Magnetic Field-Assisted Photocatalytic Degradation of Organic Pollutants over Bi1−xRxFeO3 (R = Ce, Tb; x = 0.00, 0.05, 0.10 and 0.15) Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, L.; Mi, W. Progress in BiFeO3-Based Heterostructures: Materials, Properties and Applications. Nanoscale 2020, 12, 477–523. [Google Scholar] [CrossRef]
- Haruna, A.; Abdulkadir, I.; Idris, S.O. Photocatalytic Activity and Doping Effects of BiFeO3 Nanoparticles in Model Organic Dyes. Heliyon 2020, 6, e03237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Chen, X.Y.; Yin, K.B.; Dong, S.; Ren, Z.F.; Yuan, F.; Yu, T.; Zou, Z.G.; Liu, J.-M. Visible-Light Photocatalytic Properties of Weak Magnetic BiFeO3 Nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Gao, T.; Chen, Z.; Huang, Q.; Niu, F.; Huang, X.; Qin, L.; Huang, Y. A Review: Preparation of Bismuth Ferrite Nanoparticles and Its Applications in Visible-Light Induced Photocatalysis. Rev. Adv. Mater. Sci. 2015, 40, 97–109. [Google Scholar]
- Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. A Newly Emerging Visible Light-Responsive BiFeO3 Perovskite for Photocatalytic Applications: A Mini Review. Mater. Res. Bull. 2017, 90, 15–30. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic Materials for Environmental Applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Rong, N.; Chu, M.; Tang, Y.; Zhang, C.; Cui, X.; He, H.; Zhang, Y.; Xiao, P. Improved Photoelectrocatalytic Properties of Ti-Doped BiFeO3 Films for Water Oxidation. J. Mater. Sci. 2016, 51, 5712–5723. [Google Scholar] [CrossRef]
- Senne, J.K. Electrochemical and Nuclear Magnetic Resonance Spectroscopy Studies of Phenol Red and Related Compounds. Ph.D. Thesis, Lowa State University, Ames, IA, USA, 1969. [Google Scholar]
