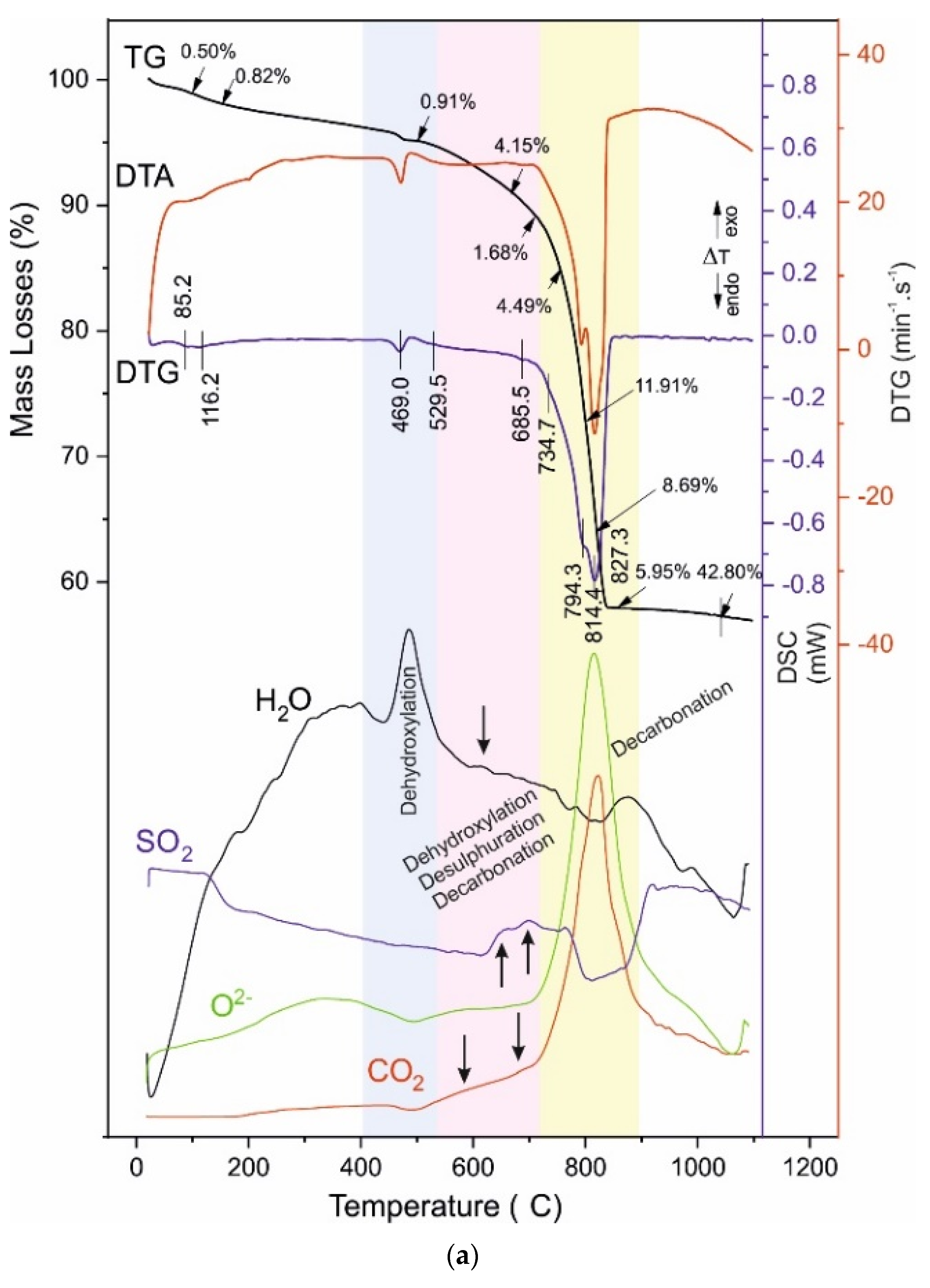
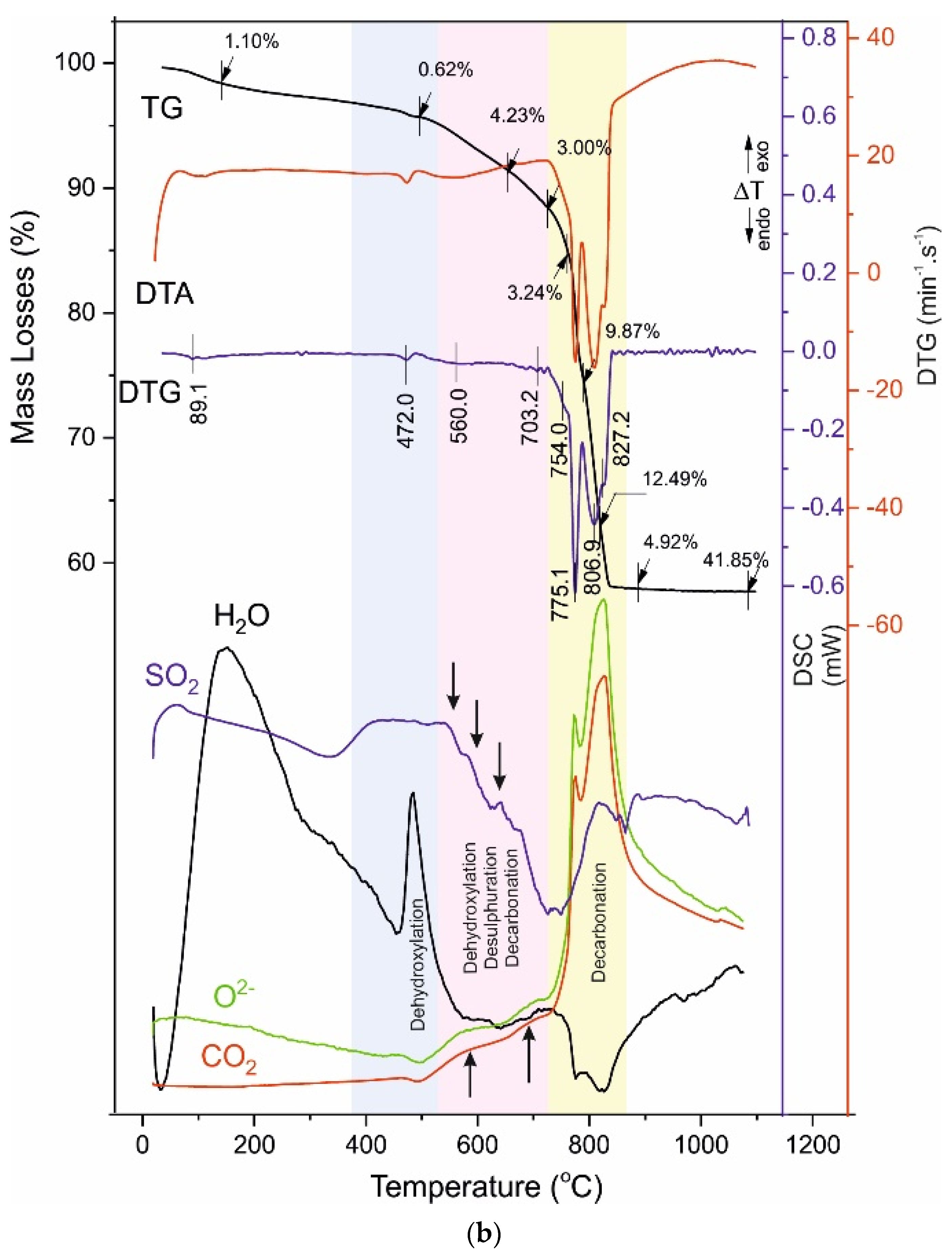
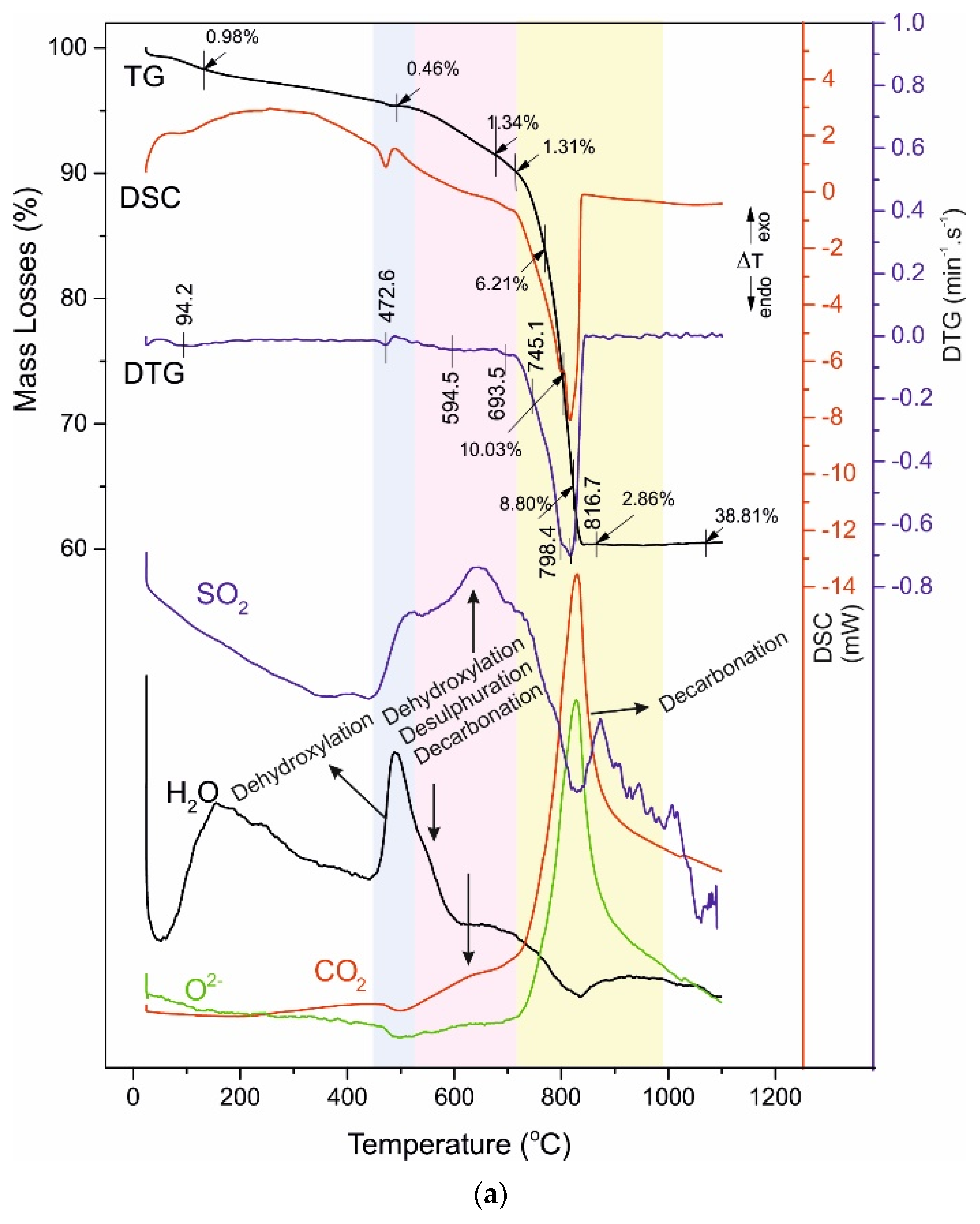
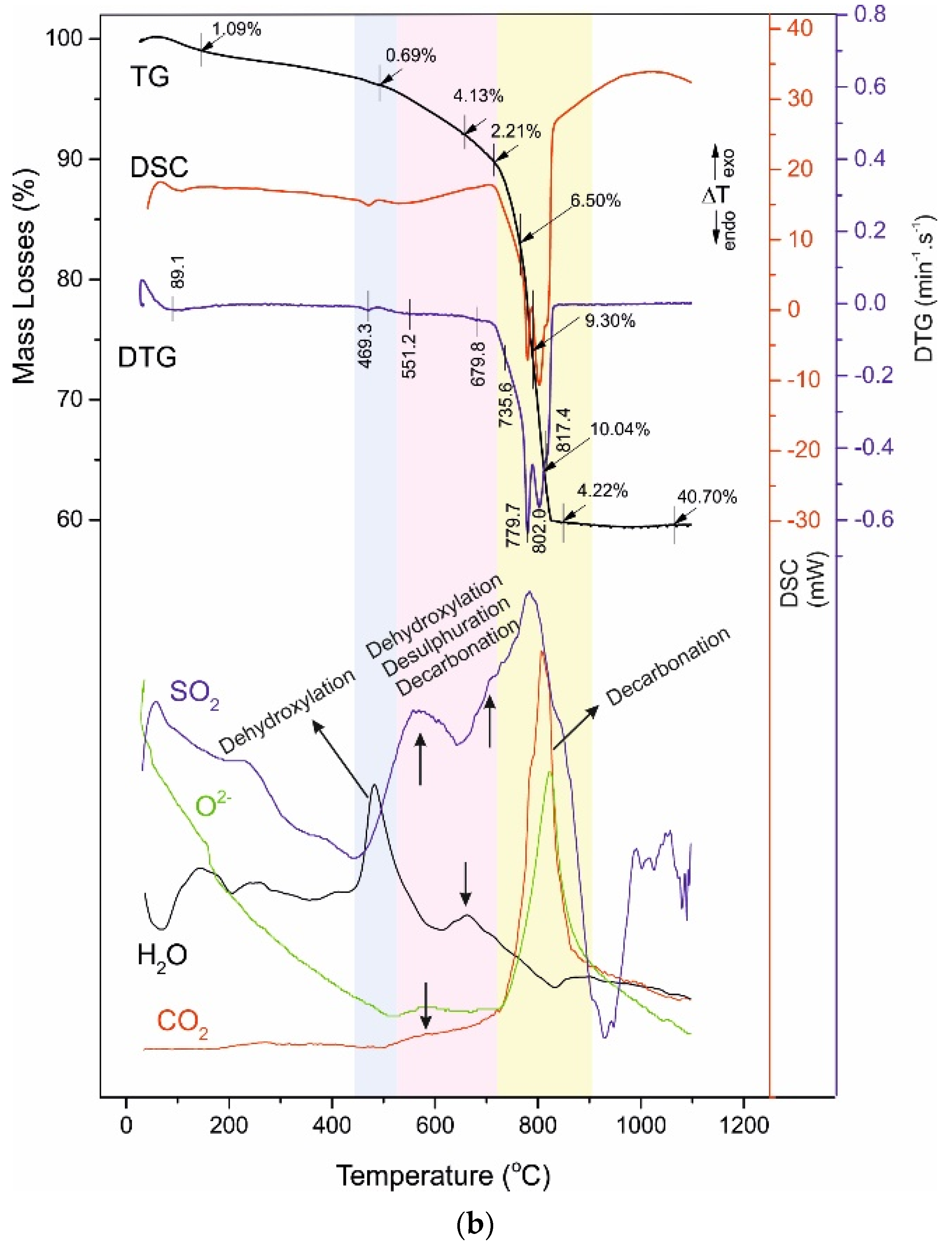
4.3. Thermal Analysis
- -
- -
- -
- -
4.4. Reaction Mechanism of Thermal Decomposition of Decorative Cement Composites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| A | white Portland cement + sand + water |
| B | white Portland cement + marble powder + water |
| C | white Portland cement + marble powder + HRWR + water |
| A028 | Sample A after water curring of 28 days |
| A120 | Sample A after water curring of 120 days |
| B028 | Sample B after water curring of 28 days |
| B120 | Sample B after water curring of 120 days |
| C028 | Sample C after water curring of 28 days |
| C120 | Sample C after water curring of 120 days |
| C-S-H | calcium silicate hydrate |
| HRWR | polycarboxylate-based admixture |
| PXRD | Powder X-ray diffraction |
| FTIR | Fourier Transformed Infrared |
| TG/DTG–DSC | Thermal analysis |
| ML, ΔGtotal | Mass Losses, ΔG |
| Tinfl | Temperature of the point of inflection |
| RT | Room temperature |
| EnT | Endothermal effect |
| ExT | Exothemal effect |
References
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data Discuss. 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Cloete, S.; Giuffrida, A.; Romano, M.C.; Zaabout, A. Economic assessment of the swing adsorption reactor cluster for CO2 capture from cement production. J. Clean. Prod. 2020, 275, 123024. [Google Scholar] [CrossRef]
- Korhonena, J.; Honkasalob, A.; Seppälä, J. Circular Economy: The Concept and its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Agyabeng-Mensah, Y.; Tang, L.; Afum, E.; Baah, C.; Dacosta, E. Organisational identity and circular economy: Are inter and intra organisational learning, lean management and zero waste practices worth pursuing? Sustain. Prod. Consum. 2021, 28, 648–662. [Google Scholar] [CrossRef]
- Kechagia, P.; Koutroumpi, D.; Bartzas, G.; Peppas, A.; Samouhos, M.; Deligiannis, S.; Tsakiridis, P.E. Waste marble dust and recycled glass valorization in the production of ternary blended cements. Sci. Total Environ. 2020, 761, 143224. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Ming, X.; He, K.; Li, L.; Shen, S. Effect of Macro-, Micro- and Nano-Calcium Carbonate on Properties of Cementitious Composites—A Review. Materials 2019, 12, 781. [Google Scholar] [CrossRef]
- Aïtcin, P.C. Lea’s Chemistry of Cement and Concrete, 5th ed.; Hewlett, P.C., Liska, M., Eds.; Elsevier: Oxford, UK, 2019; ISBN 978-0-08-100773-0. [Google Scholar]
- Hamad, B.S. Investigations of chemical and physical properties of white cement concrete. Adv. Cem. Based Mater. 1995, 2, 161–167. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. Properties of architectural mortar prepared with recycled glass with different particle sizes. Mater. Des. 2011, 32, 2675–2684. [Google Scholar] [CrossRef]
- Sanytsky, M.; Kropyvnytska, T.; Fic, S.; Ivashchyshyn, H. Sustainable low-carbon binders and concretes. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 166, p. 06007. [Google Scholar] [CrossRef]
- Aïtcin, P.-C.; Mindess, S. Sustainability of Concrete; Taylor & Francis: Abingdon, UK, 2011; ISBN 9781138075689. [Google Scholar]
