Effect of Imidazole as Corrosion Inhibitor on Carbon Steel Weldment in District Heating Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens and Solution Preparation
2.2. Electrochemical Measurement
2.3. Surface Analysis
3. Results and Discussion
3.1. Electrochemical Measurement
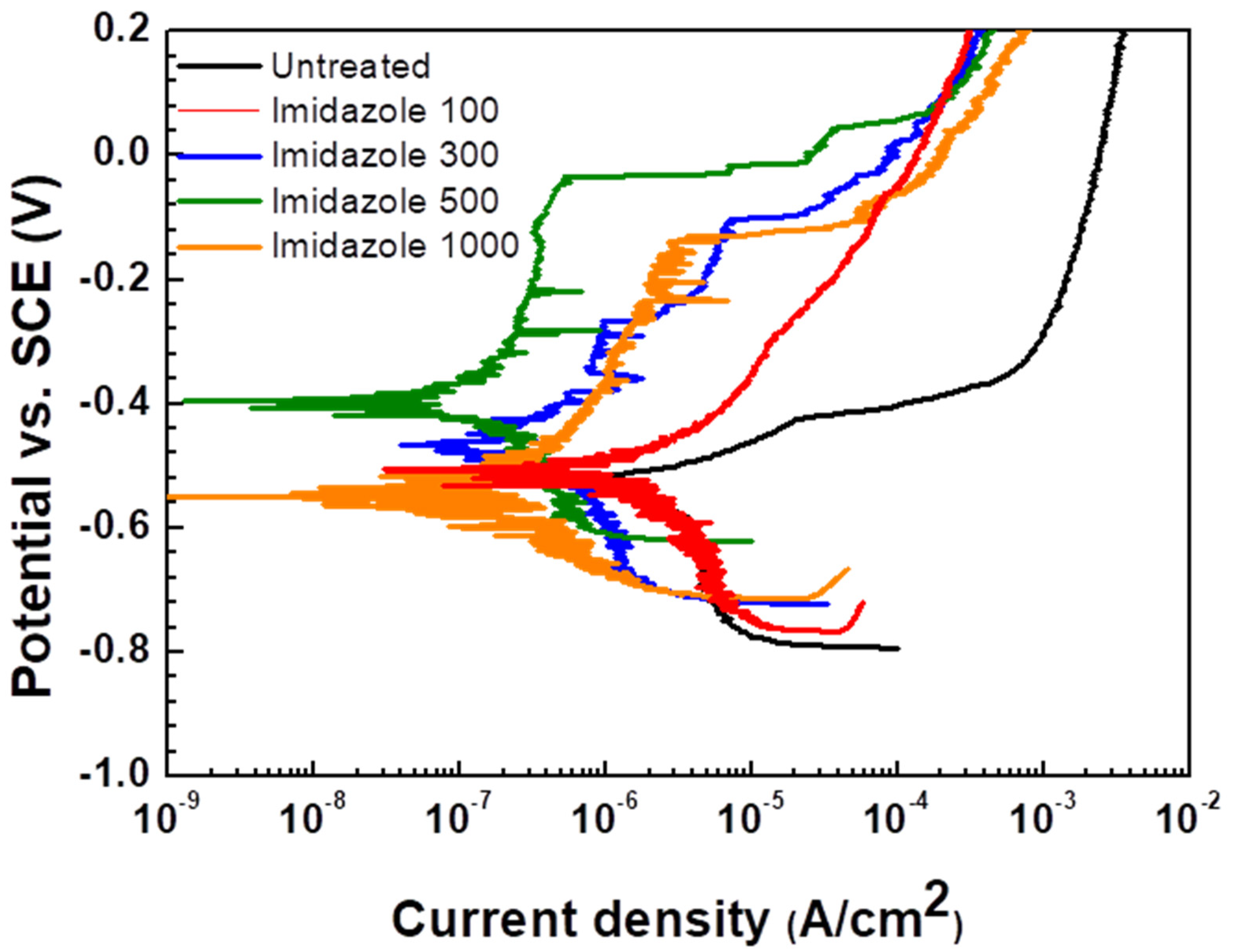
3.1.1. Potentiodynamic Polarization Tests
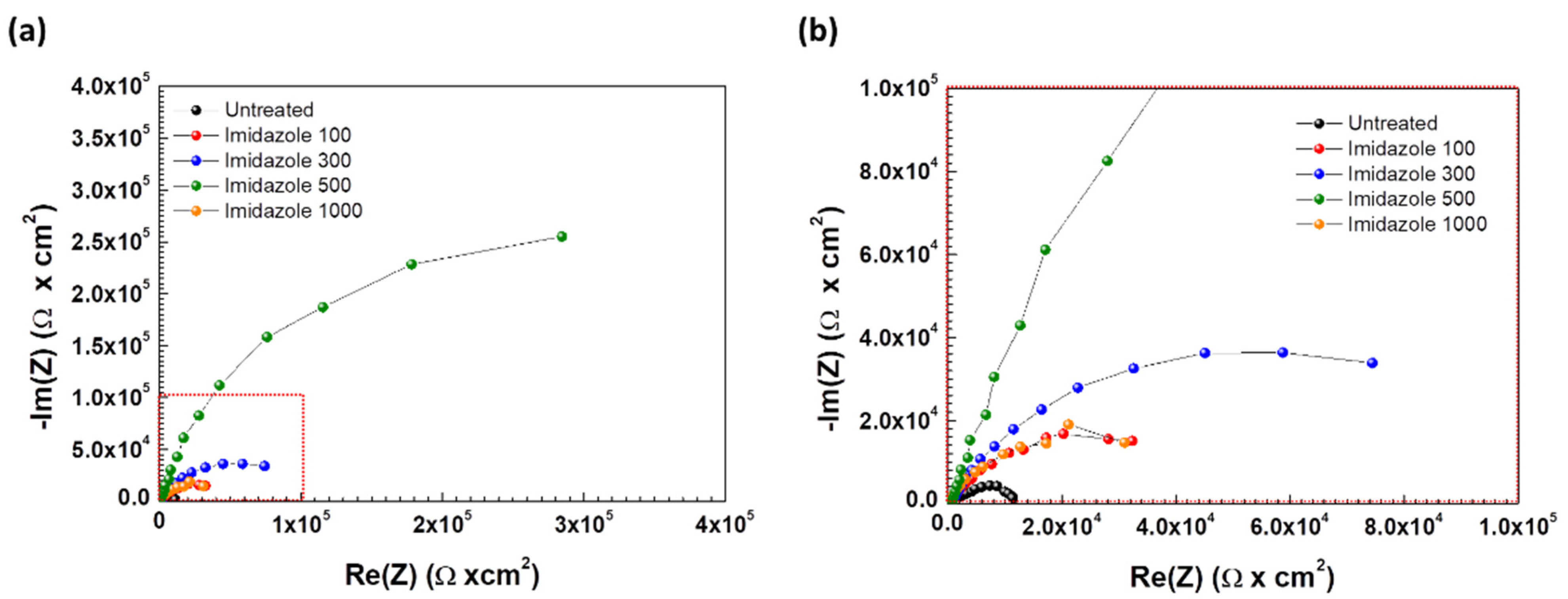
3.1.2. Electrochemical Impedance Spectroscopy
3.2. Surface Analysis
3.2.1. Optical Microscopy (OM)
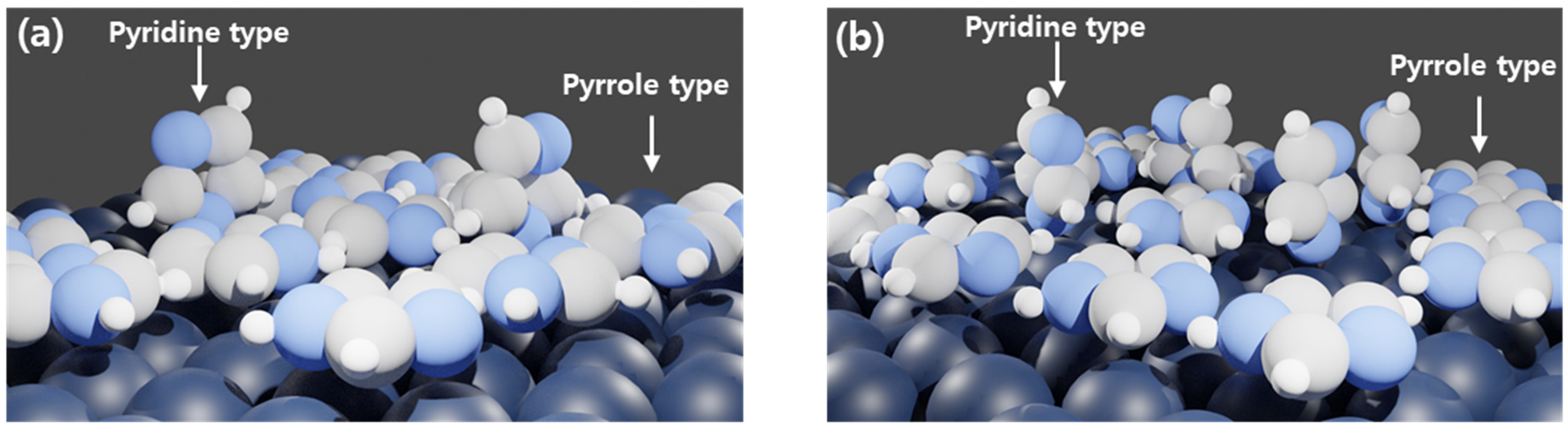
3.2.2. X-ray Photoelectron Spectroscopy (XPS)
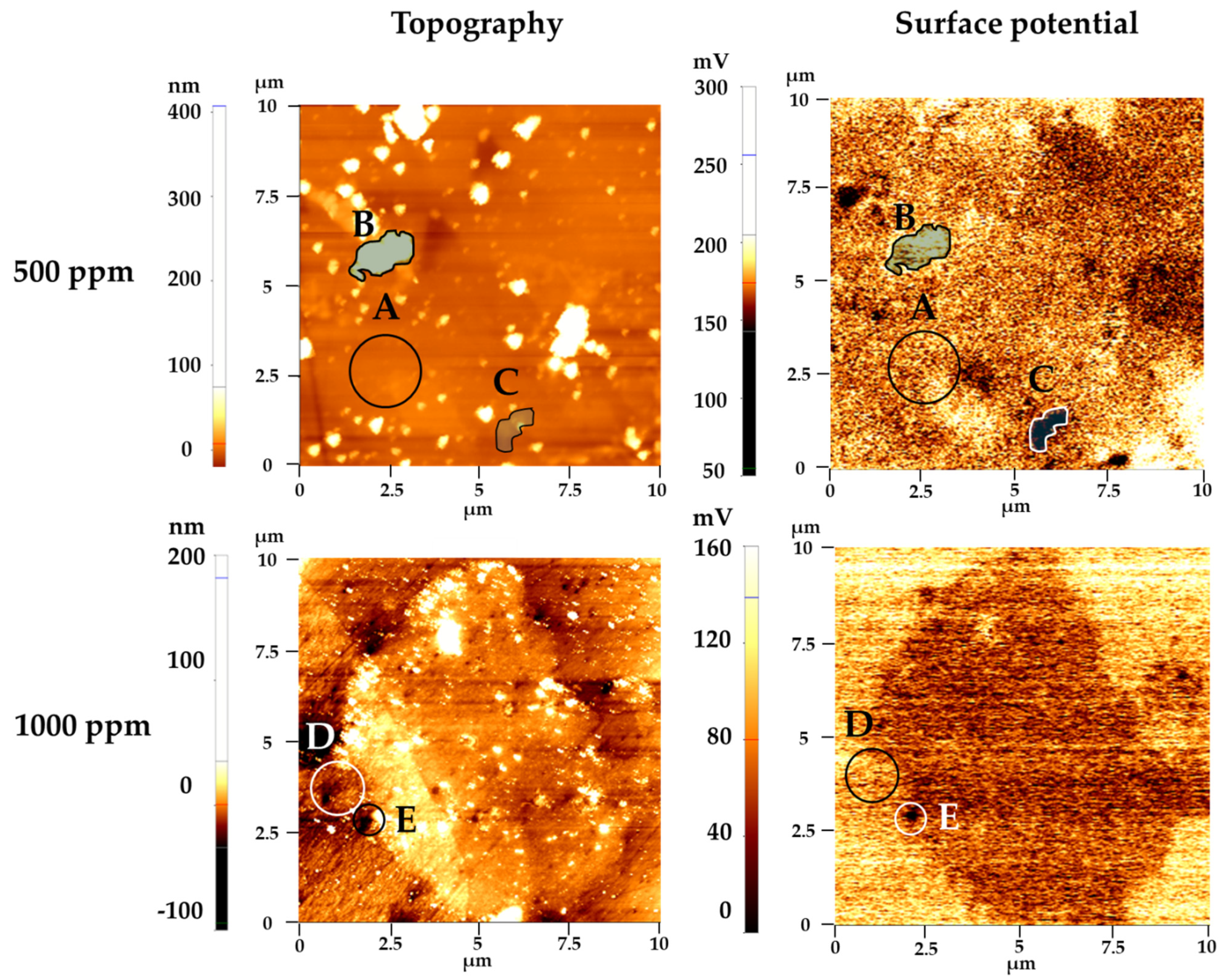
3.2.3. Atomic Force Microscopy (AFM)
3.3. Inhibition Mechanism
4. Conclusions
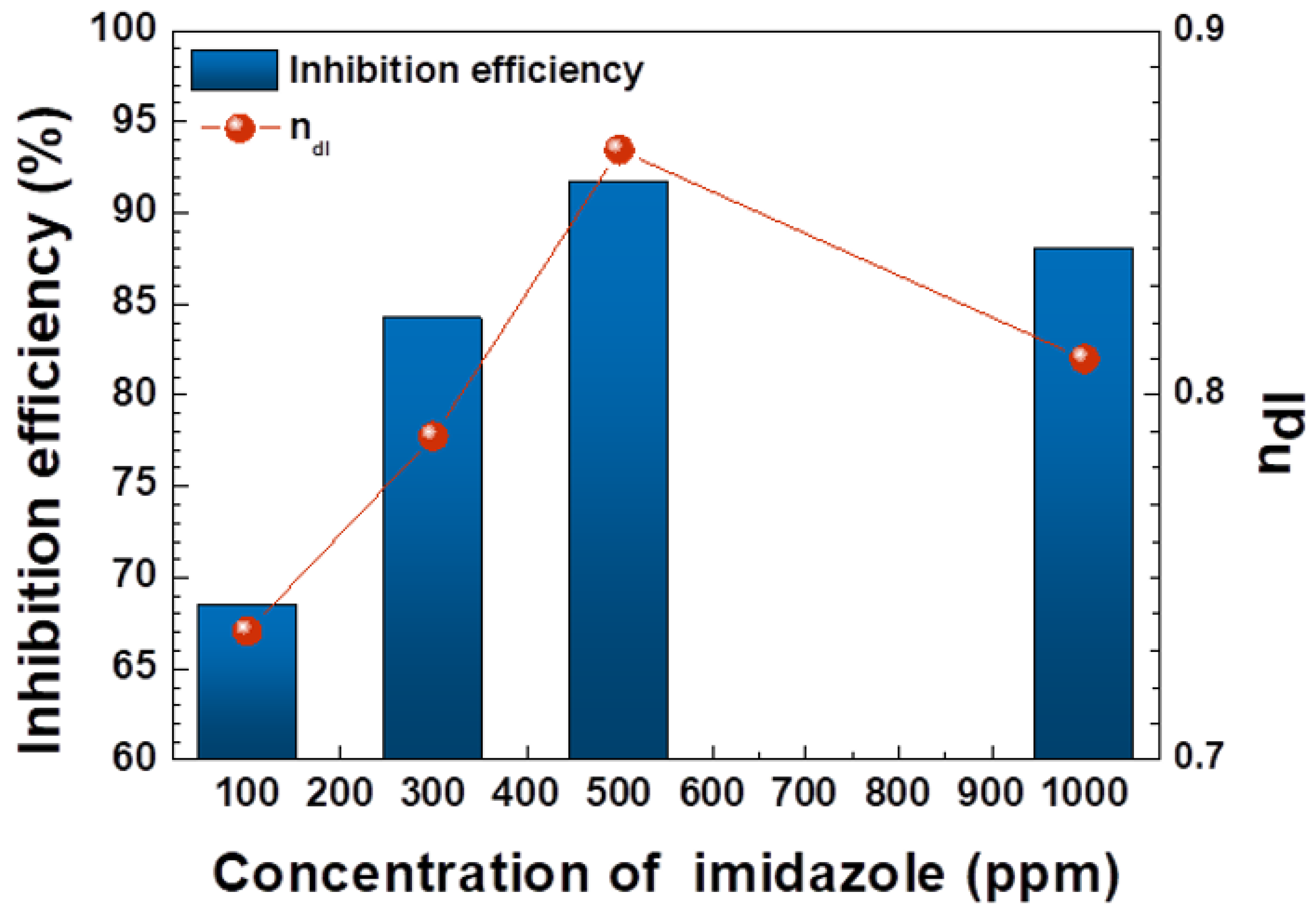
- In electrochemical measurement, the inhibition efficiency of imidazole on weldment of carbon steel increased as the concentration of imidazole increased up to peak inhibition efficiency at 500 ppm in this experiment;
- Inhibition efficiency decreased from peak value when imidazole concentration reached 1000 ppm;
- OM measurement after 40 h immersion showed that 500 ppm imidazole solution offered the highest inhibition efficiency and corrosion resistance. In 300 and 1000 ppm solution samples, micro-scale pittings were observed implying the lower inhibition efficiency and corrosion resistance;
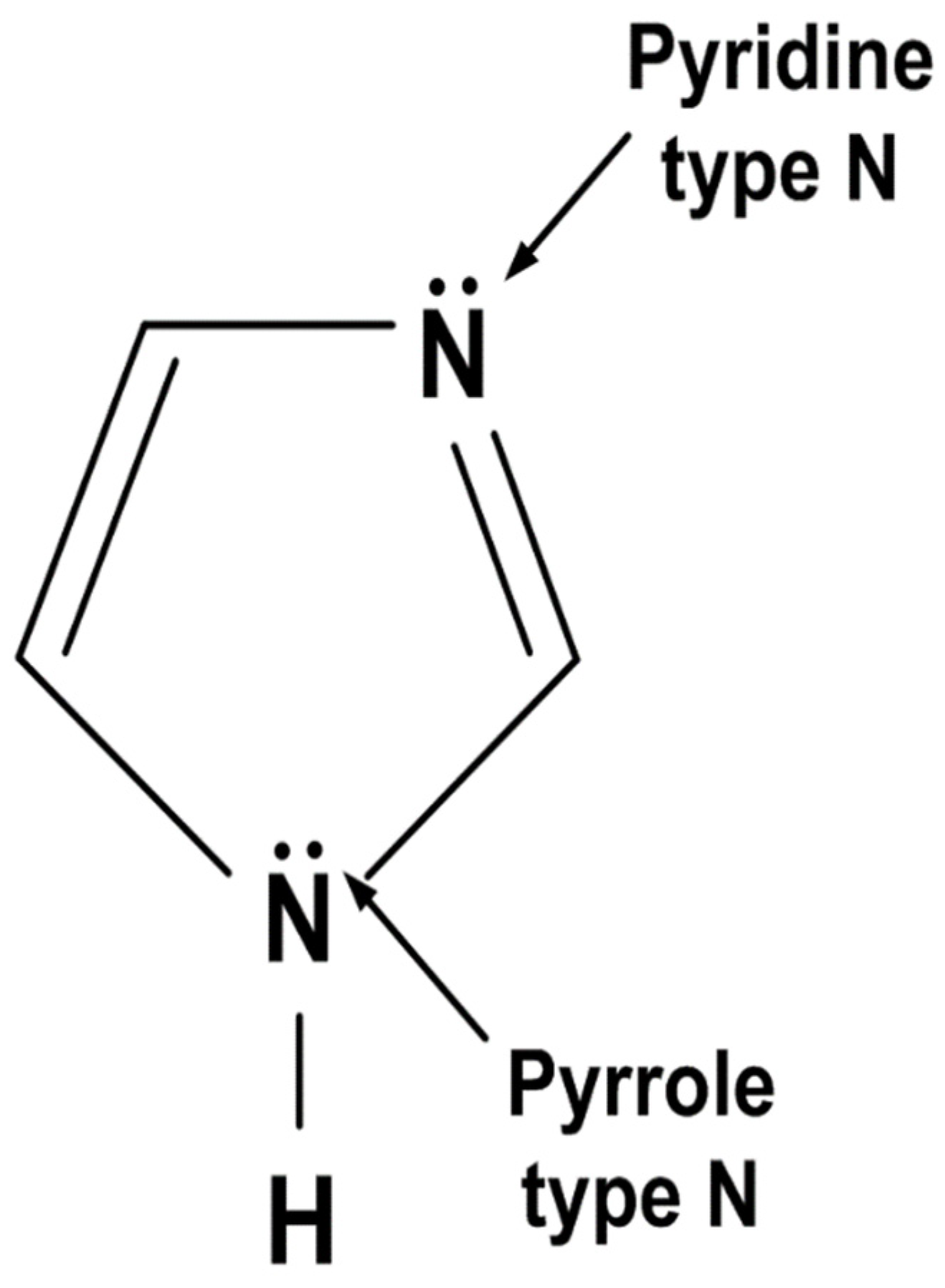