- Senne, J.K.; Marple, L.W. Electrochemical Reduction of Phenol Red. Anal. Chem. 1970, 42, 1147–1150. [Google Scholar] [CrossRef]
- Thanasekaran, P.; Rajendran, T.; Rajagopal, S.; Srinivasan, C.; Ramaraj, R.; Ramamurthy, P.; Venkatachalapathy, B. Marcus Inverted Region in the Photoinduced Electron Transfer Reactions of Ruthenium(II)-Polypyridine Complexes with Phenolate Ions. J. Phys. Chem. A 1997, 101, 8195–8199. [Google Scholar] [CrossRef]
- Li, C.; Hoffman, M.Z. One-Electron Redox Potentials of Phenols in Aqueous Solution. J. Phys. Chem. B 1999, 103, 6653–6656. [Google Scholar] [CrossRef]
- Sires, I.; Guivarch, E.; Oturan, N.; Oturan, M.A. Efficient Removal of Triphenylmethane Dyes from Aqueous Medium by In Situ Electrogenerated Fenton’s Reagent at Carbon-Felt Cathode. Chemosphere 2008, 72, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, W.; Chan, Y.; Leung, C.; Mak, C.; Ploss, B. Studies of Rare-Earth Doped BiFeO3 Ceramics. Int. J. Appl. Ceram. Technol. 2011, 8, 1246–1253. [Google Scholar] [CrossRef]
- Hou, Y.; Qu, J.; Zhao, X.; Lei, P.; Wan, D.; Huang, C.P. Electro-Photocatalytic Degradation of Acid Orange II Using A Novel TiO2/ACF Photoanode. Sci. Total Environ. 2009, 407, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Tomioka, M.; Matsukata, M.; Kikuchi, E. Catalytic Degradation of Ethanol in An Electric Field. Catal. Today 2009, 146, 183–187. [Google Scholar] [CrossRef]
- Jara, C.C.; Fino, D.; Spinelli, P. Bio-Refractory Organics Degradation over Semiconductor Foam under A Superimposed Electric Field. Catal. Today 2007, 124, 273–279. [Google Scholar] [CrossRef]
- Aragones, A.C.; Haworth, N.L.; Darwish, N.; Ciampi, S.; Bloomfield, N.J.; Wallace, G.G.; Diez-Perez, I.; Coote, M.L. Electrostatic Catalysis of A Diels–Alder Reaction. Nature 2016, 531, 88–91. [Google Scholar] [CrossRef]
