Photocurable Epoxy Acrylate Coatings Preparation by Dual Cationic and Radical Photocrosslinking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Epoxy-Scrylate Pre-Polymer
2.3. Characterization Methods
2.4. Preparation of Coating Compositions and Cured Films
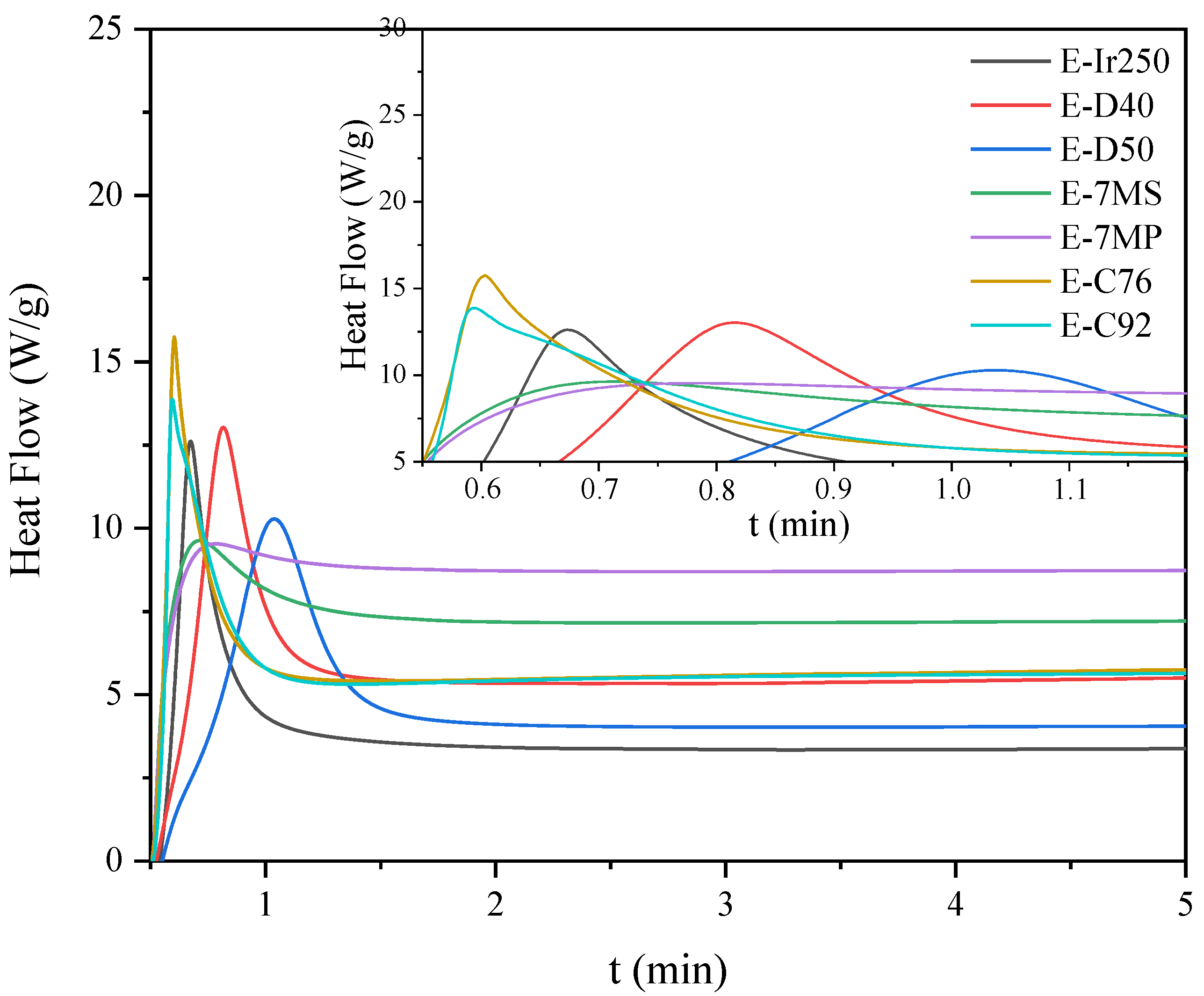
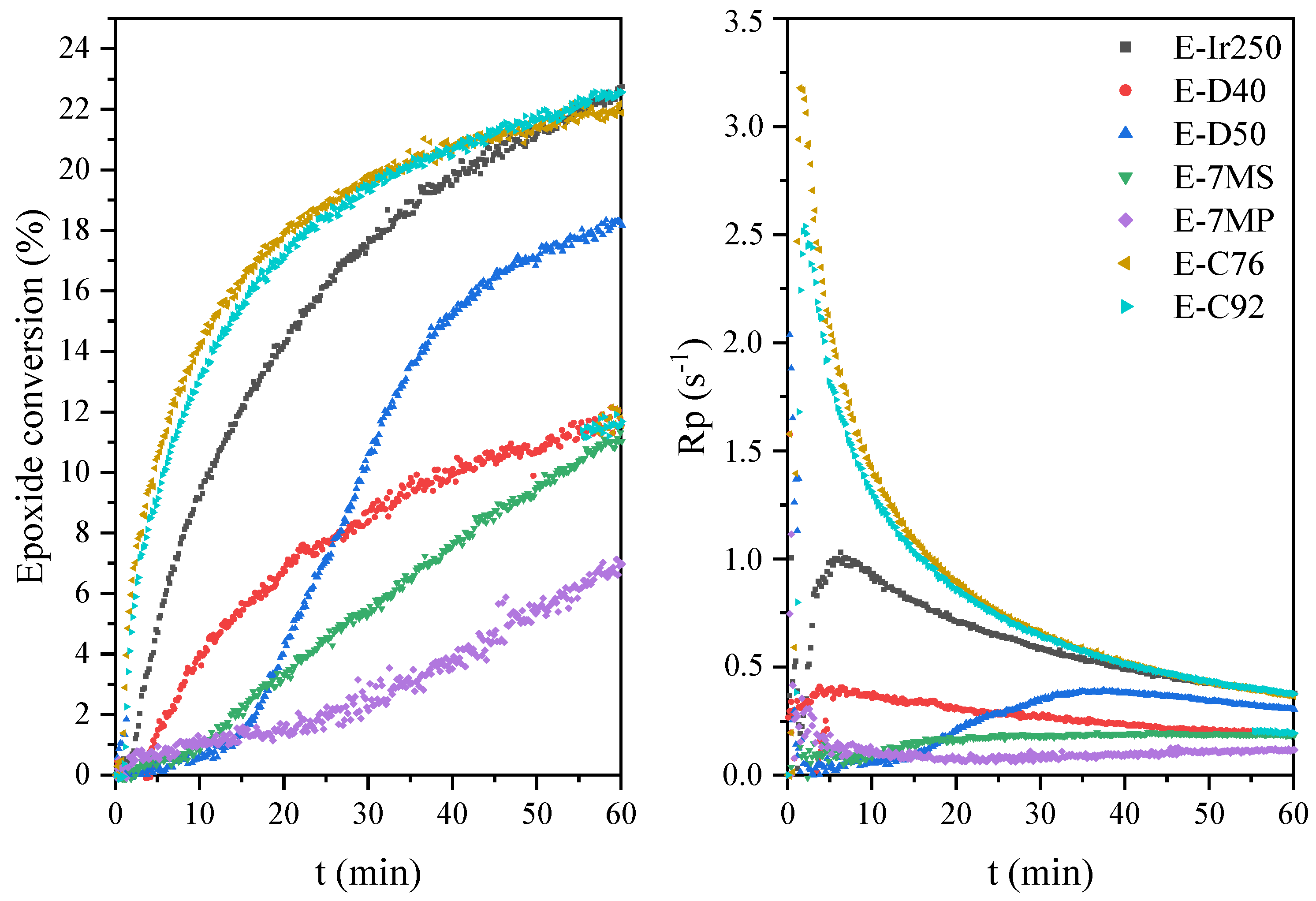
2.5. Characteristics of the Photopolymerization Process and Properties of Cured Coatings
3. Results
3.1. The Approach to the Development of the EA Prepolymer Synthesis Parameters
3.2. The Properties of Photocurable Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, L.; Li, Y.; Zheng, J.; Lu, M.; Wu, K. Modified epoxy acrylate resin for photocurable temporary protective coatings. Prog. Org. Coat. 2015, 89, 17–25. [Google Scholar] [CrossRef]
- Gorsche, C.; Seidler, K.; Knaack, P.; Dorfinger, P.; Koch, T.; Stampfl, J.; Moszner, N.; Liska, R. Rapid formation of regulated methacrylate networks yielding tough materials for lithography-based 3D printing. Polym. Chem. 2016, 7, 2009–2014. [Google Scholar] [CrossRef]
- Wang, K.; Wu, W.; Zhu, X.; Yu, Q. Synthesis and Photopolymerization of 2,2-Di((Acryloyloxy)Methyl)Butyl Bis(2-(Acryloyloxy)Ethyl)Carbamate. Des. Monomers Polym. 2012, 15, 31–39. [Google Scholar] [CrossRef][Green Version]
- Decker, C. Kinetic Study and New Applications of UV Radiation Curing. Macromol. Rapid Commun. 2002, 23, 1067–1093. [Google Scholar] [CrossRef]