- De Schutter, G.; Bartos, P.J.M.; Domone, P.; Gibbs, J. Self-Compacting Concrete; Whittles Publishing: Dunbeath, UK, 2008. [Google Scholar]
- Amziane, S.; Lanos, C.; Mouret, M. Self Compacting Concrete; Loukili, A., Ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-1-848-21290-9. [Google Scholar]
- Kubatova, D.; Zezulova, A.; Rybova, A.; Bohac, M. Synthesis of b-C2S-based binder from limestone and calcium silicate wastes. J. Therm. Anal. Calorim. 2019, 138, 1901–1912. [Google Scholar] [CrossRef]
- Stoyanov, V.; Kostova, B.; Petkova, V.; Pelovski, Y. Structure of white cement mortars with high content of marble powder. J Therm. Anal. Calorim. 2012, 110, 405–412. [Google Scholar] [CrossRef]
- Petkova, V.; Stoyanov, V.; Kostova, B.; Kalinkin, A.; Zvereva, I.; Tzvetanova, Y.; Serafimova, E. Spectroscopic Investigations of Phase Formation in Cement Mortars with a High Content of Mineral Fillers. In Proceedings of the Thirteenth International Scientific Conference Dedicated to the 60-th Anniversary of the First Artificial Satellite of the Earth and 45 Years of Bulgaria in Space with International Participation, Space, Ecology, Safety—SES 2017, Sofia, Bulgaria, 2–4 November 2017; Mardirossian, G., Srebrova, T., Jelev, G., Eds.; Space Research and Technology Institute, Bulgarian Academy of Sciences: Sofia, Bulgaria, 2017; pp. 297–303, ISSN 1313–3888. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997; ISBN 0-7277-2592-0. [Google Scholar]
- BDS EN 196-1:2016. Methods of Testing Cement—Part 1: Determination of strength; Bulgarian Institute for Standardization: Sofia, Bulgaria, 2016; 35p. [Google Scholar]
- Stoyanov, V. Investigation of properties of decorative mortars besed on design of experiments. In Proceedings of the International Conference on Civil Engineering Design and Construction. (Eurocodes—Science and Practice) DCB 2010, Varna, Bulgaria, 9–11 September 2010; pp. 463–470. (In Bulgarian). [Google Scholar]
- Mandrino, D.; Paulin, I.; Kržmanc, M.M.; Škapin, S.D. Physical and chemical treatments influence on the thermal decomposition of a dolomite used as a foaming agent. J. Therm. Anal. Calorim. 2018, 131, 1125–1134. [Google Scholar] [CrossRef]
- Nežerka, V.; Slížková, Z.; Tesárek, P.; Plachý, T.; Frankeová, D.; Petráňová, V. Comprehensive study on mechanical properties of lime-based pastes with additions of metakaolin and brick dust. Cem. Concr. Res. 2014, 64, 17–29. [Google Scholar] [CrossRef]
- Powder Diffraction File™ (PDF®) Search; International Centre for Diffraction Data (ICDD): Newtown Square, PA, USA; pp. 1973–3273.
- Taylor, H. Reactions and reactivities of compounds in hydraulic cements. Solid State Ion 1990, 43, 31–35. [Google Scholar] [CrossRef]
- Bizzozero, J.; Scrivener, K.L. Limestone reaction in calcium aluminate cement–calcium sulfate systems. Cem. Concr. Res. 2015, 76, 159–169. [Google Scholar] [CrossRef]
- Glasser, F.P. The formation and thermal stability of spurrite, Ca5(SiO4)2CO3. Cem. Concr. Res. 1973, 3, 23–28. [Google Scholar] [CrossRef]
- Grice, J.D. The structure of spurrite, tilleyite and scawtite, and relationships to the other silicate-carbonate minerals. Can. Mineral. 2005, 43, 1489–1500. [Google Scholar] [CrossRef]
- Bolio-Arceo, H.; Glasser, F.P. Formation of spurrite, Ca5(SiO4)2CO3. Cem. Concr. Res. 1990, 20, 301–307. [Google Scholar] [CrossRef]
