A Proteomic Analysis of Discolored Tooth Surfaces after the Use of 0.12% Chlorhexidine (CHX) Mouthwash and CHX Provided with an Anti-Discoloration System (ADS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
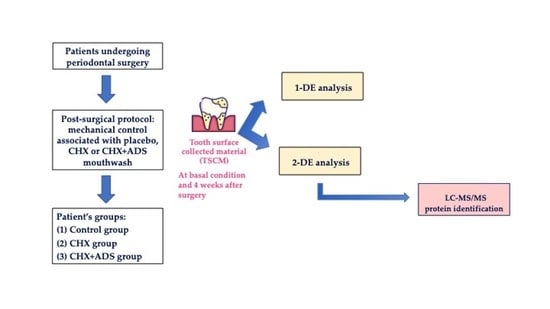
2.2. Patient Recruitment and TSCM Collection
2.3. Protein Extraction from TSCM
2.4. Proteomic Analysis
2.5. Protein Identification by Mass Spectrometry
2.6. Statistics
3. Results
3.1. 1-DE Analysis
3.2. 2-DE Analysis
3.3. LC-MS/MS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuboniwa, M.; Tribble, G.D.; Hendrickson, E.L.; Amano, A.; Lamont, R.J.; Hackett, M. Insights into the virulence of oral biofilms: Discoveries from proteomics. Expert Rev. Proteom. 2012, 9, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef]
- Chenicheri, S.; Usha, R.; Ramachandran, R.; Thomas, V.; Wood, A. Insight into oral biofilm: Primary, secondary and residual caries and phyto-challenged solutions. Open Dent. J. 2017, 11, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.D. Dental plaque as a biofilm and microbial community—Implications for health and disease. BMC Oral Health 2006, 6 (Suppl. 1), S14. [Google Scholar] [CrossRef] [Green Version]
- Valm, A.M. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef]
- Donos, N. The periodontal pocket. Periodontology 2000 2018, 76, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Flemmig, T.F. Periodontitis. Ann. Periodontol. 1999, 4, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Tonetti, M.S. Diagnosis and epidemiology of periodontal osseous lesions. Periodontology 2000 2000, 22, 8–21. [Google Scholar] [CrossRef]
- Qasim, S.S.B.; Al-Otaibi, D.; Al-Jasser, R.; Gul, S.S.; Zafar, M.S. An evidence-based update on the molecular mechanisms underlying periodontal diseases. Int. J. Mol. Sci. 2020, 21, 3829. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A. Disease progression: Identification of high-risk groups and individuals for periodontitis. J. Clin. Periodontol. 2005, 32, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; John, R.P.; Laine, M.L. Identification of genetic risk factors for periodontitis and possible mechanisms of action. J. Clin. Periodontol. 2005, 32, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.; Wilson, R.F.; Hasan, A.S.; Scott, D.A. Mechanisms of action of environmental factors-tobacco smoking. J. Clin. Periodontol. 2005, 32, 180–195. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Bissett, S.M. Periodontitis and diabetes. Br. Dent. J. 2019, 227, 577–584. [Google Scholar] [CrossRef]
- Carnevale, G.; Kaldahl, W.B. Osseous resective surgery. Periodontology 2000 2000, 22, 59–87. [Google Scholar] [CrossRef] [Green Version]
- Faveri, M.; Gursky, L.C.; Feres, M.; Shibli, J.A.; Salvador, S.L.; de Figueiredo, L.C. Scaling and root planing and chlorhexidine mouthrinses in the treatment of chronic periodontitis: A randomized, placebo-controlled clinical trial. J. Clin. Periodontol. 2006, 33, 819–828. [Google Scholar] [CrossRef]
