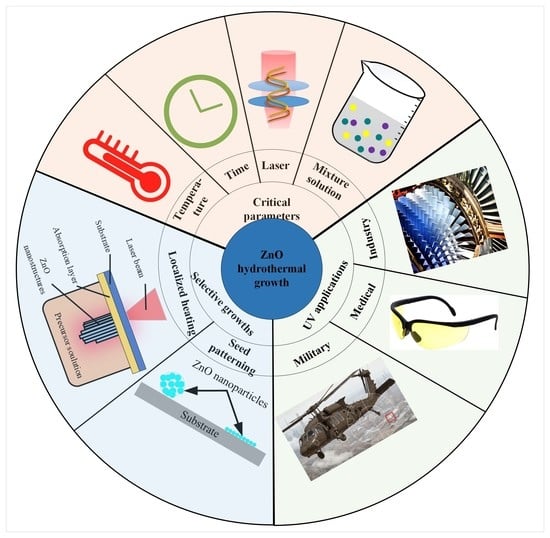
Economic Friendly ZnO-Based UV Sensors Using Hydrothermal Growth: A Review
Abstract
1. Introduction
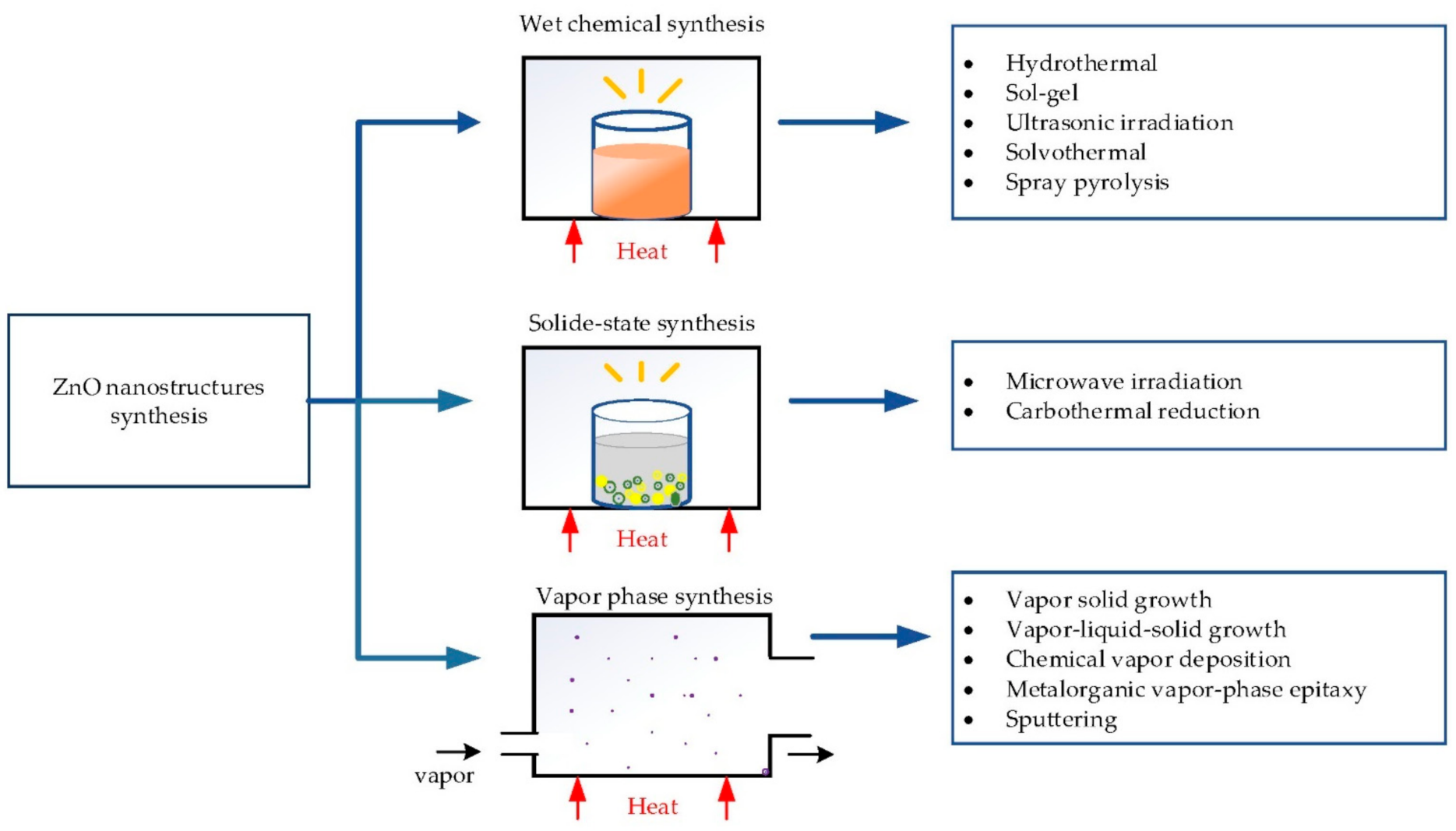
2. ZnO Nanostructures’ Hydrothermal Growth
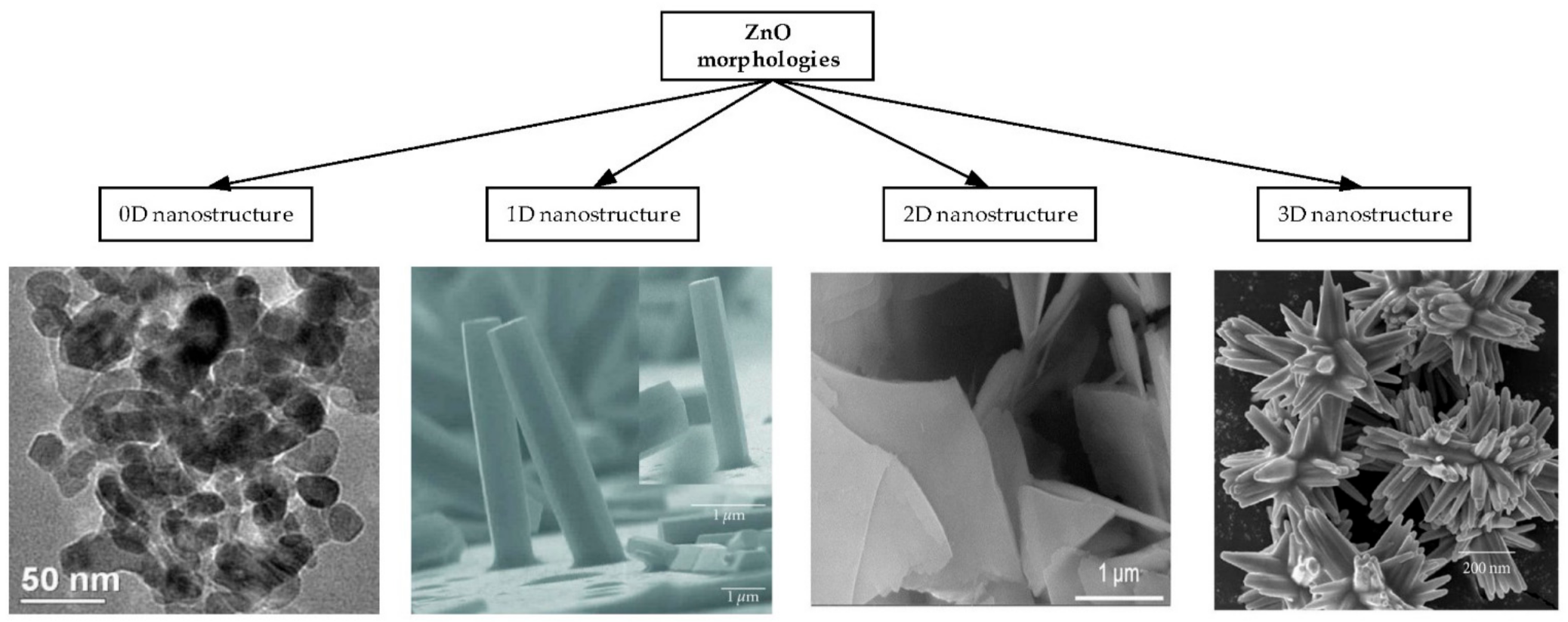
2.1. Different Types of ZnO Nanostructures
- Three dimensional (3D) ZnO nanostructures are built with the agglomeration of 0D, 1D and 2D nanostructures [78]. They are structures in which electrons can move freely on three non-nano scales such as nanoflowers [32,47,80,94,95], nanorings [17,18,25], nanospheres [96], nanohelix [18], nanocombs [97], superstructures [80] and sea urchin [37].