- Tuttle, O.F.; Harker, R.I. Synthesis of spurrite and the reaction wollastonite+calcite spurrite+carbon dioxide. Am. J. Sci. 1957, 255, 226–234. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Wenhong, Y.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Trevor, H.L.; Methven, C.M.; Jones, T.G.J.; Pelham, S.E.; Fletcher, P.; Hall, C. Determining Cement composition by Fourier transform infrared spectroscopy. Adv. Cem. Based Mater. 1995, 2, 91–104. [Google Scholar]
- Chukanov, N.V. Infrared Spectra of Mineral Species: Extended Library; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK,, 2014; ISBN 978-94-007-7127-7. [Google Scholar]
- Chukanov, N.V.; Chervonnyi, A. Infrared Spectroscopy of Minerals and Related Compounds; Springer: Cham, Switzerland; Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK,, 2016. [Google Scholar] [CrossRef]
- Burdick, V.L.; Day, D.E. Coordination of Aluminum Ions in Tricalcium Aluminate. J. Am. Ceram. Soc. 1967, 50, 97–101. [Google Scholar] [CrossRef]
- Min, Y.; Ren, G.; Gao, T.; Li, J.; Qiu, D.; Yi, H.; Han, H. Uses of Near-Infrared Spectra for the Identification of Calcite and Dolomite in Carbonate Rocks. J. Comput. Theor. Nanosci. 2015, 12, 5854–5858. [Google Scholar] [CrossRef]
- Trezza, M.A.; Lavat, A.E. Analysis of the system 3CaO.Al2O3-CaSO4.2H2O-CaCO3-H2O by FT-IR spectroscopy. Cem. Concr. Compos. 2001, 31, 869–872. [Google Scholar] [CrossRef]
- Lane, M.D. Mid-infrared emission spectroscopy of sulfate and sulfate-bearing minerals. Am. Mineral. 2007, 92, 1–18. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J. Infrared and infrared emission spectroscopy of nesquehonite Mg(OH)(HCO3)2H2O—Implications for the formula of nesquehonite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1255–1260. [Google Scholar] [CrossRef]
- Steiner, S.; Lothenbach, B.; Proske, T.; Borgschulte, A.; Winnefeld, F. Effect of relative humidity on the carbonation rate of portlandite, calcium silicate hydrates and ettringite. Cem. Concr. Res. 2020, 135, 106116. [Google Scholar] [CrossRef]
- Fernández, J.M.; Duran, A.; Navarro-Blasco, I.; Lanas, J.; Sirera, R.; Alvarez, J.I. Influence of nanosilica and a polycarboxylate ether superplasticizer on the performance of lime mortars. Cem. Concr. Res. 2013, 43, 12–24. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Infrared Spectroscopy in the Analysis of Building and Construction Materials. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophile, T., Ed.; InthechOpen: Rijeka, Croatia, 2011; ISBN 978-953-51-0537-4. [Google Scholar] [CrossRef]
- Ryskin, Y.I. The Vibrations of Protons in Minerals: Hydroxyl, water and ammonium. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society: London, UK, 1974. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Montes, M.; Pato, E.; Fernández-Jiménez, A.; Blanco-Varela, M.T. Study on the activation of ternesite in CaOAl2O3 and 12CaO7Al2O3 blends with gypsum for the development of low-CO2 binders. J. Clean. Prod. 2021, 291, 125726. [Google Scholar] [CrossRef]
- De Weerdt, K.; Plusquellec, G.; Revert, A.B.; Geiker, M.R.; Lothenbach, B. Effect of carbonation on the pore solution of mortar. Cem. Concr. Res. 2019, 118, 38–56. [Google Scholar] [CrossRef]
