Nano Zero Valent Iron (nZVI) as an Amendment for Phytostabilization of Highly Multi-PTE Contaminated Soil
Abstract
1. Introduction,
2. Materials and Methods
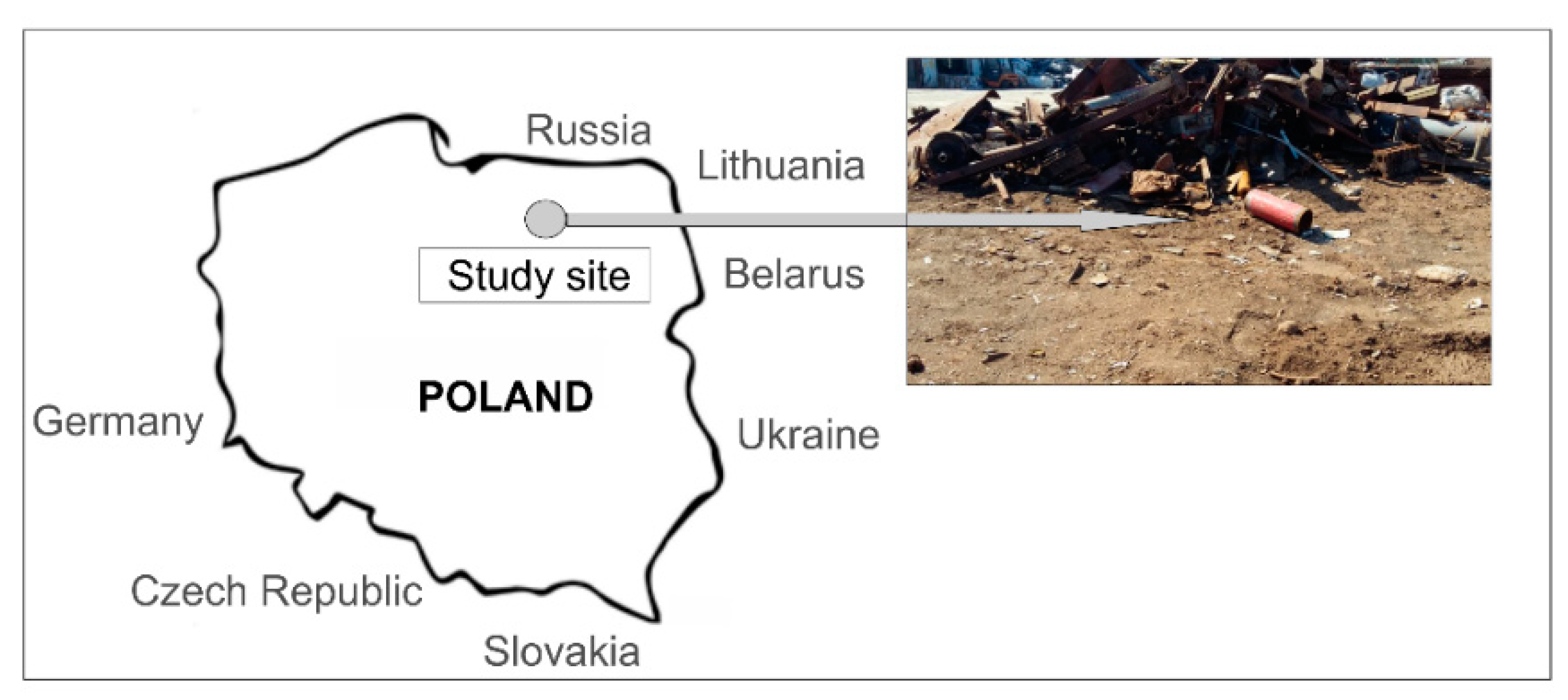
2.1. Study Site and Soil Sampling Procedure
2.2. Experiment Description
2.3. Soil Analysis
2.4. Analysis of Plant Biomass
2.5. Statistical Analysis
3. Results and Discussion
3.1. Initial Soil Characterization
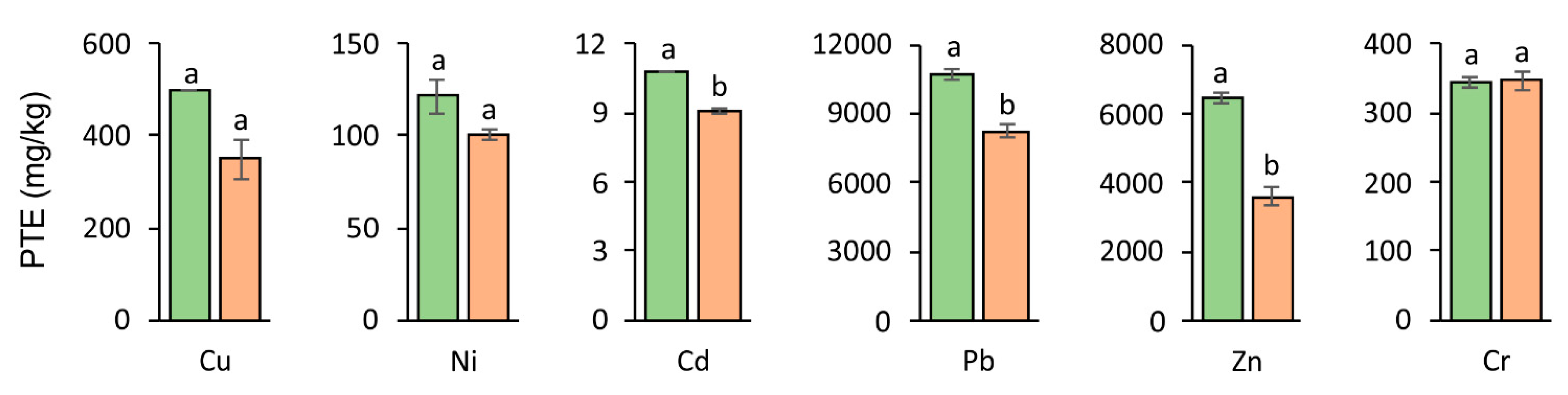
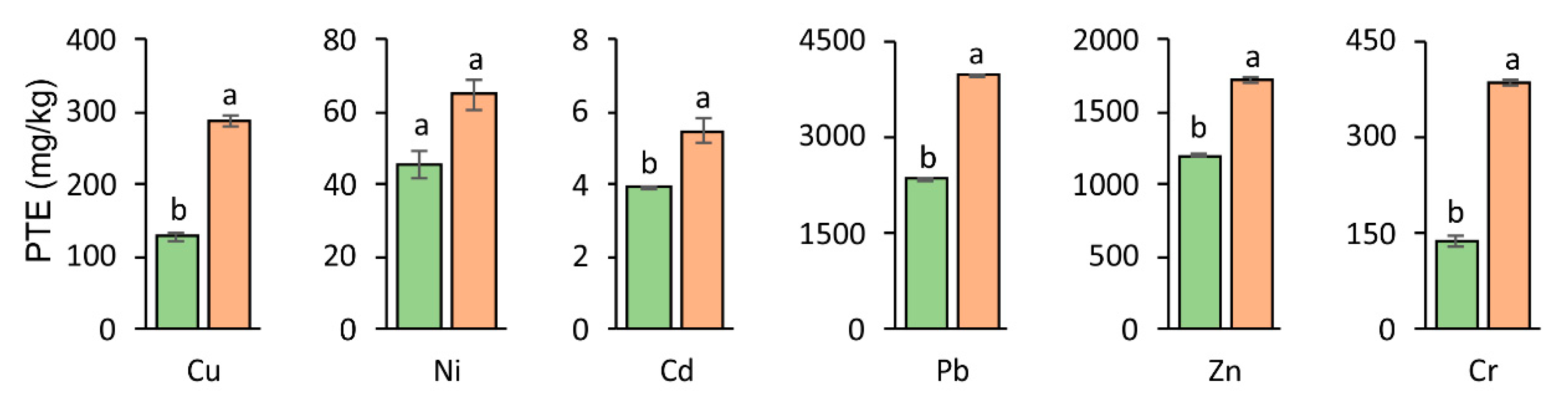
3.2. Accumulation of PTEs in Soil After Experiment
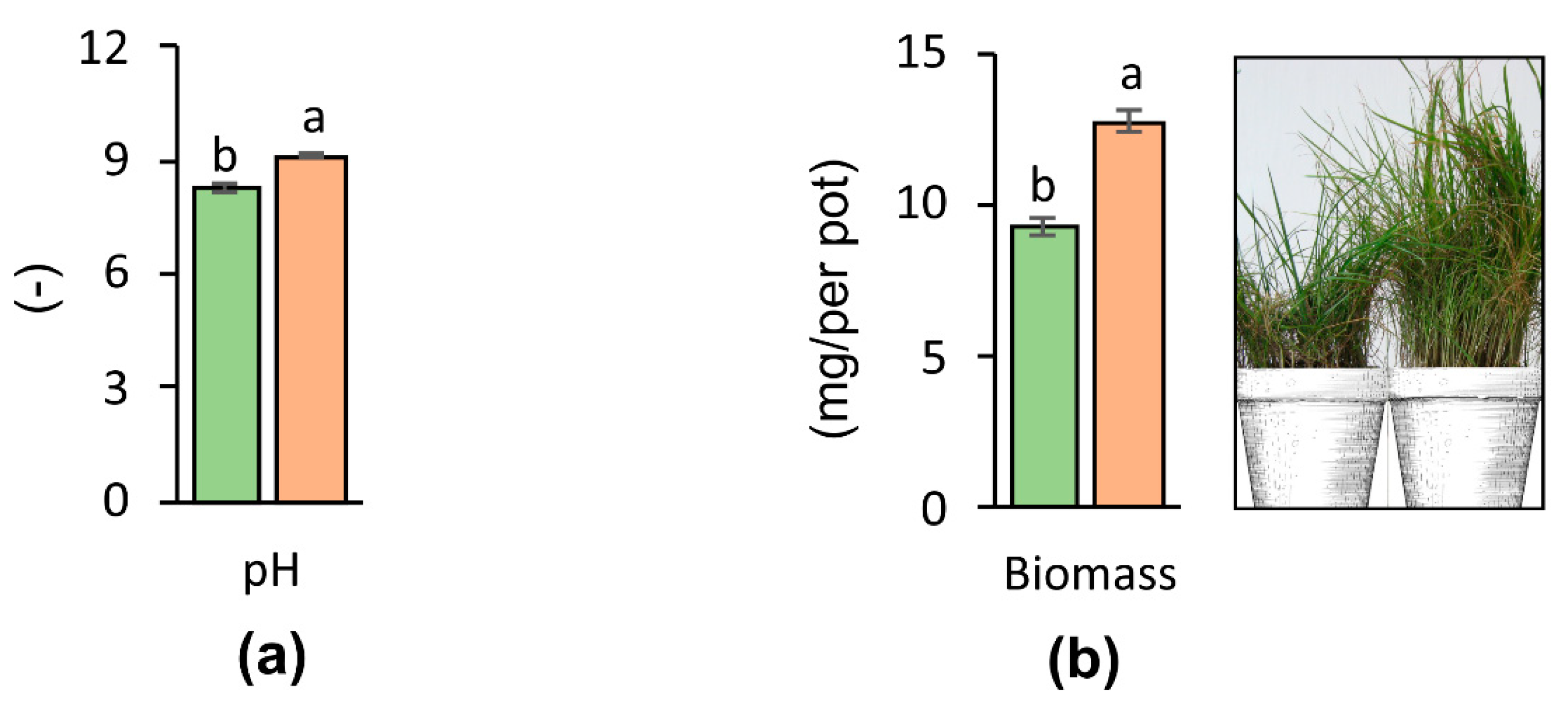
3.3. Soil pH After Application on nZVI
3.4. Effect of nZVI on the Plant Biomass Yield
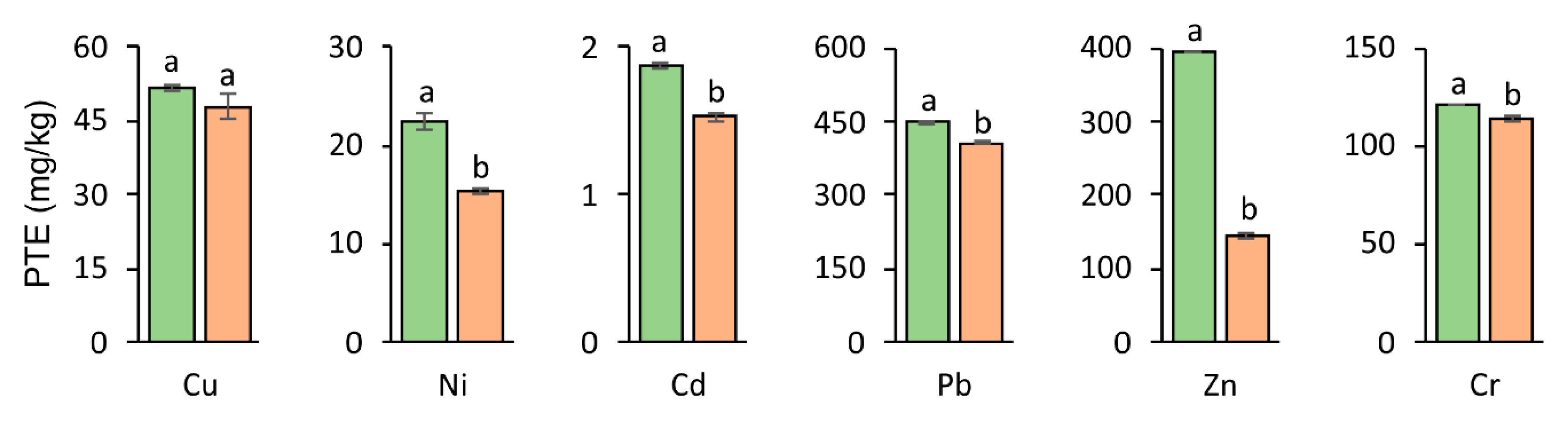
3.5. PTEs Accumulation in Aboveground Parts and Roots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nascimento, C.M.; de Sousa Mendes, W.; Silvero, N.E.Q.; Poppiel, R.R.; Sayão, W.M.; Dotto, A.C.; Santos, N.V.; Amorim, M.T.A.; Demattê, J.A.M. Soil degradation index developed by multitemporal remote sensing images, climate variables, terrain and soil attributes. J. Environ. Manag. 2021, 277. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Comino, J.; López-Vicente, M.; Kumar, V.; Rodríguez-Seijo, A.; Valkó, O.; Rojas, C.; Pourghasemi, H.R.; Salvati, L.; Bakr, N.; Vaudour, E.; et al. Soil science challenges in a new era: A transdisciplinary overview of relevant topics. Air Soil Water Res. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Mazur, Z.; Radziemska, M.; Fronczyk, J.; Jeznach, J. Heavy metal accumulation in bioindicators of pollution in urban areas of northeastern Poland. Fresenius Environ. Bull. 2015, 24, 216–223. [Google Scholar]
- Lebrun, M.; Miard, F.; Nandillon, R.; Hattab-Hambli, N.; Léger, J.C.; Scippa, G.S.; Morabito, D.; Bourgerie, S. Influence of biochar particle size and concentration on Pb and As availability in contaminated mining soil and phytoremediation potential of poplar assessed in a Mesocosm Experiment. Water Air Soil Pollut. 2021, 232, 3. [Google Scholar] [CrossRef]