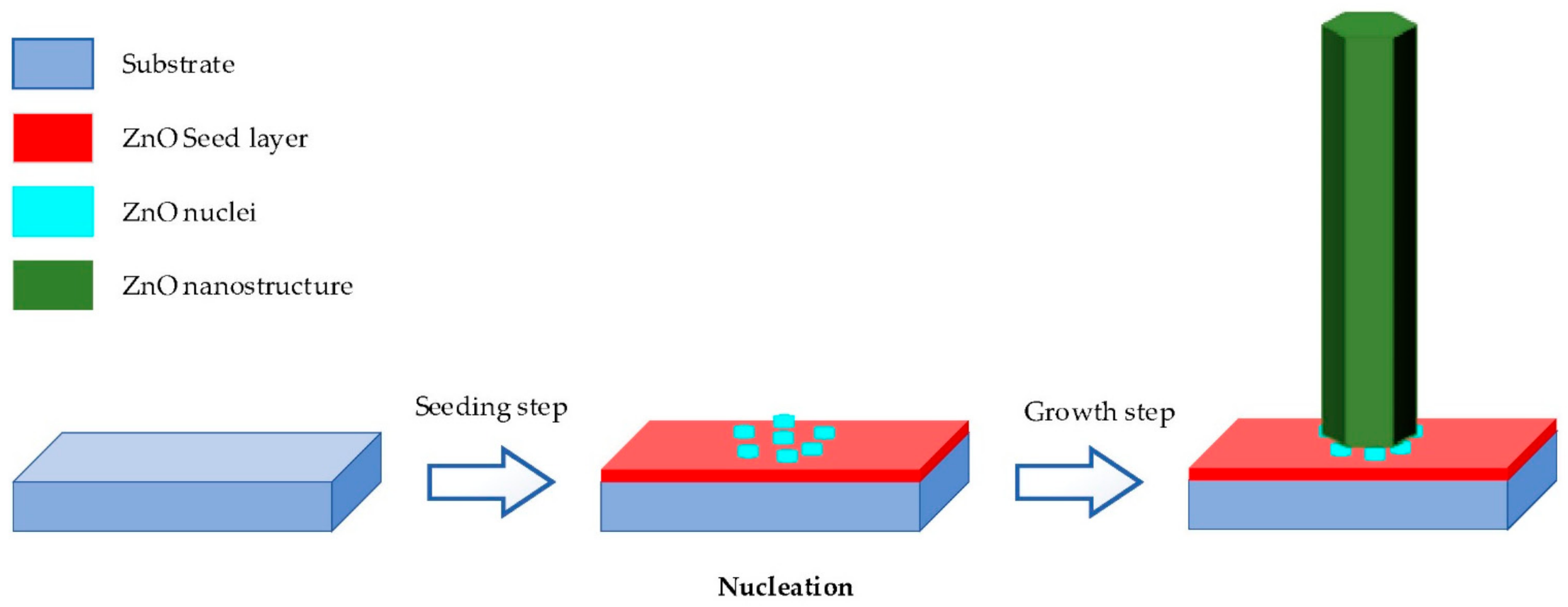
2.2. Hydrothermal Growth Process
- Seeding: The subtract is seeded with a layer of ZnO nanoparticles. The seeded nanoparticles play a role in promoting nucleation for nanostructure growth by decreasing the thermodynamic barrier. The less the nucleation of ZnO is, the bigger ZnO growth and the better the crystallinity of ZnO [98].
- Growing: The seeded subtract is kept in the precursor at a certain temperature for a fixed period to ensure stable growing regimes. The precursor is a mixture of aqueous solutions containing alkaline reagent (such as NaOH, KOH and hexamethylenetetramine (HMTA)) and zinc ion salt (such as Zn (NO3)2 and ZnCl2) [39,40,47,99]. In addition to the precursor, a guiding agent (such as polyethyleneimine (PEI)) is inserted to decrease the lateral growth and maximize the length of nanostructures.
3. ZnO Selective Hydrothermal Growth
3.1. Localized Heat
3.1.1. Joule Heating Growth
3.1.2. Laser-Induced Growth
3.2. Seed Patterning
3.2.1. Microcontact Printing
3.2.2. Inkjet Printing
4. Influence of Fabrication Parameters on ZnO Hydrothermal Growth
4.1. Influence of Laser Power
4.2. Influence of Precursor Solution
4.3. Influence of Base Concentration
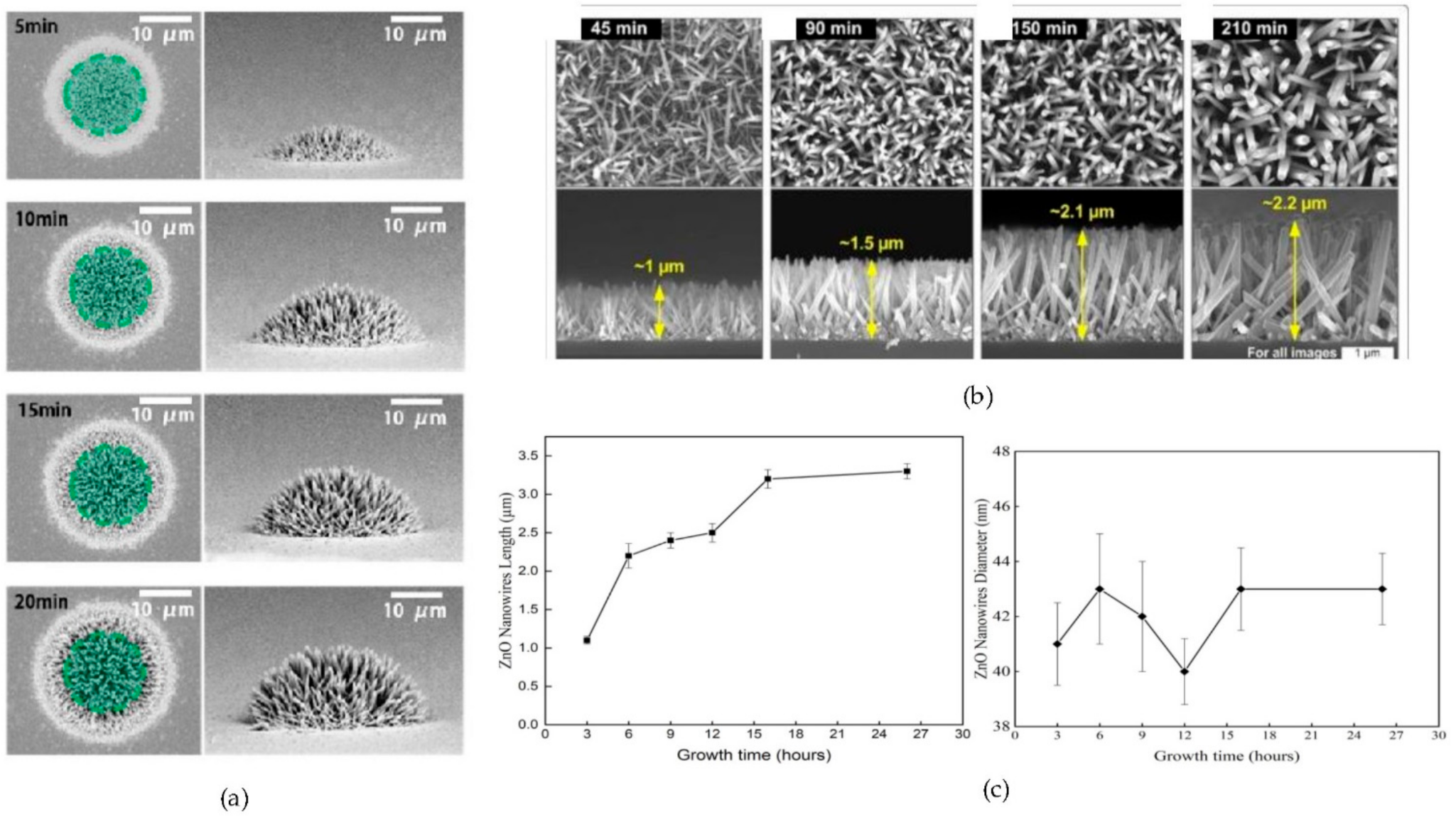
4.4. Influence of Growth Time
4.5. Influence of Growth Temperature
4.6. Influence of the Seed Solution
5. ZnO-Based UV Sensors
5.1. Pure ZnO Nanostructures for UV Sensing
5.2. ZnO-Based Composites for UV Sensing
6. Conclusions and Perspectives
- (1)
- The synthesis of well-controlled ZnO nanostructures via the hydrothermal method remains uncertain. The stability of their morphology, geometry and size are varied with the experimental conditions. The precious controlling architecture of ZnO nanostructures is still challenging.
- (2)
- Composite materials with ZnO nanostructures can regulate the defects of ZnO-grown nanostructures and enhance the quality of UV sensors. However, comparative research for these composite materials is needed.
- (3)
- The sensitivity and the photoresponse speed of UV devices are still limited. Therefore, the improvement of their performance is of vital importance for the development of UV applications.