- Gziut, K.; Kowalczyk, A. Influence of radical photoinitiators on features of polyacrylate syrups and self-adhesives. Polimery 2020, 65, 268–274. [Google Scholar] [CrossRef]
- Sanai, Y.; Kubota, K. Effect of UV-curing conditions on the polymer structures: A comparison between coating and adhesive. Polym. J. 2020, 52, 1153–1163. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Sangermano, M. Advances in cationic photopolymerization. Pure Appl. Chem. 2012, 84, 2089–2101. [Google Scholar] [CrossRef]
- Kabatc, J.; Jurek, K. Free radical formation in three-component photoinitiating systems. Polymer 2012, 53, 1973–1980. [Google Scholar] [CrossRef]
- Rocco, C.; Karasu, F.; Croutxé-Barghorn, C.; Allonas, X.; Lecompère, M.; Riess, G.; Zhang, Y.; Esteves, A.; Van Der Ven, L.; Van Benthem, R.; et al. Highly-interpenetrated and phase-separated UV-cured interpenetrating methacrylate–epoxide polymer networks: Influence of the composition on properties and microstructure. Mater. Today Commun. 2016, 6, 17–27. [Google Scholar] [CrossRef]
- Chen, Z.; Webster, D.C. Study of cationic UV curing and UV laser ablation behavior of coatings sensitized by novel sensitizers. Polymer 2006, 47, 3715–3726. [Google Scholar] [CrossRef]
- Malik, M.S.; Schogl, S.; Wolfahrt, M.; Sangermano, M. Review on UV-induced cationic frontal polymerization of epoxy monomers. Polymer 2020, 12, 2146. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T.; Thi, H.P. Photoinitiated cationic polymerization of epoxides. Polym. Int. 2001, 50, 986–997. [Google Scholar] [CrossRef]