- Yan, K.; Maark, T.A.; Khorshidi, A.; Sethuraman, V.A.; Peterson, A.A.; Guduru, P.R. The Influence of Elastic Strain on Catalytic Activity in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 6175–6181. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, S.; Tsai, C.; Li, Y.; Liu, C.; Zhao, J.; Liu, Y.; Yuan, H.; Abild-Pedersen, F.; Prinz, F.B.; et al. Direct and Continuous Strain Control of Catalysts with Tunable Battery Electrode Materials. Science 2016, 354, 1031–1036. [Google Scholar] [CrossRef]
- Horikoshi, S.; Matsubara, A.; Takayama, S.; Sato, M.; Sakai, F.; Kajitani, M.; Abe, M.; Serpone, N. Characterization of Microwave Effects on Metal-Oxide Materials: Zinc Oxide and Titanium Dioxide. Appl. Catal. B Environ. 2009, 91, 362–367. [Google Scholar] [CrossRef]
- Ai, Z.; Yang, P.; Lu, X. Degradation of 4-Chlorophenol by A Microwave Assisted Photocatalysis Method. J. Hazard. Mater. 2005, 124, 147–152. [Google Scholar] [CrossRef]
- Entezari, M.H.; Heshmati, A.; Sarafraz-yazdi, A. A Combination of Ultrasound and Inorganic Catalyst: Removal of 2-Chlorophenol from Aqueous Solution. Ultrasonics Sonochem. 2005, 12, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, J.; Kumar, P.S.S.; Anandan, S.; Grieser, F.; Ashokkumar, M. Sonophotocatalytic Degradation of Monocrotophos Using TiO2 and Fe3+. J. Hazard. Mater. 2010, 177, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Futamura, S.; Einaga, H.; Kabashima, H.; Hwan, L.Y. Synergistic Effect of Silent Discharge Plasma and Catalysts on Benzene Decomposition. Catal. Today 2004, 89, 89–95. [Google Scholar] [CrossRef]
- Hao, X.L.; Zhou, M.H.; Lei, L.C. Non-Thermal Plasma-Induced Photocatalytic Degradation of 4-Chlorophenol in Water. J. Hazard. Mater. 2007, 141, 475–482. [Google Scholar] [CrossRef]