- (4)
- Some preparations of ZnO-based UV devices are still inconsistent and time consuming. More affordable and easily manufactured ZnO nanostructures will be revolutionary for UV devices in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozel, K.; Yildiz, A. SnO2/ZnO/p-Si and SnO2/TiO2/p-Si heterojunction UV photodiodes prepared using a hydrothermal method. Sens. Actuators A Phys. 2020, 315, 112351. [Google Scholar] [CrossRef]
- Asib, N.A.M.; Husairi, F.S.; Eswar, K.A.; Afaah, A.N.; Mamat, M.H.; Rusop, M.; Khusaimi, Z. Developing high-sensitivity UV sensors based on ZnO nanorods grown on TiO2 seed layer films using solution immersion method. Sens. Actuators A Phys. 2020, 302, 111827. [Google Scholar] [CrossRef]
- Zou, W.; González, A.; Jampaiah, D.; Ramanathan, R.; Taha, M.; Walia, S.; Sriram, S.; Bhaskaran, M.; Dominguez-Vera, J.M.; Bansal, V. Skin color-specific and spectrally-selective naked-eye dosimetry of UVA, B and C radiations. Nat. Commun. 2018, 9, 3743. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, Y.; Hu, Y.; Gu, H. Ultraviolet detectors based on wide bandgap semiconductor nanowire: A review. Sensors 2018, 18, 2072. [Google Scholar] [CrossRef]
- Razeghi, M.; Rogalski, A. Semiconductor ultraviolet detectors. J. Appl. Phys. 1996, 79, 7433–7473. [Google Scholar] [CrossRef]
- Shi, L.; Nihtianov, S. Comparative study of silicon-based ultraviolet photodetectors. IEEE Sens. J. 2012, 12, 2453–2459. [Google Scholar] [CrossRef]
- Shen, T.; Dai, X.; Zhang, D.; Wang, W.; Feng, Y. ZnO composite graphene coating micro-fiber interferometer for ultraviolet detection. Sensors 2020, 20, 1478. [Google Scholar] [CrossRef]
- AlZoubi, T.; Qutaish, H.; Al-Shawwa, E.; Hamzawy, S. Enhanced UV-light detection based on ZnO nanowires/graphene oxide hybrid using cost-effective low temperature hydrothermal process. Opt. Mater. 2018, 77, 226–232. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Z.; Yuan, S.; Xu, L.; Xin, Y.; Duan, M.; Yao, S.; Yang, Y.; Xia, Z. Enhanced Ultra-violet Photodetection Based on a Heterojunction Consisted of ZnO Nanowires and Single-Layer Graphene on Silicon Substrate. Electron. Mater. Lett. 2020, 16, 81–88. [Google Scholar] [CrossRef]
- Rai, S.C.; Wang, K.; Ding, Y.; Marmon, J.K.; Bhatt, M.; Zhang, Y.; Zhou, W.; Wang, Z.L. Piezo-phototronic Effect Enhanced UV/Visible Photodetector Based on Fully Wide Band Gap Type-II ZnO/ZnS Core/Shell Nanowire Array. ACS Nano 2015, 9, 6419–6427. [Google Scholar] [CrossRef]
- Kou, L.Z.; Guo, W.L.; Li, C. Piezoelectricity of ZnO and its nanostructures. In Proceedings of the 2008 Symposium on Piezoelectricity, Acoustic Waves, and Device Applications, Nanjing, China, 5–8 December 2008; pp. 354–359. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Ditshego, N.M.J. ZnO nanowire field effect transistor for biosensing: A review. J. Nano Res. 2019, 60, 94–112. [Google Scholar] [CrossRef]
- Şerban, I.; Enesca, A. Metal Oxides-Based Semiconductors for Biosensors Applications. Front. Chem. 2020, 8, 354. [Google Scholar] [CrossRef]
- Characterization of Zinc Oxide Nanorod. Available online: https://www.azonano.com/article.aspx?ArticleID=2509 (accessed on 15 May 2021).
- Boubenia, S.; Dahiya, A.S.; Poulin-Vittrant, G.; Morini, F.; Nadaud, K.; Alquier, D. A facile hydrothermal approach for the density tunable growth of ZnO nanowires and their electrical characterizations. Sci. Rep. 2017, 7, 369. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO nanowires: A review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Rong, P.; Ren, S.; Yu, Q. Fabrications and Applications of ZnO Nanomaterials in Flexible Functional Devices-A Review. Crit. Rev. Anal. Chem. 2019, 49, 336–349. [Google Scholar] [CrossRef]
- Bagga, S.; Akhtar, J.; Mishra, S. Synthesis and applications of ZnO nanowire: A review. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2018; Volume 1989. [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, S.; Mao, Z.; Li, P.; Zhao, C.; Cohick, Z.; Huang, P.-H.; Huang, T.J. In Situ Fabrication of 3D Ag @ ZnO Nanostructures for Micro fl uidic. ACS Nano 2014, 8, 12175–12184. [Google Scholar] [CrossRef]
- Humayun, Q.; Kashif, M.; Hashim, U.; Qurashi, A. Selective growth of ZnO nanorods on microgap electrodes and their applications in UV sensors. Nanoscale Res. Lett. 2014, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Kim, D.; Ko, S.H.; Pisano, A.P.; Li, Z.; Park, I. Focused energy field method for the localized synthesis and direct integration of 1D nanomaterials on microelectronic devices. Adv. Mater. 2015, 27, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Tajik, S.; Garkani Nejad, F.; Safaei, M. Recent advances in ZnO nanostructure-based electrochemical sensors and biosensors. J. Mater. Chem. B 2020, 8, 5826–5844. [Google Scholar] [CrossRef] [PubMed]
- Noothongkaew, S.; Thumthan, O.; An, K.S. UV-Photodetectors based on CuO/ZnO nanocomposites. Mater. Lett. 2018, 233, 318–323. [Google Scholar] [CrossRef]
- Paeng, D.; Lee, D.; Yeo, J.; Yoo, J.H.; Allen, F.I.; Kim, E.; So, H.; Park, H.K.; Minor, A.M.; Grigoropoulos, C.P. Laser-induced reductive sintering of nickel oxide nanoparticles under ambient conditions. J. Phys. Chem. C 2015, 119, 6363–6372. [Google Scholar] [CrossRef]