- XPS analysis showed that the pyrrole type interaction between imidazole and metal surface was more abundant in 500 ppm solution and pyridine type interaction was more abundant in 1000 ppm solution. Pyrrole-type interaction is known to offer higher inhibition efficiency due to higher coverage;
- AFM measurement also showed that surface coverage of imidazole is lower in 1000 ppm samples;
- Electrochemical measurement, immersion tests, and AFM showed the lower inhibition efficiency in the solution with 1000 ppm of imidazole. XPS and prior studies showed that as the concentration of imidazole increased, pyridine interaction between imidazole and Fe substrate also increased, which result in the decrease of coverage and inhibition efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groysman, A. Corrosion Problems and Solutions in Oil Refining and Petrochemical Industry; Springer International Publishing: Gewerbestrasse, Switzerland, 2017; pp. 101–168. [Google Scholar]
- Tiu, B.D.B.; Advincula, R.C. Polymeric corrosion inhibitors for the oil and gas industry: Design principles and mechanism. React. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Wang, Y.; Han, D.; Li, D.; Cao, Z. A Complex imidazoline corrosion inhibitor in hydrochloric acid solutions for refinery and petrochemical plant equipment. Pet. Sci. Technol. 2009, 27, 1836–1844. [Google Scholar] [CrossRef]
- Miksic, B.M.; Furman, A.Y.; Kharshan, M.A. Effectiveness of the corrosion inhibitors for the petroleum industry under various flow conditions. In Proceedings of the CORROSION, Atlanta, GA, USA, 22–26 March 2009; p. NACE-09573. [Google Scholar]