- Benjeddou, O.; Alyousef, R.; Mohammad, H.; Soussi, C.; Khadimallah, M.A.; Alabduljabbar, H.; Tahir, M.M. Utilisation of waste marble powder as low-cost cementing materials in the production of mortar. J. Build. Eng. 2020, 32, 101642. [Google Scholar] [CrossRef]
- Cametti, G.; Danisi, R.M.; Armbruster, T.; Nagashima, M. De- and re-hydration of scolecite revisited: An in situ single-crystal X-ray study under low and high humidity conditions. Microporous Mesoporous Mater. 2015, 208, 171–180. [Google Scholar] [CrossRef]
- Ma, B.; Lothenbach, B. Thermodynamic study of cement/rock interactions using experimentally generated solubility data of zeolites. Cem. Concr. Res. 2020, 135, 106149. [Google Scholar] [CrossRef]
- Eisinas, A.; Dambrauskas, T.; Baltakys, K.; Ruginyte, K. The peculiarities of mayenite formation from synthetic katoiteand calcium monocarboaluminate samples in temperature range 25–1150 °C. J. Therm. Anal. Calorim. 2019, 138, 2275–2282. [Google Scholar] [CrossRef]
- Svatovskaya, L.; Sychov, M.; Sychova, A.; Gravit, M. New Geoecoprotective Properties of the construction materials for underground infrastructure development. Procedia Eng. 2016, 165, 1771–1775. [Google Scholar] [CrossRef]
- Morón Barrios, A.; Ferrández Vega, D.; Saiz Martínez, P.; Atanes-Sánchez, E.; Morón Fernández, C. Study of the properties of lime and cement mortars made from recycled ceramic aggregate and reinforced with fibers. J. Build. Eng. 2021, 35, 102097. [Google Scholar] [CrossRef]
- Menéndez, E.; Andrade, C.; Vega, L. Study of dehydration and rehydration processes of portlandite in mature and young cement pastes. J. Therm. Anal. Calorim. 2012, 110, 443–450. [Google Scholar] [CrossRef]
- Romano, R.C.O.; Bernardo, H.M.; Maciel, M.H.; Pileggi, R.G.; Cincotto, M.A. Hydration of Portland cement with red mud as mineral addition. J. Therm. Anal. Calorim. 2017, 131, 2477–2490. [Google Scholar] [CrossRef]
- Huang, G.; Pudasainee, D.; Gupta, R.; Liu, W.V. Thermal properties of calcium sulfoaluminate cement-based mortars incorporated with expanded perlite cured at cold temperatures. Constr. Build. Mater. 2021, 274, 122082. [Google Scholar] [CrossRef]
- Baldissera, A.F.; Schütz, M.K.; Dos Santos, L.M.; Vecchia, F.D.; Seferin, M.; Ligabue, R.; Costa, E.M.; Chaban, V.V.; Menezes, S.C.; Einloft, S. Epoxy resin-cement paste composite for wellbores: Evaluation of chemical degradation fostered carbon dioxide. Greenh. Gases Sci. Tehnol. 2017, 7, 1065–1079. [Google Scholar] [CrossRef]
- Tavangarian, F.; Emadi, R. Mechanism of nanostructure bredigite formation by mechanical activation with thermal treatment. Mater. Lett. 2011, 65, 2354–2356. [Google Scholar] [CrossRef]
- Pacewska, B.; Wiliska, I.; Blonkowski, G. Investigations of cement early hydration in the presence of chemically activated fly ash: Use of calorimetry and infrared absorption methods. J. Therm. Anal. Calorim. 2008, 93, 769–776. [Google Scholar] [CrossRef]
- Pacewska, B.; Wilinska, I.; Nowacka, M. Studies on the influence of different fly ashes and Portland cement on early hydration of calcium aluminate cement. J. Therm. Anal. Calorim. 2011, 106, 859–868. [Google Scholar] [CrossRef][Green Version]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Hou, P.; Chen, Y.; Gong, C.; Lu, L. Mechanisms and kinetics of the decomposition of calcium barium sulfoaluminate. J. Therm. Anal. Calorim. 2015, 119, 1731–1737. [Google Scholar] [CrossRef]