- Steiner, U.E.; Ulrich, T. Magnetic Field Effects in Chemical Kinetics and Related Phenomena. Chem. Rev. 1989, 89, 51–147. [Google Scholar] [CrossRef] [Green Version]
- Wakasa, M.; Suda, S.; Hayashi, H.; Ishii, N.; Okano, M. Magnetic Field Effect on the Photocatalytic Reaction with Ultrafine TiO2 Particles. J. Phys. Chem. B 2004, 108, 11882–11885. [Google Scholar] [CrossRef]
- Krenke, T.; Duman, E.; Acet, M.; Wassermann, E.F.; Moya, X.; Manosa, L.; Planes, A. Inverse Magnetocaloric Effect in Ferromagnetic Ni–Mn–Sn Alloys. Nat. Mater. 2005, 4, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaauw, C.; Woude, F.V.D. Magnetic and Structural Properties of BiFeO3. J. Phys. C Solid State Phys. 1973, 6, 1422. [Google Scholar] [CrossRef]
- Dhanalakshmi, R.; Vanga, P.R.; Ashok, M.; Giridharan, N.V. The Effect of A 0.5 T Magnetic Field on the Photocatalytic Activity of Recyclable Nd-modified BiFeO3 Magnetic Catalysts. IEEE Magn. Lett. 2016, 7, 2106904. [Google Scholar] [CrossRef]
- Kiwi, J. Magnetic Field Effects on Photosensitized Electron Transfer Reactions in the Presence of TiO2 and CdS Loaded Particles. J. Phys. Chem. 1983, 87, 2274–2276. [Google Scholar] [CrossRef]
- Wakasa, M.; Ishii, N.; Okano, M. Magnetic Field Effect on Photocatalytic Decomposition Reaction of Tert-Butanol with Platinized TiO2 Particles. C. R. Chim. 2006, 9, 836–840. [Google Scholar] [CrossRef]
- Okumura, H.; Endo, S.; Joonwichien, S.; Yamasue, E.; Ishihara, K.N. Magnetic Field Effect on Heterogeneous Photocatalysis. Catal. Today 2015, 258, 634–647. [Google Scholar] [CrossRef]