- Škola, O.; Jašúrek, B.; Veselý, D.; Němec, P. Mechanical properties of polymer layers fabricated via hybrid free radical-cationic polymerization of acrylate, epoxide, and oxetane binders. Prog. Org. Coat. 2016, 101, 279–287. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers: Part I: Comparison of the performance of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2017, 64, 313–320. [Google Scholar] [CrossRef]
- Park, C.-H.; Lee, S.-W.; Park, J.-W.; Kim, H.-J. Preparation and characterization of dual curable adhesives containing epoxy and acrylate functionalities. React. Funct. Polym. 2013, 73, 641–646. [Google Scholar] [CrossRef]
- Jaswal, S.; Gaur, B. New trends in vinyl ester resins. Rev. Chem. Eng. 2014, 30, 567–568. [Google Scholar] [CrossRef]
- Liu, P.; Gu, A.; Liang, G.; Guan, Q.; Yuan, L. Preparation and properties of novel high performance UV-curable epoxy acrylate/hyperbranched polysiloxane coatings. Prog. Org. Coat. 2012, 74, 142–150. [Google Scholar] [CrossRef]
- Asif, A.; Huang, C.; Shi, W. Structure?property study of waterborne, polyurethane acrylate dispersions based on hyperbranched aliphatic polyester for UV-curable coatings. Colloid Polym. Sci. 2004, 283, 200–208. [Google Scholar] [CrossRef]
- Oprea, S.; Vlad, S.; Stanciu, A.; Macoveanu, M. Epoxy urethane acrylate. Eur. Polym. J. 2000, 36, 373–378. [Google Scholar] [CrossRef]