- Liao, S.; Jin, G.; Khan, M.A.; Zhu, Y.; Duan, L.; Luo, W.; Jia, J.; Zhong, B.; Ma, J.; Ye, Z.; et al. The quantitative source apportionment of heavy metals in peri-urban agricultural soils with UNMIX and input fluxes analysis. Environ. Technol. Innov. 2020, 21, 101232. [Google Scholar] [CrossRef]
- Tan, K.; Ma, W.; Chen, L.; Wang, H.; Du, Q.; Du, P.; Yan, B.; Liu, R.; Li, H. Estimating the distribution trend of soil heavy metals in mining area from HyMap airborne hyperspectral imagery based on ensemble learning. J. Hazard. Mater. 2021, 401, 123288. [Google Scholar] [CrossRef] [PubMed]
- Radziemska, M.; Mazur, Z.; Fronczyk, J.; Jeznach, J. Effect of zeolite and halloysite on accumulation of trace elements in maize (Zea mays L.) in nickel contaminated soil. Fresenius Environ. Bull. 2014, 23, 3140–3146. [Google Scholar]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Resour. 2018, 147, 440–460. [Google Scholar] [CrossRef]
- Tiodar, E.D.; Văcar, C.L.; Podar, D. Phytoremediation and microorganisms-assisted phytoremediation of mercury-contaminated soils: Challenges and perspectives. Int. J. Environ. Res. Public Health 2021, 18, 2435. [Google Scholar] [CrossRef]
- Xie, L.; van Zyl, D. Distinguishing reclamation, revegetation and phytoremediation, and the importance of geochemical processes in the reclamation of sulfidic mine tailings: A review. Chemosphere 2020, 252, 126446. [Google Scholar] [CrossRef]
- Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Arsenic and iron speciation and mobilization during phytostabilization of pyritic mine tailings. Geochim. Cosmochim. Acta 2020, 286, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, M.; Peuble, S.; Pereira, F.; Paran, F.; Moutte, J.; Menad, N.; Faure, O. Aided-Phytostabilization of steel slag dumps: The key-role of pH adjustment in decreasing chromium toxicity and improving manganese, phosphorus and zinc phytoavailability. J. Hazard. Mater. 2020, 405, 124225. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowski, M.; Radziemska, M. The effect of chromium content in soil on the concentration of some mineral elements in plants. Fresenius Environ. Bull. 2009, 18, 1039–1045. [Google Scholar]