- Nurfani, E.; Lailani, A.; Kesuma, W.A.P.; Anrokhi, M.S.; Kadja, G.T.M.; Rozana, M. UV sensitivity enhancement in Fe-doped ZnO films grown by ultrafast spray pyrolysis. Opt. Mater. 2021, 112, 110768. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kim, J.; Jeong, H.E.; Park, Y.B.; Park, H.W. Fabrication of the piezoresistive sensor using the continuous laser-induced nanostructure growth for structural health monitoring. Carbon N. Y. 2019, 152, 376–387. [Google Scholar] [CrossRef]
- Henley, S.J.; Fryar, J.; Jayawardena, K.D.G.I.; Silva, S.R.P. Laser-assisted hydrothermal growth of size-controlled ZnO nanorods for sensing applications. Nanotechnology 2010, 21, 365502. [Google Scholar] [CrossRef][Green Version]
- Yeasmin, M.; Das, T.; Baruah, S. Study on the hydrothermal growth of ZnO nanorods for piezotronic. ADBU J. Eng. Technol. 2019, 8, 1–5. [Google Scholar]
- Samouco, A.; Marques, A.C.; Pimentel, A.; Martins, R.; Fortunato, E. Laser-induced electrodes towards low-cost flexible UV ZnO sensors. Flex. Print. Electron. 2018, 3, 044002. [Google Scholar] [CrossRef]
- Mai, H.H.; Tran, D.H.; Janssens, E. Non-enzymatic fluorescent glucose sensor using vertically aligned ZnO nanotubes grown by a one-step, seedless hydrothermal method. Microchim. Acta 2019, 186, 245. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, M.; He, Y.; Zhang, M.; Shen, R.; Zhang, Y.; Wang, M.; Wu, G. Fabrication and potential applications of highly durable superhydrophobic polyethylene terephthalate fabrics produced by in-situ zinc oxide (ZnO) nanowires deposition and polydimethylsiloxane (pdms) packaging. Polymers 2020, 12, 2333. [Google Scholar] [CrossRef]
- Filip, A.; Musat, V.; Tigau, N.; Polosan, S.; Pimentel, A.; Ferreira, S.; Gomes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. ZnO nanostructures grown on ITO coated glass substrate by hybrid microwave-assisted hydrothermal method. Optik 2020, 208, 164372. [Google Scholar] [CrossRef]
- Sanjeev, S.; Kekuda, D. Effect of annealing temperature on the structural and optical properties of zinc oxide (ZnO) thin films prepared by spin coating process. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Temple Circus Temple Way, Bristol, UK, 2015; Volume 73. [Google Scholar] [CrossRef]
- Kiriarachchi, H.D.; Abouzeid, K.M.; Bo, L.; El-Shall, M.S. Growth Mechanism of Sea Urchin ZnO Nanostructures in Aqueous Solutions and Their Photocatalytic Activity for the Degradation of Organic Dyes. ACS Omega 2019, 4, 14013–14020. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhai, J.; Wang, Z.L. Piezotronics and Piezo-phototronics of Third Generation Semiconductor Nanowires. Chem. Rev. 2019, 119, 9303–9359. [Google Scholar] [CrossRef]
- Ghoderao, K.P.; Jamble, S.N.; Kale, R.B. Influence of pH on hydrothermally derived ZnO nanostructures. Optik 2018, 156, 758–771. [Google Scholar] [CrossRef]
- Young, S.-J.; Yang, C.-C.; Lai, L.-T. Review—Growth of Al-, Ga-, and In-Doped ZnO Nanostructures via a Low-Temperature Process and Their Application to Field Emission Devices and Ultraviolet Photosensors. J. Electrochem. Soc. 2017, 164, B3013–B3028. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, M.; Vasant Kumar, R.; Ali, N. A Review on Preparation of ZnO Nanorods and Their Use in Ethanol Vapors Sensing. Gas Sens. 2020, 1–24. [Google Scholar] [CrossRef]
- Basnet, P.; Chatterjee, S. Structure-directing property and growth mechanism induced by capping agents in nanostructured ZnO during hydrothermal synthesis—A systematic review. Nano Struct. Nano Objects 2020, 22, 100426. [Google Scholar] [CrossRef]
- Yeo, J.; Hong, S.; Kim, G.; Lee, H.; Suh, Y.D.; Park, I.; Grigoropoulos, C.P.; Ko, S.H. Laser-Induced Hydrothermal Growth of Heterogeneous Metal-Oxide Nanowire on Flexible Substrate by Laser Absorption Layer Design. ACS Nano 2015, 9, 6059–6068. [Google Scholar] [CrossRef]
- Syed, A.; Kalloudis, M.; Koutsos, V.; Mastropaolo, E. Controlled hydrothermal growth of vertically-aligned zinc oxide nanowires using silicon and polyimide substrates. Microelectron. Eng. 2015, 145, 86–90. [Google Scholar] [CrossRef]
- Ryu, Y.Y.; Kim, T.; Han, H.S. Synthesis of porous ZnO nanosheets and carbon nanotube hybrids as efficient photocatalysts via pulsed laser ablation. Catalysts 2019, 9, 787. [Google Scholar] [CrossRef]
- Zhou, Q.; Xie, B.; Jin, L.; Chen, W.; Li, J. Hydrothermal Synthesis and Responsive Characteristics of Hierarchical Zinc Oxide Nanoflowers to Sulfur Dioxide. J. Nanotechnol. 2016, 2016, 6742104. [Google Scholar] [CrossRef]
- Oh, D.K.; Choi, H.; Shin, H.; Kim, K.; Kim, M.; Ok, J.G. Tailoring zinc oxide nanowire architectures collectively by catalytic vapor-liquid-solid growth, catalyst-free vapor-solid growth, and low-temperature hydrothermal growth. Ceram. Int. 2021, 47, 2131–2143. [Google Scholar] [CrossRef]
- Ameer, A.A.; Suriani, A.B.; Jabur, A.R.; Hashim, N.; Zaid, K. The fabrication of zinc oxide nanorods and nanowires by sol gel immersion methods. J. Phys. Conf. Ser. 2019, 1170, 012005. [Google Scholar] [CrossRef]
- Demes, T.; Ternon, C.; Riassetto, D.; Stambouli, V.; Langlet, M. Comprehensive study of hydrothermally grown ZnO nanowires. J. Mater. Sci. 2016, 51, 10652–10661. [Google Scholar] [CrossRef]
- Hjiri, M.; Bahanan, F.; Aida, M.S.; El Mir, L.; Neri, G. High Performance CO Gas Sensor Based on ZnO Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4063–4071. [Google Scholar] [CrossRef]
- Rai, P.; Kwak, W.K.; Yu, Y.T. Solvothermal synthesis of ZnO nanostructures and their morphology-dependent gas-sensing properties. ACS Appl. Mater. Interfaces 2013, 5, 3026–3032. [Google Scholar] [CrossRef]