- Tasic, S.; Bozic, B.; Dunjic, B. Synthesis of new hyperbranched urethane-acrylates and their evaluation in UV-curable coatings. Prog. Org. Coat. 2004, 51, 320–327. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Ossowicz-Rupniewska, P. Novel Multifunctional Epoxy (Meth)Acrylate Resins and Coatings Preparation via Cationic and Free-Radical Photopolymerization. Polymers 2021, 13, 1718. [Google Scholar] [CrossRef] [PubMed]
- Duran, H.; Meng, S.; Kim, N.; Hu, J.; Kyu, T.; Natarajan, L.V.; Tondiglia, V.P.; Bunning, T.J. Kinetics of photopolymerization-induced phase separation and morphology development in mixtures of a nematic liquid crystal and multifunctional acrylate. Polymer 2008, 49, 534–545. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, J. A novel photocurable modified epoxy resin for high heat resistance coatings. Colloid Polym. Sci. 2020, 298, 1303–1312. [Google Scholar] [CrossRef]
- Park, Y.-J.; Lim, D.-H.; Kim, H.-J.; Park, D.-S.; Sung, I.-K. UV- and thermal-curing behaviors of dual-curable adhesives based on epoxy acrylate oligomers. Int. J. Adhes. Adhes. 2009, 29, 710–717. [Google Scholar] [CrossRef]
- Bajpai, M.; Shukla, V.; Kumar, A. Film performance and UV curing of epoxy acrylate resins. Prog. Org. Coat. 2002, 44, 271–278. [Google Scholar] [CrossRef]