- Zhan, J.; Huang, H.; Yu, H.; Zhang, X.; Zheng, Z.; Wang, Y.; Liu, T.; Li, T. The combined effects of Cd and Pb enhanced metal binding by root cell walls of the phytostabilizer Athyrium wardii (Hook.). Environ. Pollut. 2020, 258, 13663. [Google Scholar] [CrossRef] [PubMed]
- Wetle, R.; Bensko-Tarsitano, B.; Johnson, K.; Sweat, K.G.; Cahill, T. Uptake of uranium into desert plants in an abandoned uranium mine and its implications for phytostabilization strategies. J. Environ. Radioact. 2020, 220–221, 106293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Gao, B.; Zhang, M. Stimulation of peanut seedling development and growth by zero-valent iron nanoparticles at low concentrations. PLoS ONE 2015, 10, e0122884. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, B. Hysiological effects of magnetite (Fe3O4) nanoparticleson perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2011, 5, 30–42. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wan, J.; Zhang, C.; Cheng, M.; Qin, X.; Xue, W. Stabilized nanoscale zerovalent Iron mediated cadmium accumulation and oxidative damage of Boehmeria nivea (L.) Gaudich cultivated in cadmium contaminated sediments. Environ. Sci. Technol. 2017, 51, 11308–11316. [Google Scholar] [CrossRef]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef]
- Teodoro, M.; Clemente, R.; Ferrer-Bustins, E.; Martínez-Fernández, D.; Pilar Bernal, M.; Vítková, M.; Vítek, P.; Komárek, M. Nanoscale zero-valent iron has minimum toxicological risk on the germination and early growth of two grass species with potential for phytostabilization. Nanomaterials 2020, 10, 1537. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Pinilla, P.; Alonso, J.; Lobo, M. Viability of a nanoremediation process in single or multi-metal (loid) contaminated soils. J. Hazard. Mater. 2017, 321, 812–819. [Google Scholar] [CrossRef]