- Li, J.; Pei, Q.; Wang, R.; Zhou, Y.; Zhang, Z.; Cao, Q.; Wang, D.; Mi, W.; Du, Y. Enhanced Photocatalytic Performance through Magnetic Field Boosting Carrier Transport. ACS Nano 2018, 12, 3351–3359. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, R.; Muneeswaran, M.; Vanga, P.R.; Ashok, M.; Giridharan, N.V. Photocatalytic Activity of BiFeO3 Nanoparticles Synthesized through Hydrothermal Method. AIP Conf. Proc. 2015, 1665, 130014. [Google Scholar]
- Dhanalakshmi, R.; Muneeswaran, M.; Vanga, P.R.; Ashok, M.; Giridharan, N.V. Enhanced Photocatalytic Activity of Hydrothermally Grown BiFeO3 Nanostructures and Role of Catalyst Recyclability in Photocatalysis Based on Magnetic Framework. Appl. Phys. A 2016, 122, 13. [Google Scholar] [CrossRef]
- Dhanalakshmi, R.; Muneeswaran, M.; Shalini, K.; Giridharan, N.V. Enhanced Photocatalytic Activity of La-Substituted BiFeO3 Nanostructures on the Degradation of Phenol Red. Mater. Lett. 2016, 165, 205–209. [Google Scholar] [CrossRef]
- Bhushan, B.; Wang, Z.; Tol, J.V.; Dalal, N.S.; Basumallick, A.; Vasanthacharya, N.Y.; Kumar, S.; Das, D. Tailoring the Magnetic and Optical Characteristics of Nanocrystalline BiFeO3 by Ce Doping. J. Am. Ceram. Soc. 2012, 95, 1985–1992. [Google Scholar] [CrossRef]
- Arora, M.; Kumar, M. Structural, Magnetic and Optical Properties of Ce Substituted BiFeO3 Nanoparticles. Ceram. Int. 2015, 41, 5705–5712. [Google Scholar] [CrossRef]
- Lotey, G.S.; Verma, N.K. Multiferroic Properties of Tb-Doped BiFeO3 Nanowires. J. Nanopart. Res. 2013, 15, 1553. [Google Scholar] [CrossRef]
- Simoes, A.Z.; Stojanovic, B.D.; Ramirez, M.A.; Cavalheiro, A.A.; Longo, E.; Varela, J.A. Lanthanum-Doped Bi4Ti3O12 Prepared by the Soft Chemical Method: Rietveld Analysis and Piezoelectric Properties. Ceram. Int. 2008, 34, 257–261. [Google Scholar] [CrossRef]