- Solderer, A.; Kaufmann, M.; Hofer, D.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Efficacy of chlorhexidine rinses after periodontal or implant surgery: A systematic review. Clin. Oral Investig. 2019, 23, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, H.Y.; Wong, M.M.; Cheung, S.H.; Liang, L.Y.; Lam, Y.W.; Chiu, S.K. Differential actions of chlorhexidine on the cell wall of Bacillus subtilis and Escherichia coli. PLoS ONE 2012, 7, e36659. [Google Scholar] [CrossRef] [Green Version]
- Ben-Knaz Wakshlak, R.; Pedahzur, R.; Avnir, D. Antibacterial Activity of Chlorhexidine-Killed Bacteria: The Zombie Cell Effect. ACS Omega 2019, 4, 20868–20872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matesanz-Pérez, P.; Garcia-Gargallo, M.; Figuero, E.; Bascones-Martınez, A.; Sanz, M.; Herrera, D.A. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J. Clin. Periodontol. 2013, 40, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Dutt, P.; Rathore, P.K.; Khurana, D. Chlorhexidine—An antiseptic in periodontics. J. Dent. Med. Sci. 2014, 13, 85–88. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 22, 587. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Amantea, M.; Tatullo, M. A comparative, randomized, controlled study on clinical efficacy and dental staining reduction of a mouthwash containing Chlorhexidine 0.20% and Anti Discoloration System (ADS). Ann. Stomatol. 2015, 6, 35–42. [Google Scholar] [CrossRef]
- Cortellini, P.; Labriola, A.; Zambelli, R.; Pini Prato, G.; Nieri, M.; Tonetti, M.S. Chlorhexidine with an anti discoloration system after periodontal flap surgery: A cross-over, randomized, triple-blind clinical trial. J. Clin. Periodontol. 2008, 35, 614–620. [Google Scholar] [CrossRef]
- Van Swaaij, B.W.M.; Van der Weijden, G.A.; Bakker, E.W.P.; Graziani, F.; Slot, D.E. Does Chlorhexidine mouthwash, with an anti-discoloration system, reduce tooth surface discoloration without losing its efficacy? A Systematic review and meta-analysis. Int. J. Dent. Hyg. 2020, 18, 27–43. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamini, S.; Bellei, E.; Reggiani Bonetti, L.; Monari, E.; Cuoghi, A.; Borelli, F.; Sighinolfi, M.C.; Bianchi, G.; Ozben, T.; Tomasi, A. Inflammation: An important parameter in the search of prostate cancer biomarkers. Proteome Sci. 2014, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Bertoldi, C.; Bellei, E.; Pellacani, C.; Ferrari, D.; Lucchi, A.; Cuoghi, A.; Bergamini, S.; Cortellini, P.; Tomasi, A.; Zaffe, D.; et al. Non-bacterial protein expression in periodontal pockets by proteome analysis. J. Clin. Periodontol. 2013, 40, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Bellei, E.; Bergamini, S.; Monari, E.; Fantoni, L.I.; Cuoghi, A.; Ozben, T.; Tomasi, A. High-abundance protein depletion for serum proteomic analysis: Concomitant removal of non-targeted proteins. Amino Acids 2011, 40, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Bellei, E.; Rustichelli, C.; Bergamini, S.; Monari, E.; Baraldi, C.; Lo Castro, F.; Tomasi, A.; Ferrari, A. Proteomic serum profile in menstrual-related and post menopause migraine. J. Pharm. Biomed. Anal. 2020, 184, 113165. [Google Scholar] [CrossRef] [PubMed]