- Jo, D.; Leonardo, R.S.; Cartledge, F.K.; Mendoza Reales, O.A.; Filho, R.D.T. Gypsum content determination in Portland cements by thermogravimetry. J. Therm. Anal. Calorim. 2016, 123, 1053–1062. [Google Scholar] [CrossRef]
- Boris, R.; Antonovič, V.; Kerienė, J.; Stonys, R. The effect of carbon fiber additive on early hydration of calcium aluminate cement. J. Therm. Anal. Calorim. 2016, 125, 1061–1070. [Google Scholar] [CrossRef]
- Frankeová, D.; Slížková, Z. Determination of the pozzolanic activity of mortar’s components by thermal analysis. J. Therm. Anal. Calorim. 2016, 125, 1115–1123. [Google Scholar] [CrossRef]
- Sabeur, H.; Platret, G.; Vincent, J. Composition and microstructural changes in an aged cement pastes upon two heating–cooling regimes, as studied by thermal analysis and X-ray diffraction. J. Therm. Anal. Calorim. 2016, 126, 1023–1043. [Google Scholar] [CrossRef]
- Vola, G.; Bresciani, P.; Rodeghero, E.; Sarandrea, L.; Cruciani, G. Impact of rock fabric, thermal behavior, and carbonate decomposition kinetics on quicklime industrial production and slaking reactivity. J. Therm. Anal. Calorim. 2019, 136, 967–993. [Google Scholar] [CrossRef]
- Almeida, T.F.; Leite, F.H.G.; Faria, R.T.; Holanda, J.N.F. Thermal study of calcium silicate material synthesized with solid wastes. J. Therm. Anal. Calorim. 2017, 128, 1265–1272. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Blanco-Varela, M.T. Ettringite decomposition in the presence of barium carbonate. Cem. Concr. Res. 2013, 52, 140–148. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Gan, Y.; Chang, Z.; Schlangen, E.; Šavija, B. Microstructure informed micromechanical modelling of hydrated cement paste: Techniques and challenges. Constr. Build. Mater. 2020, 251, 118983. [Google Scholar] [CrossRef]
- Haigh, R.; Sandanayake, M.; Bouras, Y.; Vrcelj, Z. A review of the mechanical and durability performance of kraft-fibre reinforced mortar and concrete. Constr. Build. Mater. 2021, 297. [Google Scholar] [CrossRef]
- Kostova, B.; Petkova, V.; Stoyanov, V.; Serafimova, E. Thermal properties of self-compacting type decorative white cement composites. In Proceedings of the XV International Conference on Thermal Analysis and Calorimetry in Russia (RTAC-2016), Saint-Petersburg, Russia, 13–23 September 2016; Peter the Great St. Petersburg Polytechnic University: Saint-Petersburg, Russia, 2016; pp. 435–439. ISBN 978-5-7422-5447-8. [Google Scholar]
- Chen, B.; Horgnies, M.; Huet, B.; Morin, V.; Johannes, K.; Kuznik, F. Comparative kinetics study on carbonation of ettringite and meta-ettringite based materials. Cem. Concr. Res. 2020, 137, 106209. [Google Scholar] [CrossRef]
- Kamruddin, M.; Ajikumar, P.K.; Dash, S.; Tyagi, A.K.; Baldev, R. Thermogravimetry-evolved gas analysis-mass spectrometry system for materials research. Bull. Mater. Sci. 2003, 26, 449–460. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.Q.; Radha, A.V.; Navrotsky, A. Thermochemistry of two calcium silicate carbonate minerals: Scawtite, Ca7(Si6O18)(CO3).2H2O, and spurrite, Ca5(SiO4)2(CO3). Geochim. Cosmochim. Acta. 2013, 115, 92–99. [Google Scholar] [CrossRef]
- Goswami, G.; Padhy, B.P.; Panda, J.D. Thermal analysis of spurrite from a rotary cement kiln. J. Therm. Anal. Calorim. 1989, 35, 1129–1136. [Google Scholar] [CrossRef]