- Cho, C.H.; Son, I.; Yoo, J.Y.; Kim, J.H.; Lee, B.; Moon, G.; Lee, E.; Lee, J.H. New UV/heat dual-curable sealant containing acrylate-epoxy hybrid resin for highly adhesive liquid crystal device. Mol. Cryst. Liq. Cryst. 2019, 678, 84–90. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, J.; Wang, F.; Huang, Q.; Peng, C.; Xu, Z. Synthesizing promising epoxy acrylate prepolymers applied in ultraviolet cured adhesives based on esterification reaction. Mater. Res. Express 2018, 5, 065321. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Kahraman, M.V.; Kayaman-Apohan, N.; Güngör, A. Synthesis and characterization of UV-curable dual hybrid oligomers based on epoxy acrylate containing pendant alkoxysilane groups. Prog. Org. Coat. 2006, 57, 50–55. [Google Scholar] [CrossRef]
- Fischer, J.; Ritter, H. Oligomeric epoxide–amine adducts based on 2-amino-N-isopropylacetamide and α-amino-ε-caprolactam: Solubility in presence of cyclodextrin and curing properties. Beilstein J. Org. Chem. 2013, 9, 2803–2811. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.A.; Hosur, M.V.; Nuruddin, M.; Tcherbi-Narteh, A.; Kumar, A.; Boddu, V.; Jeelani, S. Self-healing epoxy composites: Preparation, characterization and healing performance. J. Mater. Res. Technol. 2015, 4, 33–43. [Google Scholar] [CrossRef]
- Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins—Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. Infrared Spectrosc. Mater. Sci. Eng. Technol. 2012, 2012, 261–284. [Google Scholar] [CrossRef]