- Rajagopalan, P.; Singh, V.; Palani, I.A. Investigations on the influence of substrate temperature in developing enhanced response ZnO nano generators on flexible polyimide using spray pyrolysis technique. Mater. Res. Bull. 2016, 84, 340–345. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Meng, J.; Pan, C.; Zhang, Y.; Zhang, Y.; Liu, Z.; Zou, Y.; Fan, Y.; Wang, Z.L.; et al. Triboelectric-polarization-enhanced high sensitive ZnO UV sensor. Nano Today 2020, 33, 100873. [Google Scholar] [CrossRef]
- Ma, S.; Kitai, A.H. ZnO nanowire growth by chemical vapor deposition with spatially controlled density on Zn2GeO4:Mn polycrystalline substrates. Mater. Res. Express 2017, 4, 065012. [Google Scholar] [CrossRef]
- Güell, F.; Martínez-Alanis, P.R.; Roso, S.; Salas-Pérez, C.I.; García-Sánchez, M.F.; Santana, G.; Monroy, B.M. Plasma versus thermal annealing for the Au-catalyst growth of ZnO nanocones and nanowires on Al-doped ZnO buffer layers. Mater. Res. Express 2016, 3, 065013. [Google Scholar] [CrossRef]
- Laurenti, M.; Verna, A.; Fontana, M.; Quaglio, M.; Porro, S. Selective growth of ZnO nanowires on substrates patterned by photolithography and inkjet printing. Appl. Phys. A Mater. Sci. Process. 2014, 117, 901–907. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, D.H.; Jung, S.W.; Yi, G.C. Metalorganic vapor-phase epitaxial growth of vertically well-aligned ZnO nanorods. Appl. Phys. Lett. 2002, 80, 4232–4234. [Google Scholar] [CrossRef]
- Chou, C.C.; Shih, L.H.; Chang, S.J. The study of humidity sensor based on Li-doped ZnO nanorods by hydrothermal method. Microsyst. Technol. 2020, 9, 1–5. [Google Scholar] [CrossRef]
- Dong, J.; Wang, Z.; Wang, X.; Wang, Z.L. Temperature dependence of the pyro-phototronic effect in self-powered p-Si/n-ZnO nanowires heterojuncted ultraviolet sensors. Nano Today 2019, 29, 100798. [Google Scholar] [CrossRef]
- Yeo, J.; Hong, S.; Wanit, M.; Kang, H.W.; Lee, D.; Grigoropoulos, C.P.; Sung, H.J.; Ko, S.H. Rapid, one-step, digital selective growth of ZnO nanowires on 3D structures using laser induced hydrothermal growth. Adv. Funct. Mater. 2013, 23, 3316–3323. [Google Scholar] [CrossRef]
- Ko, S.H.; Lee, D.; Hotz, N.; Yeo, J.; Hong, S.; Nam, K.H.; Grigoropoulos, C.P. Digital selective growth of ZnO nanowire arrays from inkjet-printed nanoparticle seeds on a flexible substrate. Langmuir 2012, 28, 4787–4792. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Yeo, J.; Hwang, J.O.; Hong, S.; Lee, P.; Han, S.Y.; Lee, J.H.; Rho, Y.S.; Kim, S.O.; Ko, S.H.; et al. Simple ZnO nanowires patterned growth by microcontact printing for high performance field emission device. J. Phys. Chem. C 2011, 115, 11435–11441. [Google Scholar] [CrossRef]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, precursor concentration, growth time, and temperature on the morphology of ZnO nanostructures grown by the hydrothermal method. J. Nanomater. 2011, 2011, 269692. [Google Scholar] [CrossRef]
- Alshehri, N.A.; Lewis, A.R.; Pleydell-Pearce, C.; Maffeis, T.G.G. Investigation of the growth parameters of hydrothermal ZnO nanowires for scale up applications. J. Saudi Chem. Soc. 2018, 22, 538–545. [Google Scholar] [CrossRef]
- Yeo, J.; Kim, G.; Hong, S.; Lee, J.; Kwon, J.; Lee, H.; Park, H.; Manoroktul, W.; Lee, M.T.; Lee, B.J.; et al. Single nanowire resistive nano-heater for highly localized thermo-chemical reactions: Localized hierarchical heterojunction nanowire growth. Small 2014, 10, 5015–5022. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, Y.S.; Sang, C.H.; Sheu, J.T. Localized joule heating as a mask-free technique for the local synthesis of ZnO nanowires on silicon nanodevices. Nano Lett. 2011, 11, 4736–4741. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Shim, J.; Lee, J.O.; Choi, K.; Yu, K. Localized laser-based photohydrothermal synthesis of functionalized metal-oxides. Adv. Funct. Mater. 2015, 25, 2222–2229. [Google Scholar] [CrossRef][Green Version]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- Von Philipsborn, A.C.; Lang, S.; Bernard, A.; Loeschinger, J.; David, C.; Lehnert, D.; Bastmeyer, M.; Bonhoeffer, F. Microcontact printing of axon guidance molecules for generation of graded patterns. Nat. Protoc. 2006, 1, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, Q.; Wu, H.; Li, H.; Lai, J.; Ji, G.; Yan, L.; Wang, X.; Zhang, D.; Lin, J.; et al. Roll-to-roll micro-gravure printed large-area zinc oxide thin film as the electron transport layer for solution-processed polymer solar cells. Org. Electron. 2017, 45, 190–197. [Google Scholar] [CrossRef]
- Kwon, J.; Hong, S.; Lee, H.; Yeo, J.; Lee, S.S.; Ko, S.H. Direct selective growth of ZnO nanowire arrays from inkjet-printed zinc acetate precursor on a heated substrate. Nanoscale Res. Lett. 2013, 8, 489. [Google Scholar] [CrossRef]
- Sun, L.; Yang, K.; Lin, Z.; Zhou, X.; Zhang, Y.A.; Guo, T. Effects of coffee ring via inkjet printing seed layers on field emission properties of patterned ZnO nanorods. Ceram. Int. 2018, 44, 10735–10743. [Google Scholar] [CrossRef]
- Hong, S.; Yeo, J.; Manorotkul, W.; Kim, G.; Kwon, J.; An, K.; Ko, S.H. Low-temperature rapid fabrication of ZnO nanowire UV sensor array by laser-induced local hydrothermal growth. J. Nanomater. 2013, 2013, 246328. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S.; Wu, W.; Liu, C.R. The mechanism of controlled integration of ZnO nanowires using pulsed-laser-induced chemical deposition. Nanoscale 2019, 11, 2617–2623. [Google Scholar] [CrossRef]