- Zand, A.D.; Tabrizi, A.M.; Heir, A.V. The influence of association of plant growth-promoting rhizobacteria and zero-valent iron nanoparticles on removal of antimony from soil by Trifolium repens. Environ. Sci. Pollut. Res. 2020, 27, 42815–42829. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Diego, B.; Rubén, F.; Lorena, W. Nanoremediation of As and metals polluted soils by means of graphene oxide nanoparticles. Sci. Rep. 2020, 10, 1896. [Google Scholar]
- Polish Ministry of the Environment. Ordinance of the Minister of Environment on Soil and Ground Quality Standards; Jew Lawyer; Polish Ministry of the Environment: Warsaw, Poland, 2016; Volume 395, pp. 1–86. (In Polish) [Google Scholar]
- Radziemska, M.; Bęś, A.; Gusiatin, Z.M.; Cerda, A.; Mazur, Z.; Jeznach, J.; Kowal, P.; Brtnický, M. The combined effect of phytostabilization and different amendments on remediation of soils from post-military areas. Sci. Total Environ. 2019, 688, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Baragaño, D.; Alonso, J.; Gallego, J.R.; Lobo, M.C.; Gil-Díaz, M. Zero valent iron and goethite nanoparticles as new promising remediation techniques for As-polluted soils. Chemosphere 2020, 238, 124624. [Google Scholar] [CrossRef] [PubMed]
- Baragaño, D.; Forján, R.; Fernández, B.; Ayala, J.; Afif, E.; Gallego, J.L.R. Application of biochar, compost and ZVI nanoparticles for the remediation of As, Cu, Pb and Zn polluted soil. Environ. Sci. Pollut. Res. 2020, 27, 33681–33691. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, K. Charakterystyka właściwości mechanicznych wybranych materiałów reaktywnych. Acta Sci. Pol. Archit. 2015, 14, 57–66. (In Polish) [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 9 March 2021).