- Paes Leme, A.F.; Bellato, C.M.; Bedi, G.; Del Bel Cury, A.A.; Koo, H.; Cury, J.A. Effects of sucrose on the extracellular matrix of plaque-like biofiom formed in vivo, studied by proteomic analysis. Caries Res. 2008, 42, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrose, J.A.; Chapman, E. Function, therapeutic potential, and inhibition of Hsp70 chaperones. J. Med. Chem. 2021, 64, 7060–7082. [Google Scholar] [CrossRef] [PubMed]
- Schramm, F.D.; Heinrich, K.; Thuring, M.; Bernhardt, J.; Jonas, K. An essential regulatory function of the DnaK chaperone dictates the decision between proliferation and maintenance in Caulobacter crescentus. PLoS Genet. 2017, 13, e1007148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahn, M.; Berthold, N.; Kieslich, B.; Knappe, D.; Hoffmann, R.; Strater, N. Structural studies on the forward and reverse binding modes of peptides to the chaperone DnaK. J. Mol. Biol. 2013, 425, 2463–2479. [Google Scholar] [CrossRef]
- Mitra, S.; Bagchi, A.; Dasgupta, R. Molecular level insight into the involvement of heat shock proteins in oxidative-stress-mediated human diseases. In Role of Oxidative Stress in Pathophysiology of Diseases; Maurya, P., Dua, K., Eds.; Springer Nature: Singapore, 2020; pp. 195–207. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Guzhova, I.V.; Margulis, B.A. Glyceraldehyde-3-phosphate dehydrogenase is a multifaceted therapeutic target. Pharmaceutics 2020, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Winram, S.B.; Lottenberg, R. The plasmin-binding protein Plr of group A streptococci is identified as glyceraldehyde-3-phosphate dehydrogenase. Microbiology 1996, 142, 2311–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israelsen, W.J.; Vander Heiden, M.G. Pyruvate kinase: Function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Lu, Z. Pyruvate kinase M2 at a glance. J. Cell Sci. 2015, 128, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Susorov, D.; Zakharov, N.; Shuvalova, E.; Ivanov, A.; Egorova, T.; Shuvalov, A.; Shatsky, I.N.; Alkalaeva, E. Eukaryotic translation elongation factor 2 (eEF2) catalyzes reverse translocation of the eukaryotic ribosome. J. Biol. Chem. 2018, 293, 5220–5229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, M.E.; Malecki, J.; Falnes, P.O. Regulation of eukaryotic elongation factor 1 alpha (eEF1A) by dynamic lysine methylation. RNA Biol. 2018, 15, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Steeves, C.H.; Potrykus, J.; Barnett, D.A.; Bearne, S.L. Oxidative stress response in the opportunistic oral pathogen Fusobacterium nucleatum. Proteomics 2011, 11, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, C.; Lusuardi, D.; Battarra, F.; Sassatelli, P.; Spinato, S.; Zaffe, D. The maintenance of inserted titanium implants: In-vitro evaluation of exposed surfaces cleaned with three different instruments. Clin. Oral Implants Res. 2017, 28, 57–63. [Google Scholar] [CrossRef] [PubMed]


| Groups (a) | Total OD Intensity (b) |
|---|---|
| C group-B | 6400 ± 1221 |
| C group-T | 16,250 ± 848 |
| CHX group-B | 13,909 ± 3379 |
| CHX group-T | 25,987 ± 3233 |
| CHX+ADS group-B | 13,704 ± 4018 |
| CHX+ADS group-T | 10,623 ± 3198 |
| Comparisons Between Groups in Baseline Conditions (B) | Total OD Intensity (a) | p-Value |
|---|---|---|
| C group-B vs. CHX group-B | 6400 ± 1221 vs. 13,909 ± 3379 | p < 0.05 |