- Deka Boruah, B. Zinc oxide ultraviolet photodetectors: Rapid progress from conventional to self-powered photodetectors. Nanoscale Adv. 2019, 1, 2059–2085. [Google Scholar] [CrossRef]
- Hu, H.; Huang, X.; Deng, C.; Chen, X.; Qian, Y. Hydrothermal synthesis of ZnO nanowires and nanobelts on a large scale. Mater. Chem. Phys. 2007, 106, 58–62. [Google Scholar] [CrossRef]
- Napi, M.L.M.; Ahmad Noorden, A.F.; Loong Peng Tan, M.; Jamaluddin, H.; Hamid, F.A.; Ahmad, M.K.; Hashim, U.; Ahmad, M.R.; Sultan, S.M. Review—Three Dimensional Zinc Oxide Nanostructures as an Active Site Platform for Biosensor: Recent Trend in Healthcare Diagnosis. J. Electrochem. Soc. 2020, 167, 137501. [Google Scholar] [CrossRef]
- Goswami, L.; Aggarwal, N.; Verma, R.; Bishnoi, S.; Husale, S.; Pandey, R.; Gupta, G. Graphene Quantum Dot-Sensitized ZnO-Nanorod/GaN-Nanotower Heterostructure-Based High-Performance UV Photodetectors. ACS Appl. Mater. Interfaces 2020, 12, 47038–47047. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ding, J.; Yang, D.; Li, J.; Shi, Y.; Zhou, Y. Graphene quantum dots doped ZnO superstructure (ZnO superstructure/GQDs) for weak UV intensity photodetector application. Ceram. Int. 2020, 46, 17800–17808. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Q.; Zhang, Y.; Yang, Z.; Wang, Z.L. Nanowire piezo-phototronic photodetector: Theory and experimental design. Adv. Mater. 2012, 24, 1410–1417. [Google Scholar] [CrossRef]
- Lupan, O.; Magariu, N.; Khaledialidusti, R.; Mishra, A.K.; Hansen, S.; Krüger, H.; Postica, V.; Heinrich, H.; Viana, B.; Ono, L.K.; et al. Comparison of Thermal Annealing versus Hydrothermal Treatment Effects on the Detection Performances of ZnO Nanowires. ACS Appl. Mater. Interfaces 2021, 13, 10537–10552. [Google Scholar] [CrossRef]
- Shivaraj, B.W.; Manjunatha, C.; Abhishek, B.; Nagaraju, G.; Panda, P.K. Hydrothermal synthesis of ZnO nanotubes for CO gas sensing. Sens. Int. 2020, 1, 100018. [Google Scholar] [CrossRef]
- Znajdek, K.; Sibiński, M.; Lisik, Z.; Apostoluk, A.; Zhu, Y.; Masenelli, B.; Sędzicki, P. Zinc oxide nanoparticles for improvement of thin film photovoltaic structures’ efficiency through down shifting conversion. Opto-Electron. Rev. 2017, 25, 99–102. [Google Scholar] [CrossRef]
- Ok, J.G.; Lee, J.Y.; Baac, H.W.; Tawfick, S.H.; Guo, L.J.; Hart, A.J. Rapid anisotropic photoconductive response of ZnO-coated aligned carbon nanotube sheets. ACS Appl. Mater. Interfaces 2014, 6, 874–881. [Google Scholar] [CrossRef]
- Wahid, K.A.; Lee, W.Y.; Lee, H.W.; Teh, A.S.; Bien, D.C.S.; Azid, I.A. Effect of seed annealing temperature and growth duration on hydrothermal ZnO nanorod structures and their electrical characteristics. Appl. Surf. Sci. 2013, 283, 629–635. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Umar, A.; Ibrahim, A.A.; Al-Heniti, S.H.; Kumar, R.; Baskoutas, S.; Raffah, B.M. Synthesis, characterization and acetone gas sensing applications of Ag-doped ZnO nanoneedles. Ceram. Int. 2017, 43, 6765–6770. [Google Scholar] [CrossRef]
- Shen, T.; Wang, J.; Xia, Z.; Dai, X.; Li, B.; Feng, Y. Ultraviolet sensing characteristics of Ag-doped ZnO micro-nano fiber. Sens. Actuators A Phys. 2020, 307, 111989. [Google Scholar] [CrossRef]
- Feng, Y.; Shen, T.; Li, X.; Wei, X. ZnO-nanorod–fiber UV sensor based on evanescent field principle. Optik 2020, 202. [Google Scholar] [CrossRef]
- Soltabayev, B.; Mentbayeva, A.; Acar, S. Enhanced gas sensing properties of in doped ZnO thin films. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Jeon, I.S.; Bae, G.; Jang, M.; Yoon, Y.; Jang, S.; Song, W.; Myung, S.; Lim, J.; Lee, S.S.; Jung, H.K.; et al. Atomic-level mediation in structural interparameter tradeoff of zinc oxide nanowires-based gas sensors: ZnO nanofilm/ZnO nanowire homojunction array. Appl. Surf. Sci. 2021, 540, 148350. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, S.; Tang, P.; Feng, Y.; Li, D. Ultra-sensitive ethanol gas sensors based on nanosheet-assembled hierarchical ZnO-In2O3 heterostructures. J. Hazard. Mater. 2020, 391, 122191. [Google Scholar] [CrossRef]
- An, C.; Qi, H.; Wang, L.; Fu, X.; Wang, A.; Wang, Z.L.; Liu, J. Piezotronic and piezo-phototronic effects of atomically-thin ZnO nanosheets. Nano Energy 2021, 82, 105653. [Google Scholar] [CrossRef]
- Nundy, S.; Eom, T.Y.; Kang, J.G.; Suh, J.; Cho, M.; Park, J.S.; Lee, H.J. Flower-shaped ZnO nanomaterials for low-temperature operations in NOX gas sensors. Ceram. Int. 2020, 46, 5706–5714. [Google Scholar] [CrossRef]
- Farhad, S.; Tanvir, N.; Bashar, M.; Hossain, M.; Sultana, M.; Khatun, N. Facile synthesis of oriented zinc oxide seed layer for the hydrothermal growth of zinc oxide nanorods. Bangladesh J. Sci. Ind. Res. 2018, 53, 233–244. [Google Scholar] [CrossRef]
- Nundy, S.; Eom, T.Y.; Song, K.Y.; Park, J.S.; Lee, H.J. Hydrothermal synthesis of mesoporous ZnO microspheres as NOX gas sensor materials—Calcination effects on microstructure and sensing performance. Ceram. Int. 2020, 46, 19354–19364. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z. Nanostructures of zinc oxide. Int. J. Nanotechnol. 2009, 6, 245–257. [Google Scholar] [CrossRef]