- Gabbasova, Z.V.; Kuz’min, M.D.; Zvezdin, A.K.; Dubenko, I.S.; Murashov, V.A.; Rakov, D.N.; Krynetsky, I.B. Bi1−xRxFeO3 (R=rare earth): A Family of Novel Magnetoelectrics. Phys. Lett. A 1991, 158, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Muneeswaran, M.; Jegatheesan, P.; Giridharan, N.V. Synthesis of Nanosized BiFeO3 Powders by Co-Precipitation Method. J. Exp. Nanosci. 2013, 8, 341–346. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Liu, J.; Yang, X.; Yu, X. Investigation of Near Infrared Reflectance by Tuning the Shape of SnO2 Nanoparticles. J. Alloy Compd. 2010, 496, 261–264. [Google Scholar] [CrossRef]
- Sakar, M.; Balakumar, S.; Saravanan, P.; Bharathkumar, S. Compliments of Confinements: Substitution and Dimension Induced Magnetic Origin and Band-Bending Mediated Photocatalytic Enhancements in Bi1−xDyxFeO3 Particulate and Fiber Nanostructure. Nanoscale 2015, 7, 10667–10679. [Google Scholar] [CrossRef] [PubMed]
- Sakar, M.; Balakumar, S.; Saravanan, P.; Bharathkumar, S. Particulates vs. Fibers: Dimension Featured Magnetic and Visible Light Driven Photocatalytic Properties of Sc Modified Multiferroic Bismuth Ferrite Nanostructures. Nanoscale 2016, 8, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Dhir, G.; Lotey, G.S.; Uniyal, P.; Verma, N.K. Size-Dependent Magnetic and Dielectric Properties of Tb-Doped BiFeO3 Nanoparticles. J. Mater. Sci. Mater. Electron. 2013, 24, 4386–4392. [Google Scholar] [CrossRef]
- Guo, R.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Enhanced Photocatalytic Activity and Ferromagnetism in Gd Doped BiFeO3 Nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhao, J.-L.; Liang, Y. Degradation kinetics of phenol by a titanium dioxide photocatalyst coupled with a magnetic field. React. Kinet. Mech. Catal. 2013, 109, 273–283. [Google Scholar] [CrossRef]