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.M.; Yang, W.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Jia, F.; Ai, Z.; Zhang, L. Iron oxide shell mediated environmental remediation properties of nano zero-valent iron. Environ. Sci. Nano 2016, 4, 27–45. [Google Scholar] [CrossRef]
- Kim, J.S.; Shea, P.J.; Yang, J.E.; Kim, J.E. Halide salts accelerate degradation of high explosives by zero-valent iron. Environ. Pollut. 2007, 147, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Lavine, B.K.; Auslander, G.; Ritter, J. Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem. J. 2001, 70, 69–83. [Google Scholar] [CrossRef]
- Mukherjee, R.; Kumar, R.; Sinha, A.; Lama, Y.; Saha, A.K. A review on synthesis, characterization and applications of nano zero valent iron (nZVI) for environmental remediation. Crit. Rev. Environ. Sci. Technol. 2016, 46, 443–466. [Google Scholar] [CrossRef]
- Agrelli, D.; Caporale, A.G.; Adamo, P. Assessment of the bioavailability and speciation of heavy metal(loid)s and hydrocarbons for risk-based soil remediation. Agronomy 2020, 10, 1440. [Google Scholar] [CrossRef]
- Hijazin, T.; Radwan, A.; Lewerenz, L.; Abouzeid, S.; Selmar, D. The uptake of alkaloids by plants from the soil is determined by rhizosphere pH. Rhizosphere 2020, 15, 100234. [Google Scholar] [CrossRef]
- Romdhane, L.; Panozzo, A.; Radhouane, L.; Dal Cortivo, C.; Barion, G.; Vamerali, T. Root characteristics and metal uptake of maize (Zea mays L.) under extreme soil contamination. Agronomy 2021, 11, 178. [Google Scholar] [CrossRef]
- Lu, H.L.; Nkoh, N.J.; Abdulaha-Al Baquy, M.; Dong, G.; Li, J.Y.; Xu, R.K. Plants alter surface charge and functional groups of their roots to adapt to acidic soil conditions. Environ. Pollut. 2020, 267, 115590. [Google Scholar] [CrossRef] [PubMed]
- Gulio, C.; Camelin, E.; Tommasi, T.; Fino, D.; Pugliese, M. Anaerobic digestates from sewage sludge used as fertilizer on a poor alkaline sandy soil and on a peat substrate: Effects on tomato plants growth and on soil properties. J. Environ. Manag. 2020, 269, 110767. [Google Scholar]