- Asman, S.; Mohamad, S.; Sarih, N.M. Exploiting β-Cyclodextrin in Molecular Imprinting for Achieving Recognition of Benzylparaben in Aqueous Media. Int. J. Mol. Sci. 2015, 16, 3656–3676. [Google Scholar] [CrossRef]
- Nurhayati, T.; Royani, I. Synthesis and characterization of MAA-based molecularly-imprinted polymer (MIP) with D-glucose template. J. Phys. Conf. Ser. 2016, 739, 12143. [Google Scholar] [CrossRef]
- Arbain, N.H.; Salimon, J. Synthesis and Characterization of Ester Trimethylolpropane Based Jatropha Curcas Oil as Biolubricant Base Stocks. J. Sci. Technol. 2010, 2, 47–58. [Google Scholar]
- Wu, Z.; Li, S.; Liu, M.; Wang, Z.; Liu, X. Liquid oxygen compatible epoxy resin: Modification and characterization. RSC Adv. 2015, 5, 11325–11333. [Google Scholar] [CrossRef]
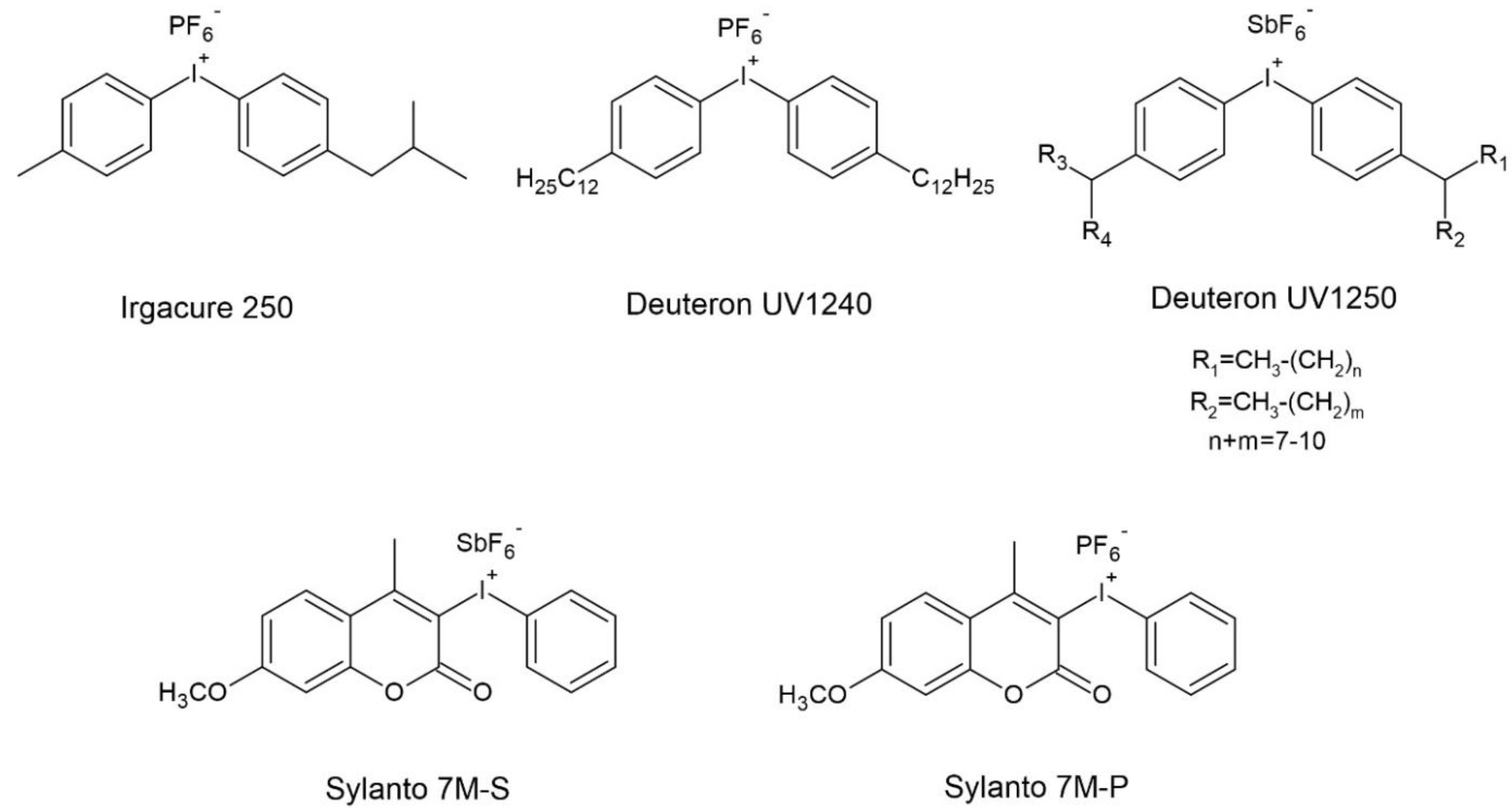
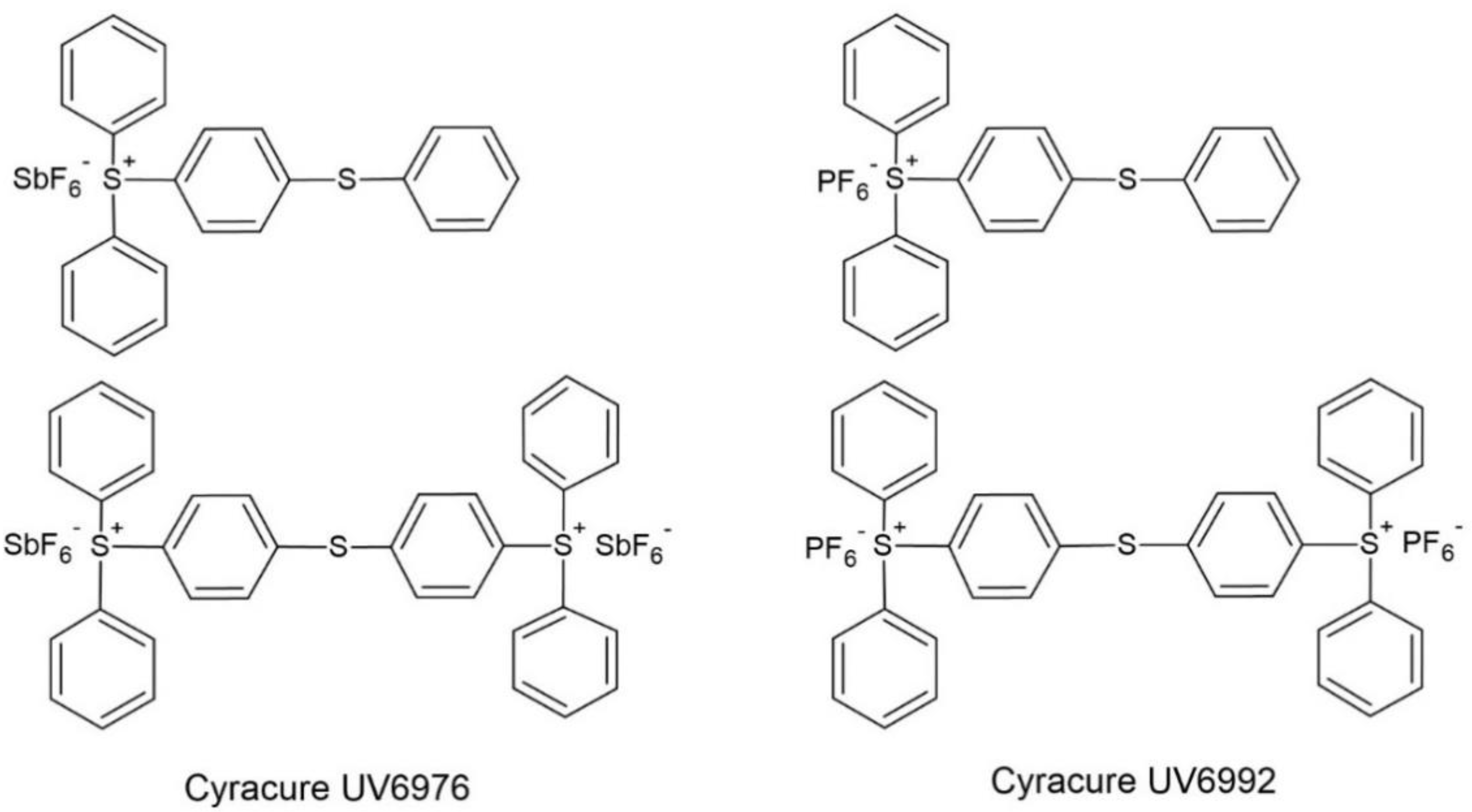
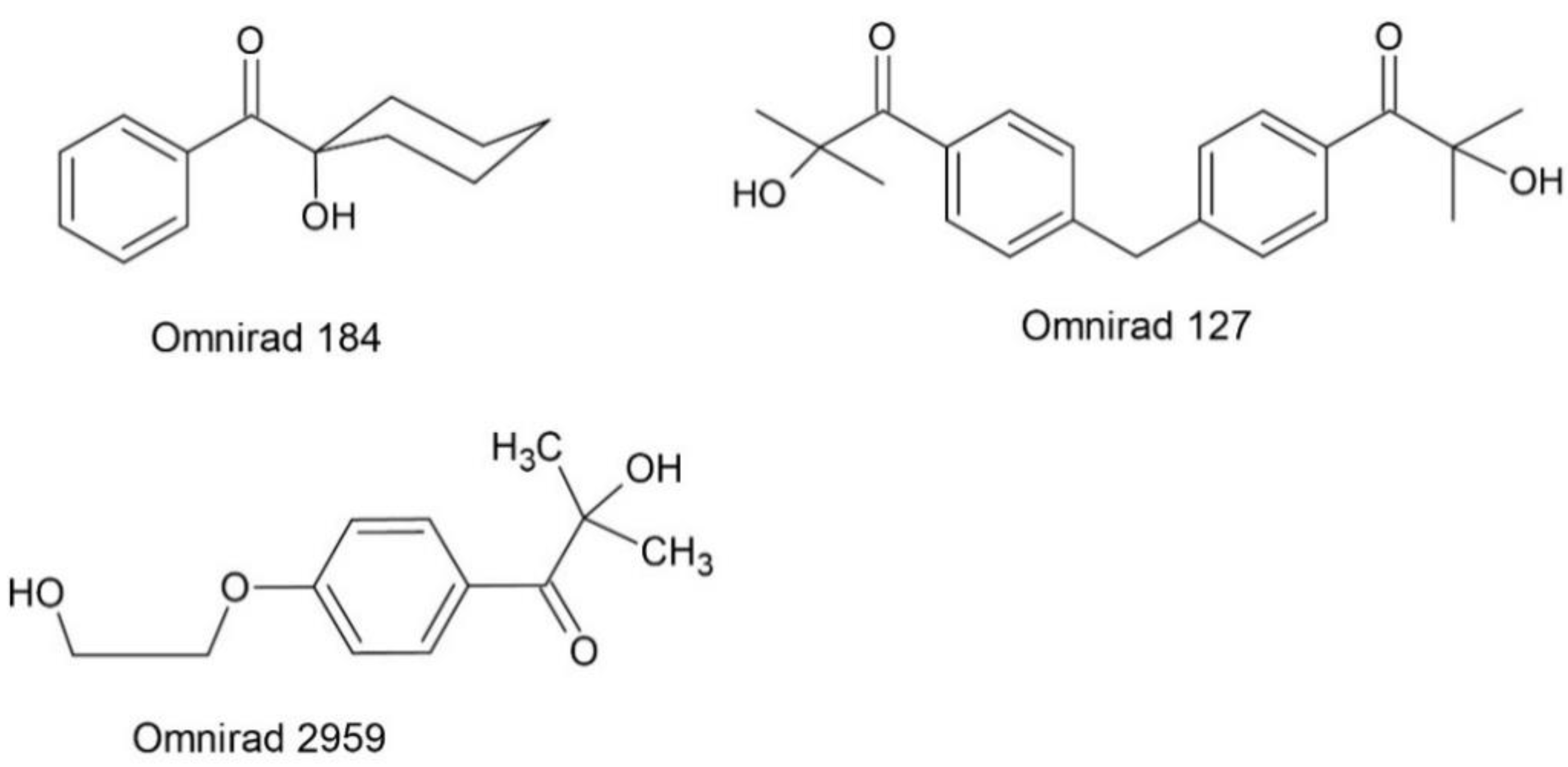

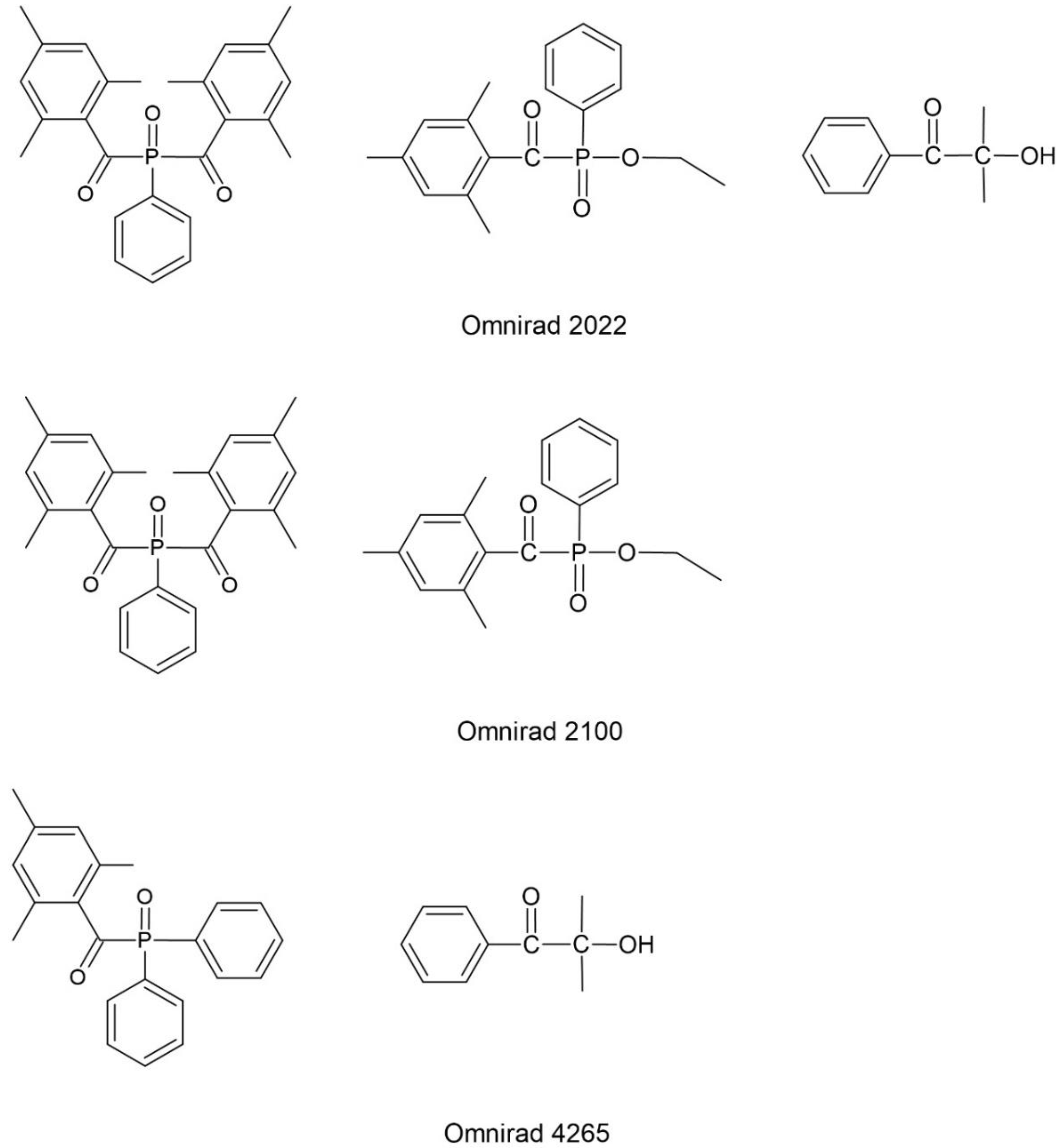
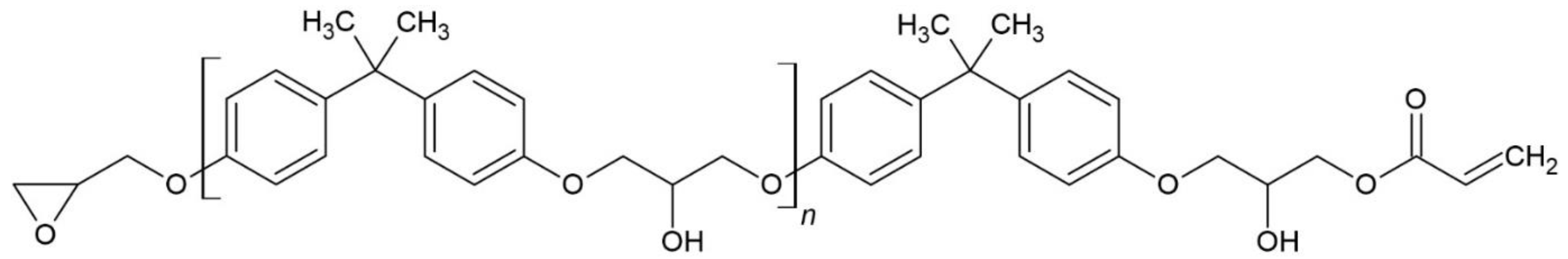
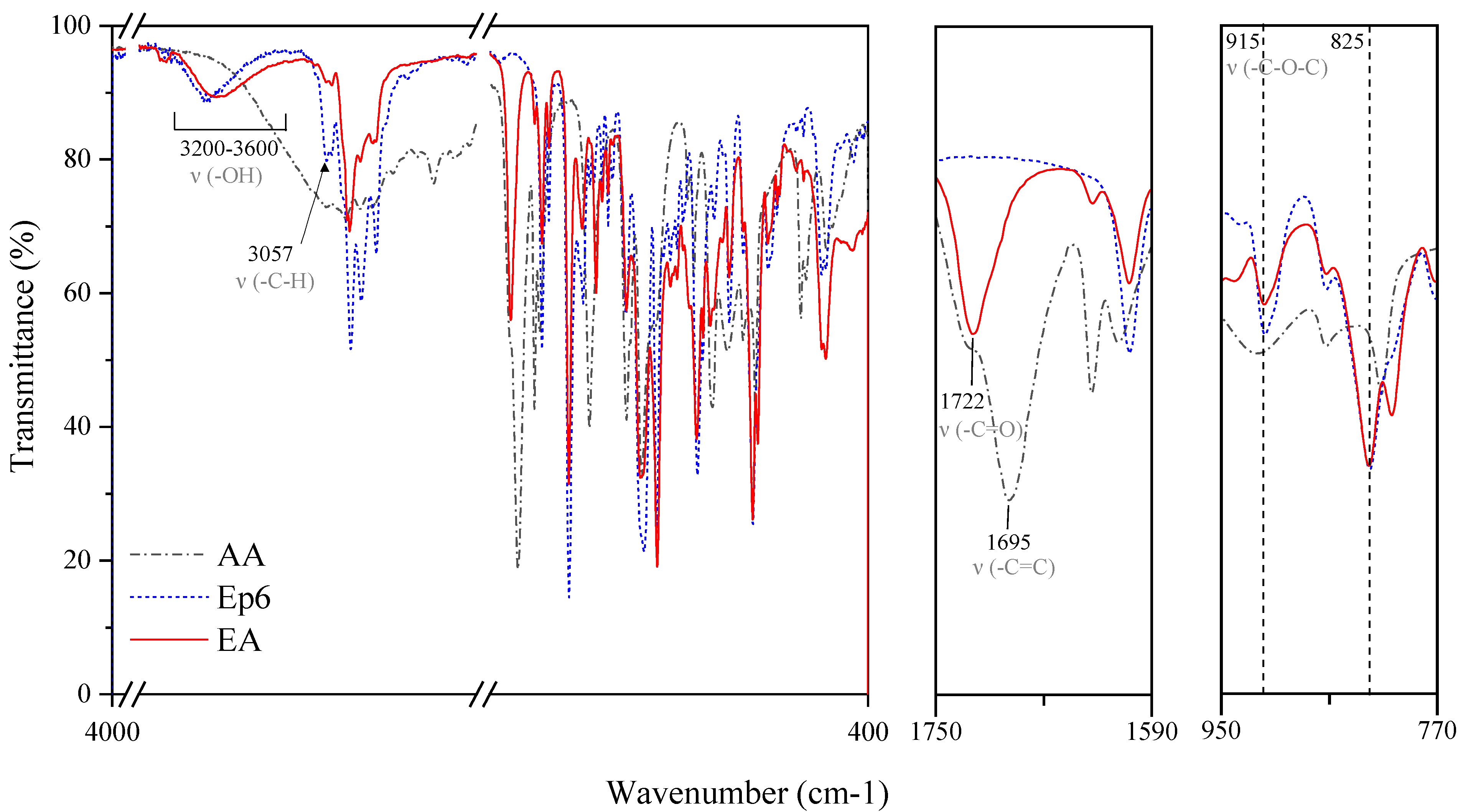
| Photoinitiator | Absorption Peaks (nm) | |||
|---|---|---|---|---|
| Type | Name | Abbreviation | ||
| Cationic | diaryliodonium cation | Irgacure 250 | Ir250 | 242 |
| Deuteron UV1240 | D1240 | 240 | ||
| Deuteron UV1250 | D1250 | 240 | ||
| Sylanto 7M-S | 7MS | 350 | ||
| Sylanto 7M-P | 7MP | 350 | ||
| triarylsulfonium cation | Cyracure UV6976 | C76 | 240,300 | |
| Cyracure UV6992 | C92 | 240,300 | ||
| Radical | α-hydroxyketones | Omnirad 184 | O184 | 246,280,333 |