- Shalaby, H.; Al-Hashem, A.; Lowther, M.; Al-Besharah, J. Industrial Corrosion and Corrosion Control Technology; KISR: Kuwait City, Kuwait, 1996; pp. 449–500. [Google Scholar]
- Lee, C.-M.; Bond, S.; Woollin, P. Preferential weld corrosion: Effects of weldment microstructure and composition. In Proceedings of the CORROSION, Huston, TX, USA, 3–7 April 2005; p. 05277. [Google Scholar]
- El-Etre, A.; Abdallah, M. Natural honey as corrosion inhibitor for metals and alloys. II. C-steel in high saline water. Corros. Sci. 2000, 42, 731–738. [Google Scholar] [CrossRef]
- Stott, J.D. Assessment and control of microbially-induced corrosion. Met. Mater. 1988, 4, 224–229. [Google Scholar]
- Thorolfsson, G. Maintenance history of a geothermal plant: Svartsengi Iceland. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Dariva, C.G.; Galio, A.F. Corrosion inhibitors–principles, mechanisms and applications. Dev. Corros. Prot. 2014, 16, 365–378. [Google Scholar]
- Rani, B.E.; Basu, B.B.J. Green inhibitors for corrosion protection of metals and alloys: An overview. Int. J. Corros. 2012, 2012, 380217. [Google Scholar] [CrossRef]
- Africa, S. Adsorption and inhibitive properties of ethanol extracts of Musa sapientum peels as a green corrosion inhibitor for mild steel in H2SO4. Afr. J. Pure Appl. Chem. 2008, 2, 46–54. [Google Scholar]