- Salim, M.U.; Mosaberpanah, M.A. Mechanical and durability properties of high-performance mortar containing binary mixes of cenosphere and waste glass powder under different curing regimes. J. Mater. Res. Technol. 2021, 13, 602–617. [Google Scholar] [CrossRef]
- Böhme, N.; Hauke, K.; Neuroth, M.; Geisler, T. In-Situ Hyperspectral Raman Imaging of Ternesite Formation and Decomposition at High Temperatures. Minerals 2020, 10, 287. [Google Scholar] [CrossRef]
- Kostova, B.; Petkova, V.; Kostov-Kytin, V.; Tzvetanova, Y.; Avdeev, G. TG/DTG-DSC and high temperature in-situ XRD analysis of natural thaumasite. Thermochim. Acta 2020, 697, 178863. [Google Scholar] [CrossRef]

| No | Chemical Composition of White Portland Cement—CEM I 52.5 N (wt%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | Free Lime | |||
| 24.3 | 2.1 | 0.2 | 68.3 | 0.3 | 0.13 | 0.02 | 1.9 | ||||
| The mineral composition of white Portland cement, (wt%) | |||||||||||
| 2 | Belite (C2S) | Alite (C2S) | Tricalcium aluminate (C3A) | Calcium aluminoferrite (C4AF) | |||||||
| 72.13 | 15.28 | 5.23 | 0.61 | ||||||||
| Chemical composition of marble powder (wt%) | |||||||||||
| 3 | CO2 + H2O | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | MnO | ||
| 45.7 | 0.12 | 0.38 | 0.14 | 32.9 | 20.0 | 0.05 | 0.19 | 0.01 | |||
| 4 | Chemical composition of sand (wt%) | ||||||||||
| Sand (SiO2) | over 85.0% | ||||||||||
| Sample | Aggregate | Ratio | ||
|---|---|---|---|---|
| Cement-to-Aggregate | Water-to-Cement | Water-to-Fines * | ||
| A028, A120 | Sand | 1:3 | 0.50 | 0.500 |
| B028, B120 | Marble powder | 1:2 | 0.60 | 0.353 |
| C028, C120 | Marble powder | 1:2 | 0.40 + HRWR | 0.235 |
| No | Description | Sample | Identified Phases (Name, ICDD Card Number; Formula) |
|---|---|---|---|
| 1. | Minerals of raw materials and their relicts in samples | ||
| white Portland cement, A028, A120; B028, B120; C028, C120 | Belite (C2S), #49-1673—Ca2SiO4 | ||
| Alite (C3S), #11-0593—Ca3SiO5 | |||
| Calcite, #47-1743—CaCO3 | |||
| white Portland cement | Tricalcium aluminate (C3A), #38-1429—Ca3Al2O6 Gypsum, #33-0311—CaSO4x2H2O | ||
| marble powder, B028, B120; C028, C120 | Dolomite, #36-0426—CaMg(CO3)2 | ||
| Mg-rich Calcite, #43-0697—CaCO3 | |||
| A028, A120 | Quartz, #46-1045—SiO2 | ||
| 2. | Newly formed phases | ||
| 2.1. | hydrate phase | A028, A120 B028, B120 C028, C120 | Portlandite (CH), #44-1481—Ca(OH)2 |
| 2.2. | C-S-H gel phases | A028, A120 B028, B120 C028, C120 | Hillebrandite, #29-0373, #42-0538—Ca2SiO3(OH)2 |
| A028, A120 | Scolecite, #41-1355—CaAl2Si3O10.3H2O | ||
| A120 C028 | Xonotlite, #23-0125—Ca6Si6O17(OH)2 | ||
| 2.3. | OH− and HCO3−/CO32− phases | B028, B120 C120 | Monocarboaluminate, #41-0219— Ca4Al2(OH)12(CO3)x5H2O |
| B028 C028 | Hemicarboaluminate, #41-0221—Ca4Al2(OH)12(OH)(CO3)0.5.4H2O | ||
| B028, B120 C028, C120 | Artinite, #72-1320—Mg2(CO3)(OH)2.3H2O | ||
| 2.4. | OH− and HSO4−/SO42− phases | A028, A120 B028, B120 C028, C120 | Ettringite, #41-1451—Ca6Al2(SO4)3(OH)12·26H2O |
| B028, B120 C028, C120 | Calcium hydrogensulphate, #85-1271—Ca(HSO4)2 | ||
| A028, A120 | Monosulphoaluminate, #50-1607—Ca4Al2(OH)12(SO4)x6H2O | ||
| No | Sample | Identified Phases (Name, ICDD Card Number; Formula) |
|---|---|---|