| Omnirad 127 | O127 | 200,260 | ||
| Omnirad 2959 | O2959 | 200,280 | ||
| acylphosphine oxides | Lucirin TPO | TPO | 295,368,380,393 | |
| Lucirin TPO-L | TPOL | 300,380 | ||
| Omnirad 819 | O819 | 295,370 | ||
| mixtures | Omnirad 2022 | O2022 | 246,282,370 | |
| Omnirad 4265 | O4265 | 275,370 | ||
| Omnirad 2100 | O2100 | 240,272,380 | ||
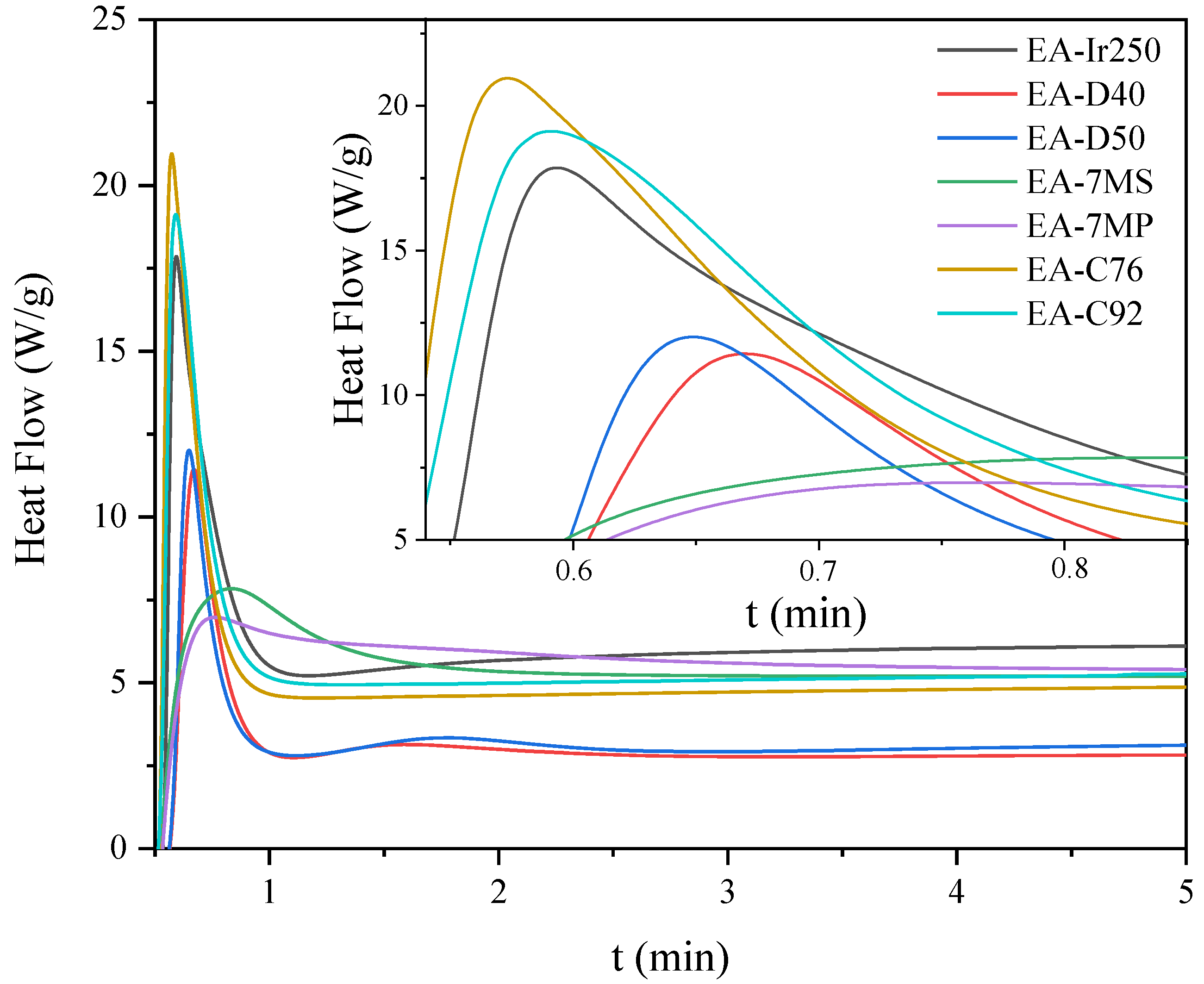
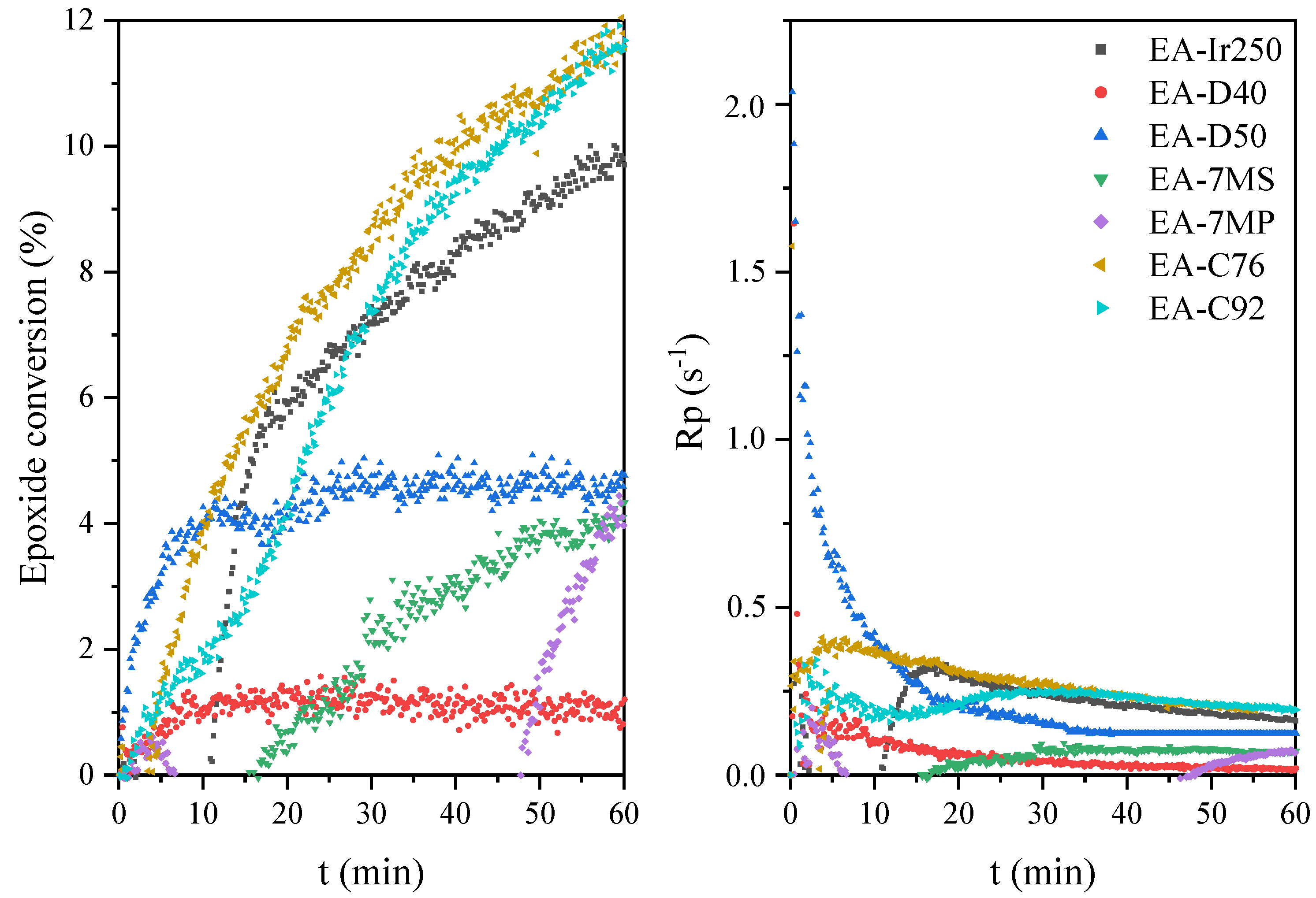
| Sample | Hmax (1) (W/g) | Rpmax (2) (s−1) | X (3) (%) | Tack-Free Time (s) | Hardness (s) | Adhesion | Gloss (GU) | Yellowness Index |
|---|---|---|---|---|---|---|---|---|
| E-Ir250 | 13 | 1.1 | 22 | 33 | 324 | 2.5 | 160 | 6.9 |
| E-D40 | 13 | 0.4 | 12 | 39 | 266 | 3.5 | 180 | 5.4 |
| E-D50 | 10 | 0.4 | 18 | 39 | 194 | 2.5 | 170 | 5.6 |
| E-7MS | 9 | 0.2 | 11 | 30 | 118 | 3.5 | 115 | 21.6 |
| E-7MP | 9 | 0.4 | 6 | 30 | 244 | 2 | 163 | 21.9 |
| E-C76 | 16 | 3.2 | 21 | 39 | 297 | 0 | 188 | 7.6 |
| E-C92 | 14 | 2.5 | 22 | 33 | 234 | 0 | 187 | 7.5 |
| EA-Ir250 | 18 | 0.3 | 10 | 57 | 333 | 1 | 89 | 6.7 |
| EA-D40 | 11 | 0.2 | 1 | 57 | 310 | 3 | 100 | 4.5 |
| EA-D50 | 12 | 2.0 | 5 | 57 | 205 | 3 | 127 | 3.7 |
| EA-7MS | 5 | 0.1 | 4 | 36 | 235 | 3 | 95 | 19.7 |
| EA-7MP | 4 | 0.2 | 4 | 39 | 267 | 4 | 163 | 15.7 |
| EA-C76 | 21 | 0.4 | 12 | 39 | 344 | 1 | 170 | 6.3 |
| EA C92 | 19 | 0.3 | 12 | 39 | 312 | 1 | 170 | 7.3 |
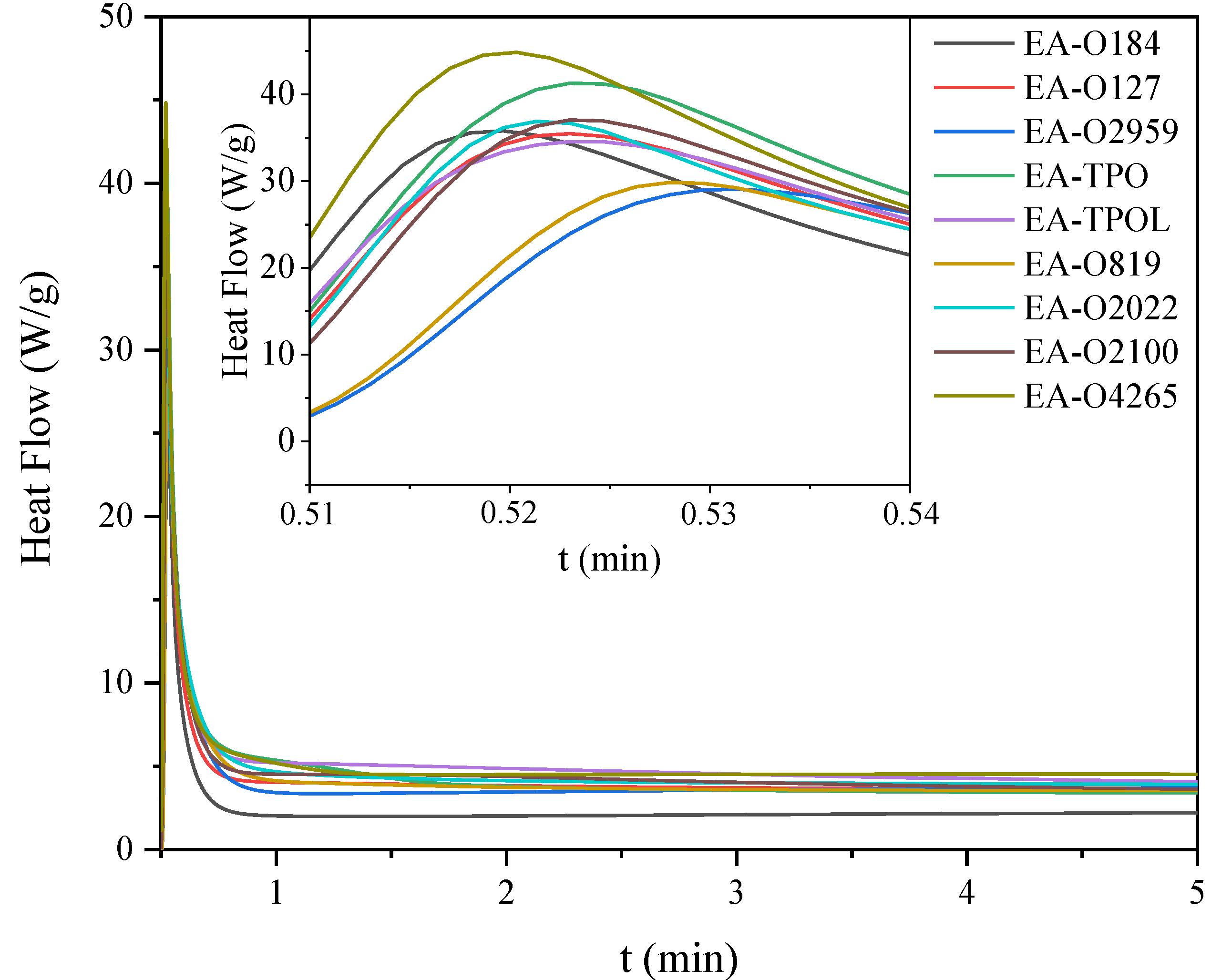
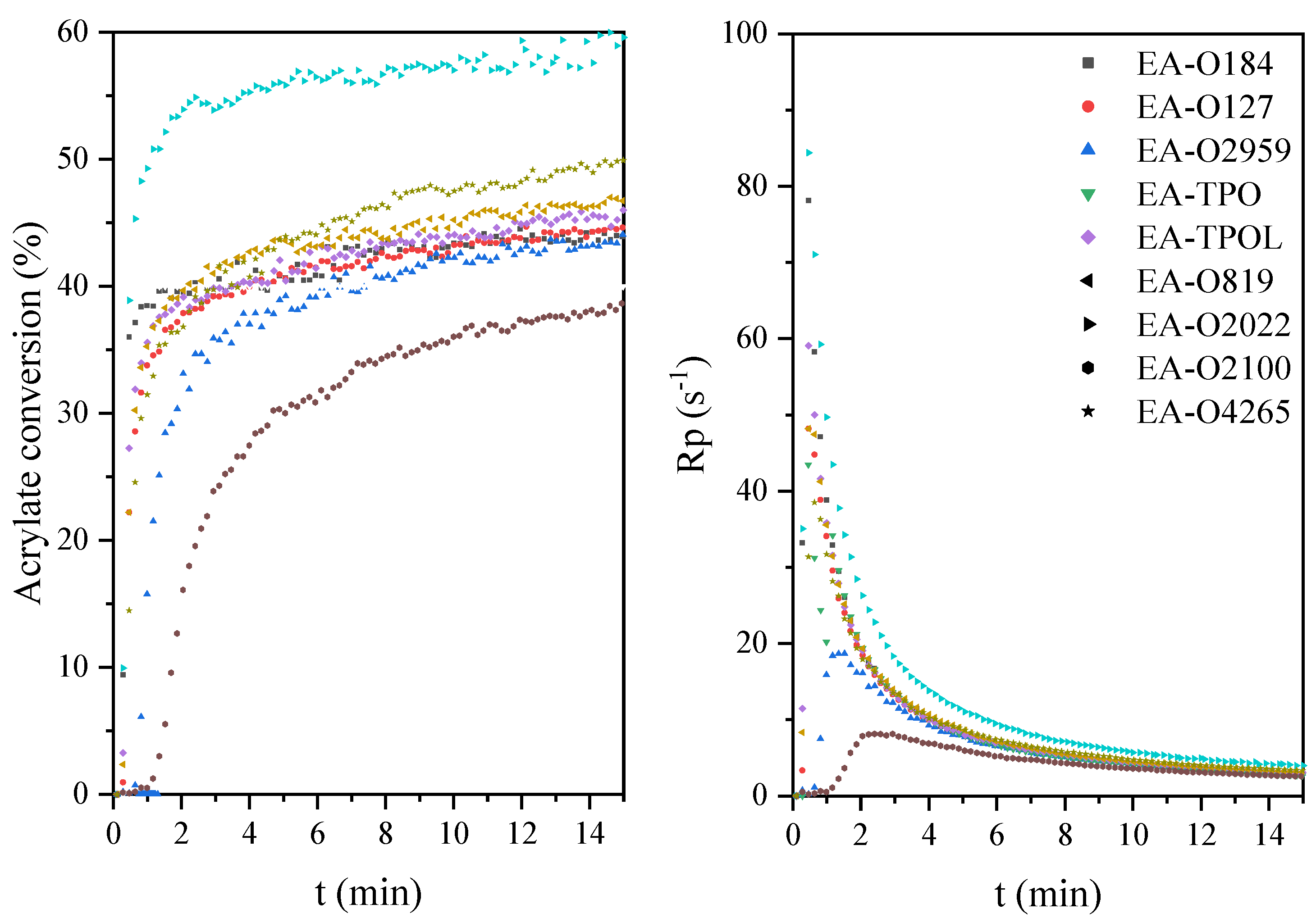