- Qiao, J.; Liu, T.; Wang, X.; Li, F.; Lv, Y.; Cui, J.; Zeng, X.; Yuan, Y.; Liu, C. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X. Bamboo—An untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere 2020, 246, 125750. [Google Scholar] [CrossRef]
- Cui, H.; Li, H.; Zhang, S.; Yi, Q.; Zhou, J.; Fang, G.; Zhou, J. Bioavailability and mobility of copper and cadmium in polluted soil after phytostabilization using different plants aided by limestone. Chemosphere 2020, 242, 125252. [Google Scholar] [CrossRef]
- Libralato, G.; Devoti, C.A.; Zanella, M.; Sabbioni, E.; Mičetić, I.; Manodori, L.; Pigozzo, A.; Manenti, S.; Groppi, F.; Ghirardini, V.A. Phytotoxicity of ionic, micro- and nano-sized iron in three plant species. Ecotoxicol. Environ. Saf. 2016, 123, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cheng, W.; Tsang, P.E.; Fang, Z. Remediation and phytotoxicity of decabromodiphenyl ether contaminated soil by zero valent iron nanoparticles immobilized in mesoporous silica microspheres. J. Environ. Manag. 2016, 166, 478–483. [Google Scholar] [CrossRef]
- Trujillo-Reyes, J.; Majumdar, S.; Botez, C.; Peralta-Videa, J.; Gardea-Torresdey, J. Exposure studies of core-shell Fe/Fe3O4 and Cu/CuO NPs to lettuce (Lactuca sativa) plants: Are they a potential physiological and nutritional hazard? J. Hazard. Mater. 2014, 267, 255–263. [Google Scholar] [CrossRef]
- Iannone, M.F.; Groppa, M.D.; de Sousa, M.E.; van Raap, M.B.F.; Benavides, M.P. Impact of magnetite ironoxide nanoparticles on wheat (Triticum aestivum L.) development: Evaluation of oxidative damage. Environ Exp. Bot. 2016, 131, 77–88. [Google Scholar] [CrossRef]
- Yoon, H.; Kang, Y.G.; Chang, Y.S.; Kim, J.H. Effects of zerovalent iron nanoparticles on photosynthesis and biochemical adaptation of soil-grown Arabidopsis thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Zine, H.; Elgadi, S.; Hakkou, R.; Papazoglou, E.G.; Midhat, L.; Ouhammou, A. Wild plants for the phytostabilization of phosphate mine waste in semi-arid environments: A field experiment. Minerals 2021, 11, 42. [Google Scholar] [CrossRef]