- Arenas, M.A.; Conde, A.; De Damborenea, J. Cerium: A suitable green corrosion inhibitor for tinplate. Corros. Sci. 2002, 44, 511–520. [Google Scholar] [CrossRef]
- De Souza, F.S.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Q.; Li, C.; Gu, N.J.M. Copper corrosion inhibition by polyaspartic acid and imidazole. Mater. Corros. 2013, 64, 347–352. [Google Scholar] [CrossRef]
- Alaoui, K.; Dkhireche, N.; Touhami, M.E.; El Kacimi, Y. Review of application of imidazole and imidazole derivatives as corrosion inhibitors of metals. In New Challenges and Industrial Applications for Corrosion Prevention and Control; IGI Global: Hershey, PA, USA, 2020; pp. 101–131. [Google Scholar]
- Zeng, X.; Zheng, X.; Guo, L.; Xu, Q.; Huang, H.; Tan, B. Three imidazole ionic liquids as green and eco-friendly corrosion inhibitors for mild steel in sulfuric acid medium. J. Mol. Liq. 2021, 324, 115063. [Google Scholar] [CrossRef]
- Zunita, M.; Wahyuningrum, D.; Bundjali, B.; Wenten, I.G.; Boopathy, R. Corrosion inhibition performances of imidazole derivatives-based new ionic liquids on carbon steel in brackish water. Appl. Sci. 2020, 10, 7069. [Google Scholar] [CrossRef]
- Zunita, M.; Wahyuningrum, D.; Buchari, B.B.; Bundjali, B. Investigation of corrosion inhibition activity of 3-butyl-2, 4, 5-triphenylimidazole and 3-butyl-2-(2-butoxyphenyl)-4, 5-diphenylimidazole toward carbon steel in 1% NaCl solution. Int. J. Electrofchem. Sci. 2012, 7, 3274–3288. [Google Scholar]