- Eom, T.H.; Han, J.I. Single fiber UV detector based on hydrothermally synthesized ZnO nanorods for wearable computing devices. Appl. Surf. Sci. 2018, 428, 233–241. [Google Scholar] [CrossRef]
- Türkyılmaz, Ş.Ş.; Güy, N.; Özacar, M. Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals. J. Photochem. Photobiol. A Chem. 2017, 341, 39–50. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, W.; Kang, K.; Kim, I.; Kang, D.; Oh, D.K.; Kim, M.C.; Choi, H.; Kim, K.; Kim, M.; et al. Low-temperature large-area fabrication of ZnO nanowires on flexible plastic substrates by solution-processible metal-seeded hydrothermal growth. Nano Converg. 2020, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Jin, L.; Zhang, B.; Chen, Y.; Mao, L.; Zhang, H.; Yang, W. A flexible field-limited ordered ZnO nanorod-based self-powered tactile sensor array for electronic skin. Nanoscale 2016, 8, 16302–16306. [Google Scholar] [CrossRef]
- Fujiwara, H.; Suzuki, T.; Niyuki, R.; Sasaki, K. Realization of low threshold ZnO nanorod array random lasers using a laser-induced hydrothermal synthesis. In Australian Conference on Optical Fibre Technology; Optical Society of America: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Xiong, G.; Jia, J.; Zhao, L.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Non-thermal radiation heating synthesis of nanomaterials. Sci. Bull. 2021, 66, 386–406. [Google Scholar] [CrossRef]
- In, J.B.; Kwon, H.J.; Lee, D.; Ko, S.H.; Grigoropoulos, C.P. In situ monitoring of laser-assisted hydrothermal growth of ZnO nanowires: Thermally deactivating growth kinetics. Small 2014, 10, 741–749. [Google Scholar] [CrossRef]
- Hong, S.; Yeo, J.; Manorotkul, W.; Kang, H.W.; Lee, J.; Han, S.; Rho, Y.; Suh, Y.D.; Sung, H.J.; Ko, S.H. Digital selective growth of a ZnO nanowire array by large scale laser decomposition of zinc acetate. Nanoscale 2013, 5, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Rahul, S.V.; Bhatnagar, P.K. Low cost UV sensor using ZnO nanorods on ITO electrodes. Eng. Res. Express 2020, 2, 025007. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, S.K.; Lim, Y.R.; Jeon, I.S.; Song, W.; Myung, S.; Lee, S.S.; Lim, J.; An, K.S. Dimensional-Hybrid Structures of 2D Materials with ZnO Nanostructures via pH-Mediated Hydrothermal Growth for Flexible UV Photodetectors. ACS Appl. Mater. Interfaces 2017, 9, 15031–15037. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Qi, L.; Yang, J.; Tang, L.; Zhang, D.; Ma, J. Hydrothermal growth of large-scale micropatterned arrays of ultralong ZnO nanowires and nanobelts on zinc substrate. Chem. Commun. 2006, 2006, 3551–3553. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Yaqoob, U.; Phan, D.T.; Chung, G.S. A novel flexible acetylene gas sensor based on PI/PTFE-supported Ag-loaded vertical ZnO nanorods array. Sens. Actuators B Chem. 2016, 222, 536–543. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Hsiao, I.P.; Chang, F.Y.; Hsu, C.L. Improving the photoelectrical characteristics of self-powered p-GaN film/n-ZnO nanowires heterojunction ultraviolet photodetectors through gallium and indium Co-doping. Mater. Sci. Semicond. Process. 2021, 121, 105295. [Google Scholar] [CrossRef]
- Babapour, A.; Yang, B.; Bahang, S.; Cao, W. Low-temperature sol-gel-derived nanosilver-embedded silane coating as biofilm inhibitor. Nanotechnology 2011, 22, 155602. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Wu, W.; Falconi, C. Piezotronics and piezo-phototronics with third-generation semiconductors. MRS Bull. 2018, 43, 922–927. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, D.; Zhao, M.; Duan, Y.; Zhou, X.; Yang, X.; Shan, C.; Qin, J.; Gao, S.; Liang, Q.; et al. Piezo-phototronic effect for enhanced sensitivity and response range of ZnO thin film flexible UV photodetectors. J. Appl. Phys. 2019, 125, 024502. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, S.; Yang, Q.; Klein, B.D.B.; Zhou, Y.S.; Wang, Z.L. Theoretical Study of Piezo-phototronic Nano-LEDs. Adv. Mater. 2014, 26, 7209–7216. [Google Scholar] [CrossRef]
- Rahimi, K.; Yazdani, A. Incremental photocatalytic reduction of graphene oxide on vertical ZnO nanorods for ultraviolet sensing. Mater. Lett. 2020, 262, 127078. [Google Scholar] [CrossRef]
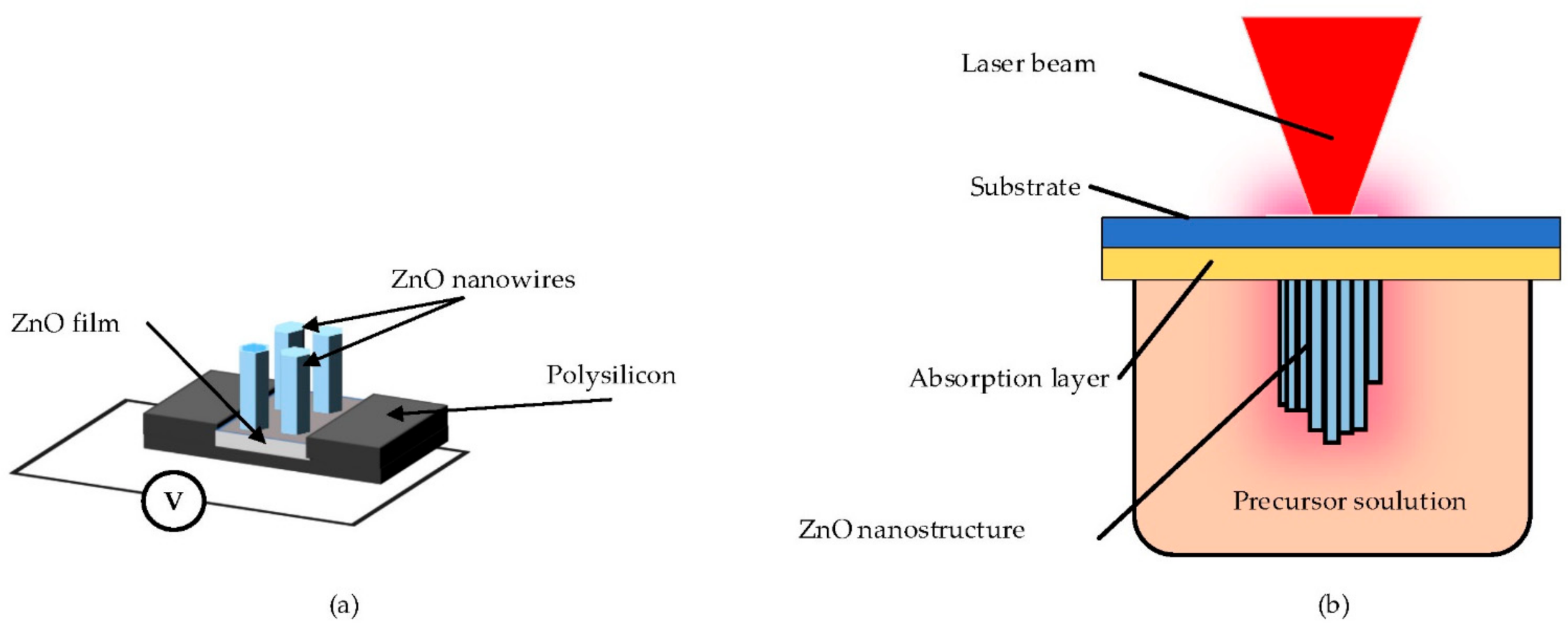

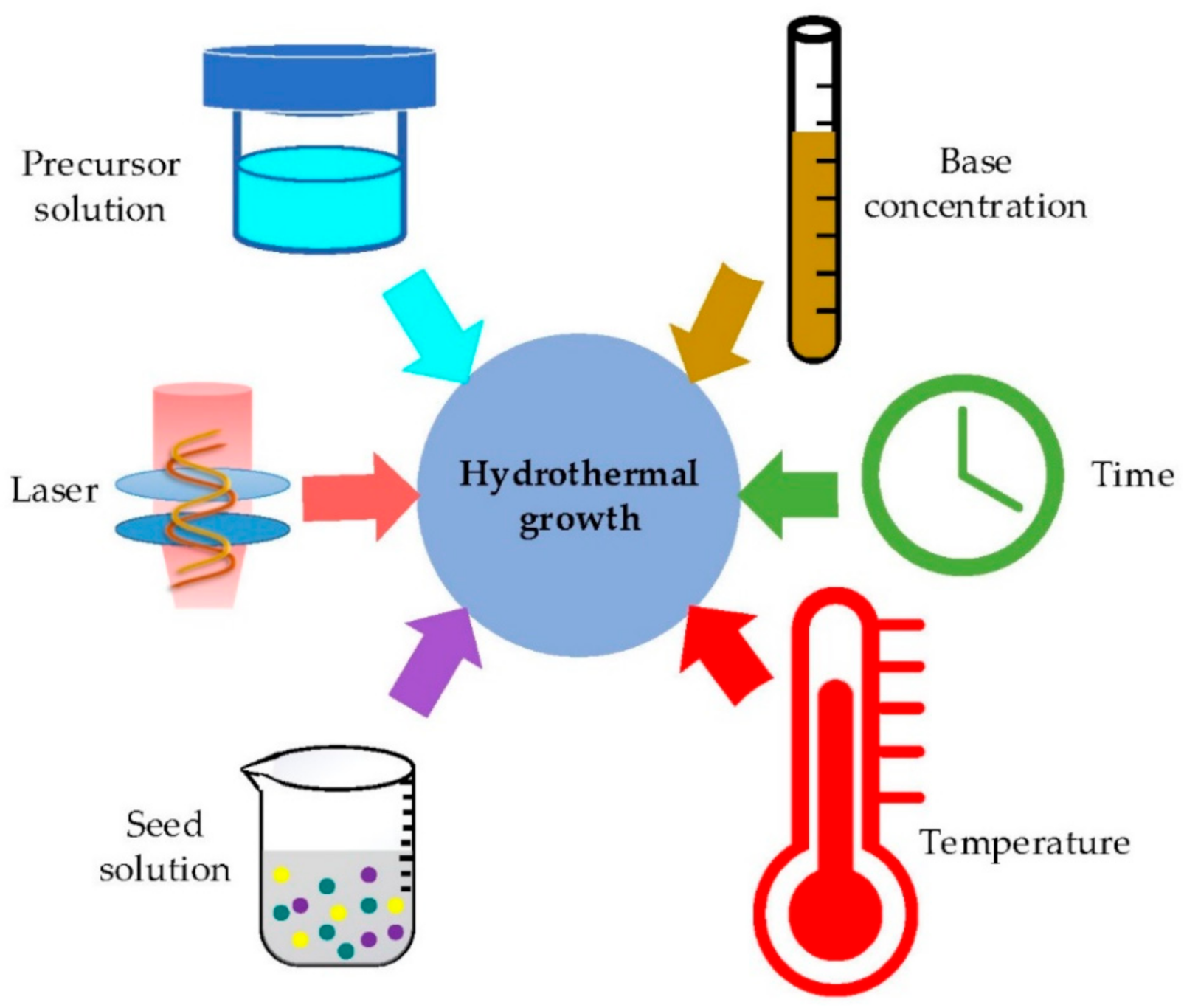
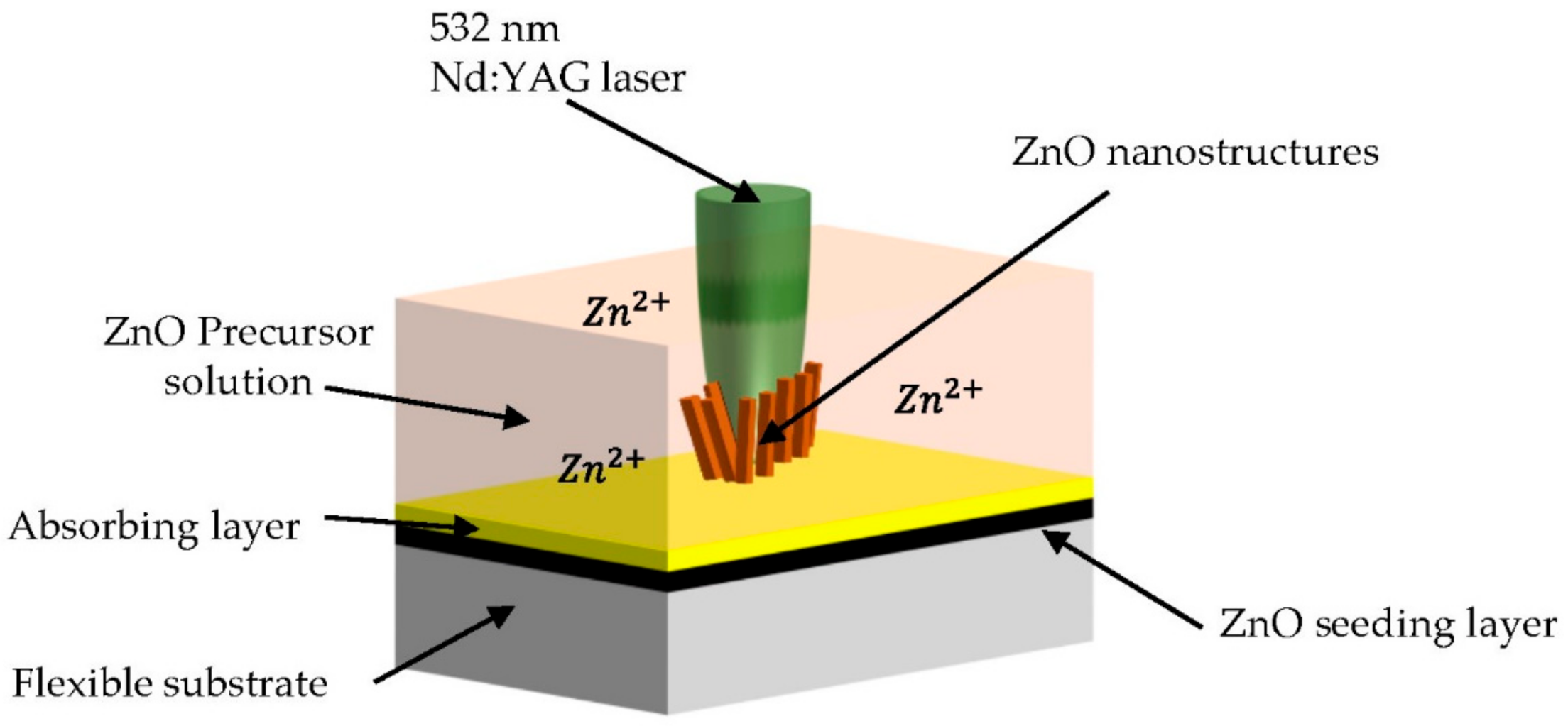
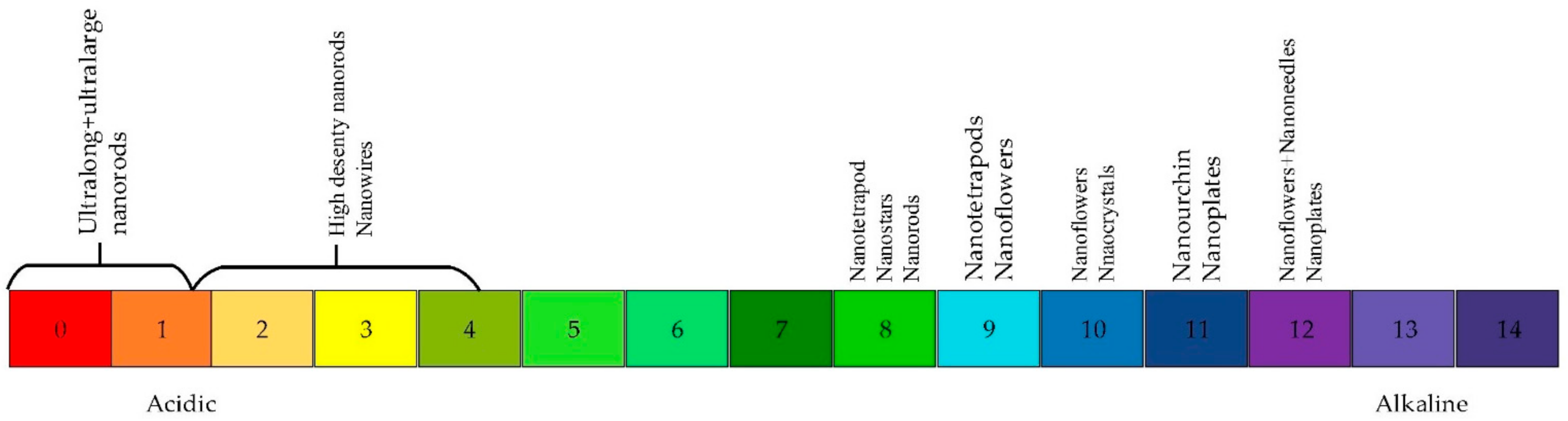

| Type of Property | Property |
|---|---|
| Preparation | Easy to grow [12]. Low- and high-temperature operation capability. Architecture and property controllability. Facility of integration on either rigid or flexible devices [20]. |
| Optical | Large bandgap [13]. Good transparency to visible light. Luminescent material [20]. Good transmissibility and reflexibility [35,36]. |
| Electrical | Semiconductor material. Good electron mobility [37]. Good chemical stability [16]. Huge piezoelectric coefficient [11,38]. |
| Biomedical | Excellent biocompatibility [13,20]. Excellent biodegradability [25]. Non-toxicity [33,37]. |
| Energy | Huge excitation binding energy [16,23]. Photocatalytic material [37,39]. |
| Group of Selective Growth | Type of Selective Growth | Advantages | Disadvantages |
|---|---|---|---|
| Localized heat | Joule heat-induced growth |
|
|
| Laser- induced growth |
|
| |
| Seed patterning | Microcontact printing |
| |
| Inkjet printing |
|
|