| 1. | A028, A120; B028, B120 C028, C120 | Larnite, #49-1673—Ca2SiO4 |
| Wollastonite, #42-0550—CaSiO3 | ||
| Anorthite, #41-1486—CaAl2Si2O8 | ||
| A028, A120 B120 C028, C120 | Anhydrite, #37-1496—CaSO4 | |
| A028, A120 B028, B120 C028 | Ternesite #49-1807—Ca5(SiO4)2SO4 | |
| A028, A120; | Quartz, #46-1045—SiO2 | |
| 2. | B028, B120 C028, C120 | Periclase, #45-0946—MgO |
| Lime, #37-1497—CaO | ||
| Akermanite, #35-0592—Ca2MgSi2O7 | ||
| Calcium Aluminium Oxide, #33-0252—Ca2Al2O5 | ||
| Spurrite, #13-0496—Ca5(SiO)2(CO3) |
| Description/ References | Bond | ν1 (cm−1) | ν2 (cm−1) | ν3 (cm−1) | ν4 (cm−1) | Raw Material/ Sample |
|---|---|---|---|---|---|---|
| 1. Minerals of raw materials and their relicts in the samples | ||||||
| Belite (C2S), Ca2SiO4 Alite (C3S), Ca3SiO5 [17,27,28,29] | Si-O in SiO44− | 520 | 923 | 657 | white Portland cement *, A028, A120, B028, B120, C028, C120 | |
| Tricalcium aluminate (C3A), Ca3Al2O6 [27,30] | Al-O in AlO45- | 754 | 435 | 856 | 545 | white Portland cement |
| Calcite, CaCO3 [27,28,29,31] | C-O in CO32− | 875 | 1411 1473 | 713 | white Portland cement *, A028, A120, B028, B120, C028, C120 | |
| Gypsum, CaSO4.2H2O [28,32,33] | S-O in SO42− | 995 | 457 | 1103, 1126, 1141 | 597, 669 | white Portland cement |
| O-H structural (1) | 3403 | 3552 | ||||
| O-H crystal (2) | 1617 | 3729 | ||||
| Dolomite, CaMg(CO3)2 [27,28,29,31] | C-O in CO32− | 879 | 1442 | 727 | marble powder *, B028, B120, C028, C120 | |
| Mg-rich Calcite, CaCO3 [28,29] | C-O in CO32− | 1081 | 879 | 1488 | 727 | marble powder *, B028, B120, C028, C120 |
| Quartz, SiO2 [28,29] | Si-O in SiO2 | 692 | 458 | 1083; 1172 | 512 | sand, A028 *, A120 |
| 2. Newly formed phases | ||||||
| 2.1. Hydrated phase | ||||||
| Portlandite (CH), Ca(OH)2 [28,29] | Ca-O-H | 3642 | 1623 | 3743; 3245 | A028 *, A120, B028, B120, C028, C120 | |
| O-H structural(1) | 3426 | 3556 | ||||
| 2.2. C-S-H gel phases—hydrosilicates formed from main oxides: CaO, Al2O3, SiO2 | ||||||
| Hillebrandite, Ca2SiO3(OH)2 [28,29] | Si-O in SiO32− | 964 | 1029; 1052 | A028, A120 B028*, B120, C028, C120 | ||
| Ca-O-H | 3642 | 1656 | 3245 | |||
| O-H structural (1) | 3424 | 3540 | ||||
| Scolecite CaAl2Si3O10.3H2O [28,29] | Al-O in AlO69- | 713 | 856 | 514 582 644 | A028 *, A120 | |
| Si-O in SiO44− | 458 | 1052; 1083; 1207; 1216 | ||||
| O-H crystal (2) | 3423 | 1623 | 3743; 3245 | |||
| Xonotlite—Ca6Si6O17(OH)2 [28,29] | Ca-O-H | 3640 | 1621 | 3733; 3247 | C028 * | |
| O-H structural (1) | 3424 | 3540 | ||||
| Si-O in SiO44− | 458 | 1041; 1052; 1083 | ||||
| 2.3. OH− and HCO3−/CO32− phases | ||||||
| Monocarboaluminate Ca4Al2(OH)12(CO3).5H2O [28,29] | Ca-O-H | 3642 | 1691 | 3731; 3247 | B120, C120 * | |
| O-H structural (1) | 3442 | 3561 | ||||
| Al-O in AlO69- | 856 | 511 667 | ||||
| C-O in CO32− | 1081 | 877 | 1444; 1481 | 700 | ||
| Hemicarboaluminate Ca4Al2(OH)13(CO3)0.5·5.5H2O [28,29] | Ca-O-H | 3642 | 1691 | 3731; 3247 | B028, C028 * | |
| O-H structural (1) | 3442 | 3548 | ||||
| Al-O in AlO69- | 856 | 511 661 | ||||
| C-O in CO32− | 1083 | 879 | 1452; 1481 | 700 | ||
| Artinite, Mg2(CO3)(OH)2.3H2O [34] | C-O in CO32− | 1083 | 877 | 1413 | 711 728 | B028, B120, C028 *, C120 |
| O-H crystal (2) | 1627 | 3731; 3235 | ||||
| O-H structural (1) | 3430 | 3548 | ||||