- Gladkikh, V.S.; Burshtein, A.I. Double-Channel Recombination of the Radical Pairs via Incoherent Δg-Mechanism of Spin-Conversion. Chem. Phys. 2006, 323, 351–357. [Google Scholar] [CrossRef]
- Veselov, A.V.; Melekhov, V.I.; Anisimov, O.A.; Molin, Y.N. The Induction of Quantum Beats by the Δg mechanism in Radical ion Pair Recombination. Chem. Phys. Lett. 1987, 136, 263–266. [Google Scholar] [CrossRef]
- Nagakura, S.; Hayashi, H.; Azumi, T. Dynamic Spin Chemistry: Magnetic Controls and Spin Dynamics of Chemical Reactions, 1st ed.; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Wu, J.M.; Zhang, T.W. Photodegradation of Rhodamine B in Water Assisted by Titania Films Prepared through A Novel Procedure. J. Photochem. Photobiol. A Chem. 2004, 162, 171–177. [Google Scholar] [CrossRef]

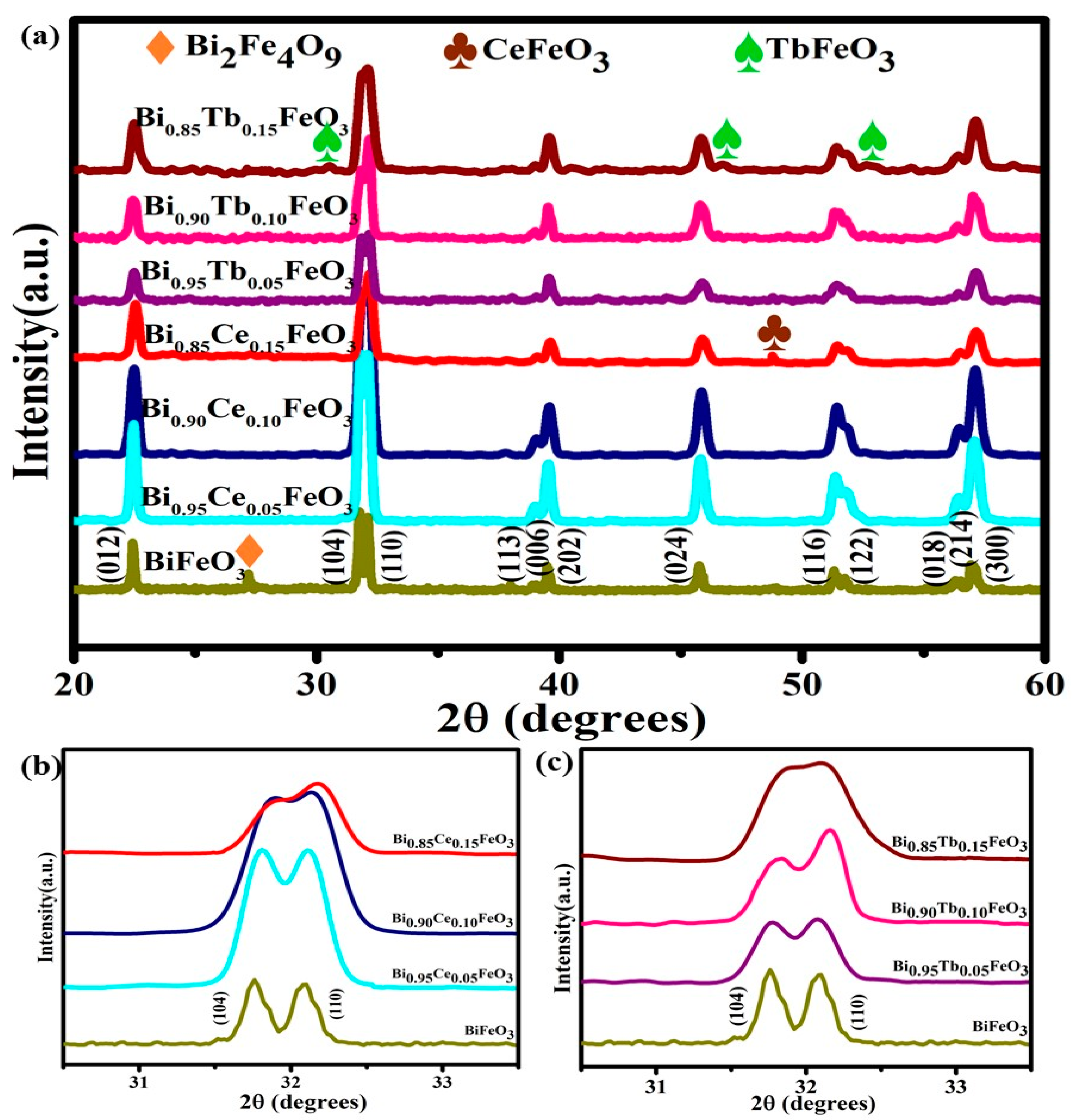
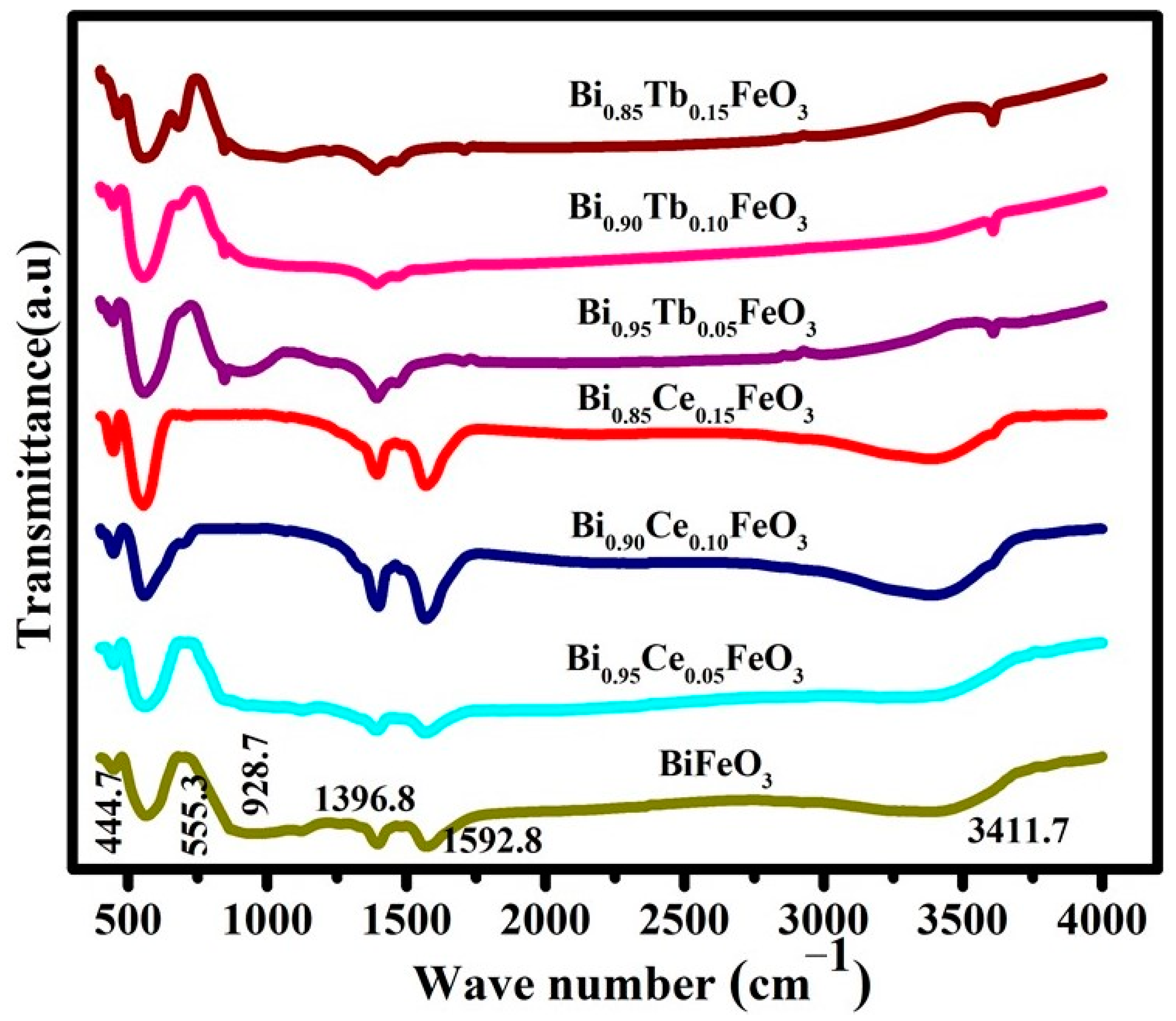
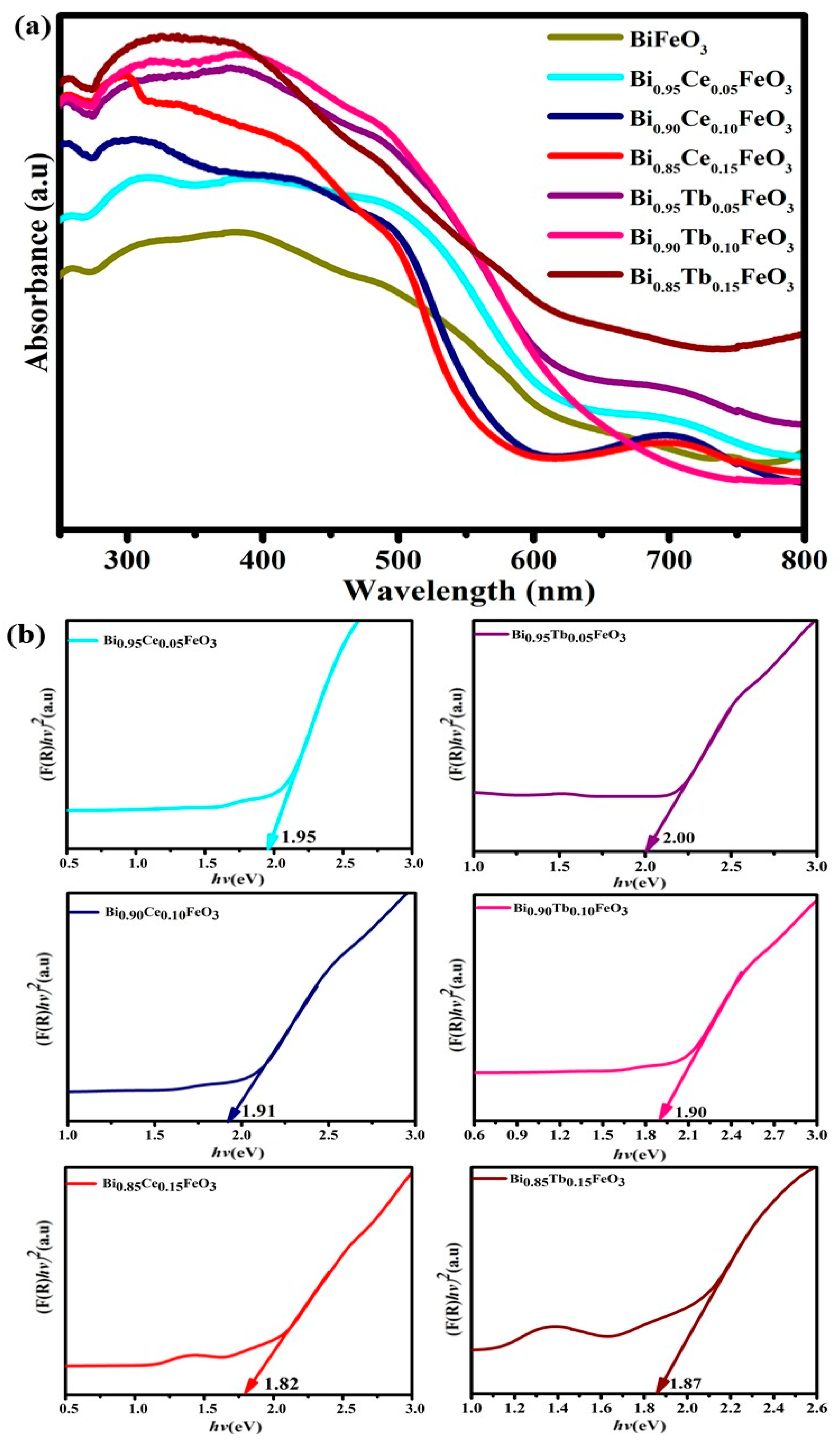

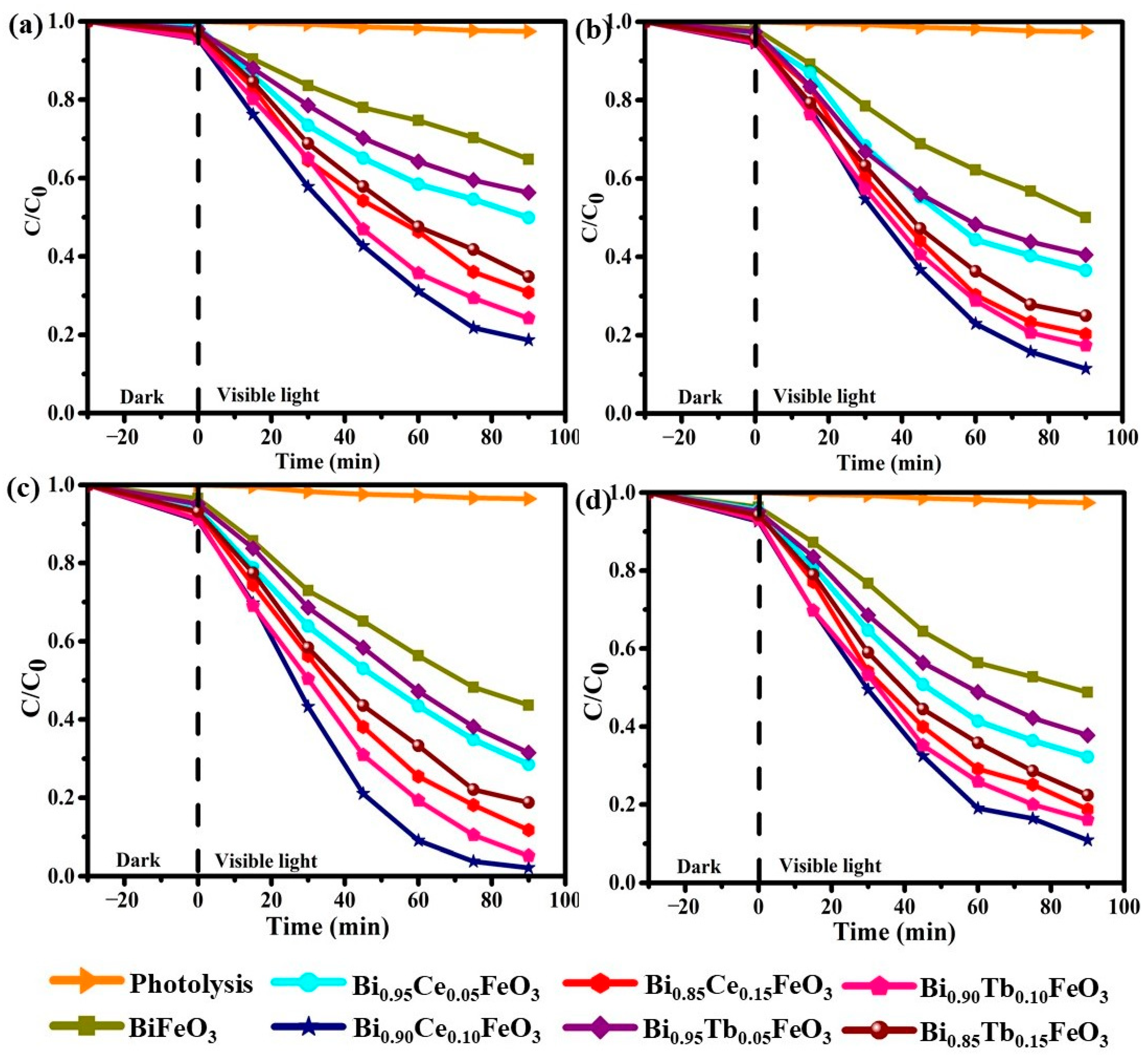

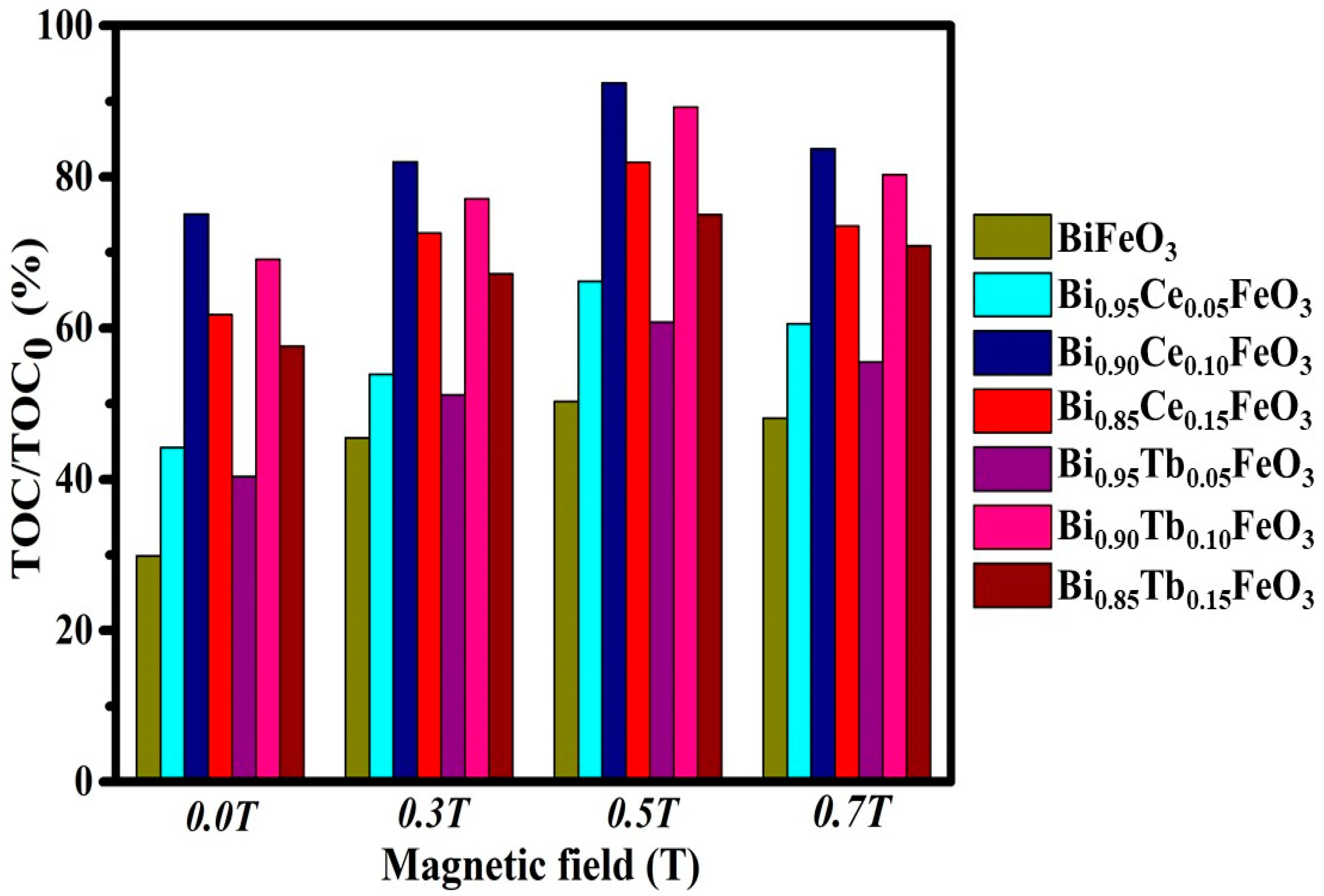
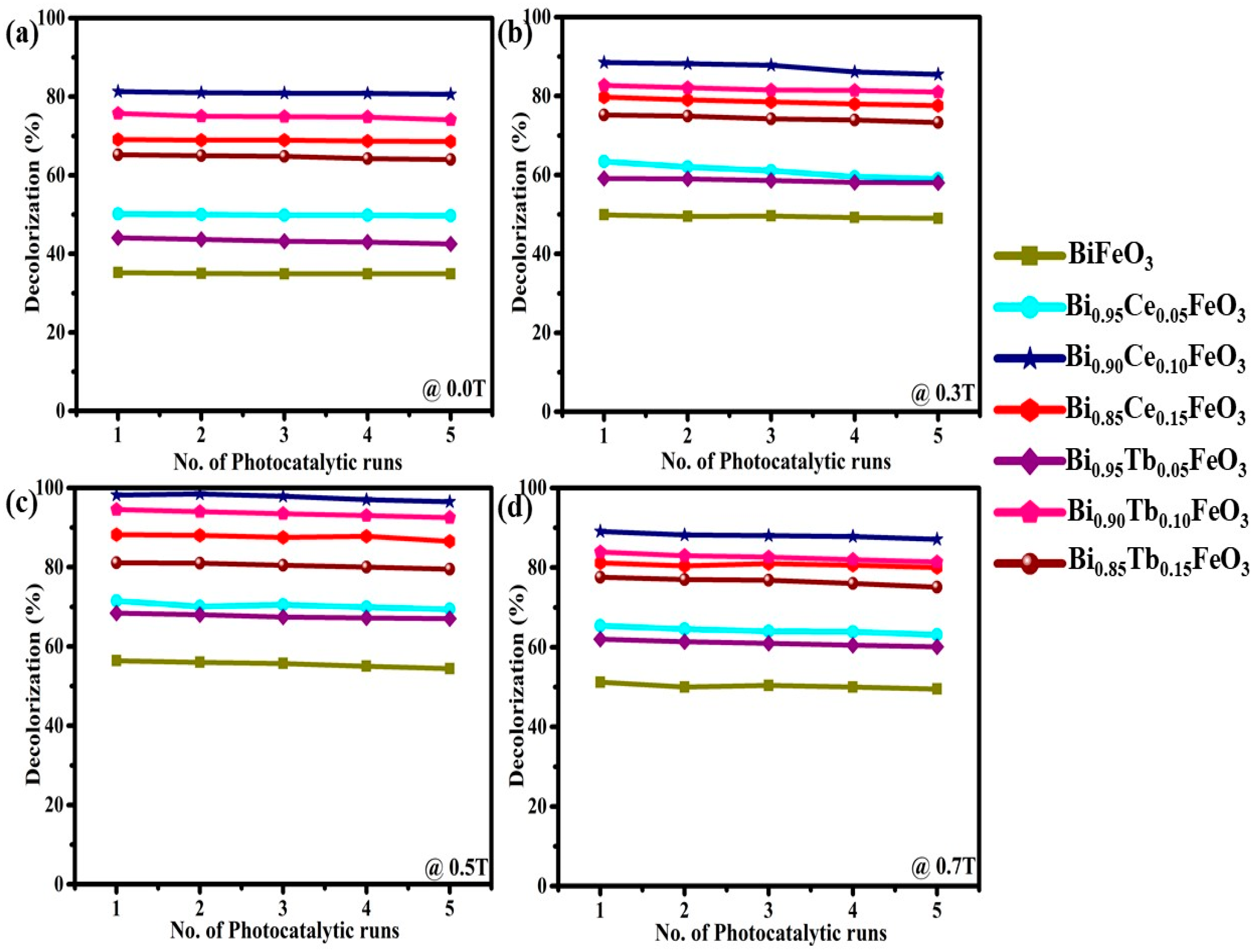
| Sample | Photodegradation Efficiencies (%) | Mineralization Efficiencies (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| @0.0T | @0.3T | @0.5T | @0.7T | @0.0T | @0.3T | @0.5T | @0.7T | |
| BiFeO3 | 35.2 | 49.9 | 56.4 | 51.2 | 29.9 | 45.5 | 50.3 | 48.1 |
| Bi0.95Ce0.05FeO3 | 50.2 | 63.4 | 71.5 | 67.8 | 44.2 | 53.9 | 66.2 | 60.6 |
| Bi0.90Ce0.10FeO3 | 81.3 | 88.5 | 97.8 | 89.1 | 75.1 | 82.0 | 92.4 | 83.7 |
| Bi0.85Ce0.15FeO3 | 69.1 | 79.7 | 88.2 | 81.2 | 61.8 | 72.6 | 81.9 | 73.5 |
| Bi0.95Tb0.05FeO3 | 43.7 | 59.4 | 68.5 | 62.2 | 40.4 | 51.2 | 60.8 | 55.5 |
| Bi0.90Tb0.10FeO3 | 75.7 | 82.6 | 94.8 | 85.9 | 69.1 | 77.1 | 89.2 | 80.3 |
| Bi0.85Tb0.15FeO3 | 65.1 | 75.0 | 81.2 | 77.6 | 57.6 | 67.2 | 75.0 | 70.9 |
| Sample | @0.0T | @0.3T | @0.5T | @0.7T | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | k (min−1 × 10−3) | R2 | k (min−1 × 10−3) | R2 | k (min−1 × 10−3) | R2 | k (min−1 × 10−3) | |