- Zhang, Z.; Chen, S.; Li, Y.; Li, S.; Wang, L. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Qin, R.; Du, Y.; Xu, Z.; Lu, M.J. Anodic Polarization Behavior of X80 Steel in Na2SO4 Solution under High Potential and Current Density Conditions. Materials 2019, 12, 394. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Chen, Z.; Zuo, Y.; Chen, Y.; Yang, W.; Xu, B. Ionic liquids with two typical hydrophobic anions as acidic corrosion inhibitors. J. Mol. Liq. 2018, 269, 886–895. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K.J. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhamid, M.A.; Ateya, B.; Pickering, H. Effect of benzotriazole on the hydrogen absorption by iron. J. Electrochem. Soc. 1997, 144, L58. [Google Scholar] [CrossRef]
- Li, J.; Zhong, T.-K.; Wadsworth, M.E. Application of mixed potential theory in hydrometallurgy. Hydrometallurgy 1992, 29, 47–60. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Guo, L. Azole-based compounds as corrosion inhibitors for metallic materials. In Azoles-Synthesis, Properties, Applications and Perspectives; IntechOpen: London, UK, 2020. [Google Scholar]
- Ergun, Ü.; Emregül, K.C.J. Azole compounds as corrosion inhibitors: Part I. J. Mater. Eng. Perform. 2014, 23, 213–221. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Al-Thani, N. A review of passivity breakdown on metal surfaces: Influence of chloride- and sulfide-ion concentrations, temperature, and pH. Emergent Mater. 2021, 1–17. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; Ying, Y.; Wang, M.; Xiao, H.; Chen, Z. Theoretical and experimental studies of structure and inhibition efficiency of imidazoline derivatives. Corros. Sci. 1999, 41, 1911–1919. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, S.-H.; Kim, G.-J. Effects of 1, 2, 3-benzotriazole on the corrosion properties of 316L stainless steel in synthetic tap water. Met. Mater. Int. 2015, 21, 1013–1022. [Google Scholar] [CrossRef]
- Liu, A.; Ren, X.; Zhang, J.; Wang, C.; Yang, P.; Zhang, J.; An, M.; Higgins, D.; Li, Q.; Wu, G. Theoretical and experimental studies of the corrosion inhibition effect of nitrotetrazolium blue chloride on copper in 0.1 MH2 SO4. RSC Adv. 2014, 4, 40606–40616. [Google Scholar] [CrossRef]
- Cramer, S.D.; Covino, B.S., Jr.; Moosbrugger, C.; Sanders, B.R.; Anton, G.J.; Hrivnak, N.; Kinson, J.; Polakowski, C.; Muldoon, K.; Henry, S.D. ASM handbook; ASM International Materials Park: Novelty, OH, USA, 2003; Volume 13A, pp. 733–741. [Google Scholar]
- Bhargava, G.; Ramanarayanan, T.A.; Gouzman, I.; Abelev, E.; Bernasek, S. Inhibition of iron corrosion by imidazole: An electrochemical and surface science study. Corrosion 2009, 65, 308–317. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John and Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Li, M.; Guo, L.; Qiao, L.; Bai, Y. The mechanism of hydrogen-induced pitting corrosion in duplex stainless steel studied by SKPFM. Corros. Sci. 2012, 60, 76–81. [Google Scholar] [CrossRef]