- Bravin, M.N.; Garnier, C.; Lenoble, V.; Gérard, F.; Dudal, Y.; Hinsinger, P. Root induced changes in pH and dissolved organic matter binding capacity affect copper dynamic speciation in the rhizosphere. Geochim. Cosmochim. Acta 2012, 84, 256–268. [Google Scholar] [CrossRef]
- Kim, K.R.; Owens, G.; Kwon, S. Influence of Indian mustard (Brassica juncea) on rhizosphere soil solution chemistry in long-term contaminated soils: A rhizobox study. J. Environ. Sci. 2010, 22, 98–105. [Google Scholar] [CrossRef]
- Vítková, M.; Puschenreiter, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal (loid) contaminated soils. Chemosphere 2018, 200, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernandez, D.; Komarek, M. Comparative effects of nanoscale zerovalent iron (nZVI) and Fe2O3 nanoparticles on root hydraulic conductivity of Solanum lycopersicum L. Environ. Exp. Bot. 2016, 131, 128–136. [Google Scholar] [CrossRef]
- Hidalgo, K.T.S.; Carrion-Huertas, P.J.; Kinch, R.T.; Betancourt, L.E.; Cabrera, C.R. Phytonanoremediation by Avicennia germinans (black mangrove) and nano zero valent iron for heavy metal uptake from Cienaga Las Cucharillas wetland soils. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100363. [Google Scholar]
- Gil-Diaz, M.; Diez-Pascuala, S.; González, A.; Alonso, J.; Rodríguez-Valdés, E.; Gallego, J.R.; Lobo, M.C. A nanoremediation strategy for the recovery of an As-polluted soil. Chemosphere 2016, 149, 137–145. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Unit | Value (Mean ± SD) | National Limit a |
|---|---|---|---|
| Cu | mg/kg | 671.1 ± 78.5 | 600 |
| Ni | mg/kg | 129.3 ± 26.7 | 300 |
| Cd | mg/kg | 22.4 ± 2.52 | 15 |
| Pb | mg/kg | 13,479 ± 669.6 | 600 |
| Zn | mg/kg | 8433 ± 1376.5 | 1000 |
| Cr | mg/kg | 602.4 ± 11.4 | 500 |