| BiFeO3 | 0.98 | 4.5 | 0.99 | 7.6 | 0.99 | 9.2 | 0.99 | 8.1 |
| Bi0.95Ce0.05FeO3 | 0.97 | 7.6 | 0.98 | 11.6 | 0.99 | 13.7 | 0.99 | 12.9 |
| Bi0.90Ce0.10FeO3 | 0.99 | 19.4 | 0.99 | 24.7 | 0.99 | 43.8 | 0.99 | 28.7 |
| Bi0.85Ce0.15FeO3 | 0.99 | 13.1 | 0.99 | 18.4 | 0.99 | 23.9 | 0.98 | 19.0 |
| Bi0.95Tb0.05FeO3 | 0.99 | 7.2 | 0.98 | 10.0 | 0.99 | 13.0 | 0.99 | 11.4 |
| Bi0.90Tb0.10FeO3 | 0.99 | 18.3 | 0.99 | 22.4 | 0.99 | 35.4 | 0.97 | 23.6 |
| Bi0.85Tb0.15FeO3 | 0.99 | 12.2 | 0.99 | 16.8 | 0.99 | 19.3 | 0.98 | 17.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhanalakshmi, R.; Giridharan, N.V.; Denardin, J.C. Magnetic Field-Assisted Photocatalytic Degradation of Organic Pollutants over Bi1−xRxFeO3 (R = Ce, Tb; x = 0.00, 0.05, 0.10 and 0.15) Nanostructures. Materials 2021, 14, 4079. https://doi.org/10.3390/ma14154079
Dhanalakshmi R, Giridharan NV, Denardin JC. Magnetic Field-Assisted Photocatalytic Degradation of Organic Pollutants over Bi1−xRxFeO3 (R = Ce, Tb; x = 0.00, 0.05, 0.10 and 0.15) Nanostructures. Materials. 2021; 14(15):4079. https://doi.org/10.3390/ma14154079
Chicago/Turabian StyleDhanalakshmi, Radhalayam, Nambi Venkatesan Giridharan, and Juliano C. Denardin. 2021. "Magnetic Field-Assisted Photocatalytic Degradation of Organic Pollutants over Bi1−xRxFeO3 (R = Ce, Tb; x = 0.00, 0.05, 0.10 and 0.15) Nanostructures" Materials 14, no. 15: 4079. https://doi.org/10.3390/ma14154079
APA StyleDhanalakshmi, R., Giridharan, N. V., & Denardin, J. C. (2021). Magnetic Field-Assisted Photocatalytic Degradation of Organic Pollutants over Bi1−xRxFeO3 (R = Ce, Tb; x = 0.00, 0.05, 0.10 and 0.15) Nanostructures. Materials, 14(15), 4079. https://doi.org/10.3390/ma14154079