| ZnO Morphology | Starting Materials | Synthesis Temperature (°C) | Growth Time | Diameter of ZnO Nanostructures | Length of ZnO Nanostructures | References |
|---|---|---|---|---|---|---|
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA and 5–7 mM PEI. | 95 | 1 h | 200–400 nm | 10–12 µm | [61] |
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA, 5–7 mM PEI and deionized (DI) water. | 95 | 1 h | >20 µm | - | [44] |
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA and 6 mM PEI. | - | - | - | 9.9 µm | [24] |
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA and 5–7 mM PEI. | 90 | 2.5 h | 100–150 nm | 1–3 µm | [72] |
| Nanowires | 25 mM of zinc nitrate hexahydrate, 25 mM HMTA and 5–7 mM PEI. | 95 | 1 h | 15 µm | 200–400 nm | [105] |
| Hemispherical bumps | Mixture of equimolar zinc nitrate hexahydrate and HMTA. | 90 | 5 h | 400 nm | 2.2 µm | [64] |
| Nanorods | Mixture of equimolar zinc nitrate hexahydrate and HMTA. | 90 | 3 h | 100 nm | 800 nm | [106] |
| Nanorods | 50 mL of solution containing 0.1 M zinc nitrate hexahydrate, 0.1 M HMTA and DI water. | 90 | 2 h | 70 nm | 15 µm | [26] |
| Nanorods | Mixture of equimolar zinc nitrate hexahydrate and HMTA | 90 | 4 h | 1.2 | - | [2] |
| Flower-like structure | Zinc acetate dehydrate and NaOH. | 120 | 15 min | 0.6 µm | 5.2 µm | [32] |
| Nanowires | ZnCl2. NaCO3 and DI water. | 140 | 6 h | 50 nm | 1 µm | [65] |
| Vertical aligned nanorods | Zn(CH3COO)2·2H2O, HMTA, absolute ethanol and distilled water. | 400 | - | 50 nm | 500 nm | [13] |
| Nanorods | 10 mL Zn(Ac)2.2H2O in 0.1 M methanol, 20 mL NaOH in 0.5 M methanol, DI water (K2SnO3,3H2O, 95%), 0.75 g of urea. | 150 | 24 h | 2.8 nm | 26 nm | [42] |
| 20 mM Zn(NO3)2 and 20 mM HMTA | 90 °C for 100 min, dried for 12 h at 60 °C and annealed 1 h at 500°C. | - | 290–330 nm | 3.2–3.4 µm |
| ZnO Morphology | UV Light (nm) | Photocurrent Iph(A) | Dark Current Idark (A) | Responsivity (A/W) | Response/Recovery Time (s) | References |
|---|---|---|---|---|---|---|
| Nanowires | 365 | 1.1 × 10−5 | ˂10−5 | - | - | [61] |
| Hemispherical bumps | 365 | 10−4 | 5 × 10−7 | - | - | [68] |
| Nanowires | 365 | 3.51 × 10−2 | 5.6 × 10−2 | - | 6.2/11 | [24] |
| Nanowires | 365 | >20 Idark | - | - | 20/40 | [72] |
| Nanorods | 300–370 | - | - | 2 | 72/110 | [23] |
| Nanowires | - | - | - | - | 4.2/4.41 and 29.32/38.86 | [74] |
| Nanorods | Sunlight | 118 Idark | - | - | 35/46 | [106] |
| Nanorods | 365 | - | - | - | 50–100/35–40 | [98] |
| Nanorods | 365 | 2.7 × 10−3 | 2 × 10−5 | 2 × 104 | - | [26] |
| Nanorods | - | 1.98 × 10−8 | 1.97 × 10−8 | 2.22 × 10−7 | 60/- | [2] |
| Nanoflowers | 184–365 | 8 × 10−4 | 10−4 | 92 | - | [32] |
| Nanowires | - | - | - | 12.4 × 10−3 | - | [110] |
| Nanowires | 365 | 2.7 × 10−6 | 1.1 × 10−6 | - | 1.18/>12.1 | [9] |
| Composite Materials | ZnO Morphology | UV Light (nm) | Photocurrent Iph (A) | Dark Current Idark (A) | Sensitivity W/(mW cm−2) | Responsivity (A/W) | Response/ Recovery Time (s) | References |
|---|---|---|---|---|---|---|---|---|
| Single-mode fiber/ZnO | nanorods | 365 | - | - | 7.096 | - | - | [89] |
| Si/ZnO | nanowires | 325 | - | - | - | 17 × 10−3 | 7 × 10−4/- | [60] |
| TiO2/ZnO | nanorods | - | 8.92 × 10−5 | 9.31 × 10−6 | - | 1.7 × 10−1 | 50/150 | [2] |
| CuO/ZnO | nanorods | 365 | 11.2 × 10−3 | 2 × 10−5 | - | 8.4 × 104 | 5/3–5 | [26] |
| Graphene Oxide/ZnO | nanowires | 365 | - | - | - | 10.13 × 103 | 11.2/81 | [8] |
| SnO2/ZnO | nanocones | 254 | - | - | - | 68 × 10−3 | - | [1] |
| Ga-doped ZnO | nanowires | 360–400 | - | - | - | 23.1 × 10−3 | 10.1/17.8 | [110] |
| In-doped ZnO | - | - | - | 34.2 × 10−3 | 10.8/13.3 | |||
| Ga+In-doped ZnO | 1.1 × 10−3 | - | - | 27.1 × 10−3 | 13.2/16.9 | |||
| Ag-doped ZnO | nanorods | 365 | - | - | 4.33 × 10−8 | - | - | [88] |
| Graphene/ZnO | nanoflakes | 365 | - | - | 4.2776 × 10−7 | - | - | [7] |
| Graphene/ZnO | nanowires | 350 | - | - | - | 1.45 × 102 | - | [107] |
| nanostars | - | - | - | 3.02 × 102 | - | |||
| nanoflowers | - | - | 3.5 × 102 | 3.5 × 102 | - | |||
| MoS2/ZnO | nanowires | - | - | - | 7.91 × 10- 6 | - | ||
| nanostars | - | - | 8.99 × 10−4 | 1.02 × 10−4 | - | |||
| nanoflowers | - | - | - | 8.99 × 10−4 | - | |||
| GaN/ZnO Graphene | nanorods | 325 | 4.6 × 10−3 | 9.73 × 10−2 | - | 1204 | 1.12/1.16 | [79] |
| Quantum dots + GaN/ZnO | 1.314 × 10−2 | 79 × 10−2 | - | 3.2 × 103 | 159/68.7 | |||
| Graphene/ZnO | nanowires | 365 | 3 × 10−6 | 2.8 × 10−7 | 10.71 | - | 1.02/0.34 | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Mawignon, F.J.; Hussain, M.; Ange, N.K.; Lu, S.; Hafezi, M.; Dong, G. Economic Friendly ZnO-Based UV Sensors Using Hydrothermal Growth: A Review. Materials 2021, 14, 4083. https://doi.org/10.3390/ma14154083
Qin L, Mawignon FJ, Hussain M, Ange NK, Lu S, Hafezi M, Dong G. Economic Friendly ZnO-Based UV Sensors Using Hydrothermal Growth: A Review. Materials. 2021; 14(15):4083. https://doi.org/10.3390/ma14154083
Chicago/Turabian StyleQin, Liguo, Fagla Jules Mawignon, Mehboob Hussain, Nsilani Kouediatouka Ange, Shan Lu, Mahshid Hafezi, and Guangneng Dong. 2021. "Economic Friendly ZnO-Based UV Sensors Using Hydrothermal Growth: A Review" Materials 14, no. 15: 4083. https://doi.org/10.3390/ma14154083
APA StyleQin, L., Mawignon, F. J., Hussain, M., Ange, N. K., Lu, S., Hafezi, M., & Dong, G. (2021). Economic Friendly ZnO-Based UV Sensors Using Hydrothermal Growth: A Review. Materials, 14(15), 4083. https://doi.org/10.3390/ma14154083