| 2.4. OH− and HSO4−/SO42− phases | ||||||
| Ettringite, Ca6Al2(SO4)3(OH)12·26H2O [27,28,29,35,36,37] | Ca-O-H | 3642 | 1643 | 3731; 3255 | A028, A120, B028, B120 *, C028, C120 | |
| O-H structural (1) | 3428 | 3561 | ||||
| S-O in SO42− | 1002 | 1139 | 599 667 | |||
| Al-O-H | 416 | 856 | ||||
| Monosulphoaluminate, Ca4Al2(OH)12(SO4).6H2O [27,28,29,37] | Ca-O-H | 3640 | 1621 | 3743; 3245 | A028 *, A120 | |
| O-H structural (1) | 3423 | 3556 | ||||
| S-O in SO42− | 1002 | 458 | 1099; 1147 | 605 | ||
| Al-O in AlO69- | 856, 1031 | 514 534 671 | ||||
| Calcium hydrogensulphate Ca(HSO4)2 [28,29,38] | O-H structural (1) | 3430 | 3548 | B028, B120, C028*, C120 | ||
| S-O in SO42− | 1004 | 468 | 1105; 1130 | 599 | ||
| Sample | Ist Range | IInd Range | IIIrd Range | IVth Range | Total Mass Loses (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 40–200 (°C) | 420–470 (°C) | 520–730 (°C) | 730–850 (°C) | ||||||
| Dehydratation of C-S-H Phases | Dehydroxylation of Ca(OH)2 | Dehydroxylation of C-S-H, Uncomplete Decarbonation and Desulphurization of Ca(HCO3)2, Ca(HSO4)2 | Uncomplete Decarbonation of MgCa(CO3)2, CaCO3 | ||||||
| Tinfl. (°C) | Mass Losses (%) | Tinfl. (°C) | Mass Losses (%) | Tinfl. (°C) | Mass Losses (%) | Tinfl. (°C) | Mass Losses (%) | ||
| white Portland cement | 65.1 98.0 | 0.30 0.39 | 429.9 | 0.25 | 539.6 | 0.15 | 711.8 | 3.68 | 5.19 |
| Marble powder | - | - | - | - | 575.7 | 1.30 | 745.0 788.5 822.4 835.9 | 8.40 14.69 15.85 7.22 | 47.83 |
| A028 | 108.5 - 177.1 | 1.01 - 0.52 | 465.3 | 0.68 | 596.7 625.1 682.8 | 1.46 1.90 1.00 | 742.5 | 3.33 | 11.44 |
| A120 | 100.4 130.2 169.5 | 0.68 0.36 0.44 | 479.2 | 0.56 | 562.9 - 675.7 | 2.46 - 1.82 | 743.3 764.6 | 1.87 0.59 | 10.48 |
| B028 | 85.2 116.2 - | 0.50 0.82 - | 469.0 | 0.91 | 529.5 - 685.5 | 4.15 - 1.68 | 734.7 794.3 814.4 827.3 | 4.49 11.91 8.69 5.95 | 42.80 |
| B120 | 89.1 - - | 1.10 - - | 472.0 | 0.62 | 560.0 - 703.2 | 4.23 - 3.00 | 754.0 775.1 806.9 827.2 | 3.24 9.87 12.49 4.92 | 41.85 |
| C028 | 94.2 - - | 0.98 - - | 472.6 | 0.46 | 585.5 - 693.5 | 1.34 - 1.31 | 798.4 816.7 827.9 830.5 | 6.21 10.03 8.80 2.86 | 38.81 |
| C120 | 89.1 - - | 1.09 - - | 469.3 | 0.69 | 551.2 - 679.8 | 4.13 - 2.21 | 735.6 779.7 802.0 817.4 | 6.50 9.30 10.04 4.22 | 40.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova, V.; Stoyanov, V.; Kostova, B.; Kostov-Kytin, V.; Kalinkin, A.; Zvereva, I.; Tzvetanova, Y. Crystal-Chemical and Thermal Properties of Decorative Cement Composites. Materials 2021, 14, 4793. https://doi.org/10.3390/ma14174793
Petkova V, Stoyanov V, Kostova B, Kostov-Kytin V, Kalinkin A, Zvereva I, Tzvetanova Y. Crystal-Chemical and Thermal Properties of Decorative Cement Composites. Materials. 2021; 14(17):4793. https://doi.org/10.3390/ma14174793
Chicago/Turabian StylePetkova, Vilma, Ventseslav Stoyanov, Bilyana Kostova, Vladislav Kostov-Kytin, Alexander Kalinkin, Irina Zvereva, and Yana Tzvetanova. 2021. "Crystal-Chemical and Thermal Properties of Decorative Cement Composites" Materials 14, no. 17: 4793. https://doi.org/10.3390/ma14174793
APA StylePetkova, V., Stoyanov, V., Kostova, B., Kostov-Kytin, V., Kalinkin, A., Zvereva, I., & Tzvetanova, Y. (2021). Crystal-Chemical and Thermal Properties of Decorative Cement Composites. Materials, 14(17), 4793. https://doi.org/10.3390/ma14174793