| C group-B vs. CHX+ADS group-B | 6400 ± 1221 vs. 13,704 ± 4018 | p < 0.05 |
| CHX group-B vs. CHX+ADS group-B | 13,909 ± 3379 vs. 13,704 ± 4018 | p ≥ 0.05 |
| Comparisons Between Groups After Treatment (T) | Total OD Intensity (a) | p-Value |
| C group-T vs. CHX group-T | 16,250 ± 848 vs. 25,987 ± 3233 | p < 0.05 |
| C group-T vs. CHX+ADS group-T | 16,250 ± 848 vs. 10,623 ± 3198 | p < 0.05 |
| CHX group-T vs. CHX+ADS group-T | 25,987 ± 3233 vs. 10,623 ± 3198 | p < 0.05 |
| Protein Name | Abbrev. Name a | Acc. Number b | DB | MW c (kDa) | Score d | Sign. Pept. e | Sign. Seq. f | emPAI g |
|---|---|---|---|---|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase * | G3PD1 | P04406-1 | NextProt | 36,201 | 70 | 4 | 2 | 0.36 |
| Glyceraldehyde-3-phosphate dehydrogenase * | G3PD2 | P04406-1 | NextProt | 36,201 | 60 | 3 | 2 | 0.36 |
| Glyceraldehyde-3-phosphate dehydrogenase * | G3PD3 | P04406-1 | NextProt | 36,201 | 94 | 3 | 2 | 0.23 |
| Pyruvate kinase PKM * | PKM2 | P14618-1 | NextProt | 58,470 | 44 | 2 | 2 | 0.14 |
| Chaperone protein DnaK ** (or Hsp70) | DnaK | Q5F6W5 | SwissProt | 68,934 | 41 | 6 | 5 | 0.31 |
| 60 kDa chaperonin 2 ** (or Hsp60) | CH602 | Q7NQX1 | SwissProt | 57,496 | 61 | 6 | 4 | 0.30 |
| Elongation factor 2 * | EF21 | P13639-1 | NextProt | 96,246 | 154 | 6 | 3 | 0.13 |
| Elongation factor 2 * | EF22 | P13639-1 | NextProt | 96,246 | 111 | 5 | 5 | 0.13 |
| Elongation factor 2 * | EF23 | P13639-1 | NextProt | 96,246 | 62 | 6 | 6 | 0.13 |
| Putative elongation factor 1-alpha-like 3 * | PEF11 | Q5VTE0-1 | NextProt | 50,495 | 279 | 8 | 2 | 0.25 |
| Putative elongation factor 1-alpha-like 3 * | PEF12 | Q5VTE0-1 | NextProt | 50,495 | 329 | 9 | 2 | 0.35 |
| Putative elongation factor 1-alpha-like 3 * | PEF13 | Q5VTE0-1 | NextProt | 50,495 | 280 | 9 | 2 | 0.25 |
| Putative elongation factor 1-alpha-like 3 * | PEF14 | Q5VTE0-1 | NextProt | 50,495 | 223 | 8 | 2 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergamini, S.; Bellei, E.; Generali, L.; Tomasi, A.; Bertoldi, C. A Proteomic Analysis of Discolored Tooth Surfaces after the Use of 0.12% Chlorhexidine (CHX) Mouthwash and CHX Provided with an Anti-Discoloration System (ADS). Materials 2021, 14, 4338. https://doi.org/10.3390/ma14154338
Bergamini S, Bellei E, Generali L, Tomasi A, Bertoldi C. A Proteomic Analysis of Discolored Tooth Surfaces after the Use of 0.12% Chlorhexidine (CHX) Mouthwash and CHX Provided with an Anti-Discoloration System (ADS). Materials. 2021; 14(15):4338. https://doi.org/10.3390/ma14154338
Chicago/Turabian StyleBergamini, Stefania, Elisa Bellei, Luigi Generali, Aldo Tomasi, and Carlo Bertoldi. 2021. "A Proteomic Analysis of Discolored Tooth Surfaces after the Use of 0.12% Chlorhexidine (CHX) Mouthwash and CHX Provided with an Anti-Discoloration System (ADS)" Materials 14, no. 15: 4338. https://doi.org/10.3390/ma14154338
APA StyleBergamini, S., Bellei, E., Generali, L., Tomasi, A., & Bertoldi, C. (2021). A Proteomic Analysis of Discolored Tooth Surfaces after the Use of 0.12% Chlorhexidine (CHX) Mouthwash and CHX Provided with an Anti-Discoloration System (ADS). Materials, 14(15), 4338. https://doi.org/10.3390/ma14154338