| EA-O184 | 36 | 78.2 | 44 | 3 | 101 | 4 | 43 | 7.1 |
| EA-O127 | 35 | 48.2 | 44 | 3 | 95 | 1 | 117 | 8.9 |
| EA-O2959 | 29 | 18.7 | 43 | 6 | 65 | 2 | 22 | 6.8 |
| EA-TPO | 41 | 43.1 | 40 | 3 | 112 | 4 | 55 | 9.6 |
| EA-TPOL | 35 | 59.1 | 45 | 3 | 88 | 4 | 120 | 7.6 |
| EA-O819 | 30 | 48.1 | 47 | 15 | 83 | 2 | 30 | 8.7 |
| EA-O2022 | 37 | 84.4 | 60 | 6 | 92 | 0 | 108 | 8.9 |
| EA-O2100 | 37 | 6.7 | 39 | 3 | 117 | 4 | 135 | 7.5 |
| EA-O4265 | 45 | 38.5 | 50 | 3 | 91 | 2 | 150 | 7.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk, P.; Mozelewska, K.; Nowak, M.; Czech, Z. Photocurable Epoxy Acrylate Coatings Preparation by Dual Cationic and Radical Photocrosslinking. Materials 2021, 14, 4150. https://doi.org/10.3390/ma14154150
Bednarczyk P, Mozelewska K, Nowak M, Czech Z. Photocurable Epoxy Acrylate Coatings Preparation by Dual Cationic and Radical Photocrosslinking. Materials. 2021; 14(15):4150. https://doi.org/10.3390/ma14154150
Chicago/Turabian StyleBednarczyk, Paulina, Karolina Mozelewska, Małgorzata Nowak, and Zbigniew Czech. 2021. "Photocurable Epoxy Acrylate Coatings Preparation by Dual Cationic and Radical Photocrosslinking" Materials 14, no. 15: 4150. https://doi.org/10.3390/ma14154150
APA StyleBednarczyk, P., Mozelewska, K., Nowak, M., & Czech, Z. (2021). Photocurable Epoxy Acrylate Coatings Preparation by Dual Cationic and Radical Photocrosslinking. Materials, 14(15), 4150. https://doi.org/10.3390/ma14154150