- Yadav, A.; Katayama, H.; Noda, K.; Masuda, H.; Nishikata, A.; Tsuru, T. Surface potential distribution over a zinc/steel galvanic couple corroding under thin layer of electrolyte. Electrochim. Acta 2007, 52, 3121–3129. [Google Scholar] [CrossRef]
- Kokalj, A. Formation and structure of inhibitive molecular film of imidazole on iron surface. Corros. Sci. 2013, 68, 195–203. [Google Scholar] [CrossRef]
- Milošev, I.; Kovačević, N.; Kokalj, A. Effect of mercapto and methyl groups on the efficiency of imidazole and benzimidazole-based inhibitors of iron corrosion. Acta Chim. Slov. 2016, 63, 544–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| pH | Cl− | Mg2+ | Ca2+ | Fe2+, Fe3+ | NH4+ |
|---|---|---|---|---|---|
| 10.0 | 14.6 | 0.2 | 1.06 | 0.2 | 1.53 |
| Imidazole Conc. (ppm) | −βc (mV/decade) | Ecorr (mVSCE) | icorr (μA/cm2) | Eb (mVSCE) | IE (%) |
|---|---|---|---|---|---|
| 0 | 698.8 | −519.875 | 2.908 | - | |
| 100 | 357.4 | −506.271 | 0.916 | 68.5 | |
| 300 | 322.5 | −466.211 | 0.46 | −268.0 | 84.2 |
| 500 | 280.4 | −402.451 | 0.24 | −33.6 | 91.7 |
| 1000 | 135.1 | −551.359 | 0.35 | −130.0 | 88.0 |
| Imidazole Conc. (ppm) | Rs (kΩ∙cm2) | Cdl (μF/cm2) | ndl | Rct (Ω∙cm2) | Rp (Ω∙cm2) | IE (%) |
|---|---|---|---|---|---|---|
| 0 | 264.25 | 2.875 × 10−5 | 0.6105 | 1.38 × 104 | 1.38 × 104 | - |
| 100 | 263.25 | 3.285 × 10−5 | 0.7353 | 4.89 × 104 | 4.89 × 104 | 71.8 |