| Sample Type | Cu | Ni | Cd | Pb | Zn | Cr | |
|---|---|---|---|---|---|---|---|
| Soil | 0.07 | 0.18 | 0.003 ** | 0.02 * | 0.01 * | 0.87 | |
| Aboveground biomass | 0.28 | 0.02 * | 0.01 * | 0.007 ** | <0.001 *** | 0.04 * | |
| Roots | 0.003 ** | 0.08 | 0.03 * | <0.001 *** | <0.001 *** | <0.001 *** | |
| * statistically significant difference at 0.05 significance level | |||||||
| ** statistically significant difference at 0.01 significance level | |||||||
| *** statistically significant difference at 0.001 significance level | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radziemska, M.; Gusiatin, Z.M.; Holatko, J.; Hammerschmiedt, T.; Głuchowski, A.; Mizerski, A.; Jaskulska, I.; Baltazar, T.; Kintl, A.; Jaskulski, D.; et al. Nano Zero Valent Iron (nZVI) as an Amendment for Phytostabilization of Highly Multi-PTE Contaminated Soil. Materials 2021, 14, 2559. https://doi.org/10.3390/ma14102559
Radziemska M, Gusiatin ZM, Holatko J, Hammerschmiedt T, Głuchowski A, Mizerski A, Jaskulska I, Baltazar T, Kintl A, Jaskulski D, et al. Nano Zero Valent Iron (nZVI) as an Amendment for Phytostabilization of Highly Multi-PTE Contaminated Soil. Materials. 2021; 14(10):2559. https://doi.org/10.3390/ma14102559
Chicago/Turabian StyleRadziemska, Maja, Zygmunt M. Gusiatin, Jiri Holatko, Tereza Hammerschmiedt, Andrzej Głuchowski, Andrzej Mizerski, Iwona Jaskulska, Tivadar Baltazar, Antonin Kintl, Dariusz Jaskulski, and et al. 2021. "Nano Zero Valent Iron (nZVI) as an Amendment for Phytostabilization of Highly Multi-PTE Contaminated Soil" Materials 14, no. 10: 2559. https://doi.org/10.3390/ma14102559
APA StyleRadziemska, M., Gusiatin, Z. M., Holatko, J., Hammerschmiedt, T., Głuchowski, A., Mizerski, A., Jaskulska, I., Baltazar, T., Kintl, A., Jaskulski, D., & Brtnicky, M. (2021). Nano Zero Valent Iron (nZVI) as an Amendment for Phytostabilization of Highly Multi-PTE Contaminated Soil. Materials, 14(10), 2559. https://doi.org/10.3390/ma14102559