| 300 | 713.25 | 1.237 × 10−5 | 0.7888 | 1.04 × 105 | 1.04 × 105 | 86.8 |
| 500 | 600.25 | 5.130 × 10−6 | 0.8673 | 6.02 × 105 | 6.02 × 105 | 97.7 |
| 1000 | 189.12 | 5.642 × 10−5 | 0.8747 | 4.01 × 104 | 4.01 × 104 | 89.7 |
| 500 ppm | 1000 ppm | |
|---|---|---|
| -C=NC (399.44 eV) | 1 | 3.49 |
| -C-NH-C (400.1 eV) | 4.8 | 1 |
| Imidazole Conc. of Solution | Location | Surface Potential Difference(mV) |
|---|---|---|
| 500 ppm | A-B | −23 |
| B-C | 63 | |
| A-C | 38 | |
| 1000 ppm | D-E | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.-J.; Choi, S.-R.; Hong, M.-S.; Kim, W.-C.; Kim, J.-G. Effect of Imidazole as Corrosion Inhibitor on Carbon Steel Weldment in District Heating Water. Materials 2021, 14, 4416. https://doi.org/10.3390/ma14164416
Ko S-J, Choi S-R, Hong M-S, Kim W-C, Kim J-G. Effect of Imidazole as Corrosion Inhibitor on Carbon Steel Weldment in District Heating Water. Materials. 2021; 14(16):4416. https://doi.org/10.3390/ma14164416
Chicago/Turabian StyleKo, Sang-Jin, Seok-Ryul Choi, Min-Sung Hong, Woo-Cheol Kim, and Jung-Gu Kim. 2021. "Effect of Imidazole as Corrosion Inhibitor on Carbon Steel Weldment in District Heating Water" Materials 14, no. 16: 4416. https://doi.org/10.3390/ma14164416
APA StyleKo, S.-J., Choi, S.-R., Hong, M.-S., Kim, W.-C., & Kim, J.-G. (2021). Effect of Imidazole as Corrosion Inhibitor on Carbon Steel Weldment in District Heating Water. Materials, 14(16), 4416. https://doi.org/10.3390/ma14164